Abstract
OBJECTIVES
This study sought to develop a novel targeted delivery therapy to ablate the major atrial ganglionated plexi (GP) using magnetic nanoparticles carrying a CaCl2 payload.
BACKGROUND
Prior studies indicated the role of hyperactivity of the cardiac autonomic nervous system in the genesis of atrial fibrillation.
METHODS
Twenty-eight male mongrel dogs underwent a bilateral thoracotomy. CaCl2-encapsulated magnetic nanoparticles (Ca-MNP) included magnetite in a sphere of biocompatible, biodegradable poly(lactic-co-glycolic acid). A custom external electromagnet focusing the magnetic field gradient (2,600 G) on the epicardial surface of the targeted GP was used to pull Ca-MNP into and release CaCl2 within the GP. The ventricular rate slowing response to high frequency stimulation (20 Hz, 0.1 ms) of the GP was used to assess the GP function.
RESULTS
The minimal effective concentration of CaCl2 to inhibit the GP function was 0.5 mmol/l. Three weeks after CaCl2 (0.5 mmol/l, n = 18 GP) or saline (n = 18 GP) microinjection into GP, the increased GP function, neural activity, and atrial fibrillation inducibility, as well as shortened effective refractory period in response to 6 h of rapid atrial pacing (1,200 beats/min) were suppressed by CaCl2 microinjection. After intracoronary infusion of Ca-MNP, the external electromagnet pulled Ca-MNP to the targeted GP and suppressed the GP function (n = 6 GP) within 15 min.
CONCLUSIONS
Ca-MNP can be magnetically targeted to suppress GP function by calcium-mediated neurotoxicity. This novel approach may be used to treat arrhythmias related to hyperactivity of the cardiac autonomic nervous system, such as early stage of atrial fibrillation, with minimal myocardial injury.
Keywords: atrial fibrillation, autonomic nervous system, calcium neurotoxicity, ganglionated plexi, magnetic nanoparticles
Pre-clinical and clinical studies indicated that a hyperactive and/or unbalanced state of the cardiac autonomic nervous system (CANS) can facilitate the initiation and maintenance of atrial fibrillation (AF) (1–3). The atrial CANS converges at several ganglionated plexi (GP) that serve as the integration centers of the CANS. Paroxysmal AF is usually initiated by pulmonary vein (PV) firing. Circumferential pulmonary vein isolation (CPVI) has been developed to isolate and eliminate the AF triggers and substrate along or within the circumferential lines. These circumferential lines also transect 3 of the 4 major atrial GP, indicating that GP ablation may underlie at least part of the therapeutic effects of CPVI (4–7).
Targeted drug delivery using supraparamagnetic nanoparticles (MNP) is a burgeoning technology in which medicines are targeted to selected tissues to maximize therapeutic efficacy and minimize side effects (8,9). Our previous study demonstrated that after intracoronary injection, MNP carrying a neurotoxic agent could be magnetically targeted to the GP and suppressed GP function (10). In the present study, we employed calcium-mediated neurotoxicity to GP to suppress AF.
METHODS
ANIMAL PREPARATION.
Twenty-eight male mongrel dogs weighing 18 to 20 kg were anesthetized with sodium pentobarbital (initial dose: 1 ml/kg; maintenance dose: 50 to 60 mg/h), followed by positive-pressure ventilation and continuous electrocardiography, intracardiac electrograms, and arterial blood pressure recording. Experimental protocols were approved by the Animal Care and Use Committees of the Renmin Hospital of Wuhan University, China, and Sinclair Research Center Inc., Auvasse, Missouri. All experiments conform to the Guide for the Care and Use of Laboratory Animals.
A bilateral thoracotomy was performed at the third intercostal space. Multielectrode catheters were sutured to different sites, including the left atrial appendage, left atrium, left superior PV, left inferior PV, right atrial appendage, right atrium, right superior PV, and right inferior PV for recording and stimulation as previously described (11–13).
EVALUATION OF GP FUNCTION.
As described previously (10–13), GP function was measured during AF by maximal prolongation of the R-R interval induced by 4 levels of high frequency stimulation of the GP (20 Hz, 0.1 ms pulse duration; Grass-S88 Stimulator, Astro-Med, West Warwick, Rhode Island) (please see the Online Appendix for details). Three major atrial GP including the anterior right ganglionated plexi (ARGP), inferior right ganglionated plexi (IRGP), and superior left ganglionated plexi (SLGP) were selected for saline or CaCl2 microinjections for their easier access.
PROTOCOL 1: DETERMINATION OF THE MINIMAL EFFECTIVE CONCENTRATION OF CaCl2.
Twelve dogs were randomly divided into 0.25 mmol/l CaCl2 group and 0.5 mmol/l CaCl2 group (Figure 1A). CaCl2 0.1 ml of 0.25 mmol/l or 0.5 mmol/l was microinjected into SLGP, ARGP, and IRGP, sequentially. GP function was evaluated at different time points: baseline and 15, 30, and 45 min after microinjection.
FIGURE 1. Experimental Design and Determination of Minimal Effective Concentration of CaCl2.

(A) The experiment design for the 3 protocols. (B, C) The minimal effective concentration of CaCl2 to suppress ganglionated plexi (GP) function was 0.5 mmol/l. Level 1: stimulating voltage from 10 to 30 V; level 2: 40 to 60 V; level 3: 70 to 90 V; level 4: 100 to 120 V. *p < 0.05 versus baseline (BS) at different stimulation levels. ERP = effective refractory period; M = mol/l; MNP = magnetic nanoparticles; RAP = rapid atrial pacing.
PROTOCOL 2: LONG-TERM EFFECTS OF CaCl2 INJECTION ON GP FUNCTION.
In protocol 2 (Figure 1A), another 12 animals were randomly divided into 2 groups: control group (0.1 ml of saline microinjection into GP) and CaCl2 group (0.1 ml, 0.5 mmol/l CaCl2). The concentration of 0.5 mmol/l CaCl2 was selected according to the results from protocol 1. A bilateral thoracotomy was done in all animals using sterile techniques; saline or CaCl2 was microinjected sequentially into SLGP, ARGP, and IRGP; the thorax was closed as previously described (11). Three weeks later, animals were anesthetized, thoracotomy was performed, and 6 h of rapid atrial pacing (RAP) (1,200 beats/min, 2× diastolic threshold) was delivered. GP function, electrophysiological parameters, and the neural activity in the ARGP were evaluated at 3 time points: before saline or CaCl2 microinjection; 3 weeks after saline or CaCl2 microinjection (new baseline); and after 6-h RAP. At the end of the experiment, GP tissue was collected for Western blot and terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) analysis.
EVALUATION OF ELECTROPHYSIOLOGICAL PARAMETERS.
Atrial programmed stimulation (S1-S1 = 330 ms, 8-beat, 2× diastolic threshold) and 1 premature beat (S2) was used to evaluate the effective refractory period at atrial and PV sites (10–13). AF was defined as irregular atrial rates >500 beats/min and a duration >5 s, associated with irregular atrioventricular conduction. The window of vulnerability (WOV), which served as a quantitative measurement of AF inducibility, was defined as the difference between the longest and the shortest S1-S2 interval (in milliseconds) at which AF was induced (12,13). The cumulative WOV (∑WOV) was the sum of the WOVs at all sites in each animal.
MEASUREMENT OF THE NEURAL ACTIVITY IN THE ARGP.
The neural activity of the ARGP was recorded as previously described (12,13). Briefly, a tungsten-coated microelectrode with an impedance of 9 to 12 MΩ at 1,000 Hz was inserted into the ARGP; neural activity was recorded, amplified (30 to 50 times), and filtered (500 Hz to 10 kHz). Spikes of neural activity, defined as deflections with a signal-to-noise ratio >3:1, were used to quantitate neural activity. Increased amplitude and frequency of neural activity indicates higher number of recruited neural elements and that more neurons were firing in a given period of time, respectively.
HISTOLOGIC STUDIES, WESTERN BLOT, AND TUNEL STAINING.
GP were resected and washed with saline at the end of the experiment. Western blot was applied to examine the expression of nerve growth factor and c-Fos. The former is a neurotrophic factor and plays a critical role in the synaptic activity, differentiation, and survival of neural elements; the latter is a marker for fast neuronal activation. TUNEL staining was used to examine apoptosis within the GP. Please see the online appendix and Yu et al. (10) for details.
PROTOCOL 3: TARGETING OF MAGNETIC NANO-PARTICLES TO THE GP BY AN EXTERNAL ELECTROMAGNET.
The left circumflex artery (LCX) is known to provide blood supply to the atrial myocardium underneath the SLGP and IRGP (14). The LCX was cannulated and a nonocclusive catheter placed in the artery and secured by a suture and tissue glue. After the magnetic field on the epicardial surface of the targeted GP was verified by a Gauss meter to be ≥2,600 G based on our prior study (10), the chest was temporarily closed to simulate the scenario that is typical for a human catheterization laboratory. Magnetic nanoparticles carrying CaCl2 payload (Ca-MNP) (1 mg in 1 ml) then was slowly infused into the circumflex artery over 3 min in 4 animals. GP functions were evaluated at different time points after Ca-MNP infusion. We performed 2 sets of control experiments (Figure 1A). First, in 4 animals, the magnetic field was focused on the epicardial surface of the ARGP whose blood supply mainly comes from the right coronary artery, and then Ca-MNP was infused into the LCX (control 1). Second, in 3 animals, MNP without the CaCl2 payload was infused into the right coronary artery and the magnetic field focused on the ARGP (control 2).
SYNTHESIS AND FUNCTIONALIZATION OF CA-MNP CARRYING CaCl2 PAYLOADS.
CaCl2-loaded poly(lactic-co-glycolic acid) (PLGA)-magnetite nanoparticles were formulated by a standard double-emulsion (water-oil-in-water) technique with slight modifications. First, mixture solution with CaCl2 (aqueous phase 1) were added into the oil phase that consisted of PLGA chloroform solution and iron oxide nanoparticles. The mixture was further emulsified using sonication. This emulsion was then added dropwise to an aqueous solution of polyvinyl alcohol containing CaCl2. The final emulsion was sonicated and then kept for stirring overnight to allow evaporation of chloroform. The CaCl2, MNP-loaded nanoparticles formed via a double emulsion were washed and purified by ultracentrifugation to remove excess surfactant, polymer, and unbound iron oxide. These nanoparticles were finally collected via lyophilization. Empty (unloaded) nanoparticles were also prepared in a same manner without the addition of CaCl2 in both phases and used as a control.
To study particle size and surface charge, Ca-MNP suspension was added to a transparent cuvette and was then inserted into the ZetaPALS dynamic light scattering detector (Brookhaven Instruments, Holtsville, New York) as previously described (15). Transmission electron microscopy (FEI Tecnai G2 Spirit BioTWIN, Hillsboro, Oregon) was also used to observe the morphology of the particles. Moreover, the iron content within the magnetite nanoparticles was assessed using a standard iron assay as detailed in our previous publications (16,17). Magnetic susceptibility of the particles was measured using a vibrating sample magnetometer (KLA-Tencor EV7, San Jose, California) and compared with bare or original MNP. The particles were embedded in wax and hysteresis loops were obtained by varying the magnetic fields at room temperature.
The loading efficiency of CaCl2 was directly determined by dissolving nanoparticles in dichloromethane and followed by precipitation of polymer via adding ethanol. The resulting solution was centrifuged and the supernatant was collected to determine the amount of loaded CaCl2. The loading efficiency is defined as the percentage of the CaCl2-loaded amount as previously determined divided by the total amount of CaCl2 used.
For drug-release studies, Ca-MNP suspensions were incubated for predetermined time points. At each time point, CaCl2 release samples were collected, stored for further analysis, and replaced with fresh deionized water. At the end of the study, the amount of Ca++ released was determined calorimetrically using a calcium-sensitive dye Arsenazo III, which undergoes color changes upon complexation with calcium (18,19). A sample solution was mixed with a Arsenazo III solution; the resulting color change was quantified based on absorbance using ultraviolet-visible spectrometer (Infinite M200 plate reader, Tecan, Durham, North Carolina).
EXTERNAL ELECTROMAGNET.
A custom electromagnet, capable of producing 2,600 G of field strength at the epicardial surface of targeted GP, was designed and constructed. The choice of 2,600 G was based on our prior study (10) in which a permanent magnet of 2,600 G placed on the epicardial surface of the GP could pull MNP out of the coronary microcirculation into the targeted GP. The magnetic field is crucial to the targeting of the magnetically active nanoparticles and provides a physical, attractive force to the particles in circulation, pulling the particles out of the local vasculature and into tissue between the vasculature and the applied magnetic field. Specific targeting is established by applying appropriate magnetic field strength and physical force on the epicardial surface of the targeted GP to increase delivery Ca-MNP to the targeted GP tissue.
STATISTICAL ANALYSIS.
We have tested for normality using the Shapiro-Wilk test of normality. All the data followed a normal distribution. All data were presented as the mean ± SD. Data in Figures 1B, 1C, 2A, and 2B were analyzed with one-way analysis of variance (ANOVA). Data in Figures 3A and 3B were analyzed with paired Student’s t-test. Data in Figures 2D and 3C were analyzed with 2-way ANOVA with repeated measures and a Bonferroni post-hoc test (SPSS Inc., Chicago, Illinois). The level of significance was set at p <0.05.
FIGURE 2. GP Function and Neural Activity Measured at BS, 3 Weeks After Microinjection, and After 6 h of RAP.

(A) Three weeks after saline injection, 6-h RAP enhanced GP function. (B) CaCl2 suppressed GP function 3 weeks after injection. Six-h RAP failed to augment the GP function. (C) Representative GP neural activity at different time points. (D) The frequency and amplitude of the anterior right GP neural activity was suppressed by CaCl2. *p < 0.05 versus group baseline at different stimulation levels in A and B; *p < 0.05 versus group baseline in D; #p < 0.05 versus control (saline injection). Abbreviations as in Figure 1.
FIGURE 3. Long-Term Effects of CaCl2 Microinjection on ERP and WOV.
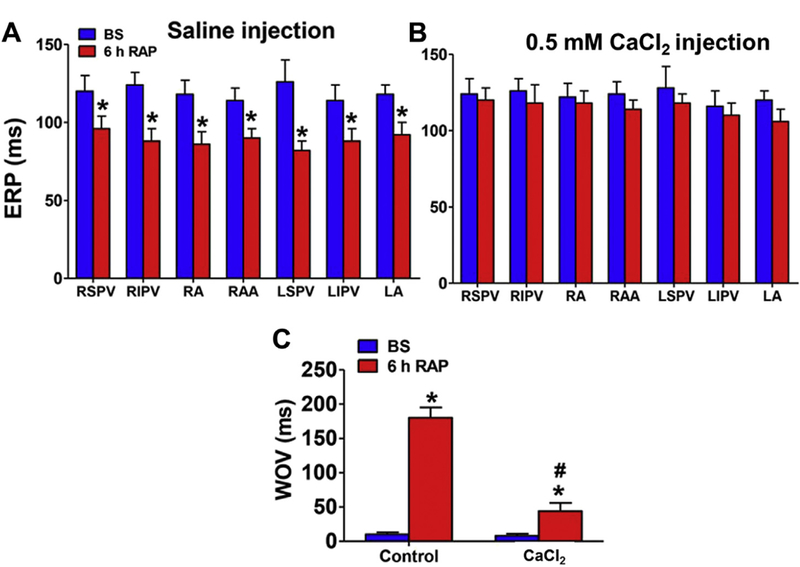
CaCl2 injection prevented ERP shortening (A, B) and window of vulnerability (WOV) widening (C) induced by 6-h RAP. *p < 0.05 versus group baseline; #p < 0.05 versus control (saline injection). LA = left atrium; LIPV = left inferior superior vein; LSPV = left superior pulmonary vein; RA = right atrium; RAA = right atrial appendage; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; other abbreviations as in Figure 1.
We have tested for normality using the Shapiro-Wilk test of normality. All the data followed a normal distribution. All data were presented as the mean ± SD. Data in Figures 1B, 1C, 2A, and 2B were analyzed with 1-way analysis of variance (ANOVA). Data in Figures 3A and 3B were analyzed with paired Student’s t-test. Data in Figures 2D and 3C were analyzed with 2-way ANOVA with repeated measures and a Bonferroni post-hoc test (SPSS Inc., Chicago, Illinois). The level of significance was set at p <0.05.
RESULTS
MINIMAL EFFECTIVE CONCENTRATION OF CaCl2 TO SUPPRESS GP FUNCTION.
Figure 1B illustrated no difference in R-R interval prolongation induced by different levels of high frequency stimulation at 15, 30, and 45 min after CaCl2 microinjection (0.25 mmol/l) into the GP (6 animals, 18 GP total). After 0.5 mmol/l of CaCl2 was microinjected into these GP (6 other animals, 18 GP), a decreased R-R interval prolongation response was found at all levels of stimulation voltage within 15 min (Figure 1C). Hence, 0.5 mmol/l CaCl2 was chosen for the chronic study (protocol 2).
LONG-TERM EFFECTS OF CaCl2 MICROINJECTION ON GP FUNCTION.
Previous studies have shown that 6 h of RAP greatly enhanced the GP function and electrical remodeling (11–13). We therefore challenged the GP with 6-h RAP to investigate whether CaCl2 could attenuate the enhanced GP activity and electrical remodeling induced by RAP. We observed that 6-h RAP augmented the R-R interval prolongation response in the saline group (Figure 2A). Decreased R-R interval prolongation was observed in the CaCl2 group both 3 weeks after CaCl2 microinjection and after 6-h RAP (Figure 2B). In Figures 2C and 2D, the frequency and amplitude of the neural activity in the ARGP was reduced 3 weeks after CaCl2 micro-injection. RAP significantly increased the neural activity only in the control group, but RAP-induced GP hyperactivity was significantly attenuated by CaCl2.
Additionally, we investigated whether CaCl2 could attenuate RAP-induced electrical remodeling. After 6-h RAP, the effective refractory period was significantly decreased at all sites in the control group but not the CaCl2 group (Figures 3A and 3B). ΣWOV, a surrogate of AF inducibility, was increased greatly in the control group but only mildly in the CaCl2 group (Figure 3C).
EFFECTS OF CA + + ON THE AUTONOMIC NEURONS IN GP.
The expression of c-Fos and nerve growth factor was evaluated to indicate activation of neurons and neural remodeling in GP in response to 6-h RAP. Figures 4A and 4B showed a significant decrease in c-Fos and nerve growth factor expression in the CaCl2 group, compared with the control group. TUNEL staining was used to evaluate apoptosis in the GP. Increased positive TUNEL staining neurons were observed in the CaCl2, but not in the control group (Figure 4C).
FIGURE 4. Long-Term Effects of CaCl2 Microinjection on the Autonomic Neurons in GP.

(A, B) The representative bands and quantitative analysis of the expression of nerve growth factor (NGF) and c-Fos in ganglionated plexi (GP). (C) Neuron apoptosis in GP. The percentage of terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL)-positive neurons in GP was 23.5 ± 7.4%. No TUNEL-positive neurons were observed in the control group. *p < 0.05 versus control. DAPI = deoxyribonucleic acid stain with 4,6-diamidino-2-phenylindole; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HE = hematoxylin and eosin stain.
FORMULATION AND RELEASE KINETICS OF CA-MNP.
The average sizes of CaCl2-loaded and blank PLGA nanoparticles were 232 ± 101 nm and 213 ± 84 nm, respectively. The zeta potential of the Ca-MNP and blank PLGA nanoparticles was –3.7 ± 2.6 mV and –11.6 ± 1.0 mV, respectively. Transmission electron microscopy images indicated that the particles are spherical in morphology and are in general uniformly dispersed.
Iron assay indicated that the iron content of Ca-MNP was 38%, and each milligram of particles contained 380 μg of bare magnetite nanoparticles. CaCl2-loaded magnetite PLGA nanoparticles had similar magnetic properties with MNP alone (Figure 5B). The CaCl2-loading efficiency was calculated to be 14%, and each milligram of Ca-MNP contained 244 μg of CaCl2, equivalent to 2.2 mmol of CaCl2. Figure 5C illustrates the release kinetics of the Ca-MNP in which approximately 20% of the CaCl2 in the Ca-MNP was released by 30 min after microinjection followed by sustained release of CaCl2 over 21 days.
FIGURE 5. Physical Properties of NP.

(A) Magnetic hysteresis loops of bare MNP and Ca-MNP exhibiting similar superparamagnetic behaviors. (B) Cumulative percentage release of CaCl2 from Ca-MNP at 37°C over 21 days. Inset, release kinetics of CaCl2 from particles in the first 10 h. emu = electromagnetic unit; NP = nanoparticle; PLGA = poly(lactic-co-glycolic acid); other abbreviations as in Figure 1.
CA-MNP INFUSION INTO LCX-ATTENUATED GP FUNCTION.
In protocol 3, the effect of Ca-MNP on GP function was evaluated using high frequency stimulation as we have already described. Figure 6A illustrates the combined results from 6 experiments (3 from SLGP and 3 from IRGP). Infusion of Ca-MNP into LCX in the presence of an electromagnetic field gradient significantly suppressed the SLGP or IRGP function. Figure 6B illustrates that in the control 1 group, Ca-MNP did not affect the function of ARGP whose main blood supply primarily comes from the right coronary artery (n = 4 GP), indicating that suppressed SLGP and IRGP function was not a time-dependent artifact. Figure 6C illustrates that in the control 2 group, MNP without CaCl2 also did not affect the ARGP function (n = 3 GP), indicating that GP suppression was not caused by embolization of the coronary microcirculation by Ca-MNP. Targeted release of CaCl2 most likely underlies the effects we observed.
FIGURE 6. The Effect of Targeted Drug Delivery on GP Function Measured by the Percentage of R-R Interval Prolongation Induced by HFS.

(A) The superior left GP or inferior right GP function was inhibited within 15 min after Ca-MNP was infused into left circumflex artery with electromagnetic (EM) field focusing on the superior left GP or inferior right GP (n = 6 GP). (B) In control 1 group (n = 4 GP), Ca-MNP were infused to the left circumflex artery not supplying the anterior right ganglionated plexi (ARGP). The ARGP function was not affected by EM field focusing on the ARGP. (C) In control 2 group, MNP without CaCl2 payload was infused into the right coronary artery and the ARGP function was not affected by EM field focusing on the ARGP (n = GP). (D) Schematic representation of the proposed targeted drug delivery system to treat AF. HFS = high frequency stimulation; other abbreviations as in Figure 1.
DISCUSSION
MAJOR FINDINGS.
We demonstrated in vivo calcium-mediated neurotoxicity of targeted GP through 3 different sets of experiments. First, acute microinjection of CaCl2 into GP identified that the minimal effective concentration of CaCl2 (0.5 mmol/l) suppressed the GP function within 15 min. Second, 3 weeks after CaCl2 microinjection, the function of the targeted GP remained suppressed and GP neural activity and electrical remodeling could not be enhanced by 6-h RAP. Apoptosis of the GP autonomic neurons also contributes to this long-term effect. Third, we demonstrated successful targeting of GP with Ca-MNP by an external electromagnetic field gradient, simulating a routine coronary angiography procedure ubiquitously performed in cardiac catheterization laboratories. The proposed targeted drug delivery system has the following novelty. First, using electromagnetic targeting to accumulate an excessive amount of calcium ion in the targeted GP transformed an endogenous substance (calcium ion) into a neurotoxin. Second, this approach may have a more favorable safety profile because the 3 components of our Ca-MNP (magnetite, PLGA polymer, and CaCl2 payload) are either endogenous substances or will be metabolized into endogenous substances. Third, this approach can be performed by all cardiologists proficient in cardiac catheterization to serve the rapidly growing number of patients with AF as the population ages.
THE CARDIAC AUTONOMIC NERVOUS SYSTEM AND AF.
Yu et al. (12,13) demonstrated a vicious cycle formed by autonomic remodeling and electrical remodeling, in which a hyperactive state of the CANS initiates paroxysmal AF. AF not only induces electrical remodeling (e.g., shortening of the effective refractory period) but also further augments the CANS activity, perpetuating AF. Selectively targeting the GP may break this vicious cycle and stop AF without collateral damage to the atrial myocardium that produces iatrogenic atrial tachycardias.
CPVI is the cornerstone of ablation treatment of drug-refractory AF; however, the 5-year success rate of paroxysmal AF ablation performed at very experienced centers was <50% (20,21). The long-term success rate of more advanced AF was even lower (21). Notably, the lesion sets of CPVI transect 3 of the 4 major atrial GP and numerous autonomic nerves, suggesting that autonomic denervation may be a major contributor to AF suppression (4). A randomized clinical trial of Katritsis et al. (6) reported improved ablation success rate if CPVI is combined with GP ablation. Notably, the success rate of CPVI and GP ablation alone was similar. On the contrary, the AFACT (Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery) trial, randomizing mostly persistent patients with AF (with markedly enlarged left atrium or failed prior ablation) to surgical CPVI versus CPVI + GP ablation, showed no additional benefits but more adverse events in the CPVI + GP group (5). Conflicting results from these 2 largest GP ablation trials suggest that GP ablation should be performed in an earlier stage of AF before inflammation and/or structural remodeling such as fibrosis becomes too advanced.
The idea of targeting GP to treat paroxysmal AF was further corroborated by 2 recent studies. Injecting botulinum toxin into the 4 major atrial GP in paroxysmal AF patients markedly suppressed AF for at least a year (7). It is intriguing that the antiarrhythmic effect of microinjected botulinum toxin lasted far beyond its neural suppression of CANS (e.g., 1 month). Lo et al. (11) provided convincing experimental evidence showing that in a canine model of paroxysmal AF simulated by daily 6-h RAP, botulinum toxin injected into 4 major atrial GP prevented occurrence of spontaneous AF and autonomic remodeling. Specifically, autonomic remodeling such as enhanced choline acetyltransferase (+) neurons in the GP and tyrosine hydroxylase (+) autonomic nerves in the atrial myocardium were not observed in the botulinum toxin-treated animals for at least 3 months (11).
AUTONOMIC DENERVATION BY CA-MNP.
Supraparamagnetic nanoparticles are nonmagnetic in the absence of an external magnetic field (8,9), making MNP suitable for targeted drug delivery in which a therapeutic payload is concentrated in target sites thereby minimizing side effects (22). We previously reported a proof-of-concept study in which MNP carrying a neurotoxin could be magnetically targeted to the IRGP and inhibit its function (10).
The Ca-MNP used in the present study has 3 important advantages. First, the encapsulated Fe3O4 will be recycled as micronutrients. Second, PLGA polymer is a biocompatible and biodegradable copolymer, widely used in U.S. Food and Drug Administration-approved products such as coronary artery stents (23). PLGA can formulate both hydrophilic and hydrophobic drugs. Importantly, PLGA biodegradation and release kinetics can be adjusted by modifying the ratio of lactide to glycolide during polymerization to achieve varying payload release rates. In humans, PLGA is metabolized into glycolic acid and lactic acid, both of which are endogenous biocompatible compounds. Third, the payload, calcium ions, are neurotoxic at high local (targeted) concentrations but are safe, ubiquitous, endogenous cations. With our targeted drug delivery protocol, the amount of CaCl2 delivered (244 μg for each infusion) is nearly negligible in comparison to the daily dose of calcium supplement (500 to 1,000 mg).
POSSIBLE MECHANISMS UNDERLYING THE AUTONOMIC INHIBITION EFFECT OF CA-MNP.
Ca2+ homeostasis is crucial in maintaining normal physiological functions of cells. In neurons, the intracellular Ca2+ level is tightly regulated at approximately 100 nmol/l to ensure the integrity of functions such as membrane excitability, synaptic transmission, and mitochondrial membrane stability (24). Excessive Ca2+ influx or release from intracellular storage causes neurotoxicity, activating a battery of second messengers and proteases as well as enables the neuronal mitochondria to generate reactive oxygen species. For example, excessive synaptic release of glutamate leads to a large influx of Ca2+ and subsequent neurotoxicity known as excitotoxicity (25). Such Ca2+-induced neurotoxicity can occur immediately or over several weeks due to apoptosis. In the present study, increased neuron apoptosis in GP was found in the CaCl2 group, which may contribute to the decreased GP function.
STUDY LIMITATIONS.
The electromagnetic field gradient of 2,600 G was chosen according to our previous data (10). It is unclear whether weaker field gradients are able to target the Ca-MNP. The electromagnetic field gradient was constantly present during the 45 min of targeting when GP function was being evaluated. The minimal targeting time requirement was not investigated in the present study. Future studies aiming at identifying the minimal effective electromagnetic field and targeting duration will greatly enhance the applicability of this novel approach to clinical practice.
The zeta potential of the Ca-MNP was low at −3.7 ± 2.6 mV due to the presence of CaCl2 on the surface of the nanoparticles. Improvement of the Ca-MNP synthetic process will be sought to increase the zeta potential and prevent agglomeration of the Ca-MNP.
We only investigated the acute effects of electromagnetic-targeted drug delivery but the results from protocol 2 demonstrated both acute and chronic GP suppression. As GP stimulation is not sensitive enough for a comprehensive evaluation of the GP function, the effects of magnetic navigation of Ca-MNP on long-term suppression of AF will need to be determined in future studies. Factors that affect MNP targeting such as the angle between the direction of the electromagnetic field gradient and blood flow, flow rate of the microcirculation underneath the targeted GP, infusion rate of the Ca-MNP, as well as the local concentration of the Ca-MNP will need to be addressed in future studies.
CLINICAL PERSPECTIVE.
AF is a progressive disease and ideally should be intervened in its very early stage. With the anticipated surge of patients with AF in the near future and limited number of ablation electrophysiologists, the procedure presented in our study can be performed by all cardiologists familiar with coronary arteriography on substantially more patients in an early stage of AF. Early intervention of AF potentially can prevent AF from progressing to more advanced stages that require expensive anticoagulation and ablative therapies.
CONCLUSIONS
CaCl2 (Ca++) is capable of inhibiting GP function and preventing RAP-induced atrial remodeling 3 weeks later. We hereby present a novel approach, whose invasiveness is similar to a routine coronary arteriography, to treat paroxysmal AF (Figure 6D). This approach uses an external electromagnet to target Ca-MNP to specific cardiac GP. Importantly, the 3 components of Ca-MNP are either endogenous substances of the human body or will be metabolized to endogenous substances, thereby minimizing toxicity and systemic side effects.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In the present study, we presented a novel targeted delivery therapy to ablate the major atrial GP using magnetic nanoparticles carrying CaCl2 payload to treat paroxysmal AF.
TRANSLATIONAL OUTLOOK:
This novel minimally invasive targeted drug delivery approach using supraparamagnetic nanoparticles enables precise ablation of cardiac autonomic nervous system elements with maximal therapeutic efficacy and minimal side effects. Further studies are warranted to determine the efficacy and safety of magnetic navigation of Ca-MNP on long-term suppression of AF.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Dale Huber at the Center for Integrated Nanotechnology, Sandia National Laboratories, Albuquerque, New Mexico, for helpful suggestions of manufacturing the nanoparticles.
Part of this study and the electromagnet were supported by NanoMed Targeting System Inc. Dr. Yu has received a grant (no. 81530011) from the National Nature Science Foundation of China. Dr. Scherlag holds a patent related to this paper; receives funding from NanoMed Targeting System; and owns stock shares of NanoMed Targeting System. Dr. Dormer holds a patent related to this paper; has received funding from NanoMed Targeting System; and owns stock shares of NanoMed Targeting System. Dr. Rutel has received funding from NanoMed Targeting Systems; serves on the Scientific Advisory Board for NanoMed Targeting Systems; and owns stock in NanoMed Targeting Systems. Dr. Po holds a patent related to this paper; has received funding from NanoMed Targeting System; owns stock shares of NanoMed Targeting System; has received research funding from NanoMed Targeting System; and has received an in-house grant of the Heart Rhythm Institute, University of Oklahoma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- ARGP
anterior right ganglionated plexi
- CANS
cardiac autonomic nervous system
- CPVI
circumferential pulmonary vein isolation
- GP
ganglionated plexi
- IRGP
inferior right ganglionated plexi
- LCX
left circumflex artery
- MNP
supraparamagnetic nanoparticle
- PLGA
poly(lactic-co-glycolic acid)
- PV
pulmonary vein
- RAP
right atrial pacing
- SLGP
superior left ganglionated plexi
- TUNEL
terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling
- WOV
window of vulnerability
Footnotes
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate.
APPENDIX For supplemental material, please see the online version of this paper.
REFERENCES
- 1.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol 2003;42:1262–8. [DOI] [PubMed] [Google Scholar]
- 2.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 2008;118:916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Po SS, Scherlag BJ, Yamanashi WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrialjunctions. Heart Rhythm 2006;3:201–8. [DOI] [PubMed] [Google Scholar]
- 4.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:1186–9. [DOI] [PubMed] [Google Scholar]
- 5.Driessen A, Berger WR, Krul S, et al. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol 2016;68: 1155–65. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62: 2318–25. [DOI] [PubMed] [Google Scholar]
- 7.Pokushalov E, Kozlov B, Romanov A, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one-year follow-up of a randomized pilot study. Circ Arrhythm Electrophysiol 2015; 8:1334–41. [DOI] [PubMed] [Google Scholar]
- 8.Angelakeris M Magnetic nanoparticles: a multifunctional vehicle for modern theranostics. Biochim Biophys Acta 2017;1861:1642–51. [DOI] [PubMed] [Google Scholar]
- 9.Usman A, Sadat U, Patterson AJ, et al. Use of ultrasmall superparamagnetic iron oxide particles for imaging carotid atherosclerosis. Nanomedicine (Lond) 2015;10:3077–87. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Scherlag BJ, Dormer K, et al. Autonomic denervation with magnetic nanoparticles. Circulation 2010;122:2653–9. [DOI] [PubMed] [Google Scholar]
- 11.Lo LW, Chang HY, Scherlag BJ, et al. Temporary suppression of cardiac ganglionated plexi leads to long-term suppression of atrial fibrillation: evidence of early autonomic intervention to break the vicious cycle of ”AF begets AF.” J Am Heart Assoc 2016;5:pii:e003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Scherlag BJ, Li S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm 2013;10:428–35. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Scherlag BJ, Sha Y, et al. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm 2012;9:804–9. [DOI] [PubMed] [Google Scholar]
- 14.Boppana VS, Castano A, Avula U, Yamazaki M, Kalifa J. Atrial coronary arteries: anatomyand atrial perfusion territories. J Atr Fibrillation 2011;4:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimi M, Yousef M, Cheng Y, Meletis EI, Eberhart RC, Nguyen K. Formulation and characterization of a covalently coated magnetic nanogel. J Nanosci Nanotechnol2009;9:4128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundaresan V, Menon JU, Rahimi M, Nguyen KT, Wadajkar AS. Dual-responsive polymercoated iron oxide nanoparticles for drug delivery and imaging applications. Int J Pharm 2014;466:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koppolu B, Rahimi M, Nattama S, Wadajkar A, Nguyen KT. Development of multiple-layer polymeric particles for targeted and controlled drug delivery. Nanomedicine 2010;6:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata ME, Delbem AC, Hall KB, Buzalaf MA, Pessan JP. Fluoride and calcium concentrations in the biofilm fluid after use of fluoridated dentifrices supplemented with polyphosphate salts. Clin Oral Investig 2017;21:831–7. [DOI] [PubMed] [Google Scholar]
- 19.Westhaus E, Messersmith PB. Triggered release of calcium from lipid vesicles: a bioinspired strategy for rapid gelation of polysaccharide and protein hydrogels. Biomaterials 2001;22: 453–62. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368–77. [DOI] [PubMed] [Google Scholar]
- 21.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57:160–6. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava P, Sharma PK, Muheem A, Warsi MH. Magnetic nanoparticles: a review on stratagems of fabrication and its biomedical applications. Recent Pat Drug Deliv Formul 2017; 11:101–13. [DOI] [PubMed] [Google Scholar]
- 23.Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol 2012;59:1362–70. [DOI] [PubMed] [Google Scholar]
- 24.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology: novel aspects of an enduring theme. FEBS J 2006;273:433–50. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Singh RL, Babu GN. Cell death mechanisms in the early stages of acute glutamate neurotoxicity. Neurosci Res 2010;66:271–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


