Abstract
Regulated cell death is a major mechanism to eliminate damaged, infected, or superfluous cells. Previously, apoptosis was thought to be the only regulated cell death mechanism; however, new modalities of caspase-independent regulated cell death have been identified, including necroptosis, pyroptosis, and autophagic cell death. As an understanding of the cellular mechanisms that mediate regulated cell death continues to grow, there is increasing evidence that these pathways are implicated in the pathogenesis of many pulmonary disorders. This review summarizes our understanding of regulated cell death as it pertains to the pathogenesis of chronic obstructive pulmonary disease, asthma, idiopathic pulmonary fibrosis, acute respiratory distress syndrome, and pulmonary arterial hypertension.
Keywords: apoptosis, necroptosis, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, asthma, pulmonary arterial hypertension, acute respiratory distress syndrome
INTRODUCTION
The health and survival of multicellular organisms rely on the ability to eliminate damaged, infected, or superfluous cells. Therefore, multicellular organisms possess genetically encoded mechanisms via which cells can undergo regulated cell death (RCD). RCD occurs as part of normal physiologic programming, including organ development and epithelial renewal. It also occurs in cells that cannot mitigate stressors that threaten tissue homeostasis, e.g., cells infected by intracellular pathogens or cells encumbered by DNA damage, oxidative stress, and misfolded proteins.
Initially, investigators held the belief that there were only two forms of cell death, apoptosis and necrosis. Apoptosis is a metabolically active process in which cell death occurs with characteristic morphologic features that include cell membrane shrinkage, nuclear chromatin condensation, nuclear fragmentation, and plasma membrane blebbing. In contrast, necrosis occurs when cells die accidentally from extreme or rapid injury, resulting in the dissolution of the plasma membrane, cellular swelling, and the release of intracellular contents that promote inflammation (1–3). While these morphologic features remain relevant to understanding RCD, this dichotomous characterization of cell death has been elaborated, and now the term RCD encompasses multiple forms of active cell death with morphologic features that resemble apoptosis or necrosis (4).
Dysregulated RCD has been implicated in human disease. Excessive RCD causes tissue injury and destruction, whereas a failure of RCD is implicated in mutagenesis, impaired immunity, and autoimmune disease (5). Nowhere is the regulation of cell death more important than in the lung, an organ required to maintain a delicately thin network of epithelial-endothelial interfaces to allow for effective exchange of CO2 for O2, all while exposed to environmental stressors such as pathogens and aerosolized toxins. In this review, we summarize the current molecular understanding of RCD and focus on the role of RCD in five lung diseases: chronic obstructive pulmonary disease (COPD), asthma, idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), and pulmonary arterial hypertension (PAH).
APOPTOSIS
Apoptosis is mediated by two signaling cascades: the intrinsic and extrinsic apoptosis pathways. Intrinsic apoptosis commonly occurs due to a disruption of cellular homeostasis (Figure 1), and extrinsic apoptosis occurs as a consequence of extracellular signaling via death receptors (Figure 2). While intrinsic and extrinsic apoptosis have unique initiating steps, both culminate in the activation of a set of cysteine proteases called caspases (6). Initiator caspases (e.g., caspase 8 or 9) begin a series of proteolytic steps that lead to activation of executioner caspases (e.g., caspase 3 or 7) (1). Executioner caspases cleave thousands of substrates and are responsible for the enzymatic degradation of organelles, DNA fragmentation, and characteristic phosphatidylserine exposure. In human lung tissue, apoptosis has been commonly assessed by ultrastructural analyses of cellular morphology via electron microscopy; measuring DNA fragmentation histologically by terminal dUTP nick end labeling (TUNEL); flow cytometry of isolated cells for assessment of phosphatidylserine exposure and cell viability with annexin V and propidium iodide, respectively; and immunohistochemistry of proteins involved in apoptosis (7, 8).
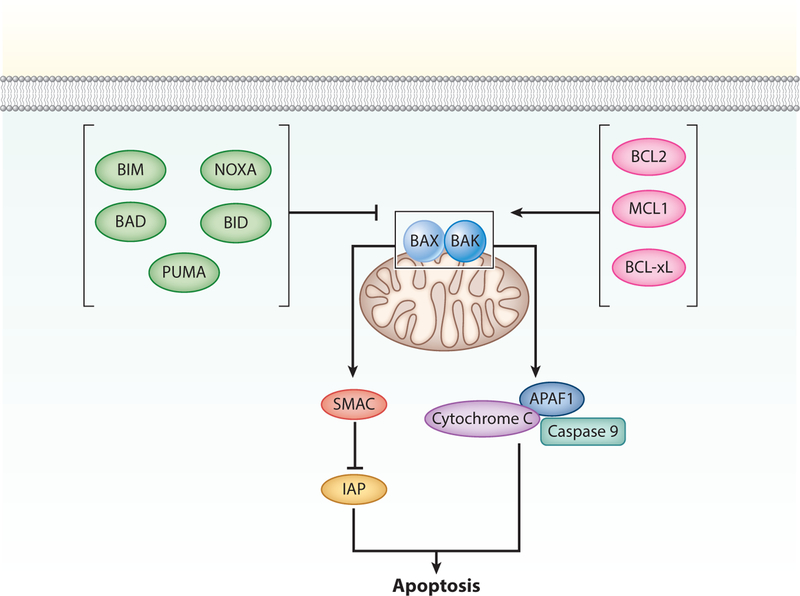
Figure 1.
Intrinsic apoptosis. The step that commits cells to undergo apoptosis is permeabilization of the mitochondrial outer membrane (MOMP). MOMP occurs when B-cell lymphoma 2 (BCL2)-associated X apoptosis regulator (BAX) and BCL2 antagonist/killer 1 (BAK) form outer mitochondrial membrane pores. This process is promoted by BH3-only proteins, including BCL2-associated death promoter (BAD), p53-upregulated binding component (PUMA), BCL2-like 11 (BIM), phorbol-12-myristate-13-acetate-induced protein (NOXA), and BH3-interacting domain death agonist (BID); and antagonized by antiapoptotic BCL2 proteins, including BCL2, B-cell lymphoma extra large (BCL-xL), and myeloid leukemia cell differentiation protein (MCL1). MOMP causes the release of cytochrome c and second mitochondria–derived activator of caspase (SMAC). Cytochrome c binds apoptotic protease activating factor 1 (APAF1) and initiator caspase 9 to form the apoptosome, where caspase 9 is activated. SMAC neutralizes the cytoplasmic proteins maintained by cells to restrain caspase activation (inhibitor of apoptosis proteins, IAPs).
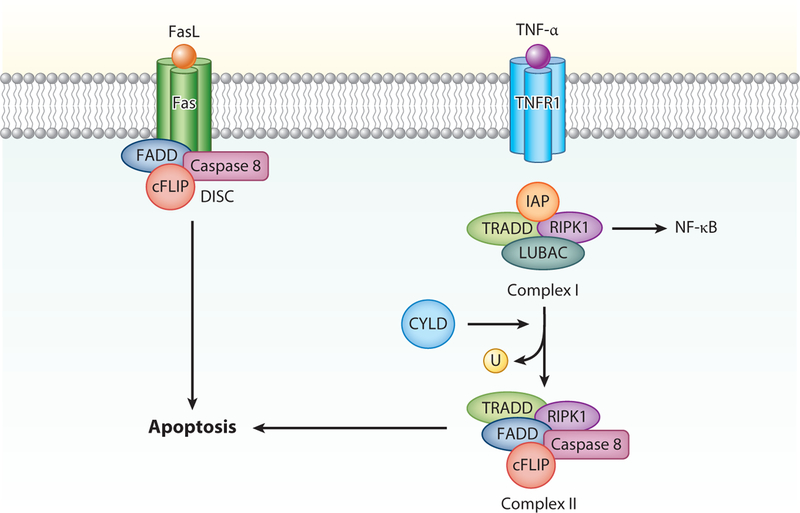
Figure 2.
Extrinsic apoptosis. Ligand binding to Fas results in the formation of the death-inducing signaling complex (DISC), composed of Fas-associated death domain (FADD), cellular FLICE-inhibitory protein (cFLIP), and caspase 8. Upon activation, cleavage of caspase 8 leads to cleavage of executioner caspases and apoptosis. The consequences of TNF receptor 1 (TNFR1) activation depends on posttranslational modifications of another protein recruited to Complex 1, receptor-interacting protein kinase 1 (RIPK1). Upon TNFR1-associated death domain (TRADD)-dependent recruitment, RIPK1 can be ubiquitinated by inhibitor of apoptosis proteins (IAPs) and linear ubiquitination chain assembly complex (LUBAC). Ubiquitinated RIPK1 (U) promotes inflammation and cell survival by activating protein kinase signaling and IκB kinase (IKK)-dependent NF-κB activation. If IAPs are absent or inhibited, RIPK1 is deubiquitinated by cylindromatosis (CYLD). Consequently, RIPK1 forms a complex with FADD and caspase 8 in the cytosol (Complex II). Similar to signaling by Fas, this complex is also regulated by cFLIP proteins and can lead to caspase 8 activation and apoptosis.
Mitochondrial outer membrane permeabilization (MOMP) commits cells to undergo intrinsic apoptosis (Figure 1). MOMP leads to the release of proapoptotic factors into the cytoplasm from the mitochondrial intermembrane space, including cytochrome c and second mitochondria-derived activator of caspase (SMAC). Cytochrome c binds apoptotic protease activating factor 1 (APAF1) which activates caspase 9, and SMAC neutralizes cytoplasmic inhibitors of apoptosis proteins (IAPs) that restrain caspase activation, e.g., X-linked inhibitor of apoptosis protein (XIAP) and survivin. Inhibition of caspases, either genetically or pharmacologically (e.g., Z-VAD-fmk), will only delay cell death, as intact mitochondria are necessary for cellular survival. Classically, MOMP was considered an all or nothing response; however, sublethal MOMP activation exists and is often associated with genomic instability (9).
MOMP is regulated by the B-cell lymphoma 2 (BCL2) family of proteins, which shares up to four BCL2 homologous domains (BH1–BH4) (4). Three categories of BCL2 proteins are involved in intrinsic apoptosis: (a) MOMP effectors that form pores across the outer mitochondrial membrane in response to apoptotic stimuli, including BCL2-associated X apoptosis regulator (BAX) and BCL2 antagonist/killer 1 (BAK); (b) proteins with only a BH3 motif (BH3-only) that promote apoptosis by binding and neutralizing prosurvival BCL2 proteins, including BCL2-associated death promoter (BAD), p53-upregulated binding component (PUMA), BCL2-like 11 (BIM), phorbol-12-myristate-13-acetate-induced protein (NOXA), and BH3-interacting domain death agonist (BID); and (c) BCL2 apoptosis inhibitors, including BCL2, B-cell lymphoma extra large (BCL-xL), and induced myeloid leukemia cell differentiation protein (MCL1). Pharmacologic BH3 mimetics have been developed to antagonize prosurvival BCL2 proteins and are currently in use for treatment of chronic lymphocytic leukemia or under investigation for treatment of other malignancies. Direct activators of BAX have also been developed (10).
In the lung, intrinsic apoptosis can occur as a consequence of growth factor withdrawal, oxidative stress, endoplasmic reticular (ER) stress, and DNA damage. Growth factors, cytokines, and other extracellular ligands can activate tyrosine kinase receptors and G protein–coupled receptors to upregulate prosurvival signaling cascades. For example, protein kinase B (AKT) is activated by the phosphorylation of phosphoinositide 3-kinase (PI3K) and negatively regulated by phosphatase and tensin homolog (PTEN) (11). Phosphorylated AKT directly inhibits BAD and caspase 9. AKT also inhibits transcription factors that upregulate proapoptotic BCL2 proteins, including the forkhead family of transcription factors (FOXO) and yes-associated protein (YAP). AKT also activates transcription factors that upregulate antiapoptotic BCL2 proteins, including cAMP response element-binding protein (CREB) and nuclear factor kappa B (NF-κB) (11, 12). Other signaling pathways that can promote cellular survival include extracellular regulated kinases (ERK1/2), Janus kinases (JAKs)/signal transducer, and activator of transcription proteins (STATs) (13). In addition to growth factor withdrawal, there is a special type of apoptosis called anoikis in which the loss of integrin-dependent anchorage to the extracellular matrix triggers caspase activation (4, 14).
Oxidative stress is an important cause of intrinsic apoptosis. Although reactive oxygen species (ROS) are important for cell signaling, excessive levels lead to macromolecular damage and cell death. In the lung, oxidative stress occurs as a consequence of inhaled toxins such as cigarette smoke, ROS generation by inflammatory cells, and mitochondrial metabolism (15). Oxidative stress can decrease mitochondrial membrane potential, promoting MOMP. Oxidative stress also leads to activation of p38 mitogen–activated protein kinase (p38) and c-Jun N-terminal kinase (JNK) via apoptosis signal–regulating kinase 1 (ASK1). Both JNK and p38 can function as pro- or antiapoptotic mediators, depending on the context. In the lung, JNK activation from oxidative stress promotes apoptosis by facilitating cytochrome c release, activating BH3-only proteins, and inhibiting BCL2. Oxidative stress also contributes to ER stress and DNA damage.
ER stress is a consequence of excess protein misfolding, often due to oxidative stress, viral infection, or genetic mutations. In response to ER stress, cells activate the unfolded protein response (UPR), a protective mechanism to facilitate protein folding that includes upregulation of protein chaperones, inhibition of mRNA translation, and promotion of the transport of misfolded ER proteins into the cytosol for ubiquitination and degradation (16). However, if ER stress is sufficiently severe or prolonged, the UPR leads to the degradation of prosurvival mRNAs, activation of JNK, upregulation of BH3-only proteins, and lowers mitochondrial membrane potential via the efflux of Ca2+ from the ER to the mitochondria.
DNA damage is also an important cause of intrinsic apoptosis. In response to DNA damage, various kinases phosphorylate and stabilize p53. The consequences of p53 stabilization are context specific and include DNA repair and cellular senescence. However, if DNA damage is sufficiently severe, p53 can mediate intrinsic apoptosis through the direct interaction with proapoptotic proteins such as PUMA and NOXA, and transcriptional upregulation of proapoptotic proteins including BIM, BAX, and APAF1.
Extrinsic apoptosis is triggered by the activation of death receptors. The best characterized death receptor is the Fas cell-surface death receptor, which can be activated by soluble or membrane-bound Fas ligand (FasL). In the lung, Fas is expressed by alveolar and bronchial epithelial cells, Clara cells, macrophages, and myofibroblasts. FasL is expressed on epithelial cells, neutrophils, monocytes, eosinophils, cytotoxic T cells, and platelets (17). Other well-studied death receptors include the tumor necrosis factor (TNF) receptor superfamily 10 (TRAILR1–4) and TNF receptor 1 (TNFR1), activated by TRAIL and TNF-α, respectively (1, 2). Following ligand activation of Fas or TRAILR, a conformational change in the cytosolic tail of the receptor leads to the recruitment of Fas-associated death domain (FADD) and procaspase 8. Here, activation of caspase 8 is regulated by cellular FLICE-inhibitory proteins (cFLIPs). Short cFLIP isomers (cFLIPs) competitively inhibit caspase 8 activation, whereas long cFLIP isomers (cFLIPL) promote caspase 8 activation (4) (Figure 2). The response to caspase 8 varies across cell types. In some cells, such as mature lymphocytes, activation of caspase 8 directly leads to proteolytic activation of executioner caspases and cell death in a mitochondrion-independent manner. In other cell types, the presence of IAPs antagonizes direct activation of apoptosis. In these cases, caspase 8 cleaves BID, and truncated BID migrates to the outer mitochondrial membrane where it promotes MOMP.
Ligand activation of TNFR1 is a variation of the Fas and TRAILR signaling pathway. Similar to Fas, activation of TRNF1 leads to the formation of a protein complex, often referred to as complex 1. This includes TNFR1-associated death domain (TRADD), the TNFR-associated factors (TRAFs), and receptor-interacting serine/threonine protein kinase 1 (RIPK1). The consequences of TNFR1 activation are dependent on RIPK1, which can be ubiquinated by IAPs and/or linear ubiquitin chain assembly complex (LUBAC). Ubiquinated RIPK1 promotes inflammation and cell survival by mitogen-activated protein kinase (MAPK) signaling and IκB kinase-dependent NF-κB activation. If IAPs are absent or inhibited, RIPK1 is deubiquitinated by cylindromatosis. Consequently, RIPK1 forms a complex with FADD and caspase 8 in the cytosol (complex 2). This complex is also regulated by cFLIP proteins and can lead to caspase 8 activation and apoptosis. However, in the absence of caspase 8, RIPK1 can lead to necroptosis (see below).
There is an alternative form of extrinsic MOMP activation. Cytotoxic T cells and natural killer cells secrete pore-forming proteins (perforin) and proteases (granzyme) into other cells to cause death. In humans, the proteolytic activity of granzymes include cleavage of BID, and granzyme-mediated cell death is characterized by MOMP and cytochrome c release.
Alternative Forms of Regulated Cell Death
Numerous alternative forms of cell death have now been described, and the number of different cell death mechanisms is likely to grow. While characterized discretely, the cellular mechanisms that underlie the various forms of RCD demonstrate a great deal of overlap and are commonly coregulated.
Necroptosis.
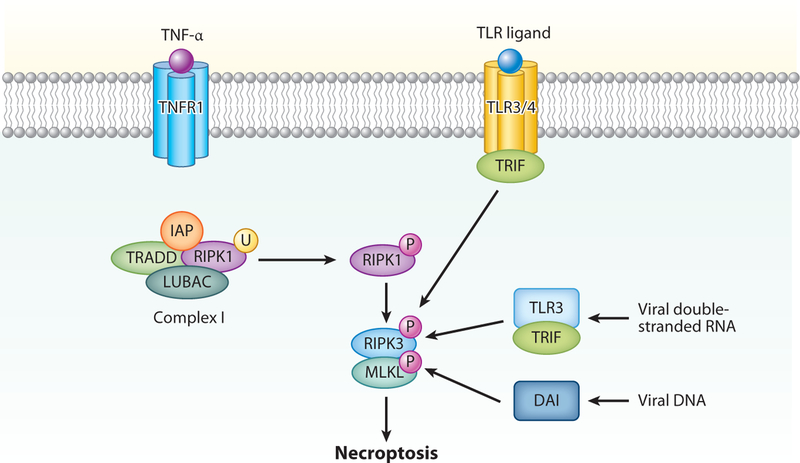
Necroptosis is a metabolically active form of RCD that results in a necrotic morphology. Necroptosis has been best characterized following ligation of death receptors, particularly TNFR1 (Figure 3) (5, 18, 19). In TNFR1-mediated necroptosis, RIPK1 phosphorylates RIPK3 to form an amyloid-like signaling complex called the necrosome. This process is aided by heat shock protein 90 and cell division cycle 37 (20). Upon assembly of the necrosome, RIPK3 phosphorylates mixed-lineage kinase domain–like pseudokinase (MLKL). Phosphorylated MLKL oligomerizes and translocates to the cell membrane to form plasma membrane pores that cause cells to swell (21). Pore formation also leads to the release of intracellular contents including danger-associated molecular patterns (DAMPs), such as high-mobility group box 1 (HMGB1), which promote inflammation (2, 22). RIPK3 also promotes inflammation during necroptosis independent of cellular pore formation through noncanonical activation of NF-κB, interleukin (IL)-1β, and IL-18. Activation of MLKL does not necessarily lead to necroptosis. Membrane damage by MLKL can be repaired by an endosomal sorting complex required for trafficking (ESCRT)-III that functions by releasing small vesicles of broken membranes (23). ESCRT-III may be important for cellular resistance to necroptotic cell death and/or a mechanism by which necroptosis can be delayed in order for cells to produce the appropriate repertoire of cytokines for immune activation.
Figure 3.
Necroptosis can occur as a consequence of TNF receptor 1 (TNFR1) activation. Similar to extrinsic apoptosis, RIPK1 ubiquitination is favored in the absence of inhibitor of apoptosis proteins (IAPs) and the presence of linear ubiquitination chain assembly complex (LUBAC). In the absence of caspase 8, RIPK1 is phosphorylated (P) and interacts with RIPK3, leading to oligomerization and phosphorylation of mixed lineage kinase domain-like pseudokinase (MLKL). DNA-dependent activator of interferon (DAI) regulatory factors can detect viral DNA and activate RIPK3-mediated necroptosis. Toll-like receptor-3 (TLR3) can also detect viral DNA and, via TLR-domain-containing adapter-inducing interferon-β (TRIF), can activate RIPK3 independent of RIPK1, also leading to necroptosis. Similarly, TLR4 can activate RIPK3 via TRIF independently of RIPK1, which also leads to necroptosis.
There is significant cross-signaling between inflammatory, apoptotic, and necroptotic signaling pathways. Necroptosis following TNFR1 activation is favored over prosurvival inflammatory signaling cascades and extrinsic apoptosis when both IAPs and caspase 8 are absent/inhibited as a consequence of genetic deletion, pharmacologic inhibition, or viral proteins. The presence of IAPs promote RIPK1 ubiquination and consequentially promote NF-κB activation and inflammation, rather than apoptosis or necrosis (as described above). The presence of caspase 8 promotes extrinsic apoptosis because the trimeric complex of FADD, cFLIPL, and caspase 8 that forms upon TNFR1 activation inhibits RIPK1 from interacting with RIPK3 (24). Necroptosis can also be inhibited by protein phosphatase 1B, which dephosphorylates RIPK3, and aurora kinase A, which inhibits RIPK1-RIPK3 interactions (5, 25). Additionally, not all cell types express RIPK3, and in these cells, caspase inhibition simply prevents cell death mediated by extracellular ligands.
Activation of RIPK3 can occur independently of RIPK1 by DNA-dependent activator of interferon regulatory factor (DAI), which detects exogenous cytosolic DNA and Toll-like receptors (TLRs) that utilize TIR-domain-containing adapter-inducing interferon-β (TRIF), including TLR3 which detects exogenous cytosolic double-stranded RNA (26, 27). Interferons can also promote necroptosis in a manner that may rely on JAK/STAT signaling (28). Unrestrained RIPK3 activation can be inhibited by inactivated RIPK1 via a molecular mechanism that is separate from its kinase function, highlighting the complex role of RIPK1 in the regulation of necroptosis (18, 29).
Pharmacologic inhibitors of necroptosis have been developed to study the role of necroptosis in inflammation and infection. The first such agent was the RIPK1 inhibitor necrostatin-1, which has been superseded by a more specific RIPK1 inhibitor 7-Cl-O-Nec-1. Other commonly used agents to study necroptosis are the RIPK3 inhibitor GSK’872 and the MLKL inhibitor necrosulfonamide (18). The use of necrostatin-1 and Ripk3-null mice have demonstrated that inhibition of necroptosis can reduce ischemia-reperfusion injury in hearts and kidneys and inflammation in murine models of colitis and sepsis (18, 30). The use of Ripk3-null mice as pharmacologic inhibitors of necroptosis has also demonstrated that necroptosis is important for the clearance of viruses that can survive in human cells by encoding antiapoptotic proteins. For example, caspase 8 inhibitors have been identified in adenoviruses and herpes family viruses; therefore, mice lacking Ripk3 have an impaired ability to control infections with these viruses. Additionally, DAMP release from cells undergoing necroptosis promotes activation of the adaptive immune responses that facilitate the elimination of viral infections, suggesting the importance of necroptosis in antiviral immunity. However, necroptosis is not universally beneficial, as certain pathogens can impair host immune responses by activating necroptosis in immune cells. It should be noted that the role of necroptosis in animal models of disease has come into question, as many studies have relied on inhibition of RIPK3 or RIPK1. Both of these proteins also promote inflammation, and subsequent studies in Mlkl-deficient mice have shown reduced effect or the opposite effect in similar animal models (30).
Pyroptosis.
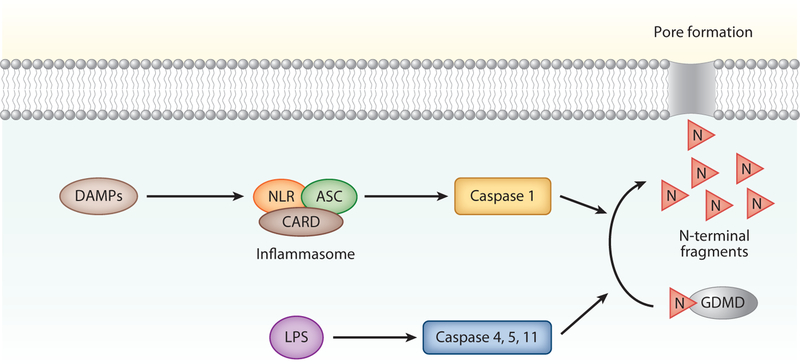
Pyroptosis is an inflammatory form of cell death that was first described in infected phagocytosing cells, such as macrophages and dendritic cells. In pyroptosis, DAMPs activate intracellular sensors, such as Nod-like receptors, leading to the formation of a multiprotein complex called the inflammasome (Figure 4). The inflammasome can include caspase 1 that cleaves and activates the precursor forms of IL-1β and IL-18 (4). Notably, an important interface between inflammasome activation and necroptosis was established when it was identified that RIPK3 can facilitate caspase-mediated cleavage of IL-1β and IL-18. If the inflammasome is sufficiently activated, caspase 1 also cleaves gasdermin D to generate N-terminal fragments that form membrane pores that lead to cell death (31). This form of RCD is associated with plasma membrane rupture, cellular swelling, and chromatin condensation. Pyroptosis can inhibit the spread of intracellular bacteria by killing the host cells and forming pores in the bacteria itself, leading to clearance via efferocytosis (32). In addition to caspase 1 activation, caspase 4 and caspase 5 (caspase 11 in mice) are also involved in pyroptosis. In these cases, caspase 4/5/11 are directly activated by intracellular lipopolysaccharide (LPS), perhaps as a consequence of cellular invasion by a pathogen; therefore, they do not require activation of the inflammasome (33). Caspase 4/5/11 activation has been described in nonimmune cells, including endothelial cells. Pyroptosis can be primed by extracytoplasmic signaling by interferons, TLRs, and TNF-α.
Figure 4.
Pyroptosis. Pyroptosis is initiated when damage-associated molecular patterns (DAMPs) activate the NOD-like receptor (NLR) leading to the formation of the inflammasome protein complex, which includes caspase activation and recruitment domain (CARD) and apoptosis-associated speck protein containing a CARD (ASC). Inflammasome activation leads to cleavage of procaspase 1, which in turn cleaves the N terminus of gasdermin D (GDMD) to generate pore forming N-terminal fragments. These fragments create pores in the cell membrane that kill the cells. Pyroptosis can also be initiated when lipopolysaccharide (LPS) activates caspases 4, 5, and 11 directly, which also leads to GDMD cleavage and pyroptosis.
Mitochondrial permeability transition–driven necrosis.
Mitochondrial permeability transition (MPT)–driven necrosis is an alternative form of cell death that can occur in the setting of extreme oxidative stress and calcium influx. Similar to intrinsic apoptosis, mitochondrial permeabilization occurs and is regulated by the BCL2 family of proteins. However, in MPT-driven necrosis, permeabilization occurs after the opening the permeability transition precomplex at the junction between the inner and outer mitochondrial membrane, resulting in a necrotic morphology. The exact mechanism via which MPT-driven necrosis occurs is unclear, but it is thought to involve cyclophilin D, as inhibitors of cyclophilin D protect against triggers of MPT-driven necrosis.
Lysosome-dependent cell death.
Lysosome-dependent cell death occurs when lysosomes release cathepsins into the cytosol, often due to oxidative damage to the lysosome membrane. These proteolytic cathepsins often activate proapoptotic proteins, leading to MOMP and intrinsic apoptosis. However, lysosome-dependent cell death can occur independently of MOMP and lead to a necrotic morphology.
Ferroptosis.
Ferroptosis is a form of cell death with a necrotic morphology and mitochondrial abnormalities that is triggered by severe lipid peroxidation; it occurs independently of apoptosis, necroptosis, and autophagy. Ferroptosis is negatively regulated by glutathione peroxidase 4 through its ability to prevent lipid peroxidation. Ferroptosis is also dependent on the presence of iron, as iron chelators can prevent this form of RCD.
Autophagy-dependent cell death.
Autophagy is a catabolic cellular mechanism in which proteins and/or organelles are targeted for lysosomal degradation as a cell-protective response. During conditions of nutrient deprivation or cellular stress, autophagy functions to promote homeostasis and bioenergetic efficiency through the recycling of cellular components. Autophagic cell death was initially coined to describe cell death associated with morphologic evidence of active autophagy, including extensive cytoplasmic vacuolization. However, these initial descriptions likely described cells in which autophagy and apoptosis occurred simultaneously, as similar conditions that induce autophagy can also induce apoptosis, including p53 activation, oxidative stress, and depletion of nutrients and growth factors. True autophagic cell death in which autophagy occurs independent of apoptosis or necroptosis is an extremely rare event. Though autophagy is an adaptive process, under certain conditions it can promote RCD, including Fas-mediated cell death, necroptosis, and ferroptosis (4).
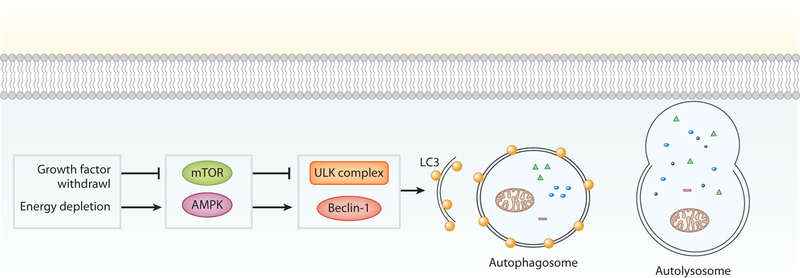
In a low-nutrient environment, the presence of adenosine monophosphate (AMP) activates AMP-activated protein kinase (AMPK), which inhibits the molecular target of rapamycin (mTOR) complex 1 and activates autophagy proteins Beclin-1 and UNC-51-like kinase 1 (ULK1) (Figure 5). This process is negatively regulated by growth factors that activate mTOR via PI3K/AKT. Upon initiation, a membrane, called a phagophore, is formed around the cargo that is targeted for degradation to isolate it from the cytoplasm. Elongation of the phagophore requires the cooperation of multiple autophagy-related proteins. Ultimately, a double-membraned autophagosomal structure around the cargo is formed, which fuses with the lysosome for degradation. Autophagy refers to the nonselective degradation of cytoplasmic components. However, specific degradation of organelles and proteins can occur, as in mitophagy in which damaged and depolarized mitochondria are targeted for lysosomal degradation. Mitophagy is regulated by Parkin and PTEN-induced putative kinase protein 1 (PINK1) (34).
Figure 5.
Autophagy. Autophagy occurs under conditions of nutrient deprivation or cellular stress. This cellular stress activates UNC-51-like kinase 1 (ULK) complex and Beclin-1, via molecular target of rapamycin (mTOR) and 5’ adenosine monophosphate-activated protein kinase (AMPK). When activated, a membrane forms that includes the autophagic protein light chain 3 (LC3). Elongation of the membrane results in the formation of an autophagosome which engulfs the cellular components to be degraded. The autophagosome fuses with a lysosome, forming the autolysosome, which ultimately degrades the cellular components.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is the third leading disease-related cause of death in the United States and is characterized by chronic bronchitis, the narrowing and loss of small airways (<2 mm), and parenchymal tissue destruction (emphysema). The most frequent modifiable risk factor for COPD is chronic exposure to cigarette smoke. However, COPD is a heterogeneous disease, and susceptibility among current and former smokers is modified by host factors that include genetics and age.
Multiple studies have demonstrated increased apoptosis in the lungs of patients with COPD. These studies have shown that apoptosis is more closely associated with emphysema and tissue destruction than it is to cigarette smoke exposure alone (7, 35, 36). In emphysema, apoptosis commonly occurs in the alveolar septum, in both epithelial cells and endothelial cells, although the relative burden of endothelial and epithelial apoptosis varies across studies. Rates of apoptosis in COPD also vary across studies but are commonly reported at approximately 1–5% of total parenchymal cells and/or at least twice the rate of apoptosis in normal lung tissue. This degree of cell death is too high for the decades-long trajectory of cigarette smoke–mediated lung disease, suggesting a role for inadequate tissue repair in emphysema pathogenesis. The high percentage of observed apoptotic cells may also occur as a consequence of ineffective macrophage efferocytosis in COPD (37).
Animal models have demonstrated the importance of apoptosis in the pathogenesis of COPD. Apoptosis is increased in cigarette smoke-exposed mice that develop emphysema (38). Intratracheal instillation of caspases is sufficient to cause murine emphysema, and the use of caspase inhibitors or the genetic deletion of caspase 3 mitigates cigarette smoke–induced inflammation and air space enlargement (39, 40). Induction of either endothelial or epithelial cell apoptosis is sufficient to cause murine emphysema, but it is not known whether epithelial or endothelial apoptosis is more relevant to the pathogenesis of emphysema in humans (41, 42).
Oxidative stress from cigarette smoke causes apoptosis in COPD. Measurements of pulmonary oxidative stress are increased in patients with COPD, and mice with a genetic deletion of Nrf2, a transcription factor for many antioxidant genes, are susceptible to cigarette smoke–mediated apoptosis and emphysema (43). Similarly, a genetic polymorphism in the Hedgehog-interacting protein (HHIP) has been identified as a risk factor for COPD, and Hhip-deficient mice are susceptible to oxidative stress–mediated apoptosis and airspace enlargement (44). Epigenetic changes due to reduced histone deacetylase activity may also contribute to susceptibility to oxidative stress-mediated apoptosis in COPD (45).
Cigarette smoke causes DNA damage and ER stress which can cause apoptosis. Lung cells from patients with COPD have increased DNA damage, shortened telomeres, and diminished capacity for DNA repair (46). In one study, telomerase-deficient mice were prone to emphysema, however, a difference in apoptosis was not observed (47). Various DNA repair genes are downregulated in COPD, and inhibition of genes involved in DNA repair can increase susceptibility to cigarette smoke–mediated apoptosis (48). Cigarette smoke also causes protein misfolding, impaired proteostasis, and activation of the UPR. However, it is unclear whether inadequate UPR or excess UPR-mediated apoptosis contributes to the pathogenesis of COPD (16, 49).
Inadequate growth factor signaling also contributes to apoptosis and emphysema pathogenesis. Vascular endothelial growth factor (VEGF) is decreased in COPD lungs, and VEGF inhibition in mice is sufficient to cause apoptosis and emphysema (50). Inadequate Wnt, hepatocyte growth factor, and adiponectin signaling have also been implicated (51, 52). Simultaneously, there is an upregulation of stress-induced proteins in COPD that amplify apoptosis. Cigarette smoke upregulates sphingolipids, including ceramides, that mediate proapoptotic effects in endothelial and epithelial cells of the lung (53). Cigarette smoke and caspase-3 also activate endothelial monocyte–activating protein II that promotes endothelial cell apoptosis (40). In type 2 epithelial cells, RTP801 is upregulated in response to cigarette smoke and promotes apoptosis in part via inhibition of mTOR (54). In one study, genetic depletion of RTP801 protected against cigarette smoke–mediated apoptosis and airspace enlargement in mice. However, mTOR inhibition is associated with decreased cellular senescence and increased longevity, and in other studies of the role of mTOR in COPD, mTOR inhibition protected against cigarette smoke–mediated apoptosis and emphysema, suggesting a complex role for mTOR in the pathogenesis of COPD (55).
Protease/antiprotease imbalance contributes to excess alveolar cell apoptosis in COPD. An inheritable deficiency of the neutrophil elastase inhibitor α−1 antitrysin (SERPINA1) is known to cause COPD. Even in COPD patients without SERPINA1 mutations, oxidative stress and inflammatory cells inactivate antiproteases such as tissue inhibitor of metalloproteinase-1 (TIMP1) and mediate an increase in neutrophil elastases, proteinase-3, cathepsins, and matrix metalloproteinases. Proteases can activate apoptosis via binding to proteinase-activated receptors, leading to JNK activation and AKT inhibition (56). When taken up by cells, α−1 antitrysin is capable of inhibiting executioner caspases, a process that is impaired by cigarette smoke (57). Additionally, TIMP1 promotes antiapoptotic ERK and AKT signaling (58).
Inflammation can cause apoptosis in COPD. Elevated levels of TNF-α, Fas, and TRAILR found in COPD contribute to cell death and tissue destruction (58). Interferon-γ also can induce type 2 epithelial cell apoptosis in a process that is dependent on the activation of cathepsins and is only partially abrogated by the inhibition of caspases (59). In COPD, increased neutrophils and macrophages contribute to excess proteases and oxidative stress, while adaptive immune cells, such as CD8+ T cells and natural killer cells, mediate apoptosis of alveolar epithelial cells (60). Additionally, autoimmune-mediated apoptosis of endothelial cells directed by CD4+ T cells may also contribute to emphysema pathogenesis (61). The inflammatory milieu of the COPD lung can also inhibit apoptosis of immune cells, such as the B cell–activating factor member of the TNF family of proteins that promotes B-cell survival and persistence of lymphoid follicles in the emphysematous lung (62).
Whereas inflammation contributes to COPD pathogenesis, COPD is associated with impaired innate immunity. For example, decreased macrophage migration inhibitory factor (MIF) and TLR4 signaling have been reported in patients with severe COPD. Similar to growth factor withdrawal, genetic deletion of these key innate immune proteins increases susceptibility to cigarette smoke–mediated apoptosis in mouse models (63–65). Inadequate antiviral responses also contribute to cell death in COPD. Susceptibility to viral infections contributes to COPD exacerbations and lung function decline, and Poly(I:C) (double-stranded RNA) in combination with cigarette smoke leads to accelerated alveolar cell apoptosis via the activation of the retinoic acid inducible gene-1 helicase system and downstream IL-18 signaling (66).
Autophagy has been implicated in the pathogenesis of emphysema and epithelial cell death. Increased activation of the autophagy pathway, particularly in epithelial cells and macrophages, has been identified in the lungs of patients with COPD. These studies have also identified that the autophagy-related protein LC3B associates with Fas to promote extrinsic apoptosis. Moreover, inhibition of key mediators of autophagy, including LC3B and Beclin-1, protects against cigarette smoke–mediated epithelial apoptosis and airspace enlargement (67). However, it remains unclear to what extent autophagy is detrimental in COPD or if autophagy is actually increased in COPD. Other studies have suggested that autophagy protects against cigarette smoke–mediated cellular senescence, and the observed increase in autophagy reflects a failure to complete the process of autophagy (68). These discrepant findings may reflect experiment differences or underscore the complex role of autophagy in COPD pathogenesis.
Studies of mitophagy have revealed an important role of necroptosis in COPD pathogenesis. Lungs from patients with COPD have increased expression of RIPK3, and the use of necrostatin-1 can mitigate neutrophilic inflammation in cigarette smoke–exposed mice. In these studies, necroptosis occurs as a consequence of mitophagy, as mice with genetic depletion of the mitophagy protein PINK1 or treated with the mitophagy inhibitor Mdivi-1 have decreased epithelial cell death and improved lung function in animal models of disease (69). Similar to autophagy, other groups have shown a protective role for mitophagy in mitigating cigarette smoke–mediated cellular senescence and airspace enlargement (70). Taken together, the divergent findings suggest an incompletely understood complexity of these cellular processes in COPD pathogenesis.
Though the role of pyroptosis in COPD is not addressed in this study, these findings suggest that the possible role of pathogenesis should be evaluated. Cigarette smoke activates caspase 1 and its downstream target molecules, IL-1β and IL-18, and there is increased IL-1β, IL-18, and ASC in the bronchoalveolar lavage (BAL), serum, and/or sputum of patients with COPD (71, 72). Inflammasome activators are also increased in the airway of patients with COPD, including ROS, extracellular ATP, and other DAMPs (73). However, a recent study of airway brushings showed no correlation between NLRP3, caspase 1, and IL-1β and the severity of COPD (74). Inhibition of the inflammasome in rodents attenuates cigarette smoke–induced inflammation but with minimal effect on emphysema. In addition, trials of pharmacologic inhibitors of inflammasome activation have not been beneficial in COPD patients to date (73).
Recently, a polymorphism in iron-responsive element–binding protein 2 (IRP2) was identified as a risk factor for COPD. Genetic deletion of Irp2 or depletion of iron chelators protects mice from cell death and airspace enlargement (75).
ASTHMA
Asthma affects 25 million people in the United States and is characterized by variable airflow obstruction, airway hyperresponsiveness, and airway inflammation. Excess epithelial cell RCD and inadequate immune cell RCD may contribute to the pathogenesis of this disease.
Airway remodeling is a hallmark pathologic finding in asthma and occurs as a consequence of airway epithelial injury and increased airway permeability. Bronchial apoptosis has been hypothesized to contribute to airway injury and can be observed in some airway biopsies of asthmatic patients, particularly patients with severe disease (76, 77). Apoptotic bronchiole epithelial cell clusters (i.e., Creola bodies) have also been demonstrated in the BAL and sputum of asthmatic patients (78). Even in children with asthma, there is evidence of airway epithelial cell loss (79).
Epithelial apoptosis has been described in commonly used murine models of allergic asthma, including ovalbumin-challenged and house dust mite–challenged mice (80, 81), and the use of a pan-caspase inhibitor has been demonstrated to decrease airway inflammation (82). Lung epithelial cells can also recognize and internalize apoptotic cells, which may also be important in mitigating the consequences of cell death and asthma, as inhibition of epithelial clearance of apoptotic cells causes IL-33 dependent allergic inflammation in murine models of asthma (78). However, the relevance of airway epithelial apoptosis to the pathogenesis of asthma remains uncertain. Airway epithelial apoptosis has not been universally identified in murine models of asthma, and the degree to which pathologic airway epithelial cell apoptosis occurs in asthma remains unclear (83–85).
Multiple mechanisms have been implicated in mediating epithelial apoptosis in asthma, including oxidative stress and viral infection (86). Oxidative stress in asthmatic bronchial epithelial cells is a consequence of infiltrating immune cells and/or aerosolized toxins such as cigarette smoke, and airway epithelial cells from asthmatic patients have diminished antioxidant capacity (87, 88). Viral infections also contribute to asthma pathogenesis, and adenovirus, respiratory syncytial virus, and influence A virus can also induce airway epithelial cell death. However, it has also been demonstrated that asthmatic patients have an impaired antiviral response, particularly type 1 and type 2 interferons, and it has been hypothesized that this may allow certain viruses to proliferate in epithelial cells with consequential necrosis, the release of DAMPs, and persistent inflammation (89, 90).
Apoptosis via the extrinsic apoptosis pathway may also be relevant, given the presence of inflammatory cells in the airway. Fas and FasL are increased in the diseased airway of asthmatics (91, 92). However, bronchial epithelial cells are relatively apoptosis resistant in contrast to immune cells, small airway epithelial cells, and alveolar epithelial cells. Although they express Fas, maximal activation of this receptor in vitro causes only a small increase in cell death (86, 93). Similarly, while segmental allergen challenge via bronchoscopy can increase epithelial TRAIL, and Trail-deficient mice are protected from allergic airway disease, there is no difference in apoptosis between Trail-deficient and wild-type mice in murine models of asthma (94, 95). Therefore, Fas and TRAIL may play a more inflammatory role in the airway epithelium in asthma in contrast to the role they play on more distal airway epithelial cells or immune cells.
Dysregulated apoptosis of leukocytes contributes to airway inflammation in asthma. Th2 T cells elaborate type 2 inflammatory cytokines, including IL-4, IL-5, IL-9, and IL-13, and are increased in the asthmatic airway. T cells in asthmatic patients appear resistant to Fas-mediated apoptosis (96), and mice with Fas-deficient T cells are prone to persistent inflammation (97). In a murine model of asthma where resolution of allergic airway inflammation occurs as a consequence of antigen tolerance, there is an increase in TRAIL, which promotes Th2 apoptosis (98). Interestingly, Fas does not play a role in this model, suggesting that the mechanism of extrinsic apoptosis may be context dependent.
Dysregulated apoptosis of eosinophils and neutrophils are also implicated in asthma pathogenesis (99). Eosinophils have a limited life span but can persist in the airway epithelial cells. Reduced eosinophil apoptosis in BAL fluid has been shown to correlate positively with severity of asthma. Cytokines from T cells, macrophages, and epithelial cells, including IL-3, IL-5, IL-9, IL-25, and thymic stromal lymphopoietin generate an inflammatory milieu in the asthmatic airway that antagonizes eosinophil apoptosis. JAK/STAT, AKT, ERK, ROS, and FADD signaling have all been implicated in regulating eosinophil apoptosis. Inhaled steroids have long been a mainstay of asthma therapy, and they induce eosinophil apoptosis. The use of monoclonal antibodies against the IL-5 pathway, an effective therapy for asthma, is associated with eosinophil apoptosis and a rapid decrease in eosinophilia (100). Apoptosis-resistant neutrophils have also been described in asthma (101). This may be due to the presence of local factors, including TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF). Interestingly, glucocorticoids have been shown to have a prosurvival effect on neutrophils in normoxic conditions in contrast to their effect on eosinophils (101).
Other forms of cell death have also been implicated in asthma biology. Some have suggested that eosinophil cytolysis with the subsequent release of eosinophil granules may induce airway inflammation. This mechanism of eosinophil cell death is unknown, but necroptosis has been suggested to be a mechanism, as chemical inhibition of RIPK3 decreases eosinophil cytolysis (102). However, Ripk3-deficient mice have been reported to have no resistance to allergen-induced asthma (103).
Pyroptosis may also contribute to the pathogenesis of asthma, and studies have demonstrated that inflammasome activation occurs in humans with asthma and mouse models of disease; however, these studies have focused on inflammation, not pyroptosis per se (104). Recently, a splice variant in the gene that codes for gasdermin B has been associated with reduced asthma risk (105, 106). The authors found that gasdermin B was highly expressed in airway epithelial cells and that this splice variant was associated with an inability of gasdermin to mediate pyroptosis.
IDIOPATHIC PULMONARY FIBROSIS
IPF is a devastating disorder that affects approximately 3 million people worldwide. IPF is characterized by alveolar epithelial cell injury, a failure of alveolar epithelial cell repair, and consequential activation of fibroblast/myofibroblasts. Within this paradigm of IPF pathogenesis lies an apoptosis paradox, a term coined by Thannickal & Horowitz (107). In IPF, alveolar epithelial cells are apoptosis prone, but fibroblasts/myofibroblasts are apoptosis resistant.
Human IPF lung tissue exhibits heightened alveolar epithelial cell death. Histology of the IPF lung demonstrates apoptotic, and to a lesser degree, necrotic cells interspersed with hyperplastic and atypical type 2 epithelial cells (8, 108). Proof-of-concept for the contribution of epithelial cell apoptosis to the pathogenesis of IPF has been established in animal models. In mice, targeted apoptosis of type 2 epithelial cells is sufficient to cause IPF, whereas caspase inhibitors can mitigate pulmonary fibrosis (109, 110).
Transforming growth factor-beta (TGF-β) is increased in IPF lung tissue and important to the pathogenesis of the disease. The consequence of TGF-β signaling is cell-type dependent, and in lung epithelial cells, TGF-β induces apoptosis. TGF-β proapoptotic effects include (a) promotion of Fas activation of caspase 8 and JNK, (b) upregulation of proapoptotic BCL2 proteins, and (c) increased ROS. Overexpression of TGF-β causes alveolar epithelial apoptosis and murine fibrosis in animal models of disease (111).
ER stress also contributes to alveolar apoptosis in IPF. Germline mutations in surfactant genes expressed by type 2 epithelial cells cause IPF, including the SFTPA2 and SFTPC genes. The SFTPC mutation results in a truncated propeptide that cannot be targeted out of the ER, resulting in ER stress and increased caspase activation (112). ER stress is not exclusive to identifiable genetic causes of IPF, and increased proapoptotic UPR signaling in type 2 alveolar epithelial cells has also been demonstrated in nonfamilial IPF (113). Some have suggested that viral infections contribute to ER stress and have shown that latent human herpes viruses colocalize with ER stress markers (114).
Mitochondrial dysfunction contributes to the susceptibility of alveolar epithelial cell apoptosis. In IPF, type 2 epithelial cells demonstrate dysmorphic and dysfunctional mitochondria compared with age-matched controls. This has been associated with reduced PINK1, and Pink1-deficient mice are susceptible to apoptosis and bleomycin-induced fibrosis (115). Recently, thyroid hormone has been shown to protect against fibrosis in mouse models of IPF. Aerosolized thyroid hormone or the use of the thyroid hormone mimetic sobetirome improves mitochondrial function and attenuates intrinsic apoptosis in alveolar epithelial cells in a PINK1-dependent manner (116).
IPF is associated with oxidative stress, which likely contributes to increased apoptosis of alveolar epithelial cells (117). Environmental pollutants, such as cigarette smoke, are risk factors for IPF and contribute to the oxidant burden in pulmonary fibrosis. Increased ROS may arise from mitochondrial dysfunction or directly from myofibroblasts, which generate substantial amounts of extracellular ROS, to cause injury to adjacent epithelial cells (118). However, antioxidant therapies have been unsuccessful in human trials of IPF.
DNA damage and telomere shortening also contribute to alveolar epithelial cell apoptosis and IPF pathogenesis. Type 2 cells in IPF demonstrate increased p53 activation and susceptibility to oxidative DNA damage (119). Mutations in telomere maintenance genes have been found in up to 25% of familial and 1–3% of sporadic IPF patients (120). This includes two genes that code for proteins involved in telomerase function, TERT and TERC, as well as other genes involved in telomere maintenance. Even in the absence of telomere maintenance mutations, patients with IPF have shortened telomeres. Multiple studies have shown that deficiency of telomerase maintenance proteins in type 2 epithelial cells results in increased susceptibility to injury and fibrosis (121–123). Notably, all of these studies demonstrated activation of the DNA damage response, but an identifiable increase in apoptosis varied based on the type of experimental model used.
The extrinsic pathway has also been implicated in the susceptibility of alveolar epithelial cells to apoptosis. IPF is associated with increased Fas in the BAL and epithelial cells, and animal studies have demonstrated that Fas-mediated epithelial apoptosis contributes to pulmonary fibrosis. In IPF, myofibroblasts are an important source of FasL (124) and proapoptotic angiotensin peptides (125). Conversely, antiapoptotic chitinase 3-like protein 1 is reduced during acute exacerbations of IPF (126). Recently, alveolar epithelial cell death has been suggested to occur via necroptosis in IPF. RIPK3 expression is increased in IPF alveolar epithelial cells, and Ripk3-deficient mice have attenuated fibrosis and decreased HMGB1 in a bleomycin model of IPF (127).
Whereas alveolar epithelial cells are susceptible to apoptosis, lung fibroblasts/myofibroblasts are resistant to apoptosis (128). Biomechanical properties of the extracellular matrix promote fibrosis through the impairment of myofibroblast apoptosis and anoikis via activation of integrin/FAK and PI3K/AKT signaling and decreased activity of the AKT inhibitor PTEN (14, 129). In contrast to its proapoptotic role in epithelial cells, TGF-β is antiapoptotic in myofibroblasts. TGF-β and other factors like endothelin-1 mediate apoptosis resistance via signaling through these pathways (107). Consequentially, myofibroblasts have reduced Fas and FOXO activity and increased activity of XIAP and cFLIPL (107, 130). YAP and WNT pathways have also been shown to promote apoptosis resistance in myofibroblasts (130, 131).
The induction of myofibroblast apoptosis can ameliorate fibrosis in animal models. Metformin can attenuate myofibroblast resistance to apoptosis via activation of AMPK signaling, which improves mitochondrial biogenesis and decreases the threshold for apoptosis (132). The SMAC mimetic AT-406 has also been used to promote myofibroblast apoptosis (133). Cellular senescence also has an important role in mediating apoptosis resistance in myofibroblasts. For example, myofibroblasts from IPF patients have increased NAPDH oxidase 4 (Nox4) and impaired capacity to induce Nrf2 antioxidant response, which causes cellular senescence and apoptosis resistance in myofibroblasts (134). Inhibition of Nox4-mediated senescence attenuates apoptosis resistance in myofibroblasts and pulmonary fibrosis. Similarly, the induction of apoptosis in senescent fibroblasts via inhibition of BCL-xL with the BH3 mimetic ABT-26 also reverses established fibrosis in an animal model of IPF (135).
ACUTE RESPIRATORY DISTRESS SYNDROME
ARDS is characterized by acute onset and widespread pulmonary inflammation. The precipitating events are numerous, including infection, trauma, systemic inflammation, and toxins. Approximately 200,000 patients develop ARDS each year in the United States (136).
In ARDS, neutrophils are apoptosis resistant owing to the inflammatory environment of the lung (137). This was classically demonstrated in a study in which BAL fluid from patients with ARDS inhibits neutrophil apoptosis, particularly through GM-CSF (137). Subsequent studies have demonstrated that LPS, leukotrienes, cytokines, and activated complements all contribute to apoptosis resistance in neutrophils. The antiapoptotic role of GM-CSF was thought to act through PI3K signaling. However, recent transcriptomic evaluations of neutrophils in the lungs of patients with ARDS support the notion of apoptosis-resistant neutrophils, but via mechanisms distinct from PI3K/AKT activation (138).
In a variety of animal models, neutrophil apoptosis accelerates resolution of pulmonary inflammation (139). In LPS-induced lung injury, genetic deletion of p53 results in decreased neutrophil apoptosis and increased LPS-induced lung injury (140). CD4+CD25+Foxp3+Tregs promote neutrophil apoptosis and resolution of acute lung injury via TGF-β (141). Others have tried to promote apoptosis via signaling through cyclin-dependent kinase inhibitors and lipid mediators, such as lipoxins and resolvins, to counteract prosurvival signals that stem from NF-κB activation, ERK/AKT, and myeloperoxidase (139). However, the clinical relevance of the apoptotic sensitivity of neutrophils to the pathogenesis of ARDS remains unclear. One study suggested that septic patients with ARDS were less likely to have apoptotic neutrophils than those without ARDS (142). However, neutrophils are important for host defense against bacteria that cause ARDS. Furthermore, susceptibility to neutrophil apoptosis does not confer a survival benefit in patients with ARDS, and ARDS survivors have higher concentrations of BAL GM-CSF than nonsurvivors (137, 143).
In contrast to neutrophils, there is increased epithelial and endothelial cell death in ARDS. The first ultrastructural measurements of lungs from patients with ARDS demonstrated apoptosis and necrosis in type 1 epithelial cells and to a lesser degree in endothelial cells (144, 145). In murine models of ARDS, caspase inhibition mitigates lung injury (146). Many studies have focused on Fas/FasL signaling as a key contributor to apoptosis in ARDS. Elevated concentrations of Fas and FasL are associated with increased mortality in patients with ARDS, and genetic polymorphisms associated with increased Fas mRNA expression are risk factors for developing ARDS (147, 148). BAL fluid from ARDS patients induces epithelial apoptosis in vitro, a process that can be inhibited by neutralizing soluble Fas (149). Fas induces apoptosis in distal lung cells and causes ARDS in rodents, whereas inhibition of Fas/FasL signaling protects against lung injury in these models (150, 151). In ARDS, Fas is potentiated by TGF-β and angiotensin peptides that are increased in the BAL (152, 153). TNF-α–mediated apoptosis has also been described in ARDS. However, the use of caspase inhibitors does not improve outcomes in mice treated with TNF-α (154). ARDS is also associated with the upregulation of perforin/granzyme–mediated cell death (155).
Multiple antiapoptotic factors appear protective in ARDS. For example, AKT and STAT3 signaling protect against apoptosis in animal models of acute lung injury (13). ARDS is accompanied by oxidative stress, and studies in hyperoxic models of ARDS have shown an important role for BAX- and BAK-dependent alveolar epithelial cell death mediated through mitochondrial ROS (156). Interestingly, BID, BIM, and PUMA do not contribute to apoptosis and injury in this model. In LPS and hyperoxic lung injury murine models, mitophagy protects against endothelial apoptosis and lung injury (157, 158).
Necroptosis also plays a role in the pathogenesis of acute lung injury. Evidence for necroptosis has been suggested by increased HMGB1 in the BAL of patients with acute lung injury (159). In animal models of ARDS, the relative abundance of apoptotic to necroptotic cells can be influenced by LPS doses, with increased phosphorylated RIPK3 and MLK found with higher doses of LPS. Inhibition of necroptosis with necrostatin-1 or genetic deletion of Ripk3 improves outcomes in mouse models of ARDS (160). Necroptosis has been implicated as a mechanism for lung injury following red blood cell transfusion (161).
Pyroptosis also may play an important role in the pathogenesis of ARDS. In animal models of sterile lung injury, NLR-mediated activation of the inflammasome contributes to caspase 1–dependent cell death and acute lung injury (162). Endotoxemia also contributes to ARDS pathogenesis in a manner dependent on endothelial pyroptosis, where intracellular LPS directly activates caspase 11. Notably, conditional deletion of caspase 11 in endothelial cells can mitigate lung injury in murine models (33). Similarly, hemorrhagic shock, an important cause of ARDS, activates pyroptosis in lung endothelial cells (163). Permeability transition precomplex may also be involved in the pathogenesis of ARDS, as inhibition of cyclophilin D protects mice against endotoxemia-induced but not hyperoxia-induced acute lung injury (156, 164).
ARDS can occur as a consequence of bacterial and viral infections, and RCD pathways play a role in infection-mediated lung injury. RCD can be an effective method to eliminate pathogens and elicit the appropriate immune response. However, RCD pathways can cause extensive destruction or can be hijacked by pathogens to eliminate immune cells. Whether a specific RCD subroutine is beneficial or harmful is context dependent, including the type of pathogen, the pathogen burden, and the extent of injury. Extracellular bacteria, such as Streptococcus pneumoniae and Staphylococcus aureus, can be culprit organisms in ARDS, and apoptosis can be an effective mechanism of extracellular bacterial clearance by phagocytosing cells (165). Alternatively, Fas does not improve clearance of S. pneumoniae and S. aureus, but it does help with clearance of Pseudomonas aeruginosa (166, 167). Similarly, S. pneumoniae and S. aureus can induce necroptosis through pore-forming toxins (168), which can lead to tissue injury. However, recent studies have confirmed that necroptosis promotes clearance of S. aureus infection (169). As previously described, apoptosis is an effective mechanism for limiting the spread of viral infections. However, excess apoptosis can lead to extensive tissue injury and contribute to the pathogenesis of ARDS (170). Similarly, various intracellular bacteria and viruses also induce necroptosis in the lung, including Chlamydia pneumoniae and influenza (171). In some animal models, inhibition of necroptosis protects mice from influenza-mediated lung injury, but in other models, necroptosis can limit the spread of infection (171, 172). Macrophage-mediated pyroptosis is important for the clearance of certain intracellular bacteria and viral infections, including Legionella pneumophila and influenza, respectively (173). In contrast, activation of pyroptosis in experimental models of S. pneumoniae and P. aeruginosa has been associated with decreased bacterial clearance of infection (174, 175). These data highlight the complex interactions between host immunity and RCD pathways.
PULMONARY ARTERIAL HYPERTENSION
PAH is a progressive and often fatal disease characterized by vascular remodeling of pulmonary arteries, elevated pulmonary artery pressures, and eventual right heart failure. The current understanding of PAH pathogenesis postulates that the initiating phase involves susceptibility of pulmonary endothelial cells to injury and death, which is followed by activation and proliferation of apoptosis-resistant endothelial and smooth muscle cells.
Endothelial injury as the cause of PAH pathogenesis has been suggested by animal models of PAH. Mice treated with VEGF inhibitors (SU5416) or monocrotaline develop PAH that resembles human disease. In these models, there is endothelial apoptosis in the first three days of injury, but by day seven, endothelial cells become resistant to apoptosis (176). Through an improved understanding of hereditable causes of PAH, underlying mechanisms of apoptosis susceptibility have been identified. The most common hereditable cause of PAH is a loss-of-function mutation in bone morphogenetic protein receptor 2 (BMPR2). Even in non-heritable causes of PAH, there is evidence that BMPR2 signaling is impaired. BMPR2 is a member of the TGF-β super-family that is expressed on endothelial cells, and to a lesser extent on smooth muscle cells (177). In endothelial cells, BMPR2 antagonizes apoptosis, and genetic deletion of endothelial BMPR2 leads to increased endothelial apoptosis and pulmonary hypertension (178). Impaired mitochondrial function and excessive mitophagy have also been implicated as mechanisms that contribute to endothelial susceptibility to apoptosis. Mice deficient in mitochondrial uncoupling protein 2 have increased PINK1-mediated mitophagy and are susceptible to hypoxia-induced endothelial apoptosis and pulmonary hypertension (179).
Although susceptibility to endothelial injury may initiate PAH pathogenesis, the disease is characterized by proliferative and apoptosis-resistant endothelial and smooth muscle cells (180, 181). There is an increase in growth factors that inhibit endothelial and smooth muscle cell apoptosis, such as VEGF, platelet-derived growth factor (PDGF), and fibroblast growth factor 2 (FGF2), and there is also an increase in the number and/or affinity of their cognate tyrosine kinase receptors. Dysfunctional endothelial cells also under express proteins that antagonize antiapoptotic signaling in smooth muscle cells. The protein apelin is decreased in endothelial cells in patients with PAH as a consequence of impaired BMPR2 signaling (182). One of apelin’s effects is to downregulate the endothelial secretion of FGF2 via signaling through microRNAs (183). PAH is also associated with an increase in cytokines that promote endothelial resistance to apoptosis, such as IL-6 and MIF (184). Paracrine signaling by endothelin-1, serotonin, and angiotensin peptides also inhibits endothelial and smooth muscle cell apoptosis in PAH. Inhibitors of tyrosine kinase and MIF effectively mitigate pulmonary hypertension in animal models of PAH, and the use of tyrosine kinase inhibitors are under investigation in clinical trials (185, 186).
The consequence of increased extracellular signaling is the activation of antiapoptotic cellular signaling pathways, such as AKT, ERK1/2, NF-kB, and STAT3, and inactivation of proapoptotic FOXO pathways. Loss-of-function mutations in BMPR2 also contribute to apoptosis resistance in SMCs via multiple pathways, including STAT3 signaling (180, 187). Endothelial cells and smooth muscle cells have elevated cIAPs and BCL2 and decreased BAX and BIM (35, 180, 181). Inhibiting of PI3K/AKT signaling with LY294002 and triciribine, inhibiting antiapoptotic BCL2 proteins and the cIAP survivin, and upregulating FOXO signaling have all shown benefit in rodent models of PAH (188, 189). Increasing BMPR2 activity with tacrolimus may also be efficacious in patients with severe PAH (189).
Additionally, endothelial cells and smooth muscle cells demonstrate decreased oxidative phosphorylation and increased reliance on glycolysis. These changes are associated with inhibition of mitochondrial ROS and increased mitochondrial membrane potential, thereby decreasing the threshold for apoptosis activation (190). While incompletely understood, they may occur as a consequence of upregulation of targets of the hypoxia inducible factor 1α (HIF1α) transcription factor resulting from epigenetic changes and changes in SOD2 (191). Impaired autophagy may contribute to HIF1α stabilization and apoptosis resistance in pulmonary hypertension. Elevated levels of LC3B and autophagosomes have been found in murine models of PAH and patients with this disease, and LC3B-deficient mice develop elevated pulmonary arterial pressures in animal models of PAH (192).
PAH is associated with increased endothelial DNA damage and genomic instability, which may contribute to apoptosis resistance (193). The cause for genomic instability in PAH is unclear, but BMPR2 has been shown to impair DNA repair, and an impaired DNA damage response, via inhibition of p53, increases susceptibility to PAH in an animal model of disease (194, 195). It has been postulated that this genomic instability may lead to endothelial resistance to apoptosis and/or may cause the monoclonal expansion of apoptotic resistant endothelial cells in idiopathic PAH (196).
CONCLUSIONS
As our review highlights, the role of RCD in the pathogenesis of severe lung diseases is highly complex. Acute inflammation leads to epithelial and endothelial RCD in ARDS, whereas chronic inflammation may cause persistence of immune cells and susceptibility to epithelial RCD in asthma. In COPD, chronic environmental exposures and genetic factors combine to cause epithelial and endothelial RCD. Apoptosis-resistant mesenchymal cells contribute to the pathogenesis of both IPF and PAH; however, IPF is triggered by epithelial apoptosis, and PAH is triggered by endothelial apoptosis. Developing therapies that modulate RCD-mediated cell fate in lungs will be challenging. However, improving our understanding of the intracellular and extracellular processes that regulate RCD in the lung, identifying cell type–specific RCD pathways, and personalizing approaches to identify the patients most likely to benefit will launch a new era of therapeutic tools. Rigorously subclassifying RCD will facilitate the development of rational, targeted, diagnostic, preventative, and disease-modifying strategies in the lungs.
ACKNOWLEDGMENTS
M.S. is supported by NIH/NHLBI K08HL135402–01 and FAMRI YCSA 142017. I.S.B. is supported by NIH/NHLBI T32 HL007778. P.J.L. is supported by NIH/NHLBI R01 HL138386, VAORD11858595, US Department of Defense PR150809, and FAMRI 150074. We apologize to any authors whose work could not be cited due to space limitations.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Elmore S 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol 35:495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Salvesen G. 2014. Regulated cell death: signaling and mechanisms. Annu. Rev. Cell Dev. Biol 30:337–56 [DOI] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs Y, Steller H. 2011. Programmed cell death in animal development and disease. Cell 147:742–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornberry NA, Lazebnik Y. 1998. Caspases: enemies within. Science 281:1312–16 [DOI] [PubMed] [Google Scholar]
- 7.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. 2006. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res 7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. 1998. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am. J. Physiol 275:L1192–99 [DOI] [PubMed] [Google Scholar]
- 9.Kalkavan H, Green DR. 2018. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyna DE, Garner TP, Lopez A, Kopp F, Choudhary GS, et al. 2017. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32:490–505.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. 2011. Anti-apoptosis and cell survival: a review. Biochim. Biophys. Acta 1813:238–59 [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, et al. 2015. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol 308:L344–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinton LJ, Mizgerd JP. 2011. NF-κB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res 343:153–65 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, et al. 2007. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19:761–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, et al. 2007. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal 9:49–89 [DOI] [PubMed] [Google Scholar]
- 16.Kelsen SG. 2016. The unfolded protein response in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc 13(Suppl. 2):S138–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galani V, Tatsaki E, Bai M, Kitsoulis P, Lekka M, et al. 2010. The role of apoptosis in the pathophysiology of Acute Respiratory Distress Syndrome (ARDS): an up-to-date cell-specific review. Pathol. Res. Pract 206:145–50 [DOI] [PubMed] [Google Scholar]
- 18.Weinlich R, Oberst A, Beere HM, Green DR. 2017. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol 18(2):127–36 [DOI] [PubMed] [Google Scholar]
- 19.Linkermann A, Green DR. 2014. Necroptosis. N. Engl. J. Med 370(5):455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Xu T, Cao Y, Wang H, Li L, et al. 2015. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. PNAS 112(16):5017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, et al. 2014. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. PNAS 111(42):15072–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L, Kepp O, Chan FK, Kroemer G. 2017. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. Mech. Dis 12:103–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong YN, Guy C, Olauson H, Becker JU, Yang M, et al. 2017. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 69(2):286–300.e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, et al. 2011. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471:363–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Wu J, Li L, Zhang Z, Ren J, et al. 2015. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell Biol 17(4):434–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upton JW, Kaiser WJ, Mocarski ES. 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11(3):290–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, et al. 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem 288(43):31268–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, et al. 2013. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. PNAS 110(33):E3109–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, et al. 2014. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513(7516):90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, et al. 2016. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23(9):1565–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Zhao Y, Wang K, Shi X, Wang Y, et al. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–65 [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen I, Zhang Y, Krantz BA, Miao EA. 2016. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med 213:2113–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng KT, Xiong S, Ye Z, Hong Z, Di A, et al. 2017. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Investig 127:4124–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi AM, Ryter SW, Levine B. 2013. Autophagy in human health and disease. N. Engl. J. Med 368:651–62 [DOI] [PubMed] [Google Scholar]
- 35.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. 2001. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med 163:737–44 [DOI] [PubMed] [Google Scholar]
- 36.Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. 2005. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur. Respir. J 25:250–58 [DOI] [PubMed] [Google Scholar]
- 37.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. 2003. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol 81:289–96 [DOI] [PubMed] [Google Scholar]
- 38.Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, et al. 2005. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur. Respir. J 25:15–22 [DOI] [PubMed] [Google Scholar]
- 39.Aoshiba K, Yokohori N, Nagai A. 2003. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am. J. Respir. Cell Mol. Biol 28:555–62 [DOI] [PubMed] [Google Scholar]
- 40.Clauss M, Voswinckel R, Rajashekhar G, Sigua NL, Fehrenbach H, et al. 2011. Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke-induced emphysema in mice. J. Clin. Investig 121:2470–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, et al. 2008. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J. Biol. Chem 283:29447–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, et al. 2016. Targeted type 2 alveolar cell depletion. A dynamic functional model for lung injury repair. Am. J. Respir. Cell Mol. Biol 54:319–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, et al. 2004. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig 114:1248–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lao T, Jiang Z, Yun J, Qiu W, Guo F, et al. 2016. Hhip haploinsufficiency sensitizes mice to age-related emphysema. PNAS 113:E4681–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, et al. 2012. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Investig 122:2032–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, et al. 2011. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax 66:521–27 [DOI] [PubMed] [Google Scholar]
- 47.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, et al. 2011. Telomere length is a determinant of emphysema susceptibility. Am. J. Respir. Crit. Care Med 184:904–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sears CR, Zhou H, Justice MJ, Fisher AJ, Saliba J, et al. 2018. Xeroderma pigmentosum group C deficiency alters cigarette smoke DNA damage cell fate and accelerates emphysema development. Am. J. Respir. Cell Mol. Biol 58:402–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rijt SH, Keller IE, John G, Kohse K, Yildirim AO, et al. 2012. Acute cigarette smoke exposure impairs proteasome function in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol 303:L814–23 [DOI] [PubMed] [Google Scholar]
- 50.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, et al. 2000. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Investig 106:1311–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K, et al. 2011. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectin-null mice: implications for therapy. Am. J. Respir. Crit. Care Med 183:1164–75 [DOI] [PubMed] [Google Scholar]
- 52.Baarsma HA, Konigshoff M. 2017. ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax 72:746–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, et al. 2005. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med 11:491–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, et al. 2010. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat. Med 16:767–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruwanpura SM, McLeod L, Dousha LF, Seow HJ, Alhayyani S, et al. 2016. Therapeutic targeting of the IL-6 trans-signaling/mechanistic target of rapamycin complex 1 axis in pulmonary emphysema. Am. J. Respir. Crit. Care Med 194:1494–505 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, et al. 2005. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am. J. Respir. Cell Mol. Biol 33:231–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, et al. 2006. α−1 Antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am. J. Pathol 169:1155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morissette MC, Parent J, Milot J. 2009. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int. J. Chron. Obstruct. Pulmon. Dis 4:19–31 [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, et al. 2000. Interferon γ induction of pulmonary emphysema in the adult murine lung. J. Exp. Med 192:1587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majo J, Ghezzo H, Cosio MG. 2001. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur. Respir. J 17:946–53 [DOI] [PubMed] [Google Scholar]
- 61.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, et al. 2005. An animal model of autoimmune emphysema. Am. J. Respir. Crit. Care Med 171:734–42 [DOI] [PubMed] [Google Scholar]
- 62.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, et al. 2015. B cell-activating factor. An orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 192:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comer DM, Kidney JC, Ennis M, Elborn JS. 2013. Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers. Eur. Respir. J 41:1058–67 [DOI] [PubMed] [Google Scholar]
- 64.Sauler M, Leng L, Trentalange M, Haslip M, Shan P, et al. 2014. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol 306:L487–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. 2006. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Investig 116:3050–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, et al. 2008. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J. Clin. Investig 118:2771–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, et al. 2010. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. PNAS 107:18880–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bodas M, Patel N, Silverberg D, Walworth K, Vij N. 2017. Master autophagy regulator transcription factor EB regulates cigarette smoke-induced autophagy impairment and chronic obstructive pulmonary disease-emphysema pathogenesis. Antioxid. Redox Signal 27:150–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, et al. 2014. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig 124:3987–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, et al. 2015. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11:547–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, et al. 2007. IL-18 is induced and IL-18 receptor α plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J. Immunol 178:1948–59 [DOI] [PubMed] [Google Scholar]
- 72.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, et al. 2014. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol 15:727–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colarusso C, Terlizzi M, Molino A, Pinto A, Sorrentino R. 2017. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget 8:81813–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Stefano A, Caramori G, Barczyk A, Vicari C, Brun P, et al. 2014. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax 69:516–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, et al. 2016. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat. Med 22:163–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen L, Xueping E, Tarsi J, Ramkumar T, Horiuchi TK, et al. 2007. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am. J. Respir. Crit. Care Med 176(2):138–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, et al. 2002. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J. Allergy Clin. Immunol 109(2):329–37 [DOI] [PubMed] [Google Scholar]
- 78.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, et al. 2013. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493(7433):547–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, et al. 2006. Epithelial damage and angiogenesis in the airways of children with asthma. Am. J. Respir. Crit. Care Med 174(9):975–81 [DOI] [PubMed] [Google Scholar]
- 80.Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. 2003. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem. Pharmacol 66(8):1459–68 [DOI] [PubMed] [Google Scholar]
- 81.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, et al. 2013. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir. Res 14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwata A, Nishio K, Winn RK, Chi EY, Henderson WR Jr., Harlan JM. 2003. A broad-spectrum caspase inhibitor attenuates allergic airway inflammation in murine asthma model. J. Immunol 170(6):3386–91 [DOI] [PubMed] [Google Scholar]
- 83.Lambrecht BN, Hammad H. 2013. Death at the airway epithelium in asthma. Cell Res 23(5):588–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White SR. 2011. Apoptosis and the airway epithelium. J. Allergy 2011:948406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ordonez C, Ferrando R, Hyde DM, Wong HH, Fahy JV. 2000. Epithelial desquamation in asthma: Artifact or pathology? Am. J. Respir. Crit. Care Med 162(6):2324–29 [DOI] [PubMed] [Google Scholar]
- 86.Tesfaigzi Y 2006. Roles of apoptosis in airway epithelia. Am. J. Respir. Cell. Mol. Biol 34(5):537–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. 2000. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 355(9204):624. [DOI] [PubMed] [Google Scholar]
- 88.Fitzpatrick AM, Stephenson ST, Hadley GR, Burwell L, Penugonda M, et al. 2011. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J. Allergy Clin. Immunol 127(6):1604–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, et al. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med 12(9):1023–26 [DOI] [PubMed] [Google Scholar]
- 90.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, et al. 2013. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol 6(4):797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wadsworth SJ, Atsuta R, McIntyre JO, Hackett TL, Singhera GK, Dorscheid DR. 2010. IL-13 and TH2 cytokine exposure triggers matrix metalloproteinase 7–mediated Fas ligand cleavage from bronchial epithelial cells. J. Allergy Clin. Immunol 126(2):366–74.e8 [DOI] [PubMed] [Google Scholar]
- 92.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, et al. 1998. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am. J. Respir. Cell. Mol. Biol 19(4):537–42 [DOI] [PubMed] [Google Scholar]
- 93.Nakamura M, Matute-Bello G, Liles WC, Hayashi S, Kajikawa O, et al. 2004. Differential response of human lung epithelial cells to Fas-induced apoptosis. Am. J. Pathol 164(6):1949–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robertson NM, Zangrilli JG, Steplewski A, Hastie A, Lindemeyer RG, et al. 2002. Differential expression of TRAIL and TRAIL receptors in allergic asthmatics following segmental antigen challenge: evidence for a role of TRAIL in eosinophil survival. J. Immunol 169(10):5986–96 [DOI] [PubMed] [Google Scholar]
- 95.Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PA, et al. 2007. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat. Med 13(11):1308–15 [DOI] [PubMed] [Google Scholar]
- 96.Jayaraman S, Castro M, O’Sullivan M, Bragdon MJ, Holtzman MJ. 1999. Resistance to Fas-mediated T cell apoptosis in asthma. J. Immunol 162(3):1717–22 [PubMed] [Google Scholar]
- 97.Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, et al. 2006. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J. Exp. Med 203(5):1173–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Faustino L, Fonseca DM, Florsheim EB, Resende RR, Lepique AP, et al. 2014. Tumor necrosis factor-related apoptosis-inducing ligand mediates the resolution of allergic airway inflammation induced by chronic allergen inhalation. Mucosal Immunol 7(5):1199–1208 [DOI] [PubMed] [Google Scholar]
- 99.Walsh GM. 2013. Eosinophil apoptosis and clearance in asthma. J. Cell Death 6:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, et al. 201. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol 125(6):1344–53.e2 [DOI] [PubMed] [Google Scholar]
- 101.Uddin M, Nong G, Ward J, Seumois G, Prince LR, et al. 2010. Prosurvival activity for airway neutrophils in severe asthma. Thorax 65(8):684–89 [DOI] [PubMed] [Google Scholar]
- 102.Radonjic-Hoesli S, Wang X, de Graauw E, Stoeckle C, Styp-Rekowska B, et al. 2017. Adhesion-induced eosinophil cytolysis requires the receptor-interacting protein kinase 3 (RIPK3)-mixed lineage kinase-like (MLKL) signaling pathway, which is counterregulated by autophagy. J. Allergy Clin. Immunol 140(6):1632–42 [DOI] [PubMed] [Google Scholar]
- 103.Qi X, Gurung P, Malireddi RK, Karmaus PW, Sharma D, et al. 2017. Critical role of caspase-8-mediated IL-1 signaling in promoting Th2 responses during asthma pathogenesis. Mucosal Immunol 10:128–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, et al. 2017. Role for NLRP3 inflammasome-mediated, IL-1β-dependent responses in severe, steroid-resistant asthma. Am. J. Respir. Crit. Care Med 196(3):283–97 [DOI] [PubMed] [Google Scholar]
- 105.Panganiban RA, Sun M, Dahlin A, Park HR, Kan M, et al. 2018. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis.J. Allergy Clin. Immunol 142:1469–78.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chao KL, Kulakova L, Herzberg O. 2017. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. PNAS 114:E1128–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thannickal VJ, Horowitz JC. 2006. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc 3:350–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. 2001. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J. Clin. Pathol 54:132–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, et al. 2010. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med 181:254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R. 2006. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. Lung Cell. Mol. Physiol 290:L790–L6 [DOI] [PubMed] [Google Scholar]
- 111.Fernandez IE, Eickelberg O. 2012. The impact of TGF-βon lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc 9:111–16 [DOI] [PubMed] [Google Scholar]
- 112.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. 2005. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am. J. Respir. Cell Mol. Biol 32:521–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, et al. 2008. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 178:838–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, et al. 2008. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol 294:L1119–26 [DOI] [PubMed] [Google Scholar]
- 115.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, et al. 2015. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Investig 125:521–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, et al. 2018. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med 24:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. 1987. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J. Clin. Investig 79:1665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, et al. 2005. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19:854–56 [DOI] [PubMed] [Google Scholar]
- 119.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, et al. 1996. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 154:477–83 [DOI] [PubMed] [Google Scholar]
- 120.Armanios M, Blackburn EH. 2012. The telomere syndromes. Nat. Rev. Genet 13:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, et al. 2015. Telomere dysfunction causes alveolar stem cell failure. PNAS 112:5099–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, et al. 2016. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1:e86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. 2015. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep 12:286–99 [DOI] [PubMed] [Google Scholar]
- 124.Golan-Gerstl R, Wallach-Dayan SB, Amir G, Breuer R. 2007. Epithelial cell apoptosis by Fas ligand-positive myofibroblasts in lung fibrosis. Am. J. Respir. Cell Mol. Biol 36:270–5 [DOI] [PubMed] [Google Scholar]
- 125.Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, et al. 1999. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am. J. Physiol 277:L1158–64 [DOI] [PubMed] [Google Scholar]
- 126.Zhou Y, Peng H, Sun H, Peng X, Tang C, et al. 2014. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci. Transl. Med 6:240ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee JM, Yoshida M, Kim MS, Lee JH, Baek AR, et al. 2018. Involvement of alveolar epithelial cell necroptosis in IPF pathogenesis. Am. J. Respir. Cell Mol. Biol 59 10.1165/rcmb.2017-0034OC [DOI] [PubMed] [Google Scholar]
- 128.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, et al. 2004. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J. Pathol 202:486–95 [DOI] [PubMed] [Google Scholar]
- 129.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, et al. 2005. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am. J. Pathol 166:367–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kis K, Liu X, Hagood JS. 2011. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev. Mol. Med 13:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noguchi S, Saito A, Mikami Y, Urushiyama H, Horie M, et al. 2017. TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Sci. Rep 7:42595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, et al. 2018. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med 24(8):1121–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ashley SL, Sisson TH, Wheaton AK, Kim KK, Wilke CA, et al. 2016. Targeting inhibitor of apoptosis proteins protects from bleomycin-induced lung fibrosis. Am. J. Respir. Cell. Mol. Biol 54(4):482–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, et al. 2014. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med 6:231ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lagares D, Santos A, Grasberger PE, Liu F, Probst CK, et al. 2017. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med 9:eaal3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hecker L 2018. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am. J. Physiol. Lung Cell. Mol. Physiol 314:L642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matute-Bello G, Liles WC, Radella F 2nd, Steinberg KP, Ruzinski JT, et al. 1997. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med 156:1969–77 [DOI] [PubMed] [Google Scholar]
- 138.Juss JK, House D, Amour A, Begg M, Herre J, et al. 2016. Acute respiratory distress syndrome neutrophils have a distinct phenotype and are resistant to phosphoinositide 3-kinase inhibition. Am. J. Respir. Crit. Care Med 194:961–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El Kebir D, Gjorstrup P, Filep JG. 2012. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. PNAS 109:14983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. 2009. p53 Attenuates lipopolysaccharide-induced NF-κB activation and acute lung injury. J. Immunol 182:5063–71 [DOI] [PubMed] [Google Scholar]
- 141.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, et al. 2009. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig 119:2898–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fialkow L, Fochesatto Filho L, Bozzetti MC, Milani AR, Rodrigues Filho EM, et al. 2006. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit. Care 10:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matute-Bello G, Liles WC, Radella F 2nd, Steinberg KP, Ruzinski JT, et al. 2000. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit. Care Med 28:1–7 [DOI] [PubMed] [Google Scholar]
- 144.Bachofen M, Weibel ER. 1977. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am. Rev. Respir. Dis 116(4):589–615 [DOI] [PubMed] [Google Scholar]
- 145.Fujita M, Kuwano K, Kunitake R, Hagimoto N, Miyazaki H, et al. 1998. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int. Arch. Allergy Immunol 117:202–8 [DOI] [PubMed] [Google Scholar]
- 146.Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, et al. 2000. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am. J. Pathol 157:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, et al. 2002. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Pathol 161:1783–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glavan BJ, Holden TD, Goss CH, Black RA, Neff MJ, et al. 2011. Genetic variation in the FAS gene and associations with acute lung injury. Am. J. Respir. Crit. Care Med 183:356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, et al. 1999. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J. Immunol 163:2217–25 [PubMed] [Google Scholar]
- 150.Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, et al. 2005. Relationship of acute lung inflammatory injury to Fas/FasL system. Am. J. Pathol 166:685–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, et al. 2001. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am. J. Physiol. Lung Cell. Mol. Physiol 281:L328–35 [DOI] [PubMed] [Google Scholar]
- 152.Idell S, Kueppers F, Lippmann M, Rosen H, Niederman M, Fein A. 1987. Angiotensin converting enzyme in bronchoalveolar lavage in ARDS. Chest 91:52–56 [DOI] [PubMed] [Google Scholar]
- 153.Budinger GR, Chandel NS, Donnelly HK, Eisenbart J, Oberoi M, Jain M. 2005. Active transforming growth factor-β1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med 31:121–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu AN, Mohammed AZ, Rice WR, Fiedeldey DT, Liebermann JS, et al. 1999. Perforin-independent CD8+ T-cell-mediated cytotoxicity of alveolar epithelial cells is preferentially mediated by tumor necrosis factor-α: relative insensitivity to Fas ligand. Am. J. Respir. Cell Mol. Biol 20:849–58 [DOI] [PubMed] [Google Scholar]
- 155.Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. 2000. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med 161:237–43 [DOI] [PubMed] [Google Scholar]
- 156.Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, et al. 2011. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am. J. Respir. Crit. Care Med 183:1043–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, et al. 2014. Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J. Immunol 192:5296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, et al. 2014. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol 306:L604–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, et al. 2004. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med 170:1310–16 [DOI] [PubMed] [Google Scholar]
- 160.Pan L, Yao DC, Yu YZ, Li SJ, Chen BJ, et al. 2016. Necrostatin-1 protects against oleic acid-induced acute respiratory distress syndrome in rats. Biochem. Biophys. Res. Commun 478:1602–8 [DOI] [PubMed] [Google Scholar]
- 161.Qing DY, Conegliano D, Shashaty MGS, Seo J, Reilly JP, et al. 2014. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am. J. Respir. Crit. Care Med 190(11):1243–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, et al. 2012. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J. Immunol 189:2006–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiang M, Shi X, Li Y, Xu J, Yin L, et al. 2011. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J. Immunol 187:4809–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, et al. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol 171(10):5380–88 [DOI] [PubMed] [Google Scholar]
- 165.Fonai F, Priber JK, Jakus PB, Kalman N, Antus C, et al. 2015. Lack of cyclophilin D protects against the development of acute lung injury in endotoxemia. Biochim. Biophys. Acta 1852:2563–73 [DOI] [PubMed] [Google Scholar]
- 166.Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, et al. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527–30 [DOI] [PubMed] [Google Scholar]
- 167.Matute-Bello G, Frevert CW, Liles WC, Nakamura M, Ruzinski JT, et al. 2001. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun 69:5768–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ahn D, Prince A. 2017. Participation of necroptosis in the host response to acute bacterial pneumonia. J. Innate Immun 9:262–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, et al. 2016. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep 16(8):2219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, et al. 2010. Lung pathology in fatal novel human influenza A (H1N1) infection. Am. J. Respir. Crit. Care Med 181:72–79 [DOI] [PubMed] [Google Scholar]
- 171.Rodrigue-Gervais IG, Labbe K, Dagenais M, Dupaul-Chicoine J, Champagne C, et al. 2014. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 15:23–35 [DOI] [PubMed] [Google Scholar]
- 172.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, et al. 2016. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe 20(1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pulendran B, Maddur MS. 2015. Innate immune sensing and response to influenza. Curr. Top. Microbiol. Immunol 386:23–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cohen TS, Prince AS. 2013. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Investig 123:1630–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, et al. 2012. Chitinase 3-like-1 promotes Strep-tococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe 12:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. 2005. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19:1178–80 [DOI] [PubMed] [Google Scholar]
- 177.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, et al. 2002. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105:1672–78 [DOI] [PubMed] [Google Scholar]
- 178.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, et al. 2006. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ. Res 98:209–17 [DOI] [PubMed] [Google Scholar]
- 179.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, et al. 2015. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol 35:1166–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, et al. 2007. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol 293:L548–54 [DOI] [PubMed] [Google Scholar]
- 181.Tu L, Dewachter L, Gore B, Fadel E, Dartevelle P, et al. 2011. Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol 45:311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alastalo TP, Li M, Perez V, Pham D, Sawada H, et al. 2011. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Investig 121:3735–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, et al. 2013. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat. Med 19:74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, et al. 2015. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. role of the macrophage migration inhibitory factor/CD74 complex. Am. J. Respir. Crit. Care Med 192:983–97 [DOI] [PubMed] [Google Scholar]
- 185.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, et al. 2009. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J. Clin. Investig 119:512–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, et al. 2005. Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Investig 115:2811–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Long L, Ormiston ML, Yang X, Southwood M, Graf S, et al. 2015. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat. Med 21:777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, et al. 2005. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J. Clin. Investig 115:1479–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. 2017. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am. J. Respir. Crit. Care Med 195(4):425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, et al. 2004. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res 95:830–40 [DOI] [PubMed] [Google Scholar]
- 191.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. 2008. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. Heart Circ. Physiol 294:H570–78 [DOI] [PubMed] [Google Scholar]
- 192.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, et al. 2011. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med 183:649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Federici C, Drake KM, Rigelsky CM, McNelly LN, Meade SL, et al. 2015. Increased mutagen sensitivity and DNA damage in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med 192:219–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li M, Vattulainen S, Aho J, Orcholski M, Rojas V, et al. 2014. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol 50:1118–28 [DOI] [PubMed] [Google Scholar]
- 195.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, et al. 2011. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 300:L753–61 [DOI] [PubMed] [Google Scholar]
- 196.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. 1998. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J. Clin. Investig 101:927–34 [DOI] [PMC free article] [PubMed] [Google Scholar]