Abstract
Purpose:
To develop an automated optimization program to generate optimal beam settings for whole-breast radiation therapy driven by clinically oriented goals.
Materials and Methods:
Forty patients were retrospectively included in this study. Each patient’s planning images, contoured structures of planning target volumes, organs-at-risk, and breast wires were used to optimize for patient-specific–beam settings. Two beam geometries were available tangential beams only and tangential plus supraclavicular beams. Beam parameters included isocenter position, gantry, collimator, couch angles, and multileaf collimator shape. A geometry-based goal function was defined to determine such beam parameters to minimize out-of-field target volume and in-field ipsilateral lung volume. For each geometry, the weighting in the goal function was trained with 10 plans and tested on 10 additional plans. For each query patient, the optimal beam setting was searched for different gantry-isocenter pairs. Optimal fluence maps were generated by an in-house automatic fluence optimization program for target coverage and homogeneous dose distribution, and dose calculation was performed in Eclipse. Automatically generated plans were compared with manually generated plans for target coverage and lung and heart sparing.
Results:
The program successfully produced a set of beam parameters for every patient. Beam optimization time ranged from 10 to 120 s. The automatic plans had overall comparable plan quality to manually generated plans. For all testing cases, the mean target V95% was 91.0% for the automatic plans and 88.5% for manually generated plans. The mean ipsilateral lung V20Gy was lower for the automatic plans (15.2% vs 17.9%). The heart mean dose, maximum dose of the body, and conformity index were all comparable.
Conclusion:
We developed an automated goal-driven beam setting optimization program for whole-breast radiation therapy. It provides clinically relevant solutions based on previous clinical practice as well as patient specific anatomy on a substantially faster time frame.
Keywords: breast cancer, treatment planning, beam geometry, optimization, automation, whole breast radiation therapy
Introduction
The standard treatment for early-stage breast cancer is breast-conserving therapy, which consists of breast conserving surgery followed by adjuvant whole-breast radiation therapy (WBRT).1 A number of randomized clinical trials and meta-analyses2–5 demonstrate that adjuvant WBRT significantly reduces ipsilateral breast tumor recurrence rate for both invasive and noninvasive breast cancers and improves survival in patients with invasive breast cancer.
Whole-breast radiation therapy typically employs a tangential beam geometry with a medial beam and a lateral beam in order to avoid the beams diverging into the lungs and causing unnecessary irradiation to the organs-at-risk (OARs).6 Lower lung dose is desirable as it reduces the probability of complications in the lung; exposure of the heart increases the subsequent rate of ischemic heart disease, and the increase is proportional to the mean dose of the heart.7 In WBRT, the planning target volume (PTV) is the entire breast. The beams are meticulously placed to provide sufficient coverage to the PTV while avoiding the ipsilateral lung and the heart as much as possible. Hence, the beam geometry plays an important role in WBRT planning.
In previous studies by Purdie et al 8,9, an automated treatment planning methodology was developed for inversely planned tangential breast intensity-modulated radiation therapy (IMRT). For beam geometry, they utilized radiopaque breast wires for identifying the breast tissue and optimizing the beam.8 The algorithm optimizes both gantry and collimator angle based on lung and normal tissue volumes, where various gantry angle/isocenter combinations are scored until it is clinically acceptable.8
In our clinic, manual beam placement and fluence optimization are time-consuming tasks, in which the two combined could require an average of around 110.2 minutes of human effort on the sampled cases from a subsequent prospective clinical study. Beam placement plays an important role in providing adequate coverage for the PTV as well as lowering dose spill to heart and lung, which the subsequent step of fluence painting operated by dosimetrist can achieve. When suboptimal beams are used, the patient will receive either inadequate dose coverage of the PTV or unnecessary irradiation of the OARs. Often, balancing target coverage and OAR sparing is no trivial task given the patient geometry, and therefore substantial amount of physician’s valuable time has to be spent on the trial and error process of finding the optimal beam set. Sometimes due to time constraint, physician and the planning team may not necessarily generate the optimal plan although it is clinically acceptable.
In the current work, we present an automated beam geometry optimization program which can generate clinically feasible beams for WBRT based on superficial breast tissue demarcating wires as well as PTV drawn by physicians. The beam geometry optimization algorithm balances the PTV coverage and OAR sparing through beam parameters including gantry angles and isocenter location. By automating beams and fluence maps generation, the treatment planning time can be significantly reduced while the plan quality was comparable to manually generated plans.
Materials and Methods
Patient Population
Forty patients with breast cancer previously treated with WBRT at Duke University Medical Center were randomly selected for this retrospective study. The study protocol was approved by the institutional review board for clinical investigation. All patients had radiopaque demarcating wires placed to indicate the superior, inferior, midline, and lateral boarders of the breast. Among them, 20 patients were treated with only tangential beams to cover breast PTV (“tangent only geometry”); 9 patients were treated for the right breast, and 11 for the left breast. The other 20 patients, including 12 postmastectomy cases indicated by the use of chest wall PTV, were treated with tangential beams plus supraclavicular (SCV) beams, which covered SCV PTV, axillary PTV, and internal mammary node (IMN) PTV (“tangent plus SCV geometry”) in addition to breast/chest wall PTV; 8 patients were treated for the right breast and 12 for the left breast. The average breast separation (the patient thickness along the central axis of the treatment tangential beams) of all 40 patients is 22.6 cm and the standard deviation is 3.2 cm.
All patients include structure contours of breast/chest wall clinical target volume (CTV), breast/chest wall PTV, ipsilateral lung, heart, and midline wire. In addition, a structure for main PTV dose evaluation called “PTV_Eval” was also drawn. Breast/chest wall CTV were manually drawn by the attending physicians. The PTV was generated with 5 mm margin from the CTV, and the PTV_Eval was generated from the PTV by removing 5 mm skin (to remove build-up region) and excluding chest wall.10
Study Workflow
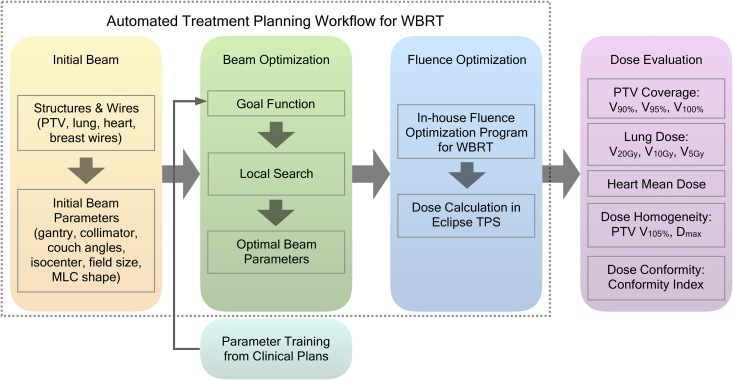
In our clinic, WBRT adopts tangential style forward planning IMRT, also known as Electronic Compensation. Attending physician is responsible for target and OAR delineation and beam placement. Planner manually edits the fluence map to achieve uniform dose distribution in the treatment volume. The plan can be used for treatment after a quality assurance (QA) plan is verified. The workflow of this study is summarized and illustrated in Figure 1. We proposed a 3-step automated workflow for WBRT: first an initial beam is placed based on wires placed on the patient body; then the beam optimization program finds the optimal beam setting for the patient geometry to meet the clinical goal; finally an in-house fluence optimization tool11 is used for generating optimal fluence maps. This tool utilizes a random forest model to predict pixel-wise fluence intensity for the tangent fields. The model takes inputs of anatomical information such as penetration depth, tissue density and outputs the fluence intensity. The model was trained using pixel-wise information from previously treated WBRT patient populations. This study focuses on first 2 steps: initial beam calculation and beam optimization. The parameters that reflect the relative balance between PTV and OAR in the goal function were trained with the training data set separate from the test data set. The automatically generated plans were compared with manually generated clinical plans to evaluate dose end points.
Figure 1.
Study workflow. The proposed automated treatment planning workflow consists of 3 steps: initial beam calculation, beam optimization, and fluence optimization. The first 2 steps and the evaluation of automatic plans are described in this work.
Program Environment
The program was developed in Eclipse (Varian Medical Systems, Palo Alto, California) Scripting Application Programming Interface (ESAPI), allowing the program to be called within the Eclipse treatment planning system (TPS) and access the patient data without exporting patient data. Eclipse version 13.6 ESAPI scripts can only read data from the TPS and do not have the permission to write or modify. After beam optimization, the program generates a human-readable file which contains the optimized beam parameters for the user to manually enter in the TPS. Separate multi-leaf collimator (MLC) files in Varian MLC format can be imported into the TPS. For Eclipse version 15.6, a write-enabled version of the program was developed, which can write an automatically generated plan directly in the TPS.
Beam Geometry Settings
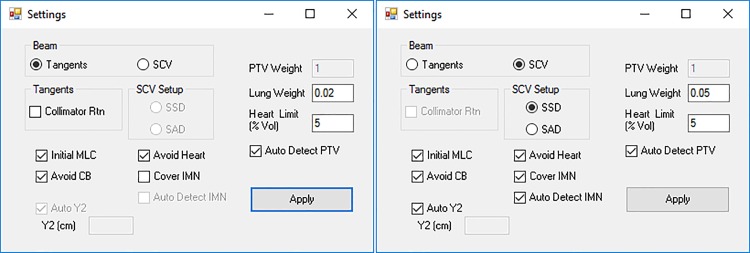
The program offers 2 types of beam geometry for clinical use, including the tangent only geometry and the tangent plus SCV geometry. The graphical user interface for beam geometry setting is shown in Figure 2. In the tangent only geometry, 2 tangential beams, which shared a matched posterior field border, were generated to cover the breast PTV. The 2 tangential beams have their gantry angles separated by approximately 180° without couch rotation and contain same degree of collimator rotation in the opposite direction. The user is offered the option to enable/disable collimator rotation and include/exclude initial MLC. The initial MLC defines the MLC shape to conform to the breast PTV in the beam’s eye view (BEV) and block the heart if needed. When the “Avoid Contralateral Breast (CB)” option is selected, the gantry angles are limited so that the lateral distance on skin (toward CB) from the midline wire to the medial beam entry point is smaller than a limit. This limit is set to 2 cm for the tangent only geometry and 3 cm for the tangent plus SCV geometry.
Figure 2.
Beam geometry settings user interface. The default settings for the tangent only geometry (left) and the tangent plus SCV geometry (right) are shown. SCV indicates supraclavicular.
In the tangent plus SCV geometry, in addition to 2 tangential beams, 1 (source to surface distance (SSD) setup, anterior beam) or 2 (source to axis distance [SAD] setup, anterior and posterior beam) SCV beams were generated to cover the SCV and axillary PTVs. The tangential beams shared both the posterior and superior field borders, which require a combination of collimator and couch rotation. The isocenter of the half-blocked (Y1 = 0) SCV beam(s) was located at the same superior-inferior location (Z coordinate) of the tangential beams’ superior field border for field matching in order to avoid overlap or gap between tangential beams and SCV beams. The anterior SCV beam was angled 15° for right-sided patients and 345° for left-sided patients to avoid irradiating trachea, esophagus, and the spinal cord.6 If SSD setup was used, only one anterior SCV beam was generated. If SAD setup was selected, another posterior SCV beam was parallel opposed to the anterior SCV beam. The field junction between tangential and SCV beams was placed around the inferior edge of the clavicle head. The SCV beam(s) was not included in the subsequent fluence optimizations and dose evaluation since it is an open static MLC field.
Initial Beam Calculation
The program requires a patient-specific set of initial beam parameters to serve as a starting point for beam optimization. With the current clinical procedures, the patient-specific anatomical information needed for the initial beam calculation was acquired through wires placed during simulation and contoured PTV. The midline and lateral wires were used to determine the initial gantry angles and isocenter location. The CT0 is defined at the middle of the breast tissue range in the superior–inferior direction. During simulation, 3 radiopaque fiducial markers are placed on the patient for laser alignment, which are used to locate the origin point (CT0) of the CT coordinates. On the axial slice of the CT0, the treatment isocenter was set at the midpoint of the midline and the lateral wires. The gantry angles were determined by the beam central axis which goes through the 2 wires. The midline wire was available as a contoured structure. The lateral wire was automatically detected from the computed tomography (CT) image using thresholding methods on local image patches.
The field size includes 4 independent jaws (X1, X2, Y1, and Y2). The X jaws were opened from the beam central axis and extended 2 cm beyond the apex of the breast as the skin flash region. In the tangent only geometry, the Y jaws were opened to cover the main PTV volume, with superior and inferior field margins; in the tangent plus SCV geometry, the superior Y jaw (Y2) was instead opened to the clavicle head (the field matching location with SCV beam) regardless of the PTV, or it could also be specified by the user. In the tangent only geometry, the collimator angles were calculated based on the slope of the midline wire seen in the BEV and the couch angles were set as 0. In the tangent plus SCV geometry, the collimator and couch angles were solved analytically given the gantry angle and Y2. The initial MLC determines the shape of the beam aperture. The initial MLC leaf positions were calculated based on the PTV and OAR projections in the BEV. Several MLC options could be set by the user, including covering the IMN and blocking the heart.
Beam Optimization
With the initial beam parameters set in the initial beam calculation, the program starts to optimize the beams to fit the PTV and OARs. The beam optimization is based on 2 parameters: the gantry angle and the isocenter location for tangential beams. Since the medial and lateral beams are parallel opposed, only one gantry angle is needed for optimization; here, the gantry angle of the medial beam was used. In theory, the isocenter location has 3 degrees of freedom: X, Y, and Z coordinates. The isocenter’s Z coordinate was fixed at zero, and its location in the X–Y plane was restricted along the perpendicular line of the initial central axis, essentially reducing the dimension from three to one. The optimization goal was to find the gantry angle-isocenter pair that minimized the goal function that penalizes PTV under coverage and lung volume being irradiated. The program automatically identifies the structures from the structure list using a template of their conventional names while also allowing the user to manually select the PTVs. The jaw size was fixed during optimization, as well as the collimator and couch angles for the tangent only geometry. Multi-leaf collimators (if used for shaping the aperture), the collimator and couch angles for the tangent plus SCV geometry were dynamically updated to match the tangent field for each gantry angle-isocenter pair.
The goal function was based on the out-of-field PTV volume and in-field lung volume. It is written as
| Eq. 1 |
where Ω is the goal function. V ptv_out is the percentage out-of-field volume of the breast/chest wall PTV. V lung_in is the percentage in-field volume of the ipsilateral lung. w lung is the weighting factor of the lung volume to reflect the relative importance of 2 terms in the goal function. H(x) is the Heaviside function which takes on value of 1 and 0 for positive and negative x respectively, and 0.5 for x = 0. V heart_in is the percentage volume of the heart in the field. V th is the threshold volume for acceptable heart volume in the field, and was set to 5% by default. The infinity sign (∞) is introduced to penalize non-negative input, which means that V heart_in has to be below V th in order to have the objective function optimized.
The lung weighting factor w lung was trained from clinical plans for the tangent only geometry and tangent plus SCV geometry separately. From the 20 patients of the same beam geometry, 10 were randomly selected as the training set, and the other 10 were reserved to test the program. For each patient in the training set, the percentage volumes in Equation 1 were calculated for the clinical beam setting and automatic beam settings with different gantry angles and isocenters. The optimal w lung was identified, which minimized the training set’s sum of goal function differences between the clinical beam setting and the optimum in the automatic beam settings. The training process essentially averaged the clinical choices of the PTV-OAR trade-offs in the training set. The trained lung weighting factors are set as default values for the program, which could be easily adjusted by the user to achieve a desired trade-off between PTV and lung. In this study, the default value for each beam geometry was used for beam optimization.
The search space was a 2-dimensional grid centered at the initial beam setting. The grid size for the gantry angles and the isocenter location was 1° and 1 mm, respectively. Due to the design of the goal function and the spatial relationship between the PTV and the lung, the search space was convex with only one global minimum, which was established when an exhaustive search method was firstly experimented on the training sets. To accelerate the optimization, a local search scheme was employed in the program, where the current beam setting was moved to the optimal setting among its 4 nearest neighbors until a local optimum was reached. After the beam is optimized, the isocenter can be slightly shifted along the central axis direction to adjust the SSDs of the 2 tangential beams. Possible collision between the patient and the gantry head can be detected, and a warning would be sent to the user.
Plan Evaluation
For plan evaluation, the beam setting choices, including the use of initial MLC, IMN coverage, the use of collimator rotation for the tangent only geometry, and the field matching location for the tangent plus SCV geometry in the automatic plans were consistent with the clinical plans.
After beam optimization, the in-house fluence optimization program11 built with ESAPI was executed to produce optimal fluence maps. Either single-energy (6 MV) beams or mixed-energy (6 MV and 15 MV) beams were used for each patient, which was clinically determined by the dosimetrist and agreed by the attending physician. The beam energy choices of the automatic plans followed the clinical choice. For plan dose comparison, the prescription doses (Rx) for all patients were set at 200 cGy per fraction for 25 fractions with the total dose of 5000 cGy. For tangent plus SCV geometry, only the dose contribution from the tangential beams was compared between the clinical and automatic plans, excluding the SCV beams.
Several dose metrics were recorded from the TPS, including PTV_Eval V105% (the relative volume receiving at least 105% of the prescription dose), V100%, V95%, V90%, ipsilateral lung V20Gy, V10 Gy, V5 Gy, heart mean dose, maximum dose of body, and conformity index (CI). The CI12 is defined as the ratio of the absolute volume (of the body) covered by the 95% isodose over the absolute volume of the PTV_Eval, expressed as
| Eq. 2 |
The mean values and standard deviations of the dose metrics were calculated for the clinical and automatic plans of the training and test sets. Wilcoxon signed-rank tests were conducted with a significance level of .05.
Results
For each patient, the calculation time for beam geometry optimization varied between 10 seconds and 120 seconds, depending on the patient anatomy. The program was able to automatically produce a set of beam parameters for every patient. The total planning time of the automated treatment planning process, including fluence optimization, was less than 5 minutes for each patient, which reflected a significant decrease from manual treatment planning.
The PTV_Eval V95%, ipsilateral lung V20Gy, heart mean dose , maximum dose of body, and CI were tabulated for comparison between clinical and automatic plans in Table 1. For all testing cases, the mean value for all dose metrics except lung dose was comparable between 2 plan groups, with automatic plan achieving lower lung mean dose than the clinical plans. It should be noted from Table 1 that the mean lung V20Gy for both beam geometries were lower for the automatic plans, and that the mean PTV_Eval V95% for tangent plus SCV geometry were higher for the automatic plans, which represents improvement in both target coverage and OAR sparing.
Table 1.
Dose Metrics Comparison Between Automatic and Clinical Plans. The Numbers in Each Cell are Reported as the Mean Value (Standard Deviation).
| PTV_Eval V95%, % | Lung V20Gy, % | Heart , % Rx | Maximum Dose, % Rx | CI | ||
|---|---|---|---|---|---|---|
| Tangent test set | Clinical | 94.4 (3.5) | 12.9 (5.4) | 2.2 (1.4) | 108.7 (1.4) | 1.40 (0.16) |
| Auto | 94.5 (2.7) | 9.8 (5.1) | 2.0 (1.0) | 109.1 (0.6) | 1.32 (0.10) | |
| P value | 0.846 | 0.049a | 0.416 | 0.516 | 0.062 | |
| SCV test set | Clinical | 82.6 (7.8) | 23.0 (8.6) | 2.4 (1.5) | 109.2 (2.9) | 1.43 (0.52) |
| Auto | 87.5 (6.2) | 20.7 (8.0) | 2.7 (1.6) | 108.5 (1.4) | 1.45 (0.54) | |
| P value | 0.037a | 0.232 | 0.049a | 0.316 | 0.977 | |
| All test sets | Clinical | 88.5 (8.4) | 17.9 (8.7) | 2.3 (1.4) | 108.9 (2.2) | 1.41 (0.38) |
| Auto | 91.0 (5.9) | 15.2 (8.6) | 2.4 (1.4) | 108.8 (1.1) | 1.38 (0.38) | |
| P value | 0.079 | 0.025a | 0.466 | 0.727 | 0.239 | |
Abbreviations: CI, conformity index; PTV, planning target volume; Rx, prescription dose; SCV, supraclavicular.
aIndicates statistical significance.
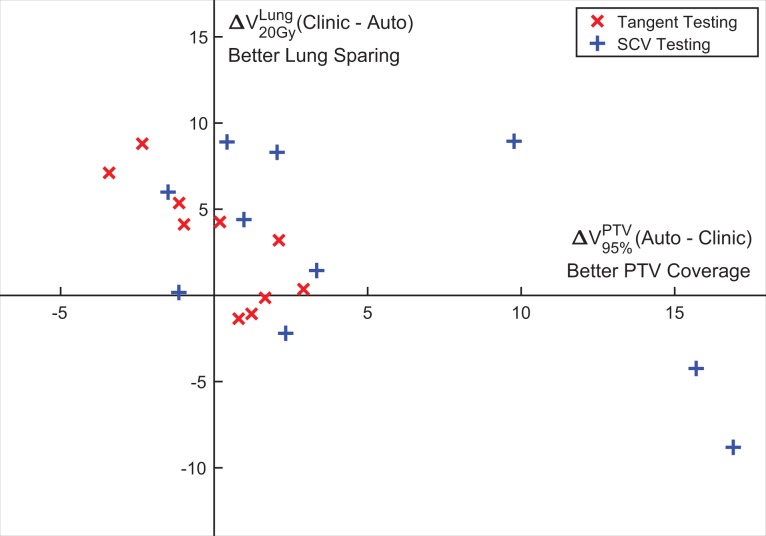
To evaluate the combined performance of the PTV coverage and lung sparing, which are typically trade-off criteria, the ipsilateral lung V20Gy difference is plotted case by case against the PTV_Eval V95% difference for all testing cases in Figure 3. As can be seen, the automatic plan achieved both superior PTV coverage and better lung sparing for 8 of 20 patients (upper-right quadrant). An example case in this quadrant is shown in Figure 4. The goal-driven beam optimization can improve lung sparing without compromising PTV coverage. With different gantry angles and isocenter location from the clinical plan, the irradiated normal tissue volume was reduced. For the 6 patients in the upper-left quadrant, trade-off favored more lung sparing than PTV coverage for the automatic plan. For the 6 patients in the lower right quadrant, lung dose was higher while better PTV coverage was provided. No automatic plan showed inferior PTV coverage and worse lung sparing. The 2 beam geometries are separated by color, with red being tangent only geometry and blue being tangent plus SCV geometry. All but 3 tangent plus SCV geometry cases achieved similar PTV coverage with PTV V95% differences within ±5%. The 3 outlier cases had considerably higher (>9%) PTV V95% for the automatic plans, and 2 of them had increased lung dose.
Figure 3.
Planning target volume (PTV) coverage (PTV_Eval V95%) and lung sparing (ipsilateral lung V20Gy) comparison between automatic plans and clinical plans. The horizontal axis is the difference (automatic-clinical) of PTV_Eval V95%, and positive direction on the axis means better PTV coverage; the vertical axis is the difference (clinical-automatic) of ipsilateral lung V20Gy, and positive direction on the axis means better lung sparing. Different patient groups are separated and denoted with different markers as shown in the legend.
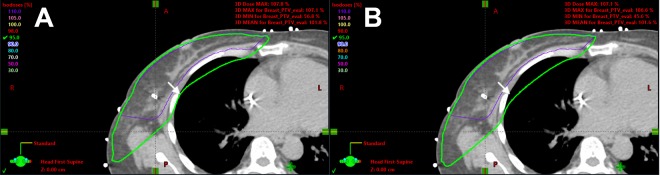
Figure 4.
Dose distribution comparison in the same axial slice of one example case between (A) the automatic plan and (B) the clinical plan. The thick green line is the 95% isodose line, and the thin purple line is the PTV_Eval contour on this slice. The white arrows point to the same position at the edge of the lung (note the distance to the 95% isodose line). This case shows how the automatic plan improves the sparing of the lung without sacrificing the PTV coverage. PTV indicates planning target volume.
One example case for demonstrating PTV coverage and lung sparing trade-off is shown in Figure 5. This case falls in the lower-right quadrant in Figure 3. It illustrates how beam geometry can affect the dose distribution and the PTV-lung balance. The posterior field border in the automatic plan (5A) was placed deeper into the ipsilateral lung than the clinical plan (5B), in exchange of better PTV coverage. By increasing the lung weighting factor from the default 0.05 to 0.2, the program produced another automatic plan (5C), which was in between the previous 2 plans (5A and 5B) in terms of PTV-lung balance.
Figure 5.
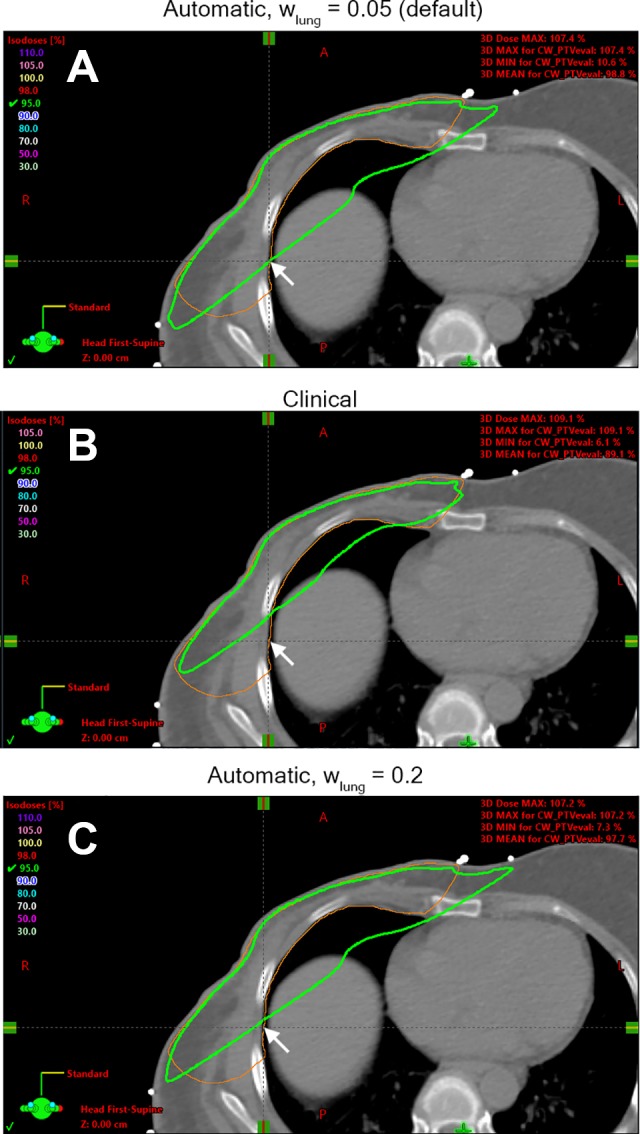
Dose distribution in the same axial slice of one example case in the automatic plan with default lung weighting factor of 0.05 (A), the clinical plan (B), and the automatic plan with increased lung weighting factor of 0.2 (C). The thick green line is the 95% isodose line, and the thin orange line is the PTV_Eval contour on this slice. The arrows point to the same position at the edge of the PTV_Eval contour. This case shows how the automatic plan improves the PTV coverage at the expense of increasing the lung dose and how the lung weighting factor affects the dose coverage. PTV indicates planning target volume.
Discussion
This study developed an automated beam geometry optimization program that enables the user to quickly generate a customizable and clinically feasible beam geometry for WBRT treatment planning. The customizable user settings add flexibility to the beam geometry and can easily adapt to most clinical scenarios. The adjustable lung weighting allows the user to fine-tune the balance between PTV coverage and lung sparing. The study is of substantial clinical significance given that it dramatically reduces the time required for WBRT treatment planning and improves the clinical efficiency. As part of a subsequent prospective clinical study which analyzes the proposed auto-planning scheme with current clinical practice in terms of quality and efficiency, 30 whole breast or chest wall cases were included. We will not expand the detail about this study since it is not the core of the current one. But it is worth mentioning that mean (standard deviation) time for beam placement and fluence optimization was 110.2 (62.8) minutes for manual planning and 6.4 (2.1) minutes for auto-planning which demonstrates substantial efficiency improvement while plan quality is similar (no statistical significance). This work also reduces interhuman variation, as its underlying parameters reflect clinical importance. Together with the automated fluence optimization program, an automatic treatment planning workflow for WBRT can be integrated into the Eclipse TPS to replace the routine breast treatment planning manual process.
From the data in Table 1, the tangent only geometry cases generally have better PTV coverage and lung sparing compared to the tangent plus SCV geometry cases. The reasons for this observation are 2-fold. Firstly, if portions of the PTV extend superior to the clavicle head in the tangent plus SCV geometry, they are covered by the SCV beam(s) instead of the tangential beams. Since the dose evaluation only included the tangential beams, this part of the PTV receives no primary dose from the plans in this study. Secondly, most clinical plans of the tangent plus SCV geometry cover the PTV including the IMN, which extends more medially than the PTV in tangent only geometry. This part of the target significantly overlaps with the OARs (lung and heart) seen from the BEV, resulting in higher lung and heart dose.
In many clinical plans of the tangent only geometry, the entire PTV was covered by the beams, because the OAR dose constraints were more easily satisfied as the PTV-OAR overlaps in the BEV were smaller. The irradiated lung volume was not an issue for these easier cases. In most clinical plans of the tangent plus SCV geometry; however, PTV coverage was compromised in order to restrict OAR doses. For these more difficult cases, the planner was required to achieve a balance between PTV coverage and OAR sparing. In the clinical plans, the average percentage out-of-field PTV volume and in-field ipsilateral lung volume are 0.93% and 13.12% for the tangent only geometry, and 4.53% and 29.85% for the tangent plus SCV geometry. The freedom to adjust the lung weighting factor allows the planner to choose different priorities as shown in Figure 5. Therefore, the target differences justify the use of different lung weighting factors in the goal function for the 2 beam geometries.
The program is designed to be customizable and flexible. It is feasible to handpick a group of similar training cases to train the lung weighting factor which tailors to a specific clinical scenario or an individual planner. Since the patients in this study were randomly selected from the same patient cohort, the clinical plans include a small range of clinical preferences (PTV-OAR balance). As the trained lung weighting factor reflects the averaged clinical preference, the size of the training data set does not significantly affect the training result in this study. To validate the default lung weighting factor and investigate the training data size impact on the model performance, for each beam geometry, the training process was repeated 10 times on 19 cases (10 original training cases and 9 testing cases, with 1 testing case left out each time). The average lung weighting factors (standard deviation) trained from 19 cases were 0.012 (0.002) for tangent only geometry and 0.059 (0.004) for tangent plus SCV geometry, while the default values trained from 10 cases are 0.02 and 0.05. A planner can also explore the automatic plan space by experimenting with different parameters and settings within minutes and choose the optimal one based on clinical judgment. However, the MLC shape algorithm is currently not affected by the lung weighting factor, which limits the variability of the beam aperture shape given different weighting factors. In general, the optimization result is not sensitive to small changes in the lung weighting factor but depends heavily on the beam geometry settings (eg, use of MLC, heart block). Even if the planner is not completely satisfied with the automatic plan, it is easy to manually fine-tune the beam parameters and/or MLC shapes within the TPS before fluence map optimization.
One important feature of the program is its dependence on the structure contours. The program calculates the 3-dimensional spatial relationship between the beams and relevant structures. For the same patient anatomy, the automatically generated beam geometry could be different if the target and OAR contours were drawn differently. As the contours were manually delineated, even if the same guideline is followed, there could be variation in contours among different operators, especially for the PTV. Therefore, automatic contouring will further improve planning efficiency and the consistency of plan quality. One limitation of current form of beam optimization is that it is looking for breast wires to start with. Breast wire is placed during CT simulation to mark the boundary of the breast tissue. However, variations in the wire placement would not significantly affect the beam optimization result as they are only used to determine the optimization starting point and the collimator angles in the tangent only geometry. In order to deploy this work, additional steps of wire placement and PTV contouring need to be added if not previously implemented. It would be of interest to develop beam optimization without wire, which offers greater efficiency and research along this line is warranted. The proposed implementation of beam optimization does not waive the necessity of other procedures in the treatment chain, such as CT simulation, chart checking, treatment simulation or IMRT QA.
Conclusion
An automated goal-driven beam setting optimization program for WBRT is developed in this study. The program is able to produce optimized beams for all patients. Together with the fluence optimization program, automated treatment planning for WBRT is made possible. Plans with automatically generated beam settings achieved comparable plan quality as manually generated clinical plans in terms of PTV coverage, OAR sparing, dose homogeneity, and dose conformity. This program offers a valuable tool for WBRT treatment planning, as it provides clinically relevant solutions based on previous clinical practice as well as patient specific anatomy under a substantially faster time frame.
Abbreviations
- BEV
beam’s eye view
- CB
contralateral breast
- CI
conformity index
- CTV
clinical target volume
- ESAPI
Eclipse scripting application programming interface
- IMN
internal mammary node
- IMRT
intensity-modulated radiation therapy
- MLC
multi-leaf collimator
- OAR
organ-at-risk
- PTV
planning target volume
- QA
quality assurance
- SAD
source to axis distance
- SCV
supraclavicular
- SSD
source to surface distance
- TPS
treatment planning system
- WBRT
whole-breast radiation therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Q. Jackie Wu received NIH R01CA201212 research grant.
ORCID iD: Wentao Wang, MS  https://orcid.org/0000-0003-4216-4938
https://orcid.org/0000-0003-4216-4938
Q. Jackie Wu, PhD  https://orcid.org/0000-0001-8235-2322
https://orcid.org/0000-0001-8235-2322
References
- 1. American College of R. Practice guideline for the breast conservation therapy in the management of invasive breast carcinoma. J Am Coll Surg. 2007;205(2):362–376. [DOI] [PubMed] [Google Scholar]
- 2. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 3. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. [DOI] [PubMed] [Google Scholar]
- 4. Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. [DOI] [PubMed] [Google Scholar]
- 5. Group EBCTC. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2006;366(9503):2087–2106. [DOI] [PubMed] [Google Scholar]
- 6. Bentel GC. Radiation Therapy Planning. 2nd ed New York, NY: McGraw-Hill, Health Professions Division; 1996. [Google Scholar]
- 7. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. [DOI] [PubMed] [Google Scholar]
- 8. Purdie TG, Dinniwell RE, Letourneau D, Hill C, Sharpe MB. Automated planning of tangential breast intensity-modulated radiotherapy using heuristic optimization. Int J Radiat Oncol Biol Phys. 2011;81(2):575–583. [DOI] [PubMed] [Google Scholar]
- 9. Purdie TG, Dinniwell RE, Fyles A, Sharpe MB. Automation and intensity modulated radiation therapy for individualized high-quality tangent breast treatment plans. Int J Radiat Oncol Biol Phys. 2014;90(3):688–695. [DOI] [PubMed] [Google Scholar]
- 10. Vicini F, Freedman GM, White JR. A phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer. RTOG 1005. [Google Scholar]
- 11. Sheng Y, Li T, Yoo S, et al. Development of an ultra-fast, high-quality whole-breast radiation therapy treatment planning system. Int J Radiat Oncol Biol Phys. 2016;96(2):S228. [Google Scholar]
- 12. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans - Technical note. J Neurosurg. 2000;93:219–222. [DOI] [PubMed] [Google Scholar]