Abstract
Background:
Patients with severe hemophilia A and inhibitors are at risk of bleeding during invasive procedures. The standard of care for preventing perioperative bleeding has been replacement therapy with FVIII concentrates or for patients with high-titer inhibitors, bypassing agents. However, there is no consensus on the appropriate management of surgery in patients receiving the novel agent emicizumab. The aim of this study was to demonstrate a case of a patient on emicizumab undergoing major surgery with bypassing agents with preoperative use of the thrombin generation assay (TGA) and thromboelastography (TEG).
Methods:
We report a patient with hemophilia A with inhibitors who had undergone a total knee replacement while on emicizumab combined with a bypassing agent. We utilized TEG and TGA to determine which bypassing agent to choose as well as to inform about the ideal dose.
Results:
We elected to use recombinant FVIIa as a bypassing agent for the surgery based upon the TGA results.
Conclusion:
The TGA can be utilized to support decision-making in patients on emicizumab undergoing major surgery to both predict efficacy and potentially minimize the risk of thrombotic events.
Keywords: bypassing agents, emicizumab, hemophilia, inhibitor, thrombin generation test, thromboelastography
Introduction
Hemophilia A is an inherited bleeding disorder caused by deficiency or dysfunction of the coagulation protein factor VIII (FVIII). The current treatment of hemophilia consists of replacing the missing factor which can be done in a prophylactic manner or on demand.1 However, factor replacement is an immunogenic process with an incidence of new FVIII inhibitors in patients with severe FVIII deficiency of approximately 30%2 with an overall inhibitor prevalence of 5–7% (12–13% when limited to patients with severe disease).3 Patients who develop inhibitors have worse morbidity than those with hemophilia who do not develop inhibitors4 and prophylactic factor replacement is not as effective as it is in patients without inhibitors. Therefore, improved therapy for patients with inhibitors remains an important challenge of hemophilia care. There are different therapeutic approaches for patients with hemophilia with inhibitors, such as immune tolerance induction (ITI) and bypassing agent therapy, however ITI does not eradicate inhibitors in at least 30% of patients with inhibitors, and bypassing agents have limited effectiveness in the prevention (and even treatment) of bleeding. Emicizumab is a recombinant, humanized, bispecific monoclonal antibody that bridges activated FIX and FX to restore the function of missing activated FVIII and has been demonstrated to be largely well tolerated and effective in patients with hemophilia with inhibitors in two clinical trials of patients with inhibitors.5–8 Importantly, standard factor assays cannot determine the pharmacodynamic effects of bypassing agents nor the effect of emicizumab. However, the thrombin generation assay (TGA) and thromboelastography (TEG), known as global hemostasis assays, may provide some data regarding the pharmacodynamics of emicizumab and bypassing agents. Surgery represents a challenge in hemophilia management due to the risk of excessive bleeding especially in inhibitor patients in whom intensive bypassing therapy is usually required. Here we report a patient with hemophilia with inhibitors who had undergone a total knee replacement while on emicizumab combined with a bypassing agent.
Case
Preoperative history
The patient was a 25-year-old male born full term with normal spontaneous vaginal delivery who was hospitalized for 1 month immediately after birth secondary to a large progressive scalp hematoma and was diagnosed with hemophilia at that time. Subsequently, his genotype demonstrated the presence of the intron 22 inversion. The patient started prophylaxis immediately after the recognition that his bleeding was due to hemophilia. There was no known family history of bleeding disorders or bleeding symptoms. He had been on prophylaxis three times a week when he developed a high-titer inhibitor (39.3 BU) at the age of 11 months. This was discovered due to bleeding episodes (an injured finger and a massive perineal and scrotal bleed) that were unresponsive to treatment with FVIII concentrate. He then began ITI approximately 3 weeks after the detection of the inhibitor with the same concentrate he had been treated with for prophylaxis, a plasma-derived FVIII with Von Willebrand factor (VWF) (pdFVIII/VWF). He was initially on a dose of 50 IU/kg/day along with activated prothrombin complex concentrate (aPCC) or recombinant activated factor VII (rFVIIa) on demand for episodic treatment of bleeding episodes. After 14 months on this ITI regimen, and with an ongoing high-titer inhibitor, the ITI was modified to 100 IU/kg/day. Of note, his ITI course had been complicated by recurrent central line infections, severe hemophilic arthropathy of the right knee, bilateral ankles, right shoulder and right elbow. He did not respond favorably after a total course of 33 months of ITI, and this approach was abandoned. Since that time (for ~20 years), he was subsequently treated with prophylaxis or episodic bypassing agents at different time periods.
During the past several years as an adult, his quality of life had significantly deteriorated, incorporating significant pain, feelings of sadness, and ongoing, repeated bleeding episodes. For his severe acute (with bleeds) and chronic (due to arthropathy) pain, he had been treated at various times with celecoxib, hydrocodone, acetaminophen, ibuprofen and vaporized medical marijuana. He also had reduced his activity to the minimum required for daily living. Additional therapies he received included radioisotopic synovectomies (right knee, right elbow, left elbow), physical therapy, and a variety of ambulation assistive devices. He also required numerous hospitalizations for management of severe bleeding episodes, pain management, port replacements, port infections, and an appendectomy which was performed at the age of 12 years. He continued having many bleeds into various joints and, in particular, he had significant deterioration in the joint function combined with severe, daily pain in his right knee.
In 2016, he was enrolled in the HAVEN 17 study at the age of 23 years. He received emicizumab per protocol with four loading doses of 3 mg/kg/dose followed by the maintenance dosing of 1.5 mg/kg/dose given subcutaneously once per week. He received aPCC or rFVIIa as needed for breakthrough bleeds as he had been doing prior to initiation of emicizumab. At 6 weeks after enrolling in the emicizumab study, he presented to the emergency department with an acute gastrointestinal (GI) bleed and was admitted to the hospital for severe anemia. His endoscopy showed gastric ulcer and biopsy was positive for Helicobacter pylori. He received red blood cell transfusions and medications for the H. pylori infection. He received rFVIIa in combination with emicizumab for his GI bleed. At 5 months after the initiation of emicizumab, he presented with left eye swelling, proptosis, and a severe headache, and was ultimately diagnosed with a cavernous sinus thrombosis and superior ophthalmic vein thrombosis (his is the first case of thrombosis described in more detail in the HAVEN 1 study).7 Notably, he had received relatively large doses of aPCC exceeding what is now in the black box warning for emicizumab to treat a right knee bleed.9 The aPCC and emicizumab were discontinued and he was observed in the hospital. He did not receive anticoagulation. His symptoms resolved within a week and his imaging demonstrated complete resolution of the thrombosis 2 weeks after diagnosis. He resumed emicizumab 5 weeks after it was initially discontinued and has remained on emicizumab ever since. He has had no further thrombotic events, nor any other adverse events related to emicizumab.
Overall, he had far fewer bleeding events on emicizumab which he has now been receiving for over nearly 3 years. His annual bleeding rate (ABR) before emicizumab was 24; whereas his ABR after emicizumab was 7 and 5 at the first and second year, respectively. However, the arthropathy of his right knee was so severe that despite a reduction in bleeding in that joint, he continued to have significant mobility problems and chronic pain. Thus, with the support of his healthcare team, he elected to undergo a total right knee arthroplasty/replacement (TKR). Notably, he was still in the HAVEN 1 study at the time of the surgery; however, at the time of his enrollment (nearly 2 years prior to the surgery), he did meet the eligibility requirement of ‘no planned surgery’ as this surgery was not planned at that time.
Preoperative laboratory testing
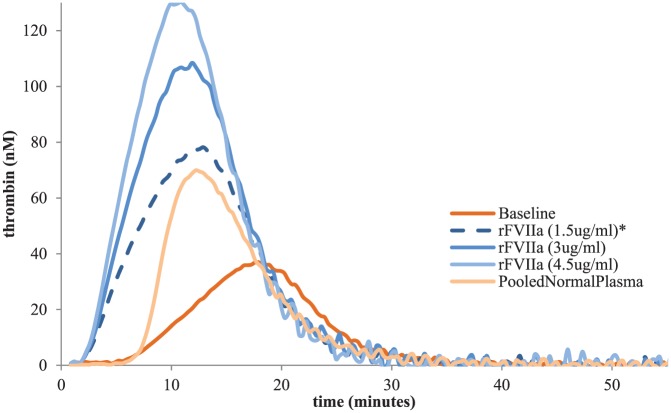
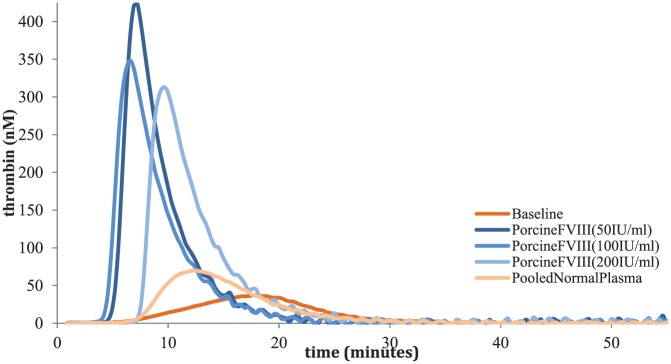
With the benefit of time to plan for his surgery as well as our laboratory’s capability to perform TGA and TEG, we elected to follow (albeit in a modified fashion) the approach of Dargaud and colleagues for the selection of bypassing agents for his surgery.10 The TGA method we used involves the calibrated automated thrombogram (Stago, Asnieres, France) with the PPP-LOW reagent which utilizes low-dose tissue factor as has been previously reported.10 We ran a commercial reference plasma purchased from Precision Biologic which was comprised of platelet-poor plasma from 20 or more screened male and female donors aged 18–66 years (catalog number CCN-10). For the TEG, whole blood was used with the kaolin activation we have previously reported.11 We spiked different concentrations of rFVIIa (1.5 ug/ml, 3 ug/ml, 4.5 ug/ml; Table 1, Figure 1), aPCC (0.05 IU/ml, 0.1 IU/ml, 0.2 IU/ml, 0.3 IU/ml, 0.5 IU/ml, 0.75 IU/ml; Table 2, Figure 2) and porcine FVIII (50 IU/ml, 100 IU/ml 200 IU/ml; Table 3, Figure 3) in vitro into the patient’s plasma and whole blood and analyzed the results. The patient had an excellent TGA response to rFVIIa demonstrating an increasing endogenous thrombin potential (ETP) and peak thrombin as expected. We elected to use the 1.5 µg/ml concentration (which approximates a dose of 90 µg/kg) which was the lowest concentration which gave results close to the pooled normal plasma (PNP) and was therefore chosen for the surgery. For aPCC, results demonstrated a very high level of thrombin generation at known therapeutic concentrations which has been previously reported.12 This in combination with his prior thrombotic event while receiving aPCC and the now known synergy between emicizumab and aPCC led us to make every effort to avoid using it for this patient’s surgery. Corresponding doses for the aPCC in vitro concentrations are 5 U/kg, 10 U/kg, 20 U/kg, 30 U/kg, 50 U/kg and 75 U/kg, respectively. We also spiked the same medications with the same concentrations in vitro before surgery to the patient’s whole blood and performed TEG. However, the TEG was not sufficiently sensitive demonstrating near normal results with emicizumab alone. For recombinant porcine FVIII, ETP results were close to PNP with all concentrations; however, we did not have the patient’s antiporcine FVIII inhibitor titer and as such it did not feel entirely safe to use porcine FVIII as the first option for his surgery. It should be pointed out that there is no antiporcine FVIII inhibitor assay that has been validated for patients on emicizumab, further clouding the potential use for this agent. Of note, the patient’s antihuman FVIII inhibitor level was 45 BU and therefore we did not consider giving a high dose of FVIII products. Given the above information, we elected to use rFVIIa exclusively to prevent bleeding for his TKR with a preoperative bolus dose of 200 µg/kg followed by repeated doses of 100 µg/kg (see details below). The choice to use a higher preoperative dose (200 µg/kg) was aimed to be a bolus dose to ensure sufficient thrombin generation during the period of highest risk and has been our center’s approach for surgery in inhibitor patients for many years.
Table 1.
Presurgery in vitro spiking of rFVIIa.
| TGA values | Baseline | rFVIIa (1.5 µg/ml) |
rFVIIa (3 µg/ml) |
rFVIIa (4.5 µg/ml) |
Pooled normal plasma* |
|---|---|---|---|---|---|
| Lagtime_(min) | 7.55 | 3.2 | 3.2 | 3.2 | 7.67 |
| ETP_(nM•min) | 491.52 | 1006.34 | 1301.41 | 1446.76 | 714.63 |
| Peak_(nM) | 36.59 | 77.48 | 107.62 | 130.55 | 69.61 |
| ttPeak_(min) | 17.69 | 12.34 | 11.45 | 10.78 | 12.67 |
| VelIndex_(nM/min) | 3.61 | 8.48 | 13.05 | 17.23 | 13.92 |
ETP, endogenous thrombin potential; TGA, thrombin generation assay; ttPeak, time to peak; VelIndex, velocity index.
The pooled normal plasma results in our lab are different than previously reported with lower peak thrombin and ETP in particular. This is due to the different reagents and methods used by the different labs. Our lab follows the manufacturer’s instructions explicitly.
Figure 1.
Presurgery in vitro spiking of rFVIIa.
rFVIIa, recombinant activated Factor VII.
Table 2.
Presurgery in vitro spiking of aPCC.
| TGA values | Baseline | aPCC (0.05 IU/ml) |
aPCC (0.1 IU/ml) |
aPCC (0.2 IU/ml) |
aPCC (0.3 IU/ml) |
aPCC (0.5 IU/ml) |
aPCC (0.75 IU/ml) |
Pooled normal plasma* |
|---|---|---|---|---|---|---|---|---|
| Lagtime_(min) | 7.55 | 4.67 | 4 | 3.33 | 3.33 | 3 | 2.67 | 7.67 |
| ETP_(nM•min) | 491.52 | 634.95 | 975.76 | 1476.13 | 1837.95 | 2596.55 | 2980.33 | 714.63 |
| Peak_(nM) | 36.59 | 40.07 | 70.32 | 135.15 | 185.7 | 283.04 | 381.6 | 69.61 |
| ttPeak_(min) | 17.69 | 15.11 | 13 | 10.22 | 9 | 7.33 | 6.33 | 12.67 |
| VelIndex_(nM/min) | 3.61 | 3.84 | 7.81 | 19.63 | 32.77 | 65.32 | 104.07 | 13.92 |
aPCC, activated prothrombin complex concentrate; ETP, endogenous thrombin potential; TGA, thrombin generation assay; ttPeak, time to peak; VelIndex, velocity index.
The pooled normal plasma results in our lab are different than previously reported with lower peak thrombin and ETP in particular. This is due to the different reagents and methods used by the different labs. Our lab follows the manufacturer’s instructions explicitly.
Figure 2.
Presurgery in vitro spiking of aPCC.
aPCC, activated prothrombin complex concentrate.
Table 3.
Presurgery in vitro spiking of porcine FVIII.
| TGA values | Baseline | Porcine FVIII (50 IU/ml) |
Porcine FVIII (100 IU/ml) |
Porcine FVIII (200 IU/ml) |
Pooled normal plasma* |
|---|---|---|---|---|---|
| Lagtime_(min) | 7.55 | 5.21 | 4.65 | 7.55 | 7.67 |
| ETP_(nM•min) | 491.52 | 1960.71 | 1818.57 | 1772.51 | 714.63 |
| Peak_(nM) | 36.59 | 422.24 | 349.62 | 312.85 | 69.61 |
| ttPeak_(min) | 17.69 | 7.21 | 6.65 | 9.77 | 12.67 |
| VelIndex_(nM/min) | 3.61 | 210.53 | 174.32 | 141.26 | 13.92 |
ETP, endogenous thrombin potential; TGA, thrombin generation assay; ttPeak, time to peak; VelIndex, velocity index.
The pooled normal plasma results in our lab are different than previously reported with lower peak thrombin and ETP in particular. This is due to the different reagents and methods used by the different labs. Our lab follows the manufacturer’s instructions explicitly.
Figure 3.
Presurgery in vitro spiking of porcine FVIII.
Perioperative history
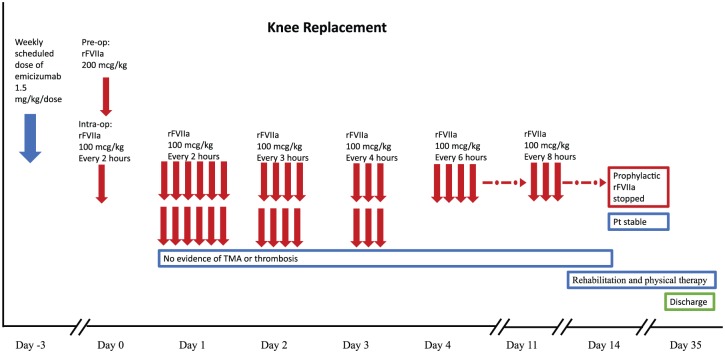
He remained on emicizumab (as part of the HAVEN 1 study) with his weekly scheduled dose given 3 days before the surgery (given the long half-life of emicizumab and the steady-state levels, we did not adjust his dosing day) and received rFVIIa 200 µg/kg just before the incision in order to provide sufficient thrombin generation for the surgery. This was followed initially by 100 µg/kg every 2 h during the operation and for the first full day postoperatively. On postoperative day 2, he received the same dose every 3 h and this was subsequently tapered on day 3 and 4 to every 4 and 6 h, respectively. He remained on every 6 h dosing through postoperative day 11. From day 11–14, his dose was further tapered to every 8 h. In addition, he was carefully monitored for symptoms and signs of thrombosis and thrombotic microangiopathy (TMA) including twice daily laboratory testing (complete blood count, reticulocyte count, chemistry panel including blood urea nitrogen, creatinine, bilirubin, and lactose dehydrogenase levels) for the first week and daily laboratory testing during the second postoperative week. He had no evidence of TMA. He was also examined daily with special emphasis on signs of thrombosis and had no symptoms or signs suggestive of thrombosis. Of note, anticoagulants are typically prescribed for TKR patients (controversially for hemophilia patients) postoperatively, but he did not receive prophylactic anticoagulation. On postoperative day 14 the patient was stable, and he was transferred to our inpatient rehabilitation unit where he remained hospitalized for 3 additional weeks specifically for intensive physical therapy during which time he received rFVIIa only prior to physical therapy sessions. The patient has continued physical therapy as an outpatient and has had an overall outstanding result (currently 11 months after surgery) with restoration of mobility in his right knee and a reduction in his pain which is currently related to his ongoing physical therapy (Figure 4).
Figure 4.
Perioperative management.
Discussion
Emicizumab is a bispecific antibody currently licensed in many countries for the prevention of bleeding in patients with hemophilia with inhibitors. Planned surgeries were excluded from the HAVEN studies although unplanned surgeries were performed though with no specific guidance in the protocol. Perioperative management in patients with inhibitors is a significant challenge in any situation, and even more so when patients are receiving a novel therapy. There are two case series reporting the outcomes of surgery in patients on emicizumab. The first describes the surgical experience for patients on emicizumab from the HAVEN 1 and 2 studies.13 There were 22 patients who underwent a total of 29 surgical procedures (24 surgical procedures in 17 patients in HAVEN 1, 5 surgical procedures in 5 patients in HAVEN 2). Most of the procedures were considered minor surgery. A total of 20 of these procedures (69%) were managed without prophylactic bypassing agents (BPA), whereas 9 (31%) were managed with prophylactic BPAs. Among the nine surgeries that were managed with prophylactic BPAs, eight were managed with rFVIIa with a mean dose 152.81 µg/kg (86.54–254.72 µg/kg) and one surgery used prophylactic aPCC at a single dose of 49.78 U/kg. Overall, eight of these surgeries (89%) did not result in postoperative bleeding. A tooth extraction resulted in a single treated postoperative bleeding event. The second was recently reported by Zimowski and colleagues which described surgeries from nine patients.14 A total of seven of the nine patients received prophylactic factor (rFVIIa in six and plasma-derived FVIII in one) for the following procedures: three central venous access device (CVAD) placements, one CVAD removal, one elbow synovectomy, one complex dental extraction, and one infected penile prosthesis removal. All the patients experienced minimal blood loss perioperatively, none required transfusions of blood products and no patient experienced thrombosis or TMA. Overall, two of the nine did not receive prophylactic factor prior to their minor procedures and did not have postoperative bleeding.
In the HAVEN 1 trial, once-weekly emicizumab prophylaxis that was administered subcutaneously in patients with hemophilia with inhibitors was reported to be associated with a bleeding rate that was 87% lower than the rate with no prophylaxis. This led us to be confident that emicizumab conferred a level of hemostatic protection including for the patient described in this report; however, that level is not easy to quantify. Nevertheless, a TKR is a major surgery with a significant risk for bleeding and postoperative complications related to bleeding such as early or late infection of the prosthesis. We thus felt that prophylactic BPA was required, however we chose to perform global assays to help determine the best choice of agent and dose.
In this patient, the in vitro TGA testing with rFVIIa demonstrated normalization of both the ETP and peak thrombin at a concentration of 1.5 µg/ml (approximating a dose of 90 µg/kg) without leading to excessive thrombin generation. This was also supported by in vivo TGA results at the day of surgery. The patient’s 52 min postoperative TGA results showed a 2.92 min lag time, 828.73 nM-min ETP and a peak thrombin of 43.27 nM, which was close to our preoperative 1.5 µg/ml of rFVIIa spiking TGA results. In contrast, in vitro aPCC spiking demonstrated a very high level of thrombin generation as has been previously reported, and we elected therefore not to use this agent unless absolutely necessary. As the in vitro spiking concentrations for aPCC escalate, lag time decreases and peak thrombin and ETP increase showing more prothrombotic results. It should be noted, however, that at rather low concentrations of aPCC (0.1 IU/ml equating to a dose of 10 IU/kg) demonstrated TGA results similar to 1.5 µg/ml of rFVIIa concentration. Thus, additional research into the use of lower than approved doses of aPCC for patients on emicizumab should be conducted.
Thus, given the above results, we elected to take a conservative course with frequent dosing of rFVIIa for the first few days followed by a tapering course similar to what has been suggested in the past.15 We elected to avoid aPCC given the patient’s prior thrombotic event and the events noted in other patients described in HAVEN 1. The patient did extremely well, and no excessive bleeding was reported either during or after the surgery. He is now much more mobile than he had been, and his chronic knee pain has resolved though he does still require occasional pain medication in association with his physical therapy. Of note, he had no thrombotic events or TMA.
There are two previous reports of major surgery in patients on emicizumab. The first was reported by Kruse-Jarres and colleagues as part of a larger series described above.13 Of the 29 surgical procedures reported, 2 were major surgeries, 1 right knee arthroscopy, synovectomy, debridement of arthrofibrosis, and chondroplasty in HAVEN 1, and a laparoscopic appendectomy in HAVEN 2. The second is a case reported from Italy by Santagostino and colleagues16 This patient (also a HAVEN 1 study patient) had a right hip replacement performed, and a preoperative rFVIIa bolus of 98 µg/kg was administered and repeated rFVIIa boluses of 82 µg/kg were given every 3 h afterwards. However, this case differs from ours as the treating physicians elected to switch to FVIII therapy the day after the surgery due to a right thigh hematoma which ended up with dropped hemoglobin levels despite packed red blood cell (pRBC) transfusions. Replacement with plasma-derived FVIII was the preferred treatment method due to their concern of a thrombotic risk. The inhibitor titer at that time was 2 BU/ml, and it was felt that the FVIII level could be achieved albeit with higher than typical doses of FVIII concentrate. He received 115 IU/kg by bolus followed by continuous infusion at 3.3–4 IU/kg/h until postoperative day 7 when FVIII levels were decreased despite increasing the FVIII infusion rate. The patient developed FVIII antibody anamnestic response (80 BU/ml) and was switched back to rFVIIa 80 µg/kg every 4–8 h with antifibrinolytic therapy until discharge on day 13. The patient received 3 Units of pRBC 6 h after the operation, 2 units of pRBC at the following morning, and then due to the above-mentioned bleeding required 3 units of pRBC between postoperative day 2 to day 7 and 1 unit of pRBC between postoperative day 8 to day 13. Our patient did not receive any blood products during or after surgery. Authors observed an improvement of thrombin generation values after the addition of bypassing therapy to emicizumab; however, they found no correlation between TGA values and clinical conditions. They have explained this with factors other than bypassing treatment influence the hemostatic system and thrombin generation.
For noninhibitor patients, the level of factor is directly proportional to the clinical response; however, the same is not true for inhibitor patients making it important to find an ideal assay that predicts the clinical response. This becomes critically important during surgical procedures.17 We utilized TEG and TGA to determine which bypassing agent to choose as well as to inform about the ideal dose.17,18 This is the first report describing the use of TEG/TGA to select BPA treatment for patients with hemophilia with inhibitors on emicizumab undergoing major surgery. In a recent paper by Dargaud and colleagues, TGA was used for individualizing the treatment of a perirenal hematoma with bypassing agents in a patient with hemophilia with inhibitors who is currently on emicizumab.19 The patient safely recovered with no thrombotic event after combining aPCC with emicizumab. We have found that TEG results (with our kaolin activation method) were not as helpful as the TGA results as the TEG parameters with emicizumab alone were essentially normal thus not offering the sensitivity that TGA offered.
In conclusion, we have demonstrated the utility of TGA for the individualization of bypassing therapy in patients on emicizumab undergoing major surgery utilizing a similar approach that has been previously published. The TGA can be utilized to support decision-making in patients on emicizumab undergoing major surgery to both predict efficacy and to potentially minimize the risk of thrombotic events. A clinical trial is underway to prospectively assess the outcomes of being on emicizumab when undergoing minor surgery and will hopefully lead to an improved understanding of the management of such patients (ClinicalTrials.gov identifier: NCT003361137). Major surgeries (both elective and urgent) in patients on emicizumab will occur and we wish to emphasize that the best outcomes require a multidisciplinary team supported by a specialized coagulation laboratory.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: HK, EMC, CLY, JL and JD have nothing to disclose. GY has received honoraria and consulting fees from Alnylam, Bioverativ, CSL Behring, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, Shire, Spark, and UniQure.
GY is a principal investigator in HAVEN 1 and managed the patient’s surgery; HK and EMC performed the laboratory testing; HK, EMC and GY analyzed the data; JL and JD edited the paper; HK, EMC, CLY and GY wrote the paper.
This is a case report and we do not give any patient information, so there is no need for informed consent nor ethics approval.
ORCID iD: Hande Kizilocak  https://orcid.org/0000-0003-0323-2571
https://orcid.org/0000-0003-0323-2571
Contributor Information
Hande Kizilocak, Hemostasis and Thrombosis Center, Children’s Hospital Los Angeles, 4650 Sunset Boulevard, Mail Stop #54, Los Angeles, CA 90027, USA.
Clara Lana Yukhtman, Western University of Health Sciences, Pomona, CA, USA.
Elizabeth Marquez-Casas, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Jeanie Lee, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Jennifer Donkin, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Guy Young, Children’s Hospital Los Angeles, Los Angeles, CA, USA; University of Southern California Keck School of Medicine, Los Angeles, CA, USA.
References
- 1. Balkaransingh P, Young G. Novel therapies and current clinical progress in hemophilia A. Ther Adv Hematol 2018; 9: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witmer C, Young G. Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol 2013; 4: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wight J, Paisley S. The epidemiology of inhibitors in hemophilia A: a systemic review. Haemophilia 2003; 9: 418–435. [DOI] [PubMed] [Google Scholar]
- 4. Franchini M, Mannucci PM. Inhibitors of propagation of coagulation (factors VIII, IX and XI): a review of current therapeutic practice. Br J Clin Pharmacol 2011; 72: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med 2012; 18: 1570–1574. [DOI] [PubMed] [Google Scholar]
- 6. Sampei Z, Igawa T, Soeda T, et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One 2013; 8: e57479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017; 377: 809–818. [DOI] [PubMed] [Google Scholar]
- 8. Young G, Sidonio RF, Liesner R, et al. HAVEN 2 updated analysis: multicenter, open-label, phase 3 study to evaluate efficacy, safety and pharmacokinetics of subcutaneous administration of emicizumab prophylaxis in pediatric patients with hemophilia A with inhibitors. Blood 2017; 130: 85. [Google Scholar]
- 9. Genentech. HEMLIBRA® prescribing information, www.gene.com/download/pdf/hemlibra_prescribing.pdf (2017, date accessed 2017).
- 10. Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood 2010; 116: 5734–5737. [DOI] [PubMed] [Google Scholar]
- 11. Salinas V1, Carmona R, Mohammed BM, et al. Is some better than none: are TEG and TGA profiles different in severe FVIII-deficient patients with inhibitors? Haemophilia 2015; 21: 398–404. [DOI] [PubMed] [Google Scholar]
- 12. Hartmann R, Feenstra T, Valentino L, et al. In vitro studies show synergistic effects of a procoagulant bispecific antibody and bypassing agents. J Thromb Haemost. Epub ahead of print 11 June 2018. DOI: 10.1111/jth.14203. [DOI] [PubMed] [Google Scholar]
- 13. Kruse-Jarres R, Callaghan MU, Croteau SE, et al. Surgical experience in two multicenter, open-label phase 3 studies of emicizumab in persons with Hemophilia A with inhibitors (HAVEN 1 and HAVEN 2). Blood 2017; 130: 89. [Google Scholar]
- 14. Zimowski KL, Batsuli GM, Reding MT, et al. Maintaining perioperative hemostasis in patients with severe Hemophilia A and inhibitors receiving emicizumab prophylaxis. Blood 2018; 132: 635.29950291 [Google Scholar]
- 15. Santagostino E, Escobar M, Ozelo M, et al. Recombinant activated factor VII in the treatment of bleeds and for the prevention of surgery-related bleeding in congenital haemophilia with inhibitors. Blood Rev 2015; 29: 9–18. [DOI] [PubMed] [Google Scholar]
- 16. Santagastino E, Mancuso ME, Novembrino C, et al. Rescue FVIII replacement to secure haemostasis in a patient with haemophilia A and inhibitors on emicizumab prophylaxis undergoing hip replacement. Haematologica. Epub ahead of print 28 March 2019. DOI: 10.3324/haematol.2018.215129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young G, Blain R, Nakagawa P, et al. Individualization of bypassing agent treatment for haemophilic patients with inhibitors utilizing thromboelastography. Haemophilia 2006; 12: 598–604. [DOI] [PubMed] [Google Scholar]
- 18. Young G, Ebbesen LS, Viuff D, et al. Evaluation of thromboelastography for monitoring recombinant activated factor VII ex vivo in haemophilia A and B patients with inhibitors: a multicentre trial. Blood Coagul Fibrinolysis 2008; 19: 276–282. [DOI] [PubMed] [Google Scholar]
- 19. Dargaud Y, Lienhart A, Janbain M, et al. Use of thrombin generation assay to personalize treatment of breakthrough bleeds in a patient with hemophilia and inhibitors receiving prophylaxis with emicizumab. Haematologica 2018; 103: 181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]