Abstract
BACKGROUND:
Sickle cell anemia (SCA) is a life-threatening blood disorder characterized by the presence of sickle-shaped erythrocytes. Hydroxyurea is currently the only US Food and Drug Administration–approved treatment and there is a need for a convenient method to monitor compliance and hydroxyurea concentrations, especially in pediatric SCA patients.
METHODS:
We describe a novel approach to the determination of hydroxyurea concentrations in dried whole blood collected on DMPK-C cards or volumetric absorptive microsampling (VAMS) devices. Hydroxyurea was quantified by electrospray ionization LC-MS/MS using [13C15N2]hydroxyurea as the internal standard. Calibrators were prepared in whole blood applied to DMPK-C cards or VAMS devices.
RESULTS:
Calibration curves for blood hydroxyurea measured from DMPK-C cards and VAMS devices were linear over the range 0.5–60 μg/mL. Interassay and intraassay CVs were < 15% for blood collected by both methods, and the limit of detection was 5 ng/mL. Whole blood hydroxyurea was stable for up to 60 days on DMPK-C cards and VAMS devices when frozen at −20 °C or −80 °C. Whole blood hydroxyurea concentrations in samples collected on DMPK-C cards or VAMS devices from SCA patients were in close agreement.
CONCLUSIONS:
This tandem mass spectrometry method permits measurement of hydroxyurea concentrations in small volumes of dried blood applied to either DMPK-C cards or VAMS devices with comparable performance. This method for measuring hydroxyurea from dried blood permits the evaluation of therapeutic drug monitoring, individual pharmacokinetics, and medication adherence using heel/finger-prick samples from pediatric patients with SCA treated with hydroxyurea.
Hydroxyurea (also referred to as hydroxycarbamide) is a cytotoxic, antimetabolic, and antineoplastic agent with a remarkable breadth of therapeutic activity for a wide variety of human diseases. Over the past 50 years, hydroxyurea has been used successfully to treat many conditions, including chronic myelogenous leukemia (1), myeloproliferative neoplasms (2), polycythemia vera (3), HIV (4), and sickle cell anemia (SCA)3 (5). In SCA, hydroxyurea exerts its clinical benefits primarily through induction of fetal hemoglobin, although additional salutary effects are also recognized (6). With excellent oral bioavailability, hydroxyurea is typically taken once daily. Pharmacokinetics demonstrate rapid peak serum concentrations 15–60 min after administration (7), a half-life of 1–3 h, and elimination primarily by renal clearance (8, 9). In the setting of children with SCA, differences in hydroxyurea pharmacokinetics have been observed with both rapid and slow absorption phenotypes (7), thus making optimal dosing difficult to determine. Consequently, there is a need for a convenient and robust method for monitoring hydroxyurea concentrations, especially using low volumes of blood typically obtained from pediatric patients to optimize dosing levels and to afford dose adjustments based on clinical response.
Published analytical techniques for the measurement of hydroxyurea in biological fluids include colorimetric spectrophotometry (10, 11), HPLC (12–15), and mass spectrometry (MS) (16, 17). These methods require relatively large volumes of whole blood, serum, or plasma, and involve extraction and derivatization procedures that are tedious and time-consuming. Furthermore, the limited analytical sensitivity of most of these methods makes them unsuitable for use in determining the pharmacokinetics of hydroxyurea in the pediatric population, for which repeated venipuncture procedures are not practical.
Our objective was to establish an analytically sensitive and specific method for the measurement of hydroxyurea in small volumes of whole blood typically obtained from pediatric patients. Here we describe an LC-MS/MS method for measuring hydroxyurea in whole blood specimens collected either as dried blood spots or on volumetric absorptive microsampling (VAMS) devices. This assay represents a substantial technical improvement over existing methods in affording the routine analysis of hydroxyurea to enable optimization of dosing and monitoring of treatment adherence in the pediatric population. Furthermore, this methodological approach is applicable for the therapeutic monitoring of hydroxyurea in the blood of patients living in remote locations where SCA is prevalent.
Material and Methods
CHEMICALS AND REAGENTS
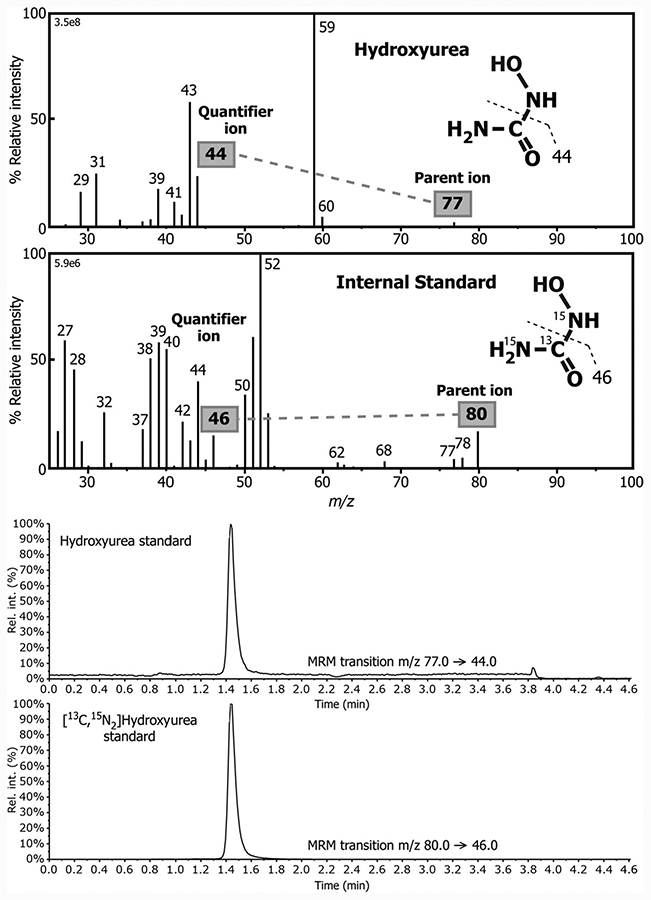
Hydroxyurea and ammonium formate were obtained from Sigma. An isotopically-labeled internal standard (IS), [13C15N2]hydroxyurea, was purchased from Toronto Research Chemicals. The chemical structures of the natural and isotopically labeled analog are shown in Fig. 1. Water, formic acid, and acetonitrile (ACN) used for the mobile phase were of HPLC-MS grade and obtained from Fischer Scientific.
Fig. 1.
Mass spectrum obtained from collision-induced dissociation of the parent ion of hydroxyurea and its stable-labeled IS obtained under electrospray ionization during constant infusion of each compound and representative LC-MS/MS mass chromatograms for the MRM transitions m/z 77→44 and m/z 80→46 used for the targeted detection of hydroxyurea and its stable-labeled IS, respectively.
PREPARATION OF STANDARD SOLUTIONS
Stock solutions of hydroxyurea and the IS were prepared at a concentration of 1.0 g/L in water and aliquots stored at −80 °C and used within a 6-month period. Working solutions of 0.5, 1.0, 5.0, 10.0, 20.0, 30.0, 40.0, and 60.0 μg/mL of hydroxyurea were prepared by appropriate dilutions of the stock solutions and working solutions with 98% ACN. These concentrations were selected because typical peak serum or plasma hydroxyurea concentrations measured by colorimetry in children with SCA reportedly range from 15 to 45 μg/mL, and the drug is detectable in most patients for 6–8 h after the daily dose (7).
SAMPLE PREPARATION
DMPK-C cards, Whatman™ 903 specimen collection paper and FTA classic cards were purchased from GE Health. VAMS devices (18, 19) were purchased from Neoteryx (Torrance, USA; marketed as Mitra®) and used according to the manufacturer’s recommendation. Venous or capillary blood samples were collected from patients undergoing treatment with hydroxyurea according to a research protocol approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board (protocol CCHMC 2014–6046). For patient samples a 10-μL volume of whole blood was applied to the DMPK-C card, or wicked by the VAMS device. Neither samples of whole dried blood on DMPK-C cards (sampling the entire blood spot) nor VAMS devices have been reported to show variations of hydroxyurea by hematocrit or evidence of nonhomogeneity (18, 19). In addition, our preliminary data using an HPLC method (20) confirmed that plasma and whole blood hydroxyurea concentrations were equivalent (see Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/ vol62/issue12), due presumably to the small size of the molecule and free exchange between the cellular and noncellular compartments. Calibrators of hydroxyurea over the dynamic range 0.5–60.0 μg/mL were prepared in a pool of whole blood, and 10-μL aliquots of these calibrators were applied to the DMPK-C cards and wicked to the VAMS devices in an identical manner to that of the patient samples (Fig. 2). Samples were allowed to dry at room temperature for 2–3 h before immediate use or stored at −80 °C. At the time of analysis, the entire dried blood spot was cut out of the filter paper card with scissors or removed from the head of the VAMS device using tweezers, and the samples were transferred to 2.0-mL Eppendorf tubes. Hydroxyurea was recovered by addition of 200 μL of acetonitrile/water (80:20 vol/vol). The IS (10 μg/mL) was then added and the samples were shaken for 15 min at 40 °C using a water bath shaker (Taitec Co. Ltd.). The solutions were then centrifuged at 14000g for 20 min and the clear supernatants then transferred to 12 × 75–mm borosilicate glass culture tubes (Cardinal Health) and evaporated under a stream of nitrogen gas. The dried extract was then dissolved in 98% ACN (100 μL) for analysis by LC-MS/MS.
Fig. 2.
Examples of the whole blood spotted to a DMPK-C card and collected with a VAMS device.
HPLC-MS/MS ANALYSIS
HPLC was performed on Shimadzu LC-20AD (Shimadzu Corporation) instrument coupled to an AB Sciex 5500 Q-Trap mass spectrometer. Chromatography was performed on an Atlantis® HILIC Silica 3μm column (2.1 × 100 mm). Hydroxyurea and the IS coeluted from the column and were separated from other potentially interfering compounds using a gradient elution mobile phase composed of a binary solvent mixture comprised of 10 mmol/L ammonium formate (solvent A) and acetonitrile with 0.1% formic acid (solvent B). The gradient started with 97% solvent B for 130 s and was then ramped down to 50% solvent B over a 140 s period, where it was maintained for 60 s before recycling back to 97% in 210 s. It was held at 97% solvent B for 150 s before initiating the next injection. Chromatography was performed at 40 °C with a flow rate of 0.4 mL/min. The total chromatographic run time was 5 min. The injection volume was 10 μL for each sample. MS/MS acquisition was carried out in multiple reaction ion monitoring mode using electrospray ionization with detection of the positive ions generated. The following electrospray ionization inlet conditions were used: gas 1, nitrogen (60 ψ); gas 2, nitrogen (50 ψ); ion spray voltage 5000 V; ion source temperature, 550 °C; curtain gas, nitrogen (30 ψ). Detection of hydroxyurea was achieved by monitoring the [M+H]+ (parent) ion and the collision-induced dissociation fragment at m/z 44 (Fig. 1). The corresponding transition m/z →46 was monitored for the stable-labeled IS (Fig. 1). Data acquisition and quantitative processing were accomplished using Multiquant™ version 3.0.
METHOD VALIDATION
A series of experiments using different extraction solvents and conditions were performed to determine optimal conditions for the extraction of hydroxyurea from whole blood spots and VAMS devices. Numerous solvents and combinations of solvents were investigated and the effect of temperature, mode of extraction, and extraction time were explored.
LINEARITY
Calibrators of hydroxyurea prepared in a pooled whole blood matrix over the concentration range 0.5–60.0 μg/mL were added to blank DMPK-C cards and VAMS devices to generate standard curves. The resulting samples on DMPK-C cards and VAMS devices were subjected to exactly the same procedures as the patient samples. The area ratio of analyte/IS was plotted against the calibrator concentration. The hydroxyurea concentration in the patient sample was determined from the peak area response ratio for hydroxyurea/IS interpolated against the calibration curve of known concentrations of hydroxyurea. The limit of quantification (LOQ) and limit of detection (LOD) was calculated based on a signal to noise ratio of 10 and 3, respectively (21).
SELECTIVITY AND CARRYOVER
To test the selectivity of the method, blood obtained from 6 healthy volunteers in the absence of any drug administration was applied to DMPK-C cards and sampled with VAMS devices and the extracts analyzed for potential interferences from endogenous compounds eluting at the retention time corresponding to hydroxyurea. Carryover was assessed by analyzing sample blanks consisting of mobile phase interspersed between patient samples and calibrators.
EXTRACTION RECOVERY AND MATRIX EFFECT
The overall efficiency of the extraction procedure was determined by analysis of whole blood samples that were spiked with hydroxyurea at concentrations of 0.5, 5, 30, and 60 μg/mL. At each concentration, 4 replicates were spotted onto DMPK-C cards or VAMS devices and extracted. The same concentrations of pure hydroxyurea standards in solvent were also analyzed identically and the peak area response for hydroxyurea obtained for the extracts of the blood samples was compared the peak area response of hydroxyurea obtained from the pure standards, and the ratio expressed as a % recovery.
Endogenous compounds like phospholipids in whole blood can produce a matrix effect (22) resulting in suppression or enhancement of ionization (23). The effect of the matrix on hydroxyurea LC-MS signal intensity was investigated at concentrations of 5 and 60 μg/mL. At each concentration 3 replicates were spiked to extracts from DMPK-C cards or VAMS devices that did not contain hydroxyurea. The matrix effect was expressed as the ratio of peak area response obtained for hydroxyurea from the blood extracts to that of pure standards of hydroxyurea in solvent at the same concentrations.
RECOVERY AND IMPRECISION
Recovery and imprecision were evaluated by adding known amounts of analyte [at 4 different concentrations, corresponding to the low LOQ (LLOQ), and at the low, medium, and high concentration QC controls] spiked to blank DMPK-C cards and VAMS devices. The recovery was expressed as the difference of the measured analyte concentrations from nominal values (% bias) and the imprecision as the CV of the measured concentrations. The intra- and interassay recovery was targeted to be within ±15%, except for the LLOQ where a deviation of ±20% was accepted. The intraassay and interassay reproducibility (relative SD) was targeted not to exceed 15%, except for the LLOQ, where 20% deviation was accepted.
STABILITY
The short- and long-term stability of hydroxyurea spotted on DMPK-C cards and VAMS devices was evaluated using low and high concentration quality control samples by comparing the hydroxyurea concentration determined in freshly prepared samples with the same samples that were stored at 4 °C, −20 °C, and −80 °C after storage for 7, 14, and 30 days.
COMPARISION OF SAMPLES COLLECTED ON DMPK-C CARDS WITH VAMS DEVICES
Blood samples from patients with SCA who were being treated with hydroxyurea were used to demonstrate the applicability of the analytical technique. Whole blood from these patients was simultaneously applied to both DMPK-C cards or collected with VAMS devices (Fig. 2) and hydroxyurea concentrations determined by the described HPLC-MS/MS methodology.
Results and Discussion
SAMPLE EXTRACTION
Hydroxyurea is a small (76 Da) and highly polar molecule, and consequently proved difficult to completely extract from dried blood samples collected on DMPK-C cards and VAMS devices. Numerous solvents and combinations of solvents were used in an attempt to obtain a quantitative recovery of hydroxyurea from the dried whole blood samples and these included methanol, acetonitrile, buffer/organic solvent, dichloromethane, ethyl acetate, isopropanol, and n-butanol. None of these solvents proved to be suitable to efficiently recover hydroxyurea from the paper. However, aqueous/organic mixtures yielded much improved extraction recoveries and ultimately a mixture of acetonitrile:water (80:20, v/v) was found to yield the highest and most consistent recovery (55%) among all the solvent mixtures and strategies tested. Sonication and shaking was performed for periods of 15, 30, 45, and 60 min to facilitate the extraction recovery of hydroxyurea and overall, shaking the sample was found to be preferable to sonication, but no differences in recovery of hydroxyurea were noted among the different duration times of shaking. For this reason we opted to shake the sample for 15 min to recover hydroxyurea from the paper.
EFFECT OF DIFFERENT PAPERS
There are currently a number of different commercial papers/cards that can be used to obtain whole dried blood specimens, so we compared the efficiency of extraction of hydroxyurea from a number of different cards with the VAMS device. Whole blood (10 μL) was pipetted onto DMPK-C cards, 903 specimen cards, and FTA classic cards and also collected with VAMS devices calibrated to absorb 10 μL of whole blood (24). After spotting, whole blood specimens were allowed to dry for 2–3 h at ambient temperature, and then the solvent extraction performed. Dried whole blood collected on DMPK-C cards and VAMS devices performed comparably well and yielded the highest recoveries of hydroxyurea when compared with the other papers and polymer tested (see online Supplemental Fig. 1). For this reason, further method validation was performed with both these sampling devices.
EFFECT OF EXTRACTION TEMPERATURE
Dried whole blood samples were analyzed under various conditions with or without heat, recognizing that heat can degrade chemical compounds or stop enzymatic degradation of analytes and stabilize them without the need for chemical additives (25). Heating the sample during extraction improved the extraction efficiency and reproducibility of hydroxyurea from the whole dried blood samples. Temperatures of 40 °C, 70 °C, and 100 °C were investigated, and heating at 40 °C gave the highest recovery of hydroxyurea from both DMPK-C cards and VAMS devices while providing highly reproducible results. In contrast, extraction at 70 °C and 100 °C yielded a lower recovery, which we assumed to be due to some degradation of the hydroxyurea (data not shown).
CHOICE OF IS
Typically, the stable-labeled analog of the compound to be measured is added directly to the sample matrix before work-up. Stable-labeled forms of hydroxyurea were commercially available and therefore used; here, 1 atom of13C and 2 atoms of 15N were incorporated into the molecule resulting in a mass shift of 3 Da (Fig. 1). In the case of blood spots collected directly from the patient, it was not possible to add the IS; furthermore, after addition of IS to the paper would not afford equilibration with endogenous hydroxyurea in the dried sample. To overcome this problem pooled whole blood samples containing known concentrations of hydroxyurea were prepared and then applied to DMPK-C cards or VAMS devices for use as calibrators. These dried blood samples were then subjected to the same extraction method to that for patient samples with addition of the stable-labeled IS taking place after extraction and recovery of hydroxyurea.
METHOD VALIDATION
Linearity.
Linearity (r2 >0.99) was obtained over the entire dynamic range of 0.5–60 μg/mL for both types of whole blood collection methods (Fig. 3).
Fig. 3. Calibration curves obtained for hydroxyurea in whole blood spotted on DMPK-C cards and VAMS devices.
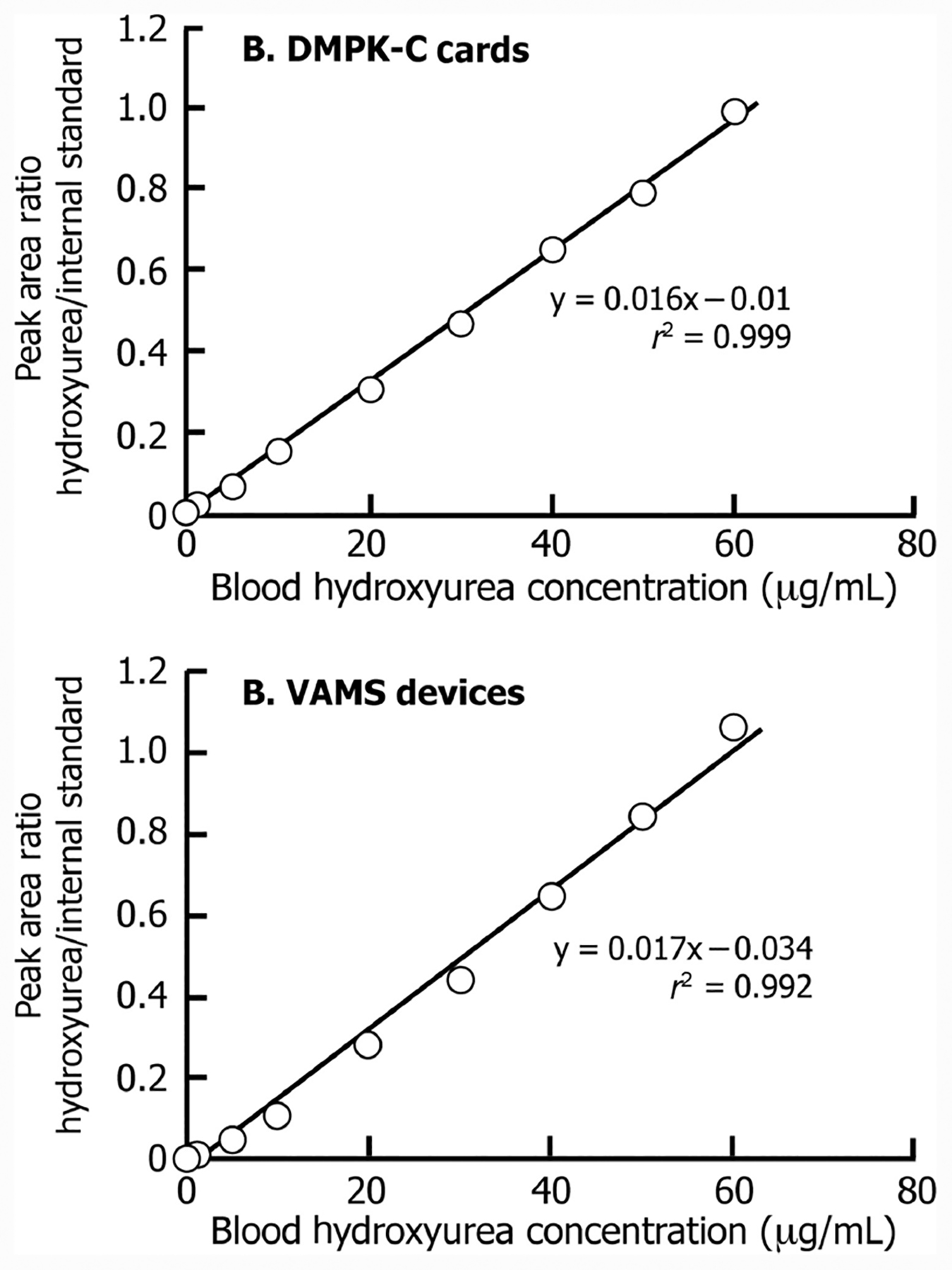
The area ratio of the hydroxyurea peak/IS is plotted against concentration of hydroxyurea in dried blood from these collection devices.
Selectivity and carryover.
In the absence of hydroxyurea, analysis of samples from healthy volunteers confirmed there were no interfering endogenous compounds in whole blood that coeluted or interfered with the measurement of hydroxyurea or its IS when monitoring the multiple reaction monitoring (MRM) transitions selected. Thus, selectivity was considered to be satisfactory (see online Supplemental Fig. 2).
EXTRACTION RECOVERY AND MATRIX EFFECT
The mean extraction recoveries of hydroxyurea from DMPK-C cards and VAMS devices were consistently 52% and 53%, respectively. The IS extraction recoveries were 57.34% for DMPK-C cards and 58.98% for VAMS devices. Although these values represented incomplete recovery rates, they were reproducible and allowed accurate comparison of hydroxyurea concentrations with calibrators that were prepared on DMPK-C cards and VAMS devices and extracted in an identical fashion to that of the patient samples. The matrix ionization suppression or enhancement of the analyte and IS was assessed by measuring the matrix factor at 2 QC concentration levels. The mean absolute matrix factors at low and high QC concentration were 0.74 and 0.75 for samples collected on DMPK-C cards and 0.72 and 0.71 for VAMS devices respectively (see online Supplemental Table 2). The mean absolute matrix factor for IS was 0.78 for DMPK-C cards and 0.77 for samples applied to VAMS devices.
RECOVERY AND IMPRECISION
Intraassay (n = 10) and interassay precision (n = 20) for samples collected on DMPK-C cards and VAMS devices spiked with 0.5, 5, 30, and 60 μg/mL of hydroxyurea standard in whole blood gave CVs ranging 5.50%–11.55% and 1.36%–7.78%, respectively. The corresponding interassay comparisons ranged from 1.29%–7.69% for DMPK-C cards and 2.79%–9.12% for the VAMS devices. The intra- and interassay recovery was 97.0%–104.0% and 92.0%–105.0% of the target concentration, respectively for hydroxyurea measured from DMPK-C cards, and correspondingly, 89.0%–100.0% and 87%–102% for measurement from VAMS devices (see online Supplemental Table 3). These results demonstrate excellent reproducibility for the determination of hydroxyurea in small volumes of whole blood.
STABILITY
The stability for hydroxyurea in processed samples maintained in the Autosampler was found to be similarly stable for up to 48 h for samples collected on DMPK-C cards and VAMS devices (Table 1). Hydroxyurea was found to be stable when collected and stored on DMPK-C cards and VAMS devices at temperatures of −20 °C and −80 °C for up to 60 days. However, there was evidence of 25% degradation observed for whole dried blood collected on DMPK-C cards and VAMS devices at a hydroxyurea concentration of 0.5 μg/mL, and 52% degradation at a concentration of 60 μg/mL when both types of samples were maintained at 4 °C, for 30 days. These results indicate that, once collected and dried, samples should be frozen to ensure stability over time.
Table 1.
Effect of temperature and duration of storage on whole blood hydroxyurea concentrations measured from dried blood collected using DMPK-C cards and VAMS devices at different concentrations.a
| Concentration, μg/mL | T = 7 days | T = 14 days | T = 30 days | |
|---|---|---|---|---|
| DMPK C cards | ||||
| Stored at 4 °C | 0.5 | 90.17 ± 0.44 | 81.75 ± 3.85 | 75.85 ± 4.08 |
| 60.0 | 65.37 ± 1.07 | 54.13 ± 3.39 | 48.58 ± 0.23 | |
| Stored at −20 °C | 0.5 | 95.08 ± 0.01 | 90.56 ± 13.29 | 96.07 ± 5.12 |
| 60.0 | 90.88 ± 1.83 | 92.99 ± 2.03 | 93.07 ± 3.11 | |
| Stored at −80 °C | 0.5 | 102.14 ± 0.59 | 97.41 ± 14.36 | 99.98 ± 3.81 |
| 60.0 | 93.69 ± 3.64 | 94.22 ± 2.34 | 100.45 ± 1.09 | |
| VAMS devices | ||||
| Stored at 4 °C | 0.5 | 87.90 ± 10.32 | 91.86 ± 7.97 | 75.11 ± 0.56 |
| 60.0 | 64.19 ± 1.28 | 51.98 ± 0.46 | 46.14 ± 0.98 | |
| Stored at −20 °C | 0.5 | 106.52 ± 12.11 | 103.15 ± 2.56 | 95.37 ± 6.01 |
| 60.0 | 83.69 ± 1.28 | 82.55 ± 1.65 | 82.48 ± 0.78 | |
| Stored at −80 °C | 0.5 | 101.92 ± 10.34 | 103.58 ± 3.20 | 106.00 ± 8.10 |
| 60.0 | 86.62 ± 1.86 | 90.21 ± 0.94 | 93.53 ± 0.68 |
The values are expressed as the % of the target concentration measured in freshly prepared samples of whole blood to which known concentrations of hydroxyurea were added.
COMPARISON OF DMPK-C CARDS AND VAMS DEVICES
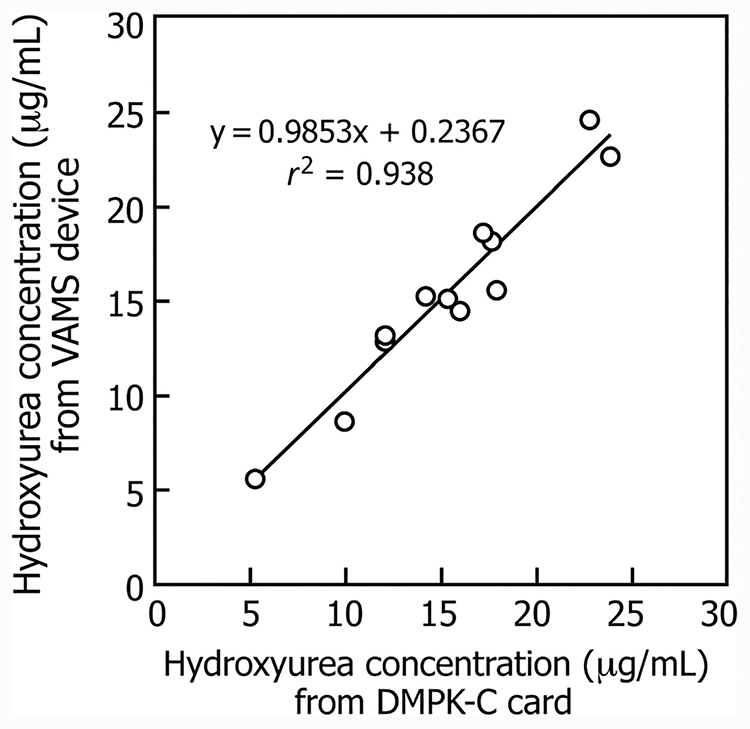
When whole blood was spiked with known and different concentrations of hydroxyurea and collected on VAMS devices or DMPK-C cards the measured concentration of hydroxyurea was similar among the different modes of sampling (see online Supplemental Table 4A). The applicability of this LC-MS/MS method was demonstrated from measurement of hydroxyurea concentrations in whole blood samples from patients treated with hydroxyurea by comparing concentrations measured from samples collected on DMPK-C cards and VAMS devices. Measurement of hydroxyurea in whole blood samples collected on DMPK-C cards and VAMS devices gave similar results with a correlation of 0.938 for whole blood hydroxyurea concentrations between the 2 methods of sampling whole blood (Fig. 4). Because blood spot collection on DMPK-C cards from patients in the clinic or outreach locations would provide variable spotted volumes of blood for analysis, we prefer the VAMS micro-sampling device for analysis of patient samples because it is calibrated to absorb exactly 10.0 μL and eliminates hematocrit variability (18). To date, collection of this small volume of blood has been very easy, even in children with SCA. It has been reported that sampling blood by finger-prick yields successive capillary drops that are heterogeneous with regard to hematocrit and other hematologic markers (26), which could lead to considerable variability in estimating the circulating concentrations of any drug despite excellent analytic imprecision. Despite this limitation of finger-prick sampling, we observed comparable concentrations for hydroxyurea in plasma and whole blood irrespective of whether blood was obtained through venipuncture or finger-prick sampling for patients with SCA treated with hydroxyurea (see online Supplemental Table 4B), presumably because hydroxyurea fully equilibrates between whole blood and plasma.
Fig. 4.
A comparison of the blood hydroxyurea concentrations measured in patients with SCA treated with the drug hydroxyurea when the samples were collected as dried blood samples on DMPK-C cards and on VAMS devices.
CLINICAL APPLICATION
This LC-MS/MS assay for the measurement of hydroxyurea in whole blood has potentially important clinical utility for the therapeutic monitoring of hydroxyurea concentrations in patients with SCA treated with this drug. Hydroxyurea is now recognized as the primary disease-modifying treatment for SCA, and new NIH guidelines recommend increased use in both children and adults (27). As more patients begin hydroxyurea treatment, it will be important to optimize and individualize therapy by measurement and monitoring of hydroxyurea concentrations, as well as assessing medication adherence.
When children with SCA begin hydroxyurea treatment, the usual method of dosing begins with standard initiation at 15–20 mg/kg/day, given as a single daily oral dose, followed by stepwise increases every 1–2 months toward a maximum tolerated dose, defined as the stable daily dose causing mild marrow suppression without hematological toxicities (6, 7). However, this dose initiation and escalation process is necessarily slow and patients often require over 6 months to achieve the maximum tolerated dose (28), which delays their clinical response. Our LC-MS/MS methodology to measure hydroxyurea could improve this process. Our previous hydroxyurea pharmacokinetics analysis indicates that peak plasma levels are achieved between 15 min and 2 h after the daily dose, but no steady-state level is achieved because of the short in vivo half-life and daily dosing regimen (7). However, area-under-the-curve calculations may help predict the hydroxyurea maximum tolerated dose, which has led us to develop a Pk-based pharmacometrics-based model of hydroxyurea dosing (29).
Another area of potential clinical application is the assessment of hydroxyurea medication adherence; because the drug is usually detectable for 6–8 h after the daily dose (7), spot clinical checks of serum or whole blood can be performed using our LC-MS/MS technique. To provide an important clinical example, plasma was recently collected on 2 young siblings with suspected medication noncompliance. Whole blood analysis using the microsampling device failed to detect any hydroxyurea a few hours after the dose was allegedly administered, which led to a family intervention to improve daily dosing. The improved analytical sensitivity of our LC-MS/MS technique to detect hydroxyurea concentrations as low as 5 ng/mL should increase the duration of drug detection, and possibly allow assessment of medication adherence for 12–24 h after the daily dose. The LC-MS/MS method based on dried blood analysis yielded hydroxyurea concentrations in blood samples that were similar to values obtained by an existing HPLC method that required much higher blood volumes (see online Supplemental Table 5).
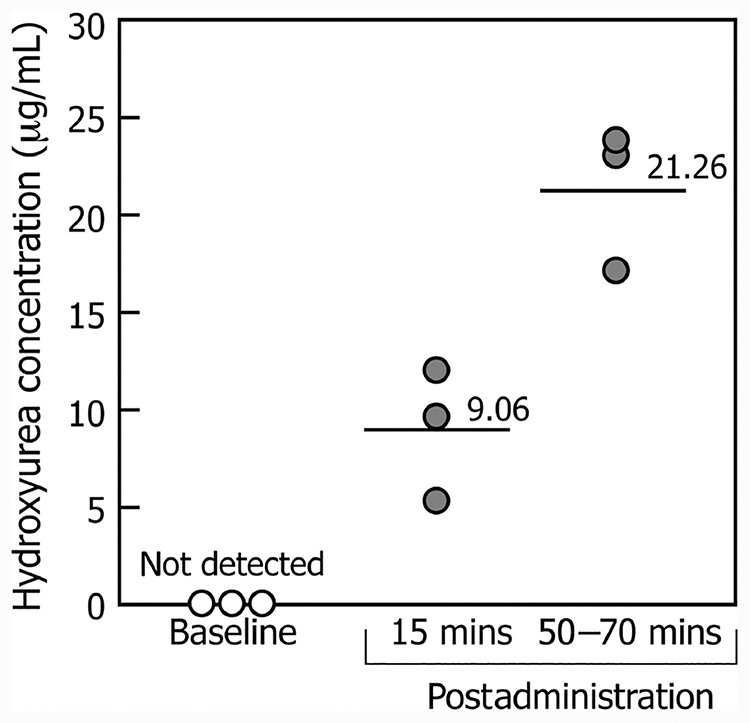
Finally, the clinical utility of this assay is exemplified by our demonstration of differences in the whole blood hydroxyurea concentrations in the samples from several patients with SCA, collected at different times after drug administration. As expected, the highest concentrations were found in blood samples collected between 50–70 min after drug administration, whereas hydroxyurea was not detected in samples collected at baseline (Fig. 5).
Fig. 5. Whole blood hydroxyurea concentrations in 3 patients with SCA treated with the drug hydroxyurea measured at baseline and at 15 min and 50–70 min after drug administration.
Blood hydroxyurea was measured by LC-MS/MS in finger-prick samples collected as dried blood on VMAS devices.
Overall, the advantages of this analytical method over previously described methods include rapid and convenient sample preparation, direct injection without derivatization, small sample volume requirements, and high reproducibility. This approach has substantial advantages over venous blood drawing in affording measurement in blood samples obtained by finger- or heel-prick, and for this reason is ideal for clinical application to pediatric patients treated with the drug. Immediate processing of the blood samples is not necessary, and the collected cards and VAMS devices can be stored and transported from remote sites. It may become possible to consider drug monitoring in remote settings such as low-resource countries in Africa, where the SCA burden is greatest.
Supplementary Material
Acknowledgments:
The authors thank Thad A. Howard and Kathryn E. McElhinney for assistance and help during sample preparation. The authors also thank Dr. Junfang Zhao for assistance with the MS instrumentation and Dr. Alexander Vinks for helpful advice on drug extracting, monitoring, and pharmacokinetics.
Research Funding: P.T. McGann, NIH (K23 HL128885 02) and Cincinnati Children’s Hospital Research Foundation (Procter Scholar Award).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, and final approval of manuscript.
Nonstandard abbreviations:
- SCA
sickle cell anemia
- MS
mass spectrometry
- LC-MS/MS
liquid chromatography tandem MS
- VAMS
volumetric absorptive microsampling
- IS
internal standard
- CAN
acetonitrile
- LOQ
limit of quantification
- LOD
limit of detection
- LLOQ
low LOQ
- MRM
multiple reaction monitoring
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Kennedy BJ, Yarbro JW. Metabolic and therapeutic effects of hydroxyurea in chronic myeloid leukemia. JAMA 1966;195:1038–43. [PubMed] [Google Scholar]
- 2.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med 2006;355:2452–66. [DOI] [PubMed] [Google Scholar]
- 3.Perrin J, Ranta D, Lesesve JF. Hydroxyurea-induced stomatocytes in a patient presenting with polycythemia vera. Am J Hematol 2015;90:573. [DOI] [PubMed] [Google Scholar]
- 4.Lori F, Malykh A, Cara A, Sun D, Weinstein JN, Lisziewicz J, Gallo RC. Hydroxyurea as an inhibitor of human immunodeficiency virus-type 1 replication. Science 1994; 266:801–5. [DOI] [PubMed] [Google Scholar]
- 5.Ware RE. Hydroxycarbamide: clinical aspects. C R Biol 2013;336:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware RE, Despotovic JM, Mortier NA, Flanagan JM, He J, Smeltzer MP, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 2011; 118:4985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22. [DOI] [PubMed] [Google Scholar]
- 9.Gwilt PR, Tracewell WG. Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin Pharmacokinet 1998; 34:347–58. [DOI] [PubMed] [Google Scholar]
- 10.Heeney MM, Whorton MR, Howard TA, Johnson CA, Ware RE. Chemical and functional analysis of hydroxyurea oral solutions. J Pediatr Hematol Oncol 2004;26:179–84. [DOI] [PubMed] [Google Scholar]
- 11.Navarra P, Del Carmine R, Ciabattoni G, D’Amato M, Ragazzoni E, Vacca M, et al. Hydroxyurea: relationship between toxicity and centrally-induced adrenal activation. Pharmacol Toxicol 1990;67:209–15. [DOI] [PubMed] [Google Scholar]
- 12.Pujari MP, Barrientos A, Muggia FM, Koda RT. Determination of hydroxyurea in plasma and peritoneal fluid by high-performance liquid chromatography using electrochemical detection.J Chromatogr B Biomed Sci Appl 1997;694:185–91. [DOI] [PubMed] [Google Scholar]
- 13.Iyamu EW, Roa PD, Kopsombut P, Aguinaga MD, Turner EA. New isocratic high-performance liquid chromatographic procedure to assay the anti-sickling compound hydroxyurea in plasma with ultraviolet detection. J Chromatogr B Biomed Sci Appl 1998;709:119–26. [DOI] [PubMed] [Google Scholar]
- 14.Yan JH, Ataga K, Kaul S, Olson JS, Grasela DM, Gothelf S, et al. The influence of renal function on hydroxyurea pharmacokinetics in adults with sickle cell disease. J Clin Pharmacol 2005;45:434–45. [DOI] [PubMed] [Google Scholar]
- 15.Dogruel M, Gibbs JE, Thomas SA. Hydroxyurea transport across the blood-brain and blood-cerebrospinal fluid barriers of the guinea-pig. J Neurochem 2003;87: 76–84. [DOI] [PubMed] [Google Scholar]
- 16.Garg U, Scott D, Frazee C, Kearns G, Neville K. Isotope-dilution gas chromatography-mass spectrometry method for the analysis of hydroxyurea. Ther Drug Monit 2015;37:325–30. [DOI] [PubMed] [Google Scholar]
- 17.Kettani T, Cotton F, Gulbis B, Ferster A, Kumps A. Plasma hydroxyurea determined by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:446–50. [DOI] [PubMed] [Google Scholar]
- 18.Spooner N, Denniff P, Michielsen L, De Vries R, Ji QC, Arnold ME, et al. A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit. Bioanalysis 2015;7: 653–9. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm AJ, den Burger JC, Swart EL. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet 2014;53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachir D, Hulin A, Huet E, Habibi A, Nzouakou R, El Mahrab M, et al. Plasma and urine hydroxyurea levels might be useful in the management of adult sickle cell disease. Hemoglobin 2007;31:417–25. [DOI] [PubMed] [Google Scholar]
- 21.Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, et al. Bioanalytical method validation–a revisit with a decade of progress. Pharm Res 2000;17: 1551–7. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Lankmayr E. Phospholipid-based matrix effects in LC-MS bioanalysis. Bioanalysis 2011;3:349–52. [DOI] [PubMed] [Google Scholar]
- 23.Annesley TM. Ion suppression in mass spectrometry. Clin Chem 2003;49:1041–4. [DOI] [PubMed] [Google Scholar]
- 24.Denniff P,Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem 2014;86:8489–95. [DOI] [PubMed] [Google Scholar]
- 25.Blessborn D, Skold K, Zeeberg D, Kaewkhao K, Skold O, Ahnoff M. Heat stabilization of blood spot samples for determination of metabolically unstable drug compounds. Bioanalysis 2013;5:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bond MM, Richards-Kortum RR. Drop-to-drop variation in the cellular components of fingerprick blood: implications for point-of-care diagnostic development. Am J Clin Pathol 2015;144:885–94. [DOI] [PubMed] [Google Scholar]
- 27.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312: 1033–48. [DOI] [PubMed] [Google Scholar]
- 28.Ware RE. Optimizing hydroxyurea therapy for sickle cell anemia. Hematology Am Soc Hematol Educ Program 2015;2015:436–43. [DOI] [PubMed] [Google Scholar]
- 29.Dong M, McGann PT, Mizuno T, Ware RE, Vinks AA. Development of a pharmacokinetic-guided dose individualization strategy for hydroxyurea treatment in children with sickle cell anaemia. Br J Clin Pharmacol 2016;81:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.