Abstract
Background
Promising efficacy and manageable toxicity of docetaxel-based concurrent chemoradiotherapy (CCRT) were reported in head and neck cancer. In addition, the effect of CCRT in combination with cisplatin and/or 5-fluorouracil on both locoregionally advanced and metastatic/recurrent nasopharyngeal carcinoma (NPC) was verified. However, CCRT with docetaxel for locoregionally advanced NPC are not well studied. This study aimed to compare effectiveness and toxicities of CCRT with weekly docetaxel versus tri-weekly cisplatin for locoregionally advanced NPC.
Methods
Clinical data of patients with locoregionally advanced NPC newly diagnosed between January 2010 and December 2014 receiving CCRT with either weekly docetaxel (15 mg/m2) or tri-weekly cisplatin (80–100 mg/m2) were reviewed. Propensity score matching at a 1:1 ratio was performed to balance baseline characteristics. Adverse events and survival were compared between the two groups.
Results
A total of 962 patients were included as the whole cohort, and 448 patients were matched and were regarded as the matched cohort. The median follow-up duration was 48 months for the whole cohort. The 3-year nodal recurrence-free survival rate was significantly increased for patients treated with docetaxel in both the whole (hazard ratio [HR] = 0.37, 95% confidence interval [CI] 0.19–0.72, P = 0.030) and matched cohorts (HR = 0.33, 95% CI 0.14–0.79, P = 0.023). However, no significant differences were observed in overall survival, local recurrence-free survival, and distant metastasis-free survival between the two groups in both cohorts. Significantly higher rates of grade 3 radiodermatitis (6.7% vs. 1.8%, P = 0.001), mucositis (74.5% vs. 37.9%, P < 0.001), and leucopenia (2.2% vs. 11.6%, P < 0.001) were observed in the docetaxel group, but any grade of renal injury (1.8% vs. 15.1%, P < 0.001), vomiting (18.8% vs. 88.3%, P < 0.001), and ALT elevation (19.2% vs. 31.3%, P = 0.027) were more common in the cisplatin group.
Conclusions
CCRT with weekly low-dose docetaxel is an effective and tolerable therapeutic regimen for locally advanced NPC. It provides a survival benefit mainly by improving the control of regional lymph node metastases, especially for patients with low pretreatment EBV DNA levels.
Electronic supplementary material
The online version of this article (10.1186/s40880-019-0380-x) contains supplementary material, which is available to authorized users.
Keywords: Concurrent chemoradiotherapy, Docetaxel, Cisplatin, Nasopharyngeal carcinoma, Propensity score matching, Intensity-modulated radiotherapy, Overall survival, Distant metastasis-free survival, Locoregional recurrence-free survival, Nodal recurrence-free survival
Background
Nasopharyngeal carcinoma (NPC) is a fairly prevalent head and neck cancer in South China [1, 2]. Radiotherapy is the only radical treatment for NPC because of its anatomical limitations and hypersensitivity to radiotherapy [3]. Compared with two-dimensional conventional radiotherapy (2D-CRT), intensity-modulated radiotherapy (IMRT) has distinct advantages of better target coverage and risk-sparing of organs, and thus has been recognized as a more sophisticated treatment.
Benefits of cisplatin-based concurrent chemoradiotherapy (CCRT) for locally advanced NPC have been repeatedly verified by a clinical trial [4] and a meta-analysis [5] since the publication of the Intergroup 0099 trial [6]. Therefore, tri-weekly high-dose cisplatin together with radiotherapy has been currently considered the standard therapeutic strategy for locally advanced NPC. However, high-dose cisplatin is frequently associated with severe vomiting, ototoxicity, and renal dysfunction [7, 8], and only about 60% of patients were able to complete three planned treatment cycles in a clinical trial [9]. As such, the selection of patients with adequate renal function as candidates for high-dose cisplatin chemotherapy is important.
Docetaxel is a taxoid-class semisynthetic agent that promotes tubulin polymerization and the assembly of stable microtubules. In addition to cytotoxic activity, docetaxel has radiosensitizing activity which can arrest the cell cycle at the G2/M phase in highly radiosensitive cells [10, 11]. Promising activity and manageable toxicity of docetaxel-based CCRT were reported in breast cancer [12], non-small cell lung cancer [13], and head and neck cancer [14]. A previous meta-analysis reported that taxane-based CCRT had equivalent efficacy and toxicities to platinum-based regimens in head and neck squamous cell carcinoma [15]. In addition, its activity was verified in both locoregionally advanced and metastatic/recurrent NPC in combination with cisplatin [16] and/or 5-fluorouracil [17, 18]. However, studies on CCRT of IMRT with docetaxel for locoregionally advanced NPC are limited. Therefore, we conducted this propensity score matching analysis to compare the curative effect and toxicity between CCRT with weekly low-dose docetaxel and tri-weekly high-dose cisplatin for locoregionally advanced NPC in the IMRT era.
Patients and methods
Patient selection
This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center. We reviewed clinical records of patients with locoregionally advanced NPC diagnosed between January 2010 and December 2014. The inclusion criteria were as follows: (1) patients had biopsy-proven, histologically confirmed non-metastatic NPC based on the 7th edition of the American Joint Committee on Cancer (AJCC) staging system according to pretreatment magnetic resonance imaging (MRI) of the nasopharynx and neck, nasopharyngoscope, chest radiography or computed tomography (CT), abdominal sonography or CT, a whole-body bone scan or [18F]-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT); (2) patients underwent Epstein–Barr virus (EBV) DNA test prior to treatment; (3) patients received radical IMRT combined with weekly docetaxel (15 mg/m2) or tri-weekly cisplatin (80–100 mg/m2) concurrent chemotherapy. Plasma EBV DNA was quantified using quantitative real-time polymerase chain reaction (q-PCR) before treatment as previously described [19, 20]. The cutoff value of plasma EBV DNA level before the initiation of treatment was as previously established (4000 copies/mL) [21].
IMRT
A detailed procedure of IMRT and target delineation has been reported previously [22]. The prescribed radiation dose to gross tumor volume of the nasopharynx (GTVnx) was 68–74 Gy, to gross tumor volume of lymph nodes (GTVnd) was 66–70 Gy, to high-risk clinical target volume (CTV1) was 60–66 Gy, and to low-risk clinical target volume (CTV2) was 50–56 Gy. All patients were treated once daily for 5 days a week, with a total of 30–33 fractions.
Concurrent chemotherapy regimens
Patients were assigned to receive concurrent chemotherapy according to the National Comprehensive Cancer Network guidelines (Version 1.2018) [23] at the start of radiotherapy.
Docetaxel (Qilu Pharmacy Co., Ltd, Jinan, Shandong, China) was administered by drip infusion at the dose of 15 mg/m2 for 30 min once per week over 3 to 6 planned cycles. Dexamethasone (10 mg by intravenous infusion), cimetidine (200 mg by intravenous infusion), and diphenhydramine (40 mg by intramuscular injection) were given 30 min before docetaxel infusion for allergy prevention. After the completion of three cycles of docetaxel, chemotherapy was terminated if the nasopharyngeal and neck masses disappeared completely (assessed with electronic nasopharyngoscopy and physical examination) or severe mucosal toxicity led to weight loss exceeding 10%.
Cisplatin (Bristol-Myers Squibb Company, New York, USA) was administered every 3 weeks via intravenous infusion at the dose of 80–100 mg/m2 on days 1, 22, and 43 for 3 cycles concurrent with radiotherapy. The third cycle of chemotherapy was not given if radiotherapy was completed before the initiation of the third cycle once severe renal and hematologic adverse events occurred.
Pre-chemotherapy protective measures for cisplatin mainly included dexamethasone (10 mg by intravenous infusion), ondansetron (8 mg by intravenous infusion), and hydration.
Adverse events and follow-up
Treatment-related adverse events were graded according to the National Cancer Institute Common Toxicity Criteria version 4.0 (CTCAE V4.0). The follow-up period was calculated from the date of diagnosis to either the latest follow-up or the date of death. Patients were examined every 3 months for the first 2 years and every 6 months thereafter. Nasopharyngoscopy, enhanced MRI of the head and neck, chest radiography, and abdominal ultrasound were routinely performed. The final date of follow-up was September 2017.
Statistical analysis
All analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). The following endpoints were assessed: overall survival (OS), distant metastasis-free survival (DMFS), local recurrence-free survival (LRFS), and nodal recurrence-free survival (NRFS). Survival was calculated as the duration from the date of treatment to the date of death/first event or the last follow-up. The Kaplan–Meier method was used to estimate survival, and the differences were compared with the log-rank test. Cox proportional hazards model were used for multivariate analyses. Propensity score matching at a ratio of 1:1 was applied to create comparable cohorts of patients receiving concurrent chemotherapy with docetaxel or cisplatin. Covariates for matching included age, sex, histological type, pretreatment EBV DNA level, T stage, N stage, and clinical stage. The Chi-square test was used to test the balance of clinical characteristics and adverse events between the two groups. Two-sided P values less than 0.05 were considered significant.
Results
Patient characteristics
A total of 962 patients were selected for this study. The median numbers of concurrent cisplatin chemotherapy cycles in the whole and the matched cohorts were both 2 cycles (range 1–3 cycles). The median numbers of concurrent docetaxel chemotherapy cycles in the whole and matched cohorts were both 3 cycles (range 2–6 cycles). Table 1 shows the baseline characteristics and treatment details before and after the propensity score matching analysis. In the cisplatin group of the whole cohort, 8 (1.1%) patients received only 1 cycle of cisplatin: the concurrent chemotherapy was terminated for 5 patients because of acute coronary heart attack, grade 3 hepatic injury, repetitive hyponatremia, and renal injury, respectively; the second cycle of chemotherapy was changed to nedaplatin for 3 patients because of renal injury. In the docetaxel group, 1 (0.4%) patient received only 2 cycles of docetaxel due to the recurrence of a duodenal ulcer. Details of the completion of chemotherapy are shown in Additional file 1: Tables S1 and S2.
Table 1.
Characteristics of the whole and propensity score-matched cohorts of patients with locoregionally advanced nasopharyngeal carcinoma
| Characteristic | Whole cohort [cases (%)] | Propensity score-matched cohort [cases (%)] | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Cisplatin group | Docetaxel group | P value | Total | Cisplatin group | Docetaxel group | P value | |
| Total | 962 | 737 | 225 | 448 | 224 | 224 | ||
| Age | 0.245 | 0.124 | ||||||
| ≤ 50 years | 663 (68.9) | 515 (69.9) | 148 (65.8) | 311 (69.4) | 163 (72.8) | 148 (66.1) | ||
| > 50 years | 299 (31.1) | 222 (30.1) | 77 (34.2) | 137 (30.6) | 61 (27.2) | 76 (33.9) | ||
| Sex | 0.428 | 0.912 | ||||||
| Male | 716 (74.4) | 544 (73.8) | 172 (76.4) | 341 (76.1) | 170 (75.9) | 171 (76.3) | ||
| Female | 246 (25.6) | 193 (26.2) | 53 (23.6) | 107 (23.9) | 54 (24.1) | 53 (23.7) | ||
| Histological type (WHO) | ||||||||
| I | 4 (0.4) | 3 (0.3) | 1 (0.4) | 0.939 | 2 (0.4) | 1 (0.4) | 1 (0.4) | 1.000 |
| II–III | 958 (99.6) | 734 (99.7) | 224 (99.6) | 446 (99.6) | 223 (99.6) | 223 (99.6) | ||
| Clinical T stagea | 0.337 | 0.278 | ||||||
| T1–T2 | 226 (23.5) | 171 (23.2) | 55 (24.4) | 114 (25.4) | 59 (26.3) | 55 (24.6) | ||
| T3 | 591 (61.4) | 461 (62.6) | 130 (57.8) | 268 (59.9) | 138 (61.6) | 130 (58.0) | ||
| T4 | 145 (15.1) | 105 (14.2) | 40 (17.8) | 66 (14.7) | 27 (12.1) | 39 (17.4) | ||
| Clinical N stagea | 0.049 | 0.959 | ||||||
| N0 | 91 (9.5) | 72 (9.8) | 19 (8.4) | 40 (8.9) | 21 (9.4) | 19 (8.5) | ||
| N1 | 524 (54.5) | 398 (54.0) | 126 (56.3) | 252 (56.3) | 126 (56.3) | 126 (56.3) | ||
| N2 | 297 (30.8) | 221 (30.0) | 76 (33.8) | 151 (33.7) | 75 (33.5) | 76 (33.9) | ||
| N3 | 50 (5.2) | 46 (6.2) | 4 (1.8) | 5 (1.1) | 2 (0.9) | 3 (1.3) | ||
| Clinical stagea | 0.109 | 0.189 | ||||||
| II | 123 (12.8) | 85 (11.5) | 38 (16.9) | 80 (17.9) | 42 (10.2) | 38 (17.0) | ||
| III | 645 (67.0) | 501 (68.0) | 144 (64.0) | 298 (66.5) | 154 (68.8) | 144 (64.3) | ||
| IV | 194 (20.2) | 151 (20.5) | 43 (19.1) | 70 (15.6) | 28 (12.5) | 42 (18.7) | ||
| Pretreatment EBV DNA | 0.258 | 0.694 | ||||||
| < 4000 copies/mL | 633 (65.8) | 492 (66.8) | 141 (62.7) | 286 (63.8) | 145 (64.7) | 141 (62.9) | ||
| ≥ 4000 copies/mL | 329 (34.2) | 245 (33.2) | 84 (37.3) | 162 (36.2) | 79 (35.3) | 83 (37.1) | ||
WHO World Health Organization, EBV Epstein–Barr virus
aThe 7th edition of the AJCC/UICC staging system was used for TNM classification
After propensity score matching analysis, 448 patients (224 pairs) were selected, and the clinical characteristics were well balanced between the docetaxel and cisplatin groups. Additional file 1: Table S3 summarizes nodal volume (the volume of positive lymph nodes) and dosimetric parameters of the two groups, and no differences were observed in the minimal dose, mean dose, maximal dose, and nodal volume.
Treatment effects
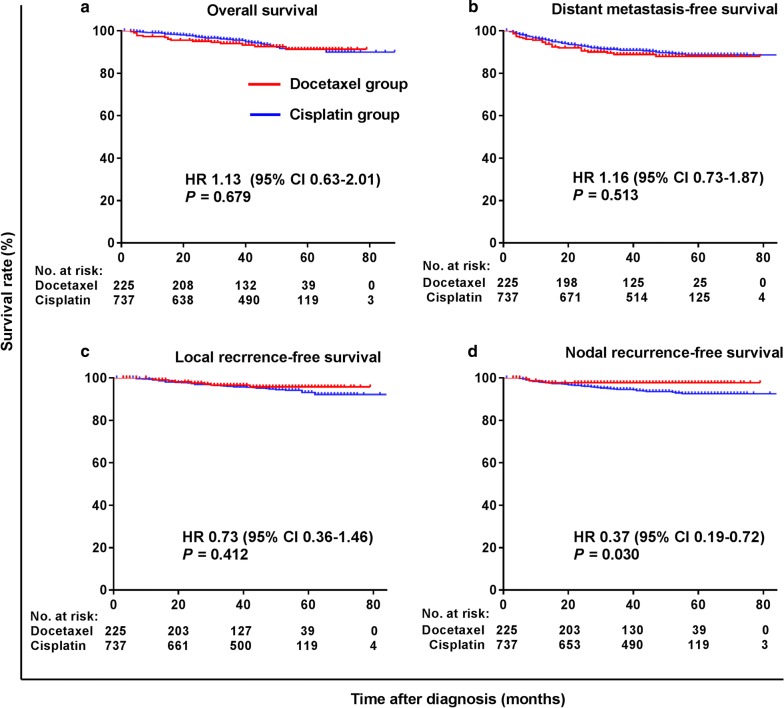
The median follow-up durations of the whole and matched cohorts were 48 months (range 1–88 months) and 47 months (range 3–89 months). Figure 1 shows the survival curves of the whole cohort. Patients in the docetaxel group had significantly higher 3-year NRFS rate than those in the cisplatin group (HR = 0.37, 95% CI 0.19–0.72, P = 0.030), whereas no significant differences in the 3-year OS, DMFS, and LRFS rates were observed between the two groups.
Fig. 1.
Kaplan–Meier survival curves of the 962 investigated patients in the whole cohort. a overall survival, b distant metastasis-free survival, c local recurrence-free survival, d Nodal recurrence-free survival
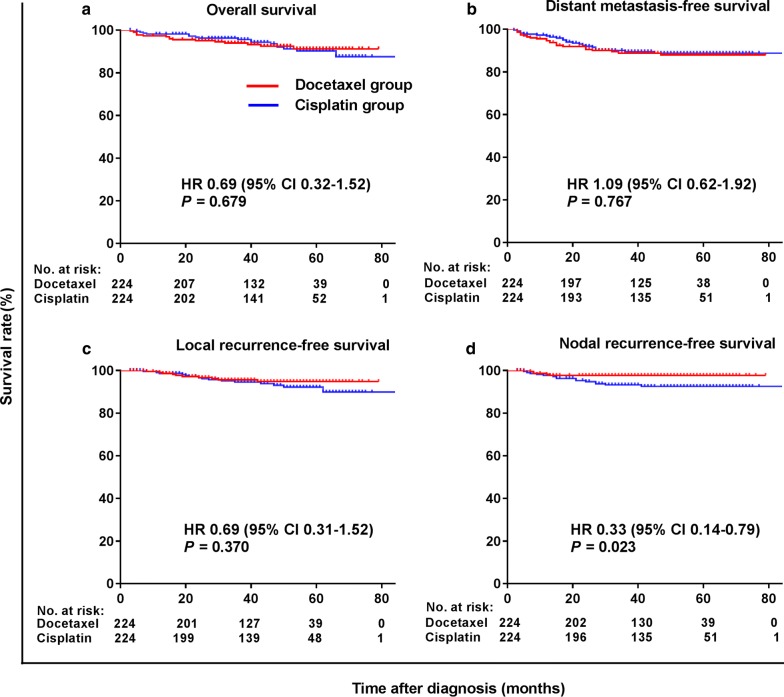
In the matched cohort, 9 (4.0%) patients experienced local recurrence, 5 (2.2%) developed regional recurrence, and 25 (11.2%) had distant metastasis in the docetaxel group; 13 (5.8%), 15 (6.7%), and 23 (10.2%) patients developed local recurrence, regional recurrence, and distant metastasis, respectively, in the cisplatin group. Survival curves for the matched cohort are shown in Fig. 2. Similarly, patients in the docetaxel group had significantly higher 3-year NRFS rate than those in the cisplatin group (HR = 0.33, 95% CI 0.14–0.79, P = 0.023), whereas the 3-year OS, DMFS, and LRFS rates did not differ significantly between the two groups.
Fig. 2.
Kaplan–Meier survival curves of the 448 patients in the propensity score-matched cohort. a Overall survival; b distant metastasis-free survival; c Local recurrence-free survival; d Nodal recurrence-free survival
In multivariate analysis (Table 2), concurrent docetaxel chemotherapy was the only independent prognostic factor for NRFS (HR = 0.34, 95% CI 0.12–0.93, P = 0.036), but not for OS, LRFS, and DMFS.
Table 2.
Adjusted Cox multivariate analyses of prognostic factors for the matched cohort
| Endpoint | Variable | P value | HR | 95% CI |
|---|---|---|---|---|
| OS | Gender (male vs. female) | 0.334 | 0.62 | 0.24–1.63 |
| Age (≤ 50 vs. > 50 years) | 0.175 | 1.63 | 0.80–3.30 | |
| Pretreatment EBV DNA (< 4000 vs. ≥ 4000 copies/mL) | 0.766 | 1.12 | 0.54–2.29 | |
| T stage (T1–2 vs. T3–4) | 0.393 | 1.54 | 0.57–4.12 | |
| N stage (N0–1 vs. N2–3) | 0.022 | 2.26 | 1.12–4.53 | |
| Clinical stage (II–III vs. IV) | 0.134 | 1.86 | 0.83–4.18 | |
| Concurrent chemotherapy (docetaxel vs. cisplatin) | 0.802 | 0.92 | 0.46–1.83 | |
| DMFS | Gender (male vs. female) | 0.885 | 0.95 | 0.48–1.88 |
| Age (≤ 50 vs. > 50 years) | 0.901 | 0.96 | 0.52–1.79 | |
| Pretreatment EBV DNA (< 4000 vs. ≥ 4000 copies/mL) | 0.039 | 1.85 | 1.030–3.31 | |
| T stage (T1–2 vs. T3–4) | 0.600 | 1.22 | 0.59–2.51 | |
| N stage (N0–1 vs. N2–3) | 0.017 | 2.01 | 1.14–3.58 | |
| Clinical stage (II–III vs. IV) | 0.530 | 1.27 | 0.61–2.64 | |
| Concurrent chemotherapy (docetaxel vs. cisplatin) | 0.827 | 1.07 | 0.60–1.88 | |
| LRFS | Gender (male vs. female) | 0.146 | 1.85 | 0.81–4.21 |
| Age (≤ 50 vs. ≥ 50 years) | 0.986 | 0.99 | 0.41–2.42 | |
| Pretreatment EBV DNA (< 4000 vs. ≥ 4000 copies/mL) | 0.852 | 0.92 | 0.39–2.17 | |
| T stage (T1–2 vs. T3–4) | 0.418 | 1.58 | 0.52–4.79 | |
| N stage (N0–1 vs. N2–3) | 0.128 | 1.86 | 0.84–4.13 | |
| Clinical stage (II–III vs. IV) | 0.278 | 0.65 | 0.29–1.45 | |
| Concurrent chemotherapy (docetaxel vs. cisplatin) | 0.288 | 0.65 | 0.29–1.45 | |
| NRFS | Gender (male vs. female) | 0.337 | 0.55 | 0.16–1.88 |
| Age (≤ 50 vs. > 50 years) | 0.146 | 0.40 | 0.12–1.38 | |
| Pretreatment EBV DNA (< 4000 vs. ≥ 4000 copies/mL) | 0.171 | 1.86 | 0.77–4.52 | |
| T stage (T1–2 vs. T3–4) | 0.491 | 0.72 | 0.28–1.86 | |
| N stage (N0–1 vs. N2–3) | 0.334 | 1.55 | 0.64–3.77 | |
| Clinical stage (II–III vs. IV) | 0.783 | 0.81 | 0.18–3.71 | |
| Concurrent chemotherapy (docetaxel vs. cisplatin) | 0.036 | 0.34 | 0.12–0.93 |
OS overall survival, EBV Epstein–Barr virus, DMFS distant metastasis-free survival, LRFS locoregional recurrence-free survival, NRFS nodal recurrence-free survival, HR hazard ratio, CI confidence interval
Subgroup analyses according to pretreatment EBV DNA levels for the matched cohort
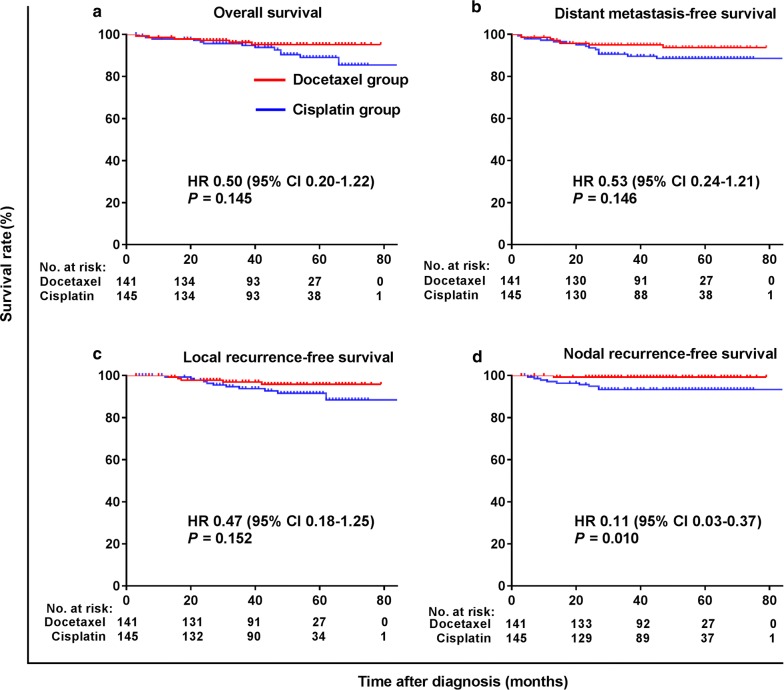
Figures 3 and 4 illustrate the subgroup survival analyses according to pretreatment levels of EBV DNA in the matched cohort. Docetaxel was associated with a significant improvement in NRFS (HR = 0.11, 95% CI 0.03–0.37, P = 0.010) for patients with pretreatment EBV DNA level < 4000 copies/mL (Fig. 3).
Fig. 3.
Kaplan–Meier survival curves of patients with pretreatment EBV DNA < 4000 copies/mL in the matched cohort. a Overall survival; b distant metastasis-free survival; c local recurrence-free survival; d Nodal recurrence-free survival
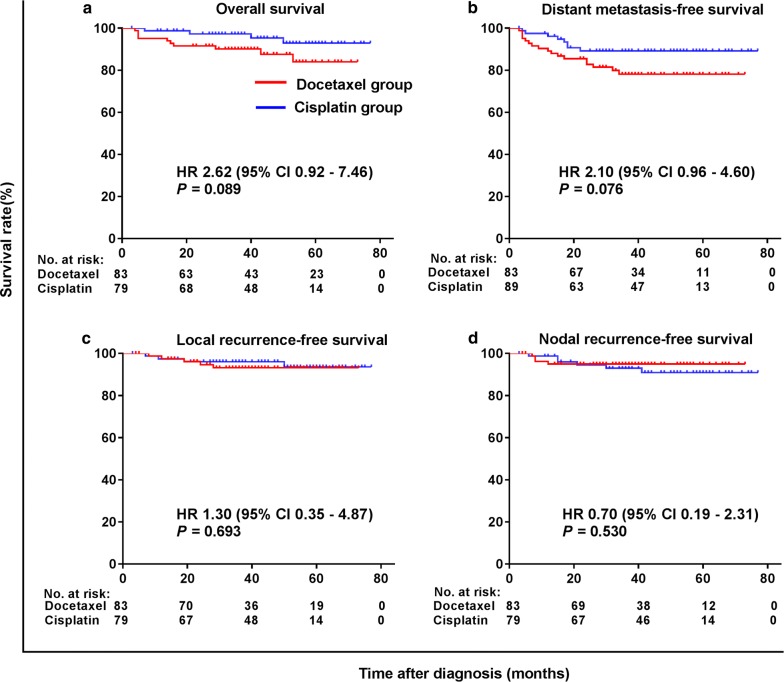
Fig. 4.
Kaplan–Meier survival curves of patients with pretreatment EBV DNA ≥ 4000 copies/mL in the matched cohort. a Overall survival; b distant metastasis-free survival; c local recurrence-free survival; d Nodal recurrence-free survival
For patients with a pretreatment EBV DNA level ≥ 4000 copies/mL, no significant differences in survival were observed between the two treatment groups (Fig. 4). This was possibly related to the small sample size that was underpowered to show statistical differences between two groups.
Adverse events
Table 3 summarizes the chemoradiotherapy-related acute adverse events in the matched cohort. Higher rates of leucopenia, anemia, and thrombocytopenia were observed in the cisplatin group. In addition, acute renal injury, vomiting, and hepatic injury were also more common in the cisplatin group.
Table 3.
Chemoradiotherapy-related acute adverse events in the matched cohort
| Adverse event | Docetaxel group [cases (%)] | Cisplatin group [cases (%)] | P value | ||
|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | ||
| Leucopenia | 90 (40.2) | 5 (2.2) | 164 (73.2) | 26 (11.6) | < 0.001 |
| Anemia | 55 (24.6) | 0 | 105 (46.8) | 2 (0.9) | < 0.001 |
| Thrombocytopenia | 3 (1.3) | 0 | 41 (18.3) | 4 (1.8) | < 0.001 |
| ALT elevation | 42 (18.8) | 1 (0.4) | 68 (30.4) | 2 (0.9) | 0.027 |
| CCR elevation | 4 (1.8) | 0 | 33 (14.7) | 1 (0.4) | < 0.001 |
| Vomitinga | 42 (18.8) | 0 | 185 (82.5) | 13 (5.8) | < 0.001 |
| Mucositisa | 45 (20.1) | 167 (74.5) | 102 (45.5) | 85 (37.9) | < 0.001 |
| Weight lossa | 171 (76.3) | 0 | 166 (74.1) | 2 (0.1) | 0.010 |
| Radiodermatitisa | 191 (85.3) | 15 (6.7) | 185 (82.6) | 4 (1.8) | 0.001 |
| Tinnitusa | 23 (10.3) | 0 | 24 (10.7) | 0 | 0.835 |
| Xerostomiaa | 171 (76.3) | 2 (0.1) | 170 (75.9) | 2 (0.1) | 0.052 |
Only one patient in the cisplatin group had grade 4 radiodermatitis
ALT Alanine transaminase, AST aspartate aminotransferase, CCR creatinine clearance
aMissing value: In the docetaxel group, medical records of 10 patients were missing for radiodermatitis, mucositis, and xerostomia, 8 for vomiting, 6 for weight loss, and 12 for tinnitus; In the cisplatin group, medical records of 35 patients were missing for radiodermatitis, 37 for mucositis, 38 for xerostomia, 9 for vomiting, 17 for weight loss, and 38 for tinnitus
Radiodermatitis and mucositis were the major adverse events observed in the docetaxel group. The rates of grade 3 radiodermatitis (6.7% vs. 1.8%, P = 0.001) and mucositis (74.5% vs. 37.9%, P < 0.001) were significantly higher in the docetaxel group than in the cisplatin group. Only one patient in the cisplatin group had grade 4 radiodermatitis. All acute adverse events were resolved with comprehensive care, and no treatment-associated deaths were observed in the two groups.
Discussion
In the present study, we used a propensity score matching analysis to eliminate influences of confounding factors while assessing the curative effect and toxicity between CCRT with weekly low-dose docetaxel and tri-weekly high-dose cisplatin for locoregionally advanced NPC in the IMRT era. The results demonstrated that concurrent chemotherapy with docetaxel significantly increased the 3-year NRFS rate as compared with cisplatin (HR = 0.33, 95% CI 0.14–0.79, P = 0.03). However, the 3-year OS, DMFS, and LRFS rates were similar between the two groups.
Wei et al. [24] reviewed records of 73 stage III–IVA NPC patients to compare the efficacy of CCRT with docetaxel and cisplatin. Similar to our findings, they found no significant difference between the two groups in 3-year OS (86.5% vs. 92.5%, P = 0.298), DMFS (87.0% vs. 92.5%, P = 0.171), and local control rates (85.6% vs. 92.3%, P = 0.264). However, the distribution of their patient characteristics between the two treatment arms was unbalanced, and 2D-CRT was administered; whereas in the present study, all patients underwent IMRT, and propensity score matching was performed to balance baseline patient characteristics.
A previous study demonstrated that patients with pretreatment EBV DNA > 4000 copies/mL had a higher risk to develop distant metastasis compared with those with EBV DNA < 4000 copies/mL [21]. In the present study, it was interesting that docetaxel significantly increased 3-year NRFS rate as compared with cisplatin (99.3% vs. 93.3%, P = 0.010) for patients with pretreatment EBV DNA < 4000 copies/mL; although the OS, DMFS, and LRFS seemed longer in the docetaxel group, the differences were not significant. It is possible that concurrent weekly low-dose docetaxel mainly improved locoregional control due to its radiosensitization or synergistic effect with radiotherapy [25], but could not eradicate micro-metastases effectively for patients with a high risk of metastasis. This indicated that choosing concurrent chemotherapy regimens according to the pretreatment EBV DNA level might help to improve the curative effect.
The optimal dose and schedule of docetaxel remain poorly defined. The schedule of docetaxel consisted of mainly 6 cycles in previous studies [14, 24, 26, 27]. In the matched cohort of the present study, all patients in the docetaxel group received 3 cycles of chemotherapy, 54 (24.1%) received 4 or more cycles; 167 (74.6%) patients in the docetaxel group suffered grade 3 mucositis, which was much higher than those in previous studies (range from 27.9% to 61.4%) [14, 24, 28]. The main reason may be related to the use of IMRT which increases the radiation dose to the target area and thus leading to an increased risk of severe mucositis. Most patients completed the planned therapy, and none experienced grade 4 mucositis. Further research is needed to identify the optimal regimen of docetaxel to balance toxicity and curative effect in the IMRT era.
Previous studies revealed that mucositis was the most common restrictive factor in CCRT with docetaxel [27]. Several phase I/II studies reported that a weekly dose of docetaxel between 10 and 20 mg/m2 demonstrated acceptable toxicity and therapeutic activity [26, 28]. Calais et al. [27] reported a phase II trial of CCRT with weekly docetaxel at 20 mg/m2 for patients with stage III/IV oropharyngeal carcinoma. The rates of grade 3–4 mucositis (84%) and radiodermatitis (53%) were high, and grade 3–4 neutropenia was observed in 5% of patients. Jomon Raphael et al. [29] found that CCRT with weekly docetaxel at 15 mg/m2 for patients with locoregionally advanced, inoperable head and neck cancer was a feasible and suitable alternative to surgery, and the major adverse events were mucosal reaction (grade 3: 57%) and radiodermatitis (grade 3: 23%). Furthermore, the rates of grade 3 dysphagia and grade 2 weight loss (> 10% of the initial weight) were 38% and 23%. In the present study, CCRT with weekly docetaxel at 15 mg/m2 was well tolerated, and 224 (99.6%) patients had completed at least 3 courses concurrent chemotherapy. The rates of hematological adverse events, vomiting, liver impairment, and renal injury were lower in the docetaxel group than in the cisplatin group, whereas the rate of acute mucositis was higher in the docetaxel group.
The present study has several drawbacks inherent to observational analyses. For instance, the accuracy of recurrence evaluation or cause of death adjudication of observational analyses was inferior to that of a prospective clinical trial, which could result in possible misclassification of survival events. In addition, adverse reactions for some patients, especially for outpatients, were unrecorded.
Conclusions
Our findings show that CCRT with weekly low-dose docetaxel is an effective and tolerable treatment for locally advanced NPC. It provides a survival benefit mainly by improving regional control, especially for patients with low pretreatment EBV DNA levels.
Additional file
Additional file 1: Table S1. Details of the completion of chemotherapy in the whole cohort (962 patients) and the matched cohort (448 patients). Table S2. Details of the dose density of cisplatin in the whole cohort (737 patients) and the matched cohort (224 patients). Table S3. Nodal volume and dosimetric parameter of the two groups in the matched cohort.
Acknowledgements
We thank the Clinical Trials Center, Sun Yat-sen University Cancer Center for trial monitoring, data management, and statistical assistance.
Abbreviations
- CI
confidence interval
- NPC
nasopharyngeal carcinoma
- 2D-CRT
two-dimensional conventional radiotherapy
- IMRT
intensity modulated radiotherapy
- CCRT
concurrent chemoradiotherapy
- MRI
magnetic resonance imaging
- q-PCR
quantitative real-time polymerase chain reaction
- EBV
Epstein–Barr virus
- CTCAE V4.0
Common Toxicity Criteria version 4.0
- GTV
gross tumor volume
- CTV
clinical tumor volume
- OS
overall survival
- DMFS
distant metastasis free survival
- LRFS
local recurrence-free survival
- NRFS
nodal recurrence-free survival
- HR
hazard ratio
- WHO
World Health Organization
- NCCN
National Comprehensive Cancer Network
Authors’ contributions
Study concepts: JFL, QZ and WL. Study design: WL, JFL and XJD. Data acquisition: JFL, QZ, ML and SL. Data analysis and interpretation: JFL, ML and YFX. Statistical analysis: JFL and XYC. Manuscript preparation: JFL, QZ and XJD. Manuscript editing: JFL. Manuscript review: WL. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used or analyzed in the present study are available from the corresponding author on reasonable request.
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the approval number of RDDA2019001011.
Ethics approval and consent to participate
The protocol of this study was approved by the institutional ethics committee of the Sun Yat-sen University Cancer Center, and consent for the use of data in research was obtained for each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Jun-Fang Liao, Email: liaojf@sysucc.org.cn.
Qun Zhang, Email: zhangqungz@163.com.
Xiao-Jing Du, Email: duxj@sysucc.org.cn.
Mei Lan, Email: merrydoctor@163.com.
Shan Liu, Email: liushan@sysucc.org.cn.
Yun-Fei Xia, Email: xiayf@sysucc.org.cn.
Xiu-Yu Cai, Email: caixy@sysucc.org.cn.
Wei Luo, Phone: +86-20-87343483, Email: luowei@sysucc.org.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):90. doi: 10.1186/s40880-017-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx. 2012;39:137–144. doi: 10.1016/j.anl.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103(23):1761–1770. doi: 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 7.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomized controlled trial. Lancet Oncol. 2016;17:1509–1520. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 10.Hennequin C, Giocanti N, Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56:1842–1850. [PubMed] [Google Scholar]
- 11.Dunne AL, Mothersill C, Robson T, Wilson GD, Hirst DG. Radiosensitization of colon cancer cell lines by docetaxel: mechanisms of action. Oncol Res. 2004;14:447–454. doi: 10.3727/0965040041791455. [DOI] [PubMed] [Google Scholar]
- 12.Rivera E, Mejia JA, Arun BK, Adinin RB, Walters RS, Brewster A, et al. Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer. 2008;112:1455–1461. doi: 10.1002/cncr.23321. [DOI] [PubMed] [Google Scholar]
- 13.Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, et al. Phase II randomized trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2005;16(1):90–96. doi: 10.1093/annonc/mdi018. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Tsukuda M, Satake B, Kubota A, Kida A, Kohno N, et al. PhaseI/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol. 2004;9:107–112. doi: 10.1007/s10147-003-0375-z. [DOI] [PubMed] [Google Scholar]
- 15.Behera M, Owonikoko TK, Kim S, Chen Z, Higgins K, Ramalingam SS, et al. Concurrent therapy with taxane versus non-taxane containing regimens in locally advanced squamous cell carcinomas of the head and neck (SCCHN): a systematic review. Oral Oncol. 2014;50(9):888–894. doi: 10.1016/j.oraloncology.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–249. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 17.Chua D, Sham JS, Au GK. A phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral Oncol. 2005;41:589–595. doi: 10.1016/j.oraloncology.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Liu GY, Xing LV, Wu YS, Mao MJ, Ye YF, Yu YH, et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. Cancer Commun. 2018;38:21. doi: 10.1186/s40880-018-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT, et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59(6):1188–1191. [PubMed] [Google Scholar]
- 20.Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162–1170. doi: 10.1002/cncr.20099. [DOI] [PubMed] [Google Scholar]
- 21.Chen WH, Tang LQ, Guo SS, Chen QY, Zhang L, Liu LT, et al. Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin-Based Concurrent Chemoradiotherapy in Intensity-Modulated Radiotherapy Era. Medicine. 2016;95(5):e2642. doi: 10.1097/MD.0000000000002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Z, Luo W, Zhou QC, Zhang QH, Kang DH, Liu MZ. Impact of changing gross tumor volume delineation of Intensity modulated radiotherapy on the dose distribution and clinical treatment outcome after induction chemotherapy for the primary locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2009;28(11):1132–1137. doi: 10.5732/cjc.009.10435. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network for Head and Neck Cancers. Version 1. 2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck. Accessed 1 Sept 2018.
- 24.Wei WH, Cai XY, Xu T, Zhang GY, Wu YF, Feng WN, et al. Concurrent weekly docetaxel chemotherapy in combination with radiotherapy for stage III and IVA-B nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2012;13(3):785–789. doi: 10.7314/APJCP.2012.13.3.785. [DOI] [PubMed] [Google Scholar]
- 25.Van Rijn J, van den Berg J, Meijer OW. Proliferation and clonal survival of human lung cancer cells treated with fractionated irradiation in combination with paclitaxel. Int J Radiat Oncol Biol Phys. 1995;33:635–639. doi: 10.1016/0360-3016(95)00216-L. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Nishimura Y, Nakamatsu K, Kanamori S, Koike R, Kawamoto M, et al. Phase I study of weekly docetaxel infusion and concurrent radiation therapy for head and neck cancer. Jpn J Clin Oncol. 2003;33:297–301. doi: 10.1093/jjco/hyg054. [DOI] [PubMed] [Google Scholar]
- 27.Calais G, Bardet E, Sire C, Alfonsi M, Bourhis J, Rhein B, et al. Radiotherapy with concomitant weekly docetaxel for stages III/IV oropharynx carcinoma. Results of the 98-02 GORTEC phase II trial. Int J Radiat Oncol Biol Phys. 2004;58:161–166. doi: 10.1016/S0360-3016(03)01370-1. [DOI] [PubMed] [Google Scholar]
- 28.Karasawa K, Matsumoto F, Ito S, Oba S, Furuya T, Hirowatari H, et al. Hyperfractionated radiotherapy with concurrent docetaxel for advanced head and neck cancer: a phase II study. Anticancer Res. 2012;32(9):4013–4018. [PubMed] [Google Scholar]
- 29.Jomon Raphael C, Rajesh I, Rajesh B, Selvamani B, John S. Feasibility and response of concurrent weekly docetaxel with radical radiotherapy in locally advanced head and neck squamous cell carcinoma. J Clin Diagn Res. 2015;9(3):XC01–XC04. doi: 10.7860/JCDR/2015/10819.5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Details of the completion of chemotherapy in the whole cohort (962 patients) and the matched cohort (448 patients). Table S2. Details of the dose density of cisplatin in the whole cohort (737 patients) and the matched cohort (224 patients). Table S3. Nodal volume and dosimetric parameter of the two groups in the matched cohort.
Data Availability Statement
The datasets used or analyzed in the present study are available from the corresponding author on reasonable request.
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the approval number of RDDA2019001011.