Abstract
Pancreatic microadenomas are benign tumors of neuroendocrine origin less than 5 mm in size. Whereas most microadenomas are non-functional; a few rare functional pancreatic microadenomas have been described in the setting of multiple endocrine neoplasia type one (MEN-1). In this report, we describe a unique case of multiple functional microadenomas of the pancreatic head in a patient who presented with persistent secretory diarrhea, refractory hypokalemia, metabolic acidosis and elevated plasma vasoactive intestinal peptide (VIP) levels. Following extensive serologic, radiographic and endoscopic work up, our patient underwent open pancreaticoduodenectomy with subsequent resolution of diarrheal symptoms and electrolyte abnormalities on postoperative follow up.
INTRODUCTION
Pancreatic vasoactive intestinal polypeptide producing tumor (VIPoma) is a rare functional neuroendocrine tumor first described in association with a well-defined syndrome characterized by secretory diarrhea, refractory hypokalemia, achlorhydria and metabolic acidosis or WDHAA (Watery Diarrhea, Hypokalemia, Achlorhydria, Acidosis) Syndrome [1, 2]. Vasoactive intestinal polypeptide is a 28 amino acid hormone encoded on chromosome 6q25.2 as a physiologically active pre-prohormone that mediates enteric neurotransmission at post-synaptic class II G-protein coupled receptors [3]. The resulting activation of different downstream signal transduction pathways helps modulate pancreatic exocrine and intestinal secretion, gastric acid secretion, glycogenolysis, calcium homeostasis and satiety [3].
Review of the Surveillance, Epidemiology, and End Results (SEER) cancer registry between 1973 and 2000, shows that VIPomas accounted for only 0.9% of all 1483 pancreatic neuroendocrine tumors reported during the study window [4]. Most VIPomas originate in the pancreas (84%) with the remainder localizing to the sympathetic chain ganglia [4–6]. Isolated pancreatic VIPomas have a predilection to the tail, range in size 1 cm or greater and present as solitary lesions [4].
CASE REPORT
A 78-year-old male was admitted for evaluation of persistent secretory diarrhea of 2 months duration after presenting with acute kidney injury. Stool studies confirmed secretory diarrhea with a corresponding fecal osmotic gap of 10 mOsm/kg. Blood chemistries on admission demonstrated severe hypokalemia (K 2.1 mmol/l), elevated plasma VIP levels of 79 pg/mL, serum bicarbonate of 13 and chloride of 117. Blood glucose and calcium levels were within normal range. Urine 24-hour 5-hydroxy indoleacetic acid (5-HIAA), serum gastrin, serum parathyroid hormone and serum chromogranin levels were all likewise within normal limits. Surgical history was significant for intractable peptic ulcer disease and subsequent antrectomy with a Billroth II reconstruction. There was no reported family history of malignancy.
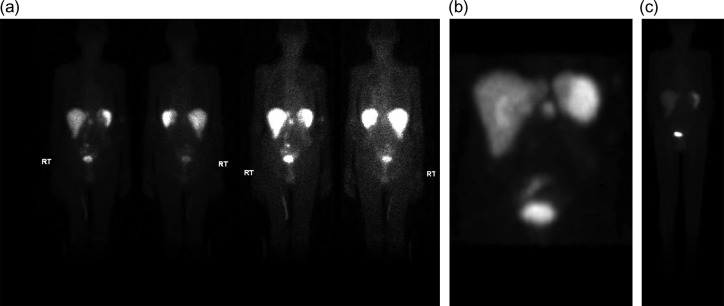
Initial inpatient evaluation started with pan endoscopy. Upper endoscopy demonstrated a Billroth II anastomosis with patent efferent and afferent limbs and no evidence of marginal ulceration or malignancy. Colonoscopy, likewise, was unremarkable. Radiographic evaluation with computed tomography of the abdomen and pelvis did not show evidence of pancreatic tumor or biliary ductal dilatation (Fig. 1). An octreotide scan, however, demonstrated increased activity near the head of the pancreas and abdominal aorta (Fig. 2 a-b). Endoscopic ultrasound did not fully evaluate the head of pancreas but showed no lesions in the left lobe of the liver, pancreatic body and tail. Capsule endoscopy then followed and was unremarkable. Despite being placed nil per Os, the patient’s diarrheal symptoms and hypokalemia persisted. Stool cultures obtained on admission demonstrated no growth.
Figure 1:
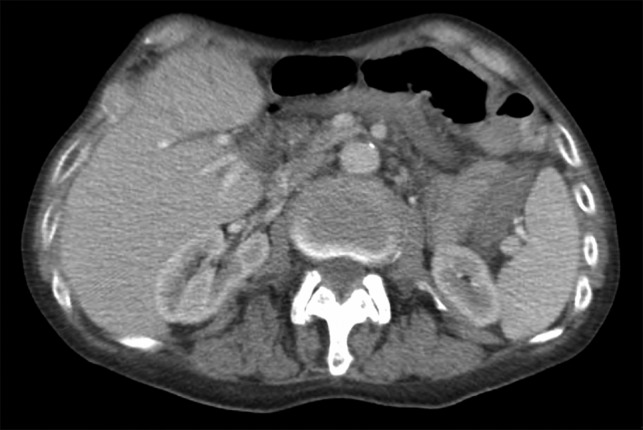
Computed tomography scan of the abdomen and pelvis with intravenous contrast (Omnipaque 350TM, iohexol, 50 mL). No evidence of pancreatic mass or biliary ductal dilation was identified.
Figure 2:
(a–c) (a and b.) Octreotide scan demonstrating increased activity in the peripancreatic region (6.6 mCi of Indium-111 labeled octreotide). (c) Repeat octreotide scan (6 of Indium-111 labeled octreotide) at 4 months post-resection demonstrated normal distribution of activity in the liver, spleen and urinary system. No other focal area of activity was identified.
After exhaustive workup, the octreotide scan, as well as the classic constellation of clinical findings consistent with VIPoma, supported our decision to proceed with operative exploration with possible pancreaticoduodenectomy. Upon entry into the abdomen, no evidence of palpable or occult metastatic disease was found; however intraoperative ultrasound with color flow doppler localized two lesions less than one cm in size at the level of the uncinate process and ampulla. At this point, an open pancreaticoduodenectomy was offered to the patient’s family versus expectant follow up and they elected for a potentially curative resection given his ongoing symptoms. A pancreaticoduodenectomy was performed and modified to the patient’s existing Billroth II anatomy; afferent and efferent limbs were re-constructed to accommodate the new pancreatojejunostomy, gastrojejunostomy and choledochojejunstomy. At the conclusion of the case, a jejunostomy tube was also placed for enteral access. Following surgery, the patient had a prolonged post-operative course complicated by persistent diarrhea and was discharged post-operative Day 14 on supplementary enteral nutrition. At 2 two-month post-operative follow up, the patient had significant improvement of his diarrhea and had been weaned from enteral nutrition. A repeat octreotide scan 4 months following resection demonstrated radiographic resolution (Fig. 2b).
DISCUSSION
Pancreatic microadenomas are benign tumors of neuroendocrine origin less than 5 mm in size [5, 7]. While most are non-functional; functional pancreatic microadenomas have been reported in patients with MEN-1 and have included insulinomas and glucagonomas [7, 8]. To the best of our knowledge, no hormonally functional pancreatic microadenomas associated with elevated plasma VIP have been described in a patient without MEN-1 [8].
Our final histopathologic evaluation is in concordance with our intraoperative sonographic findings and demonstrates pancreatic microadenomatosis throughout the pancreatic head parenchyma at the level of the uncinate process without evidence of lymphatic invasion (Fig. 3a and b). Positive staining with synaptophysin supports the neuroendocrine origin of these microadenomas (Fig. 3c).
Figure 3:
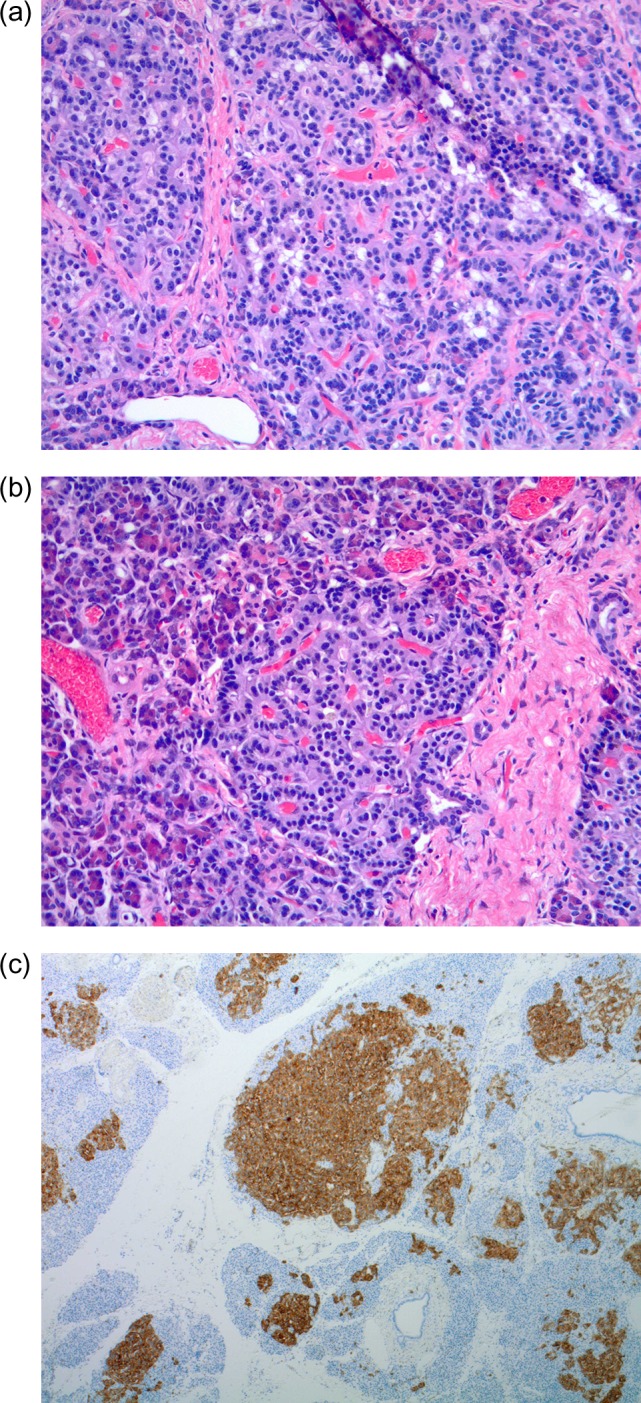
(a–c) Permanent pathology. (a and b) Multiple pancreatic microadenomas scattered throughout the pancreatic head parenchyma. (c) Immunohistochemical stains positive for synaptophysin.
Although the patient’s plasma VIP level was mildly elevated, he had presented with a functional neuroendcorine syndrome exclusively seen with VIPoma as supported by his symptomatology, stools studies and blood chemistries. We contend that the mild elevation in serum VIP is explained by a corresponding elevation in biologically active pre-prohormone VIP; which is difficult to assay by conventional means. While initial post-operative clinical and radiographic follow is encouraging, repeat serum VIP levels and continued surveillance imaging will be needed to confirm that our resection was curative.
ACKNOWLEDGMENTS
I would like to acknowledge Ms. Margaret Moutseous for her help in obtaining relevant literature.
CONFLICT OF INTEREST STATEMENT
None declared.
DISCLCOSURES
No disclosure of financial support.
REFERENCES
- 1. Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med 1958;25:374–80. [DOI] [PubMed] [Google Scholar]
- 2. Verner JV, Morrison AB. Endocrine pancreatic islet disease with diarrhea. Report of a case due to diffuse hyperplasia of nonbeta islet tissue with a review of 54 additional cases. Arch Intern Med 1974;133:492–9. [DOI] [PubMed] [Google Scholar]
- 3. Fahrenkrug J. Transmitter role of vasoactive intestinal peptide. Pharmacol Toxicol 1993;72:354–63. [DOI] [PubMed] [Google Scholar]
- 4. Ghaferi AA, Chojnacki KA, Long WD. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg 2008;12:382. [DOI] [PubMed] [Google Scholar]
- 5. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annal Oncol 2008;9:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vinik A. Vasoactive Intestinal Peptide Tumor (VIPoma) [Updated 2017 Jun 5]. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc, 2000. [Google Scholar]
- 7. Babic B, Keutgen X, Nockel P, Miettinen M, Millo C, Herscovitch P, et al. . Insulinoma due to multiple pancreatic microadenoma localized by multimodal imaging. J Clin Endocrinol Metab 2016;101:3559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klöppel G, Willemer S, Stamm B, Häcki WH, Heitz PU. Pancreatic lesions and hormonal profile of pancreatic tumors in multiple endocrine neoplasia type I. An immunocytochemical study of nine patients. Cancer 1986;57:1824–32. [DOI] [PubMed] [Google Scholar]