Abstract
Background
Pharmacological thromboprophylaxis after colorectal cancer (CRC) surgery is internationally recommended for venous thromboembolism (VTE) prevention. The aim of this retrospective study was to evaluate the risk factors of postoperative bleeding after elective surgery for patients with primary CRC receiving pharmacological thromboprophylaxis of fondaparinux or enoxaparin.
Methods
We experienced consecutive 266 patients who underwent elective surgery for CRC during the study period. Finally, the medical records of 218 patients with CRC administrated fondaparinux or enoxaparin following surgery were retrospectively reviewed to evaluate symptomatic VTE until 28 days and postoperative bleeding comparing perioperative D-dimer levels.
Results
The significant differences in TNM classification staging and type of thromboprophylaxis were observed between postoperative bleeding-negative and bleeding-positive group. There was no statistical significance among other backgrounds of patients between the two groups. One case (0.46%) of symptomatic VTE and total 11 cases (5%) of postoperative bleeding were observed. In the univariate analysis, fondaparinux thromboprophylaxis and early disease-stage CRC (stages 0 and I) were associated with risk for postoperative bleeding. Multivariate analysis revealed that fondaparinux thromboprophylaxis was identified as an independent risk factor of postoperative bleeding. Moreover, preoperative levels of D-dimer in patients with stage IV CRC were significantly higher than those with the other stages. The significant elevation in preoperative D-dimer was also observed in patients with stage II CRC compared to those with stage I CRC. Perioperative levels of D-dimer in patients with advanced disease-stage CRC (stages II, III, and IV) were significantly higher than those in patients with early disease-stage CRC.
Conclusions
Fondaparinux administration and early disease-stage CRC appeared to be risk factors for postoperative bleeding in patients with pharmacological thromboprophylaxis undergoing surgical treatment for CRC. Patients’ hypercoagulative condition depending on disease progression of CRC might be related to the occurrence of postoperative bleeding following CRC surgery.
Keywords: Bleeding, Complications, Antithrombotic prophylaxis, Fondaparinux, Anoxaparin, D-dimer
Background
Since venous thromboembolism (VTE) can result in fatal outcomes due to pulmonary thromboembolism, it is important to prevent VTE for patients undergoing abdominal surgery [1]. Recent guidelines recommended using physiological therapies such as intermittent pneumatic compression (IPC) and elastic stockings on the lower legs or pharmacological thromboprophylaxis according to patient risk level following abdominal major surgery [2]. There is a growing body of evidence supporting a symbiotic relationship between the clotting system and the biology of cancer. Colorectal cancer (CRC) leads to increased activation of the clotting system which manifests VTE; therefore, pharmacological thromboprophylaxis after CRC surgery is internationally recommended for VTE prevention [3, 4]. Two anticoagulants, fondaparinux (a synthetic Xa inhibitor) [5] and enoxaparin (a low molecular weight heparin) [6–9], are available for use in the clinical setting for such patients in Japan. A recent systematic review demonstrated that there was no difference between perioperative thromboprophylaxis with enoxaparin compared with fondaparinux in their effects on mortality, thromboembolic outcomes, major bleeding, or minor bleeding in people with cancer [10]; however, the risk factors associated with postoperative bleeding in patients with pharmacological thromboprophylaxis following CRC surgery remain unknown. Therefore, we retrospectively evaluated the risk factors of postoperative bleeding after elective surgery for patients with primary CRC receiving perioperative pharmacological thromboprophylaxis comparing perioperative D-dimer levels.
Methods
Patients
We experienced consecutive 266 patients who received elective surgery for treatment of primary CRC from June 2010 to December 2013 at the Shiga University of Medical Science Hospital. Patients received recommended VTE preventive treatments according to their classification of risk by type of surgery, found in the Japanese Circulation Society (2009) guidelines [2]. Patients with a greater than high risk received IPC and pharmacological thromboprophylaxis. Thirty-six patients who received unfractionated heparin prior to surgery due to arrhythmia and cardiac and cerebral vascular lesions were excluded in this study. Nine patients with high risk and three patients with a lesser than intermediate risk who received only IPC without anticoagulants were also excluded in this study. Finally, 218 patients were included in this retrospective analysis. Out of 218 patients, 151 patients received enoxaparin as thromboprophylaxis at a dose of 2000 IU × 2/day from August 2010 to May 2012 and from May 2013 to December 2013, and 67 patients were also administered fondaparinux at a dose of 1.5 or 2.5 mg/day according to their renal function from June 2012 to April 2013. The epidural anesthesia catheter was removed at the second postoperative day (POD2). After a minimum of 2 h post-removal of the epidural catheter, the anticoagulants were administrated until POD7. The medical records were retrospectively reviewed to evaluate symptomatic VTE and postoperative bleeding. Patients’ clinicopathological parameters and routine laboratory data (serum creatinine, alanine aminotransferase, total-bilirubin, carcinoembryonic antigen, carbohydrate antigen 19-9, and D-dimer) were also retrospectively collected. This study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. This study was approved by the ethical committee of Shiga University of Medical Science (approved IRB number R2017-021).
Evaluation of postoperative bleeding and VTE
The evaluation of postoperative bleeding was divided into two categories as described previously [11, 12]. Briefly, a major bleeding was classified as an event which met at least one of the following definitions: fatal bleeding, retroperitoneal or intracranial bleeding, bleeding of critical organs (intraocular, adrenal, endocardial, intestinal or spinal bleeding), surgical site bleeding that required surgical intervention, clinically overt bleeding with a decrease in hemoglobin by at least 20 g/L, or the need for a transfusion of two units of red blood cells or whole blood. Minor bleeding was defined as a hemorrhage that did not meet any of the major bleeding criteria.
The symptomatic VTE were reviewed until POD28 to evaluate the efficacy of VTE preventive treatments. Whole body-enhanced CT scan of the neck to the lower legs was performed to rule out VTE when the symptoms (dyspnea, chest pain, edema, pain in lower legs, etc.) of VTE were observed. The adverse events and postoperative complications were evaluated according to Common Terminology Criteria for Adverse Events version 4.0 (CTCAE ver.4) and Clavien-Dindo classification [13].
Statistical analysis
The continuous variables were expressed as median (25th–75th quartile) and analyzed with the Wilcoxon signed-rank test. The categorized variables were analyzed with Fisher’s exact chi-squared tests. Univariate and multivariate logistic regression analysis was used to identify independent risk factors for postoperative bleeding. We followed standard methods to estimate sample size for multivariate logistic regression, with at least ten outcomes needed for each included independent variable [14, 15]. An expected bleeding event rate was 10%, which was calculated from data in the previous studies that evaluated bleeding events in patients administrated fondaparinux and enoxaparin after CRC surgery [16–21]. We estimated to require 200 attempts (20 bleeding events) to appropriately perform multiple logistic regression with two variables. A p value of < 0.05 was considered to indicate statistical significance. Statistical analyses were carried out using JMP 9.0.2 (SAS Institute Inc., NC, USA)
Results
Patients’ background
Fondaparinux prophylaxis was often used compared to enoxaparin in the postoperative bleeding-positive group; therefore, we included the type of pharmacological thromboprophylaxis agents in the univariate and multivariate analysis. We also observed a statistically significant difference in TNM classification stage between two groups (Table 1). There was no statistical significance among backgrounds of patients, preoperative laboratory examinations, and the incidence of preoperative comorbidities between postoperative bleeding-negative and bleeding-positive groups. No statistical significance was observed in surgical approach (open and laparoscopic), blood loss, duration of surgery (open and laparoscopic), use of epidural anesthesia, and tumor location (right colon, left colon, and rectum) between the two groups (Table 1).
Table 1.
Clinical characteristics of the patients
| Postoperative bleeding (−), N = 207 | Postoperative bleeding (+), N = 11 | p value | |
|---|---|---|---|
| Backgrounds of patients | |||
| Age (years) | 69 (61–74) | 65 (60–74) | 0.6270 |
| Gender (male:female) | 122:85 | 8:3 | 0.3524 |
| Height (cm) | 159.8 (152.7–166.6) | 166.0 (154.1–170.8) | 0.3006 |
| Weight (kg) | 54.7 (49.7–62.8) | 64.0 (51.9–70.3) | 0.0958 |
| ASA-PS classification (1:2:3) | 80:122:5 | 4:6:1 | 0.5673 |
| Preoperative laboratory examinations | |||
| Platelet (104/mm3) | 23.3 (18.3–26.9) | 25.7 (21.5–28.6) | 0.1377 |
| eGFR | 73.3 (61.3–85.8) | 69.3 (64.2–78.3) | 0.8283 |
| Serum ALT (IU/L) | 17 (11–24) | 20 (14–26) | 0.2967 |
| Serum T-Bil (IU/L) | 0.62 (0.50–0.92) | 0.74 (0.46–0.77) | 0.6609 |
| D-dimer (μ/mL) | 0.55 (0.3–1.1) | 0.4 (0.3–0.7) | 0.5690 |
| CEA (ng/mL) | 5.3 (2.8–11.8) | 4.8 (2.5–11.3) | 0.8202 |
| CA19-9 (U/mL) | 14 (8–23) | 21 (10–40) | 0.3140 |
| Preoperative comorbidities | |||
|
Number of comorbidities (4:3:2:1:0) |
1:3:15:59:129 | 0:1:0:3:7 | 0.4854 |
| Hypertension | 162 | 4 | |
| Diabetes mellitus | 29 | 2 | |
| Cerebrovascular disease | 7 | 1 | |
| Ischemic heart disease | 2 | 0 | |
| Respiratory disease | 9 | 0 | |
| Surgical and tumor characteristics | |||
| Approach (open:laparoscopic) | 80:127 | 2:9 | 0.1505 |
| Blood loss (g) | 162 (50–403) | 170 (50–229) | 0.8022 |
| Duration of surgery | |||
| Open (min) | 223 (181–408) | 379 (162–597) | 0.7841 |
| Laparoscopic (min) | 284 (227–342) | 237 (216–313) | 0.3000 |
| Use of epidural anesthesia, n (%) | 189 (91.3%) | 10 (90.9%) | 0.9641 |
|
Type of anticoagulants (fondaparinux:enoxaparin) |
60:147 | 7:4 | 0.0374 |
|
Tumor location (Rt colon:Lt colon:Rectum) |
58:57:92 | 4:2:5 | 0.8280 |
|
TNM classification (stage 0:I:II:III:IV) |
11:51:52:58:35 | 1:6:1:0:3 | 0.0267 |
Data was presented as median (25th–75th percentile) and number (%). BMI body mass index, ASA-PS American Society of Anesthesiologists—physical status, eGFR estimated glomerular filtration rate, ALT alanine aminotransferase, T-Bil total-bilirubin, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, TNM classification UICC classification ver.7 Staging for adenocarcinoma
Postoperative VTE and bleeding
Total 11 cases (5%) of postoperative bleeding, one case (0.46%) of major bleeding and ten cases (4.6%) of minor bleeding, were observed. The details of minor bleeding were four cases of intestinal bleeding, three cases of subcutaneous hematoma, and three cases of intra-abdominal bleeding. The discontinuation of anticoagulants due to postoperative bleeding was required in nine patients (4.1%). The univariate analysis revealed that early disease stage of CRC (stages 0 and I) and fondaparinux prophylaxis were associated with risk for postoperative bleeding in our study cohort. Multivariate analysis demonstrated that fondaparinux prophylaxis was identified as an independent risk factor for postoperative bleeding (Table 2). There were no cases of fatal bleeding during the study period. One case (0.46%) of symptomatic VTE was observed in a patient with the enoxaparin prophylaxis during the observational period.
Table 2.
Univariate and multivariate analysis for risk factors of postoperative bleeding
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| Platelet | ≤ 19.8 × 104/mm3 | 1 (reference) | |||
| > 19.8 × 104/mm3 | 4.892 (0.909–90.67) | 0.0674 | |||
| Blood loss | ≤ 290 g | 1 (reference) | |||
| > 290 g | 0.209 (0.011–1.225) | 0.0720 | |||
| Approach | Laparoscopic | 1 (reference) | |||
| Open | 0.352 (0.053–1.412) | 0.1505 | |||
| TNM classification | Stages II, III, and IV | 1 (reference) | 1 (reference) | ||
| Stages 0 and I | 4.092 (1.192–16.09) | 0.0252 | 2.683 (0.737–11.00) | 0.1345 | |
| Type of anticoagulants | Enoxaparin | 1 (reference) | 1 (reference) | ||
| Fondaparinux | 4.287 (1.248–16.87) | 0.0209 | 4.864 (1.174–17.25) | 0.0310 | |
Alteration in D-dimer
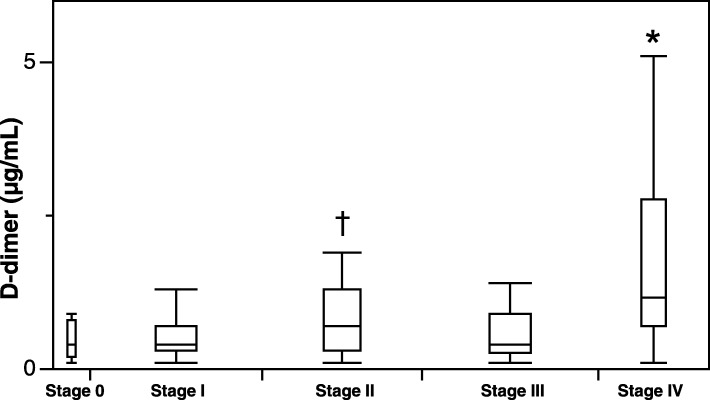
Preoperative levels of D-dimer in patients with stage IV CRC were significantly higher than those in patients with the other stages. The significant elevation in preoperative D-dimer was also observed in patients with stage II CRC compared to those in patients with stage I CRC (Fig. 1).
Fig. 1.
Preoperative levels of D-dimer according to the pathological TNM classification of CRC. *p < 0.05 vs. stages 0, I, II, and III. †p < 0.05 vs. stages I
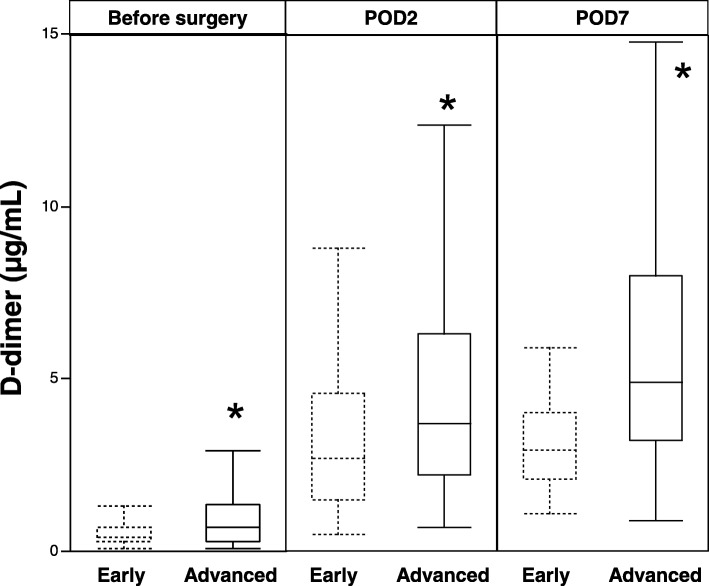
The levels of D-dimer before surgery, at second day, and at seventh day after surgery in patients with advanced disease stage of CRC (stages II, III, and IV) were significantly higher than those in patients with early disease stage of CRC (stages 0 and I) (Fig. 2).
Fig. 2.
Perioperative levels of D-dimer according to disease stage of CRC. Early, early disease-stage CRC; advanced, advanced disease-stage CRC. *p < 0.05 vs. early disease-stage CRC
Discussion
The importance of VTE prevention is widely recognized in clinical medicine, since VTE can result in severe outcomes such as pulmonary embolism. In addition, the incidence of postoperative VTE following abdominal surgery in Japan has been increasing in recent years due to the change in lifestyle to a more sedentary Western lifestyle and improvements in the diagnostic accuracy of VTE [22, 23]. Both fondaparinux and enoxaparin demonstrate efficacy and safety for the prevention of VTE after abdominal surgery for malignancies [24]. A recent systematic review concluded that the risk of VTE in Asian general surgery patients is likely to be low [25]. These studies included patients with and without in-hospital anticoagulant administration. Kim et al. reported that the incidence of VTE was 3.7% (31/840 patients) in patients undergoing colorectal cancer surgery without perioperative anticoagulant prophylaxis [26]. The incidence of symptomatic VTE in patients with enoxaparin prophylaxis reportedly ranged from 0.43 to 1.4% [13, 27, 28] and 0 to 23.3% in patients with fondaparinux prophylaxis [16, 21, 29, 30] following abdominal surgery. One case (0.46%) of VTE was observed during our observational period. Thus, our findings were almost similar to the incidences of symptomatic VTE found in previous studies. However, the number of patients enrolled in this study seemed to be too small to compare the efficacy of the two anticoagulants prophylaxis in this study.
The latest meta-analysis demonstrated that fondaparinux was significantly more effective in preventing VTE after total hip replacement therapy when compared to enoxaparin, although this effectiveness was accompanied with an increased risk of major postoperative bleeding [31]. Only a few studies have compared the efficacy and safety between fondaparinux and enoxaparin in the same study cohorts following abdominal surgery. In those studies, postoperative bleeding events were reported as 21.2 % in the enoxaparin group and 37.6% in the fondaparinux group after major hepatobiliary-pancreatic surgery in Japanese patients [32]. Total postoperative bleeding was also observed at 11.5 % in the enoxaparin group and 22.2% in the fondaparinux group among the adult cancer population [19]. The postoperative transfusions for major postoperative bleeding increased by 0.9% in the fondaparinux group compared to 0.2% in the enoxaparin group following thoracic surgery [27]. According to previous comparison studies, postoperative bleeding after surgery was likely to be more frequent in the fondaparinux group than in the enoxaparin group. The incidence of postoperative bleeding following colorectal surgery in previous studies ranged from 4.6 to 11.5% in patients with enoxaparin prophylaxis [17–20, 33] and 9.4 to 22.2% in patients with fondaparinux prophylaxis [16, 19, 21]. Our findings of major and minor bleeding in fondaparinux (10.4%) and enoxaparin (2.6%) prophylaxis are comparable to results from previous studies.
A recent study demonstrated the risk factors of postoperative bleeding in CRC patients administrated fondaparinux as thromboprophylaxis after surgery. They have shown that, in multivariate analysis, male gender, intraoperative blood loss of less than 25 mL, and a preoperative platelet count below 15 × 104/μL were found to be independent risk factors for postoperative bleeding in the laparoscopic surgery group. Only the preoperative platelet count was an independent risk factor in the open surgery group [34]. We could not evaluate risk factors of postoperative bleeding differently between open and laparoscopic surgery due to the small number of patients in the present study. Moreover, they interestingly showed intraoperative blood loss as a negative impact for postoperative bleeding complication; however, we could not also find that intraoperative blood loss was significantly associated with negative risk for postoperative bleeding in univariate analysis.
The abnormalities of coagulation and fibrinolysis are frequently observed in cancer patients. A recent meta-analysis suggested that plasma D-dimer and fibrinogen levels were correlated with tumor stage and prognosis in digestive cancer patients [35–37]. High pretreatment plasma D-dimer was reported to predict poor survival of colorectal cancer [38, 39]. We also observed the elevation in preoperative and postoperative D-dimer levels as the increase in disease stage in our patients. Previous clinical studies have suggested that preoperative D-dimer may also predict the development of postoperative VTE [40, 41]. Therefore, advanced CRC patients with elevated levels of D-dimer have a high risk of VTE following colorectal surgery. On the contrary, we did not observe such hypercoagulation status in early disease-stage CRC patients in their clinical course. Our findings revealed that early disease-stage CRC was one of the risk factors for postoperative bleeding by univariate analysis. The hypercoagulation status accompanied with progression of CRC appeared to be related to postoperative bleeding following CRC surgery in patients with pharmacological thromboprophylaxis.
We acknowledge several limitations of the current study that was a retrospective, non-randomized, and small-number cohort study. First, generally, the number of events per variable (EPV) analyzed in logistic regression analysis is recommended as 10 or greater in the previous studies [14, 15]. The statistical power appeared to be relatively weak, since EPV in the present study (11 events per two variables) was lower than 10 in multiple logistic regression analysis. However, recent research has reported the possibility of relaxing the rule of 10 EPV in logistic and Cox regression analysis. Moreover, power calculation (1-β err prob) of multiple regression in this study was 0.8439 (effect size f2 0.05, α err prob 0.05, sample size 218, number of predictors 2), which was analyzed by G3*Power 3.1 [42]. Therefore, we believe that logistic regression analysis with 5-9 EPV in the present study was acceptable. Second, we found the significant difference in the incidence of postoperative bleeding between the two anticoagulants. We adapted enoxaparin as a pharmacological thromboprophylaxis for high-risk patients following CRC surgery in 2010. We switched enoxaparin to fondaparinux from June 2012 to April 2013, because enoxaparin must be administrated twice injection daily in contrast to once injection daily of fondaparinux in Japan. However, we often observed minor bleeding complications with the administration of fondaparinux. Therefore, we reinstated enoxaparin prophylaxis and found that the incidence of minor hemorrhagic complications with the enoxaparin was similar to the first term of enoxaparin administration. We could not continuously use fondaparinux for patients ethically after our finding the significant risk of bleeding events in patients administrated fondaparinux until the achievement in enough statistical power between two drugs. Although fondaparinux prophylaxis was identified as independent risk factors for postoperative bleeding in our study, additional prospective studies are needed to clarify the significant and true risk factors for bleeding complications between the two drugs.
Conclusion
Fondaparinux administration and early disease-stage CRC appeared to be risk factors for postoperative bleeding in patients with pharmacological thromboprophylaxis undergoing surgical treatment for CRC. The coagulation status might be related to the occurrence of postoperative bleeding in patients with early disease-stage CRC according to perioperative D-dimer levels.
Acknowledgements
Not applicable
Abbreviations
- CRC
Colorectal cancer
- IPC
Intermittent pneumatic compression
- POD2
Second postoperative day
- VTE
Venous thromboembolism
Authors’ contributions
HO, TM, TS, HS, TU, SK, TY, HI, and MT analyzed and interpreted the patient data regarding colorectal cancer. HO, TM, and TS were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the funding body of the Department of Surgery, Shiga University of Medical Science.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethical committee of Shiga University of Medical Science (approved IRB number R2017-021).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bergqvist D. Low molecular weight heparin for the prevention of venous thromboembolism after abdominal surgery. Br J Surg. 2004;91:965–974. doi: 10.1002/bjs.4639. [DOI] [PubMed] [Google Scholar]
- 2.Group JCSJW Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009) Circ J. 2011;75:1258–1281. doi: 10.1253/circj.CJ-88-0010. [DOI] [PubMed] [Google Scholar]
- 3.Khalil J, Bensaid B, Elkacemi H, Afif M, Bensaid Y, Kebdani T, Benjaafar N. Venous thromboembolism in cancer patients: an underestimated major health problem. World J Surg Oncol. 2015;13:204. doi: 10.1186/s12957-015-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees PA, Clouston HW, Duff S, Kirwan CC. Colorectal cancer and thrombosis. Int J Colorectal Dis. 2018;33:105–108. doi: 10.1007/s00384-017-2909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handschin AE, Trentz O, Kock HJ, Wanner GA. Low molecular weight heparin-induced skin necrosis-a systematic review. Langenbecks Arch Surg. 2005;390:249–254. doi: 10.1007/s00423-004-0522-7. [DOI] [PubMed] [Google Scholar]
- 6.Ercan M, Bostanci EB, Ozer I, Ulas M, Ozogul YB, Teke Z, Akoglu M. Postoperative hemorrhagic complications after elective laparoscopic cholecystectomy in patients receiving long-term anticoagulant therapy. Langenbecks Arch Surg. 2010;395:247–253. doi: 10.1007/s00423-009-0483-y. [DOI] [PubMed] [Google Scholar]
- 7.Imamura H, Adachi T, Kitasato A, Tanaka T, Soyama A, Hidaka M, Fujita F, Takatsuki M, Kuroki T, Eguchi S. Safety and efficacy of postoperative pharmacologic thromboprophylaxis with enoxaparin after pancreatic surgery. Surg Today. 2017;47:994–1000. doi: 10.1007/s00595-017-1471-4. [DOI] [PubMed] [Google Scholar]
- 8.Yanagita T, Kusanagi H. Safety and effectiveness of enoxaparin as venous thromboembolism prophylaxis after gastric cancer surgery in Japanese patients. Am Surg. 2016;82:1232–1237. [PubMed] [Google Scholar]
- 9.Ernits M, Mohan PS, Fares LG, 2nd, Hardy H., 3rd A retroperitoneal bleed induced by enoxaparin therapy. Am Surg. 2005;71:430–433. [PubMed] [Google Scholar]
- 10.Matar CF, Kahale LA, Hakoum MB, Tsolakian IG, Etxeandia-Ikobaltzeta I, Yosuico VE, Terrenato I, Sperati F, Barba M, Schunemann H, Akl EA. Anticoagulation for perioperative thromboprophylaxis in people with cancer. Cochrane Database Syst Rev. 2018;7:CD009447. doi: 10.1002/14651858.CD009447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 15.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 16.Hata T, Yasui M, Murata K, Okuyama M, Ohue M, Ikeda M, Ueshima S, Kitani K, Hasegawa J, Tamagawa H, et al. Safety of fondaparinux to prevent venous thromboembolism in Japanese patients undergoing colorectal cancer surgery: a multicenter study. Surg Today. 2014;44:2116–2123. doi: 10.1007/s00595-014-0911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho YH, Seow-Choen F, Leong A, Eu KW, Nyam D, Teoh MK. Randomized, controlled trial of low molecular weight heparin vs. no deep vein thrombosis prophylaxis for major colon and rectal surgery in Asian patients. Dis Colon Rectum. 1999;42:196–202. doi: 10.1007/BF02237127. [DOI] [PubMed] [Google Scholar]
- 18.McLeod RS, Geerts WH, Sniderman KW, Greenwood C, Gregoire RC, Taylor BM, Silverman RE, Atkinson KG, Burnstein M, Marshall JC, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438–444. doi: 10.1097/00000658-200103000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves D, Liu CY. Retrospective evaluation of venous thromboembolism prophylaxis in the adult cancer population. J Oncol Pharm Pract. 2010;16:27–31. doi: 10.1177/1078155209104379. [DOI] [PubMed] [Google Scholar]
- 20.Simonneau G, Laporte S, Mismetti P, Derlon A, Samii K, Samama CM, Bergman JF, Investigators FXS. A randomized study comparing the efficacy and safety of nadroparin 2850 IU (0.3 mL) vs. enoxaparin 4000 IU (40 mg) in the prevention of venous thromboembolism after colorectal surgery for cancer. J Thromb Haemost. 2006;4:1693–1700. doi: 10.1111/j.1538-7836.2006.02083.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka Y, Ikeda M, Ikenaga M, Haraguchi N, Miyake M, Sekimoto M. Safety and efficacy of fondaparinux for prophylaxis of venous thromboembolism after colorectal cancer resection: a propensity score matched analysis. Dig Surg. 2015;32:190–195. doi: 10.1159/000381034. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Nakamura M, Sakuma M, Yamada N, Sakon M, Fujita S, Seo N. Incidence of pulmonary thromboembolism (PTE) and new guidelines for PTE prophylaxis in Japan. Clin Hemorheol Microcirc. 2006;35:257–259. [PubMed] [Google Scholar]
- 23.Sakon M, Kakkar AK, Ikeda M, Sekimoto M, Nakamori S, Yano M, Monden M. Current status of pulmonary embolism in general surgery in Japan. Surg Today. 2004;34:805–810. doi: 10.1007/s00595-004-2842-1. [DOI] [PubMed] [Google Scholar]
- 24.Sakon M, Ikeda M. Venous thromboembolism in abdominal cancer surgery. Clin J Gastroenterol. 2009;2:247–251. doi: 10.1007/s12328-009-0092-x. [DOI] [PubMed] [Google Scholar]
- 25.Yeo DX, Junnarkar S, Balasubramaniam S, Tan YP, Low JK, Woon W, Pang TC. Incidence of venous thromboembolism and its pharmacological prophylaxis in Asian general surgery patients: a systematic review. World J Surg. 2015;39:150–157. doi: 10.1007/s00268-014-2763-0. [DOI] [PubMed] [Google Scholar]
- 26.Kim DS, Park KM, Won YS, Kim JY, Lee JK, Kim JG, Oh ST, Jung SS, Kang WK. Occurrence and prognosis of symptomatic venous thromboembolism in colorectal cancer surgery patients. Vasc Specialist Int. 2014;30:49–55. doi: 10.5758/vsi.2014.30.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girard P, Demaria J, Lillo-Le Louet A, Caliandro R, Le Guillou JL, Crespin M, de Sanctis A, Stern JB. Transfusions, major bleeding, and prevention of venous thromboembolism with enoxaparin or fondaparinux in thoracic surgery. Thromb Haemost. 2011;106:1109–1116. doi: 10.1160/TH11-06-0433. [DOI] [PubMed] [Google Scholar]
- 28.Sakon M, Kobayashi T, Shimazui T. Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: results from a multicenter, randomized, open-label study. Thromb Res. 2010;125:e65–e70. doi: 10.1016/j.thromres.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M, investigators P Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92:1212–1220. doi: 10.1002/bjs.5154. [DOI] [PubMed] [Google Scholar]
- 30.Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE, Apollo I. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost. 2007;5:1854–1861. doi: 10.1111/j.1538-7836.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 31.Dong WJ, Qian HJ, Qian Y, Zhou L, Hu SL. Fondaparinux vs. enoxaparin for the prevention of venous thromboembolism after total hip replacement: a meta-analysis. Exp Ther Med. 2016;12:969–974. doi: 10.3892/etm.2016.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi H, Morikawa T, Yoshida H, Motoi F, Okada T, Nakagawa K, Mizuma M, Naitoh T, Katayose Y, Unno M. Safety of postoperative thromboprophylaxis after major hepatobiliary-pancreatic surgery in Japanese patients. Surg Today. 2014;44:1660–1668. doi: 10.1007/s00595-014-0890-8. [DOI] [PubMed] [Google Scholar]
- 33.Tokimitsu I, Ohyama K, Tajima S, Nishikawa T. Secretion of a unique collagen by spontaneously transformed murine keratinocytes (PAM cells) in vitro. J Invest Dermatol. 1991;96:267–272. doi: 10.1111/1523-1747.ep12464465. [DOI] [PubMed] [Google Scholar]
- 34.Yasui M, Ikeda M, Miyake M, Ide Y, Okuyama M, Shingai T, Kitani K, Ikenaga M, Hasegawa J, Akamatsu H, et al. Comparison of bleeding risks related to venous thromboembolism prophylaxis in laparoscopic vs open colorectal cancer surgery: a multicenter study in Japanese patients. Am J Surg. 2017;213:43–49. doi: 10.1016/j.amjsurg.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Liu Z, Qiu Y, Zhang J, Wu H, Liang R, Chen G, Qin G, Li Y, Zou D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:1494–1503. doi: 10.1016/j.ejso.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, Fu Z, Gao L, Wang X, Cheng S, Wang X, Ren H. Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol. 2017;15:48. doi: 10.1186/s12957-017-1104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durczynski A, Skulimowski A, Hogendorf P, Szymanski D, Kumor A, Marski K, Juliebo SO, Poznanska G, Strzelczyk J. The concentration of D-dimers in portal blood positively correlates with overall survival in patients with non-resectable pancreatic cancer. World J Surg Oncol. 2017;15:223. doi: 10.1186/s12957-017-1291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu SL, Ye ZH, Ling T, Liang SY, Li H, Tang XZ, Xu YS, Tang WZ. High pretreatment plasma D-dimer predicts poor survival of colorectal cancer: insight from a meta-analysis of observational studies. Oncotarget. 2017;8:81186–81194. doi: 10.18632/oncotarget.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stender MT, Larsen TB, Sorensen HT, Thorlacius-Ussing O. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost. 2012;10:2027–2031. doi: 10.1111/j.1538-7836.2012.04887.x. [DOI] [PubMed] [Google Scholar]
- 40.Clouston HW, Shaker H, Duff S, Kirwan CC. PO-09 - Incidence of pre-operative and post-operative deep vein thrombosis in colorectal cancer surgery. Interim results of a prospective clinical study. Thromb Res. 2016;140(Suppl 1):S179–S180. doi: 10.1016/S0049-3848(16)30142-6. [DOI] [PubMed] [Google Scholar]
- 41.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–2018. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 42.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.