Abstract
Quorum sensing is an important regulatory factor of P. aeruginosa virulence induction such as BF, motility, formations of proteases, pyocyanin, and some toxins. The aim of the current study is to detect the effect of the pet.ether extract from onion husk and compound drive from it on quorum sensing and virulence formations of P. aeruginosa. Quorum sensing inhibiting effect of the pet.ether extract of onion husk and a compound drive from it, was evaluated by C. violaceum reporter using dilution method as well as an antioxidant by using DPPH. The efficacious of: Quorum sensing inhibiting on pet.ether fraction and compound derived from it, were investigated for their activities toward biofilm and pyocyanin synthesis as well as motility from P. aeruginosa. The pet.ether fraction and compound derived from it of onion husk exhibited potent antimicrobial, antioxidant and Quorum sensing inhibiting effects. The pet.ether fraction and compound derived from it possesses significant reduction on pyocyanin and biofilm induction of P. aeruginosa. Moreover, they significantly inhibited swimming motilities of P. aeruginosa. For the first time, our study showed the medical importance of Allium cepa L. as antimicrobial, antioxidant as well as Quorum sensing inhibiting and virulence suppressors of P. aeruginosa. Thus, these might emphasized on Allium cepa L as a natural source for attenuating toxins of the Pseudomonas.
Keywords: C. violaceum, P. aeruginosa, Quorum sensing inhibitory activity, VFs
1. Introduction
The majority of medical research of plants focused on components that possess antimicrobial and antioxidant activities with low concern about antipathogenic effects (Wallace, 2004). Nowadays, medical studies are concentrated on managing infection of bacteria via improving antipathogenic agents that control bacterial infections by prohibiting communication process of bacteria named bacteria quorum sensing (QS), this communication system controls the production of Pseudomonas virulence formations (VFs) like pyocyanin, elastase, protease, biofilm formation (BF), bacterial motility, toxins induction, etc. (Zhang and Dong, 2004). QS in P. aeruginosa is managed by signaling agents named N-acylated homoserine lactones (AHLs). The concentration of AHLs auto-producers enhances in relation to enhance in bacterial accumulation till threshold, those signaling agents in return to the bacteria to manage pathogenicity of the bacteria (Fuqua and Greenberg, 2002). So, removal of QS represents an imperative advance to control antimicrobial resistance and bacterial virulence (Hong et al., 2012). Plants are represented as a rich natural resource of quorum inhibiting agents (QSI) (Mohamed et al., 2014). Recently, many researches dealing with the QSI from natural resources, this prompted us to investigate the QSI effect of Allium cepa L. (Onion Husks) Pet. Ether fraction (ONP-PE) and its active constituent 7-Keto-(5-6-dihydro)-β-Sitosterol (ONP) by using the reporter Chromobacterium violaceum (CVO26). Pet.ether extract and active component derived from it that showed QSI activity were investigated for anti-pathogenic potential toward P. aeruginosa PAO1, also, their influence on the virulence of P. aeruginosa was examined, like elastase, pyocyanin production (PP), swarming motility (SM), and exopolysaccharide; EPS.
2. Experimental
2.1. Plant material
Red onion (Allium cepa L.), was bought from Hail garden, a voucher number is ATA32-1 was kept in Pharmacognosy Department, COP, KSU. The husks was combined, and grinded to coarsely powder.
2.2. Extraction and isolation of ONP
Onion husk powder (1 kg) extracted by hydro-acetone (70%) using dist·H2O at 18 °C for 48 h, till exhaustion. All filtrates were combined and evaporated to get dry residue, using rotary vacuum device at 40–45 °C, to obtain a dark mucilage residue (156.2 g, yield 15.62% w/w). This residue was dissolved in CH3OH-H2O (30–70 mL), then it was fractionated successively via pet.ether (3×0.5L), pet.ether fraction was evaporated to afford ONP-PE (1.2 g).
ONP-PE (1.2 g) was subjected to column chromatographic separation (50 g Si gel, 30 cm × 1.5 cm). Elution started with pet.ether and increase the polarity by using EtOAc, 47 fractions (100 mL each) were collected and monitored the behavior by TLC using develop solvent system pet.ether:EtOAc (8.5:1.5). TLC plates were sprayed with Ceric sulfate Ce(SO4)2 spray reagent. A major compound eluted by 10% EtOAc in pet.ether was detected as white powder that was purified by recrystallization to obtain pure compound ONP (30 mg).
2.3. Characterization and structure elucidation of ONP
ONP has Rf = 0.73 using solvent system [Si gel TLC, Pet.ether: Ethylacetate (8:2)] was obtained as white needle-shaped crystals. It is freely soluble in ether and chloroform while insoluble in methanol. It gave positive Libermann's test indicating its steroidal nature. The ion peak appearing at m/z 431 in the EIMS was consistent with [M + 1]+ and a molecular formula C29H50O2. Further, the compound showed fragment ion peaks at m/z 416 [M-CH3]+, 413 [M-H2O]+, 399 [M-H2O-CH3]+, 291 [M –side chain]+, and 273 [M-side chain-H2O]+ characteristic for 7-keto-5-6 dihydro-β-sitosterol, (ONP). 1H&13C NMR spectral data of ONP were detected in CDCl3 Bruker AM 500 instrument (Bruker Biospin GmbH, Rheinstetten, Germany) operating at 500 MHz for protons, and 125 MHz for carbons. The chemical shift values were recorded in δ (ppm) units and the coupling constant (J) were expressed in Hertiz (Hz). 2-D experiments were used for recording COSY, HSQC, and HMBC spectra. The electron impact ionization mass spectra EI-MS were generated using Shimadzu QP-2010 plus.
2.4. Antibacterial assay
The agar well diffusion method was described in detail by Al-Yousef (2018). Detection of minimum inhibitory concentration (MIC) of ONP&ONP-PE toward some of drug resistant clinical strains was detected by broth dilution method, using p-iodonitro tetrazolium violet dye as an indicator of bacterial growth as described by Al-Yousef and Musarat (2018).
2.4.1. Bacterial strains
Different strains used in this study were C. violaceum CV026 (a mini-Tn5 mutant of C. violaceum 31532 that cannot synthesize its own AHL but responds to exogenous C4 and C6 AHLs), and P. aeruginosa PAO1 (C4 and 3-oxo-C12 HSL producer, McLean et al., 2004), both bacterial strains were grown in Luria-Bertani (LB) medium at 30 °C for 24 h. When required, the medium for C. violaceum CVO26 was supplemented with hexanoyl homoserine lactone (C6-HSL; Sigma-Aldrich, St Louis, MO, USA).
2.4.2. MIC detection
MIC of the ONP&ONP-PE were determined against CVO26 and PAO1 by broth macro-dilution method (CLSI, 2004). Sub-MICs were chosen for detecting the anti-virulence and anti-biofilm activities in the chosen bacterial strains.
2.5. DPPH radical scavenging assay
ONP&ONP-PE radical scavenging activities was detected spectrophotometrically against 1,1-diphenyl-2-picrylhydrazyl (DPPH), method of Gyamfi et al. (1999) as described by Al-Yousef and Musarat (2018).
2.6. Quantitative determination of violacein
Extent of violacein induction by Chromobacterium violaceum (CVO26) in presence of Sub-MICs of ONP&ONP-PE was studied by extracting violacein and quantifying photometrically using procedure of Blosser and Gray (2000) with modification (Husain et al., 2015a, Husain et al., 2015b), the procedure is described in detailed by Al-Yousef et al., 2017.
2.7. Effect on virulence factor production
Sub-MICs effect on VFs of PAO1 like LasB elastase, pyocyanin, swarming motility, EPS procedure was estimated previously by Husain et al. (2013).
2.8. Biofilm inhibition (BI) assay
ONP&ONP-PE effect on BF was determined using assay of microtitre plate (O’Toole and Kotler, 1998). The procedure demonstrated previously by Al-Yousef et al. (2017).
2.9. Molecular docking analysis
Schrodinger 2015 suite (Schrodinger, LLC, New York, NY, USA) was used for in silico docking studies, the methodology has been discussed previously in detail by Sheikh (2016).
2.9.1. Data retrieval
Chembl database was used for retrieving the three dimensional structures of compound. The 2-D of the ligand structures is presented. It was prepared by using LigPrep module of Schrodinger. The methodology is previously described in detail (Sheikh, 2016).
2.9.2. Selection and preparation of protein
The three dimensional structure of Quorum sensing control repressor (QSCR) from Pseudomonas aeruoginosa (PDB ID: 3SZT) was acquired from the PDB (Protein Data Bank) (http://www.rcsb.org) and was processed using protein preparation wizard as described previously in detail (Sheikh, 2016).
2.9.3. Docking and binding affinity calculation
Motivated fit docking was prepared using Prime module of Schrodinger 2015 suite and binding affinity of ligand molecule with receptor (3SZT) was detected using MM-GBSA. The methodology has been described previously (Sheikh, 2016).
2.9.4. Ligand preparation and conformational search
The methodology has been described previously (Sheikh, 2016).
2.9.5. Induced fit docking (IFD)
The three stereoisomers prepared by the ligprep module were submitted as starting geometries to IFD by using Prime programin Schrodinger’s IFD module. The IFD has the capability to sample both the little changes in the backbone structure and robust conformational changes inside chains. In the early stage of IFD, a softened-potential docking is performed where the ligand is docked into an ensemble of receptor conformations. This is followed by complex reduction for highest rank edpose, where both ligand and receptor binding sites are free to move. For calculations of binding energy by using the MM-GBSA continuum solvent model present in Prime module of Schrodinger 2015-3, binding affinity of ligand against the receptor molecule was also calculated.
2.10. Statistical analysis
All results were performed in triplicates, and the data obtained from experiments were plotted as mean (M) values, and the difference between control and test were assessed using student’s t test.
3. Results and discussion
3.1. Compound ONP
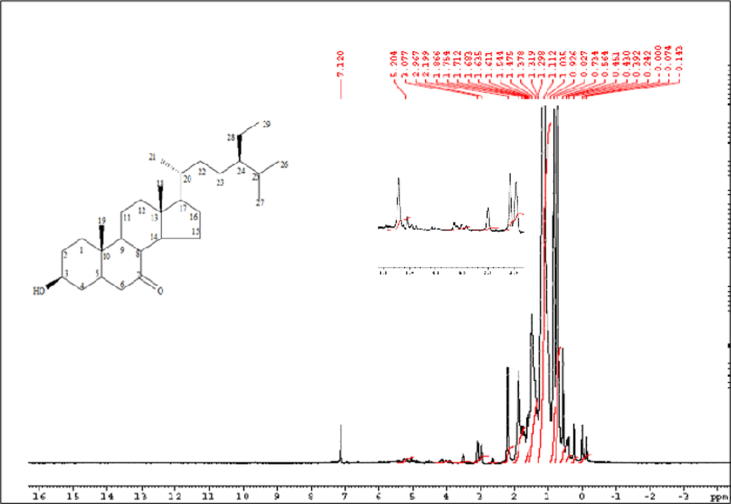
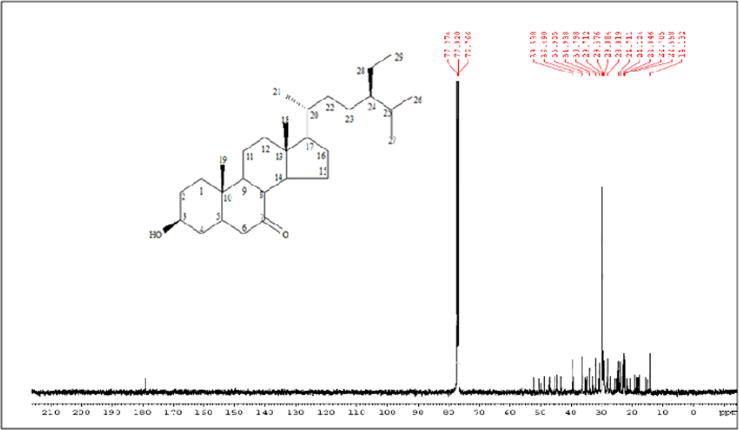
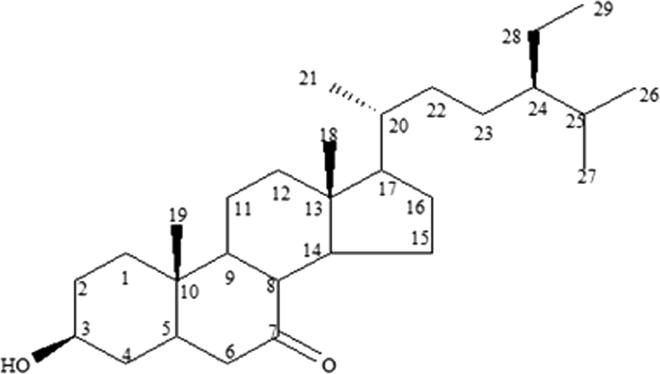
Pet. ether active fraction (ONP-PE) and its active component ONP; was further tested to assess its potential identity. ONP has Rf = 0.73 using solvent system [Si gel TLC, Pet.ether: Ethylacetate (8:2)] was obtained as white needle-shaped crystals. It is freely soluble in ether and chloroform while insoluble in methanol. It gave positive Libermann's test indicating its steroidal nature. The structure of this compound was established by 13C NMR spectrum which showed twenty nine carbon resonances that confirming the presence of a compound with formula C29H50O2 in which all the 29 carbon atoms were assigned by comparison with β-sitosterol published data. The 1H NMR spectrum, Fig. 2, is characterized by the presence of two methine protons resonating at δH 3.1 (m, H-3) and δH 2.19 (br. s, H-5). In addition, six methyl peaks appeared at δH 0.56 (3H, s, Me-18), δH 0.84 (3H, t, Me-29), δH 0.83 (3H, m, Me-27), δH 0.82 (3H, d, Me-26), δH 0.92 (3H, d, Me-21) and δH 1.0 (3H, s, Me-19). The absence of an unsaturation bond (Δ 5-6) which was in agree with absence of signals approximately at δC 140.7 and δC 121.7 for olefinic carbons (C-5&C-6) and presence of double bond ketone at δC 180 as well as presence of signals at δH 2.19 (br. s, H-5), δC 30.7 (C-5); and δH 1.9 (br. s, H-6), δC 30.7 (C-6), Fig. 3. Further, HSQC experiment was helpful in correlating each proton to the corresponding carbon. The attachment of carbonyl group at C-7 was confirmed by HMBC correlation between the methine carbon at δH 2.19 (br. s, H-5) with C-7 carbon absorption at δC 180. All these data confirmed the skeleton to be 7-Keto-(5-6-dihydro)-β-Sitosterol, Fig. 1. The above mentioned NMR analysis of compound ONP suggested the presence of 7-Keto-(5-6-dihydro)-β-Sitosterol which was isolated at the first time from Allium cepa L.
Fig. 2.
1H NMR spectrum of ONP, 500 MHz, CDCl3.
Fig. 3.
13C NMR spectrum of ONP, 125 MHz, CDCl3.
Fig. 1.
ONP (7-Keto-5, 6-dihydro)-β-Sitosterol.
3.2. Antimicrobial activity
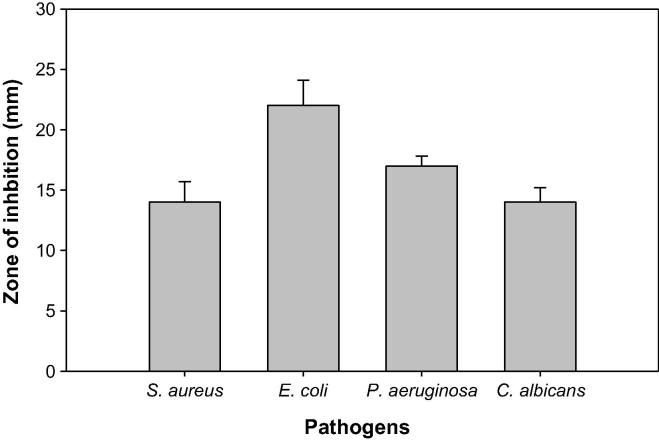
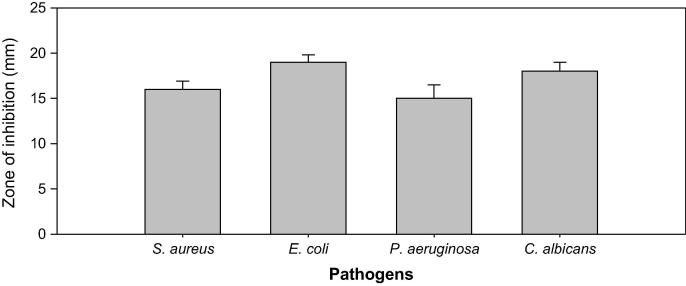
Antimicrobial screening of onion husks was determined against S. aureus, E. coli, P. aeruginosa and C. albicans 100 µL of ONP-PE&ONP at a dose of 100 mg/mL test by determination of the zone of inhibition. ONP-PE showed higher inhibitory activity against gram negative bacteria E. coli and P. aeruginosa by 22 and 17 mm in comparison with the ONP compound by 19 and 15 mm respectively. Moreover, ONP has more inhibitory activities against gram positive bacteria S. aureus as well as fungus C. albicans by 16 and 18 mm in comparison with ONP-PE by 14 mm and 14 mm respectively when compare with control, Table, 1; Figs. 4 and 5, with MIC values between 0.4 and 0.8 mg/mL for components of ONP&ONP-PE, Table 1. The results of this study appear to be emphasize on the potential antimicrobial activity of onion husks pet.erher fraction and chemical compound derived from it. Therefore, this fraction may be helpfulness in the management of multi-diseases that might be a cause of infection.
Fig. 4.
Antimicrobial activity of ONP PE.
Fig. 5.
Antimicrobial activity of ONP.
Table 1.
Antimicrobial activity (MIC) of ONP-PE&ONP.
| S. No | Samples | Inhibition zone (mm) |
|||
|---|---|---|---|---|---|
| 100 mg/mL | S. aureus | E. coli | P. aeruginosa | C. albicans | |
| 1 | ONP PE MIC (mg/mL) |
14 + 1.7 (0.4) |
22 ± 2.1 (0.4) |
17 ± 0.8 (0.8) |
14 ± 1.2 (0.4) |
| 2 | ONP MIC (mg/mL) |
16 ± 0.9 (0.4) |
19 ± 0.8 (0.8) |
15 ± 1.5 (0.8) |
18 ± 1.0 (0.8) |
| 3 | Ampicillin | 21 | – | – | – |
| 4 | Doxycycline | – | 25 | 24 | – |
| 5 | Nystatin | – | – | – | 23 |
3.3. Antioxidant activity
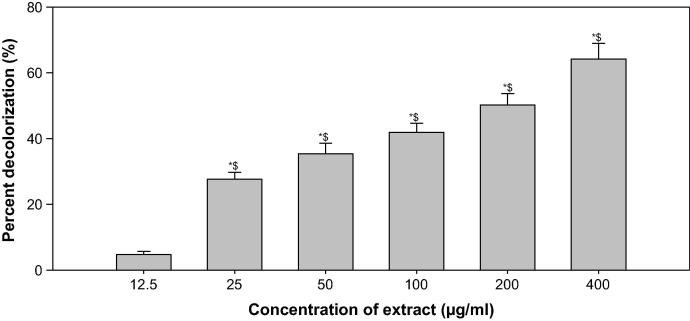
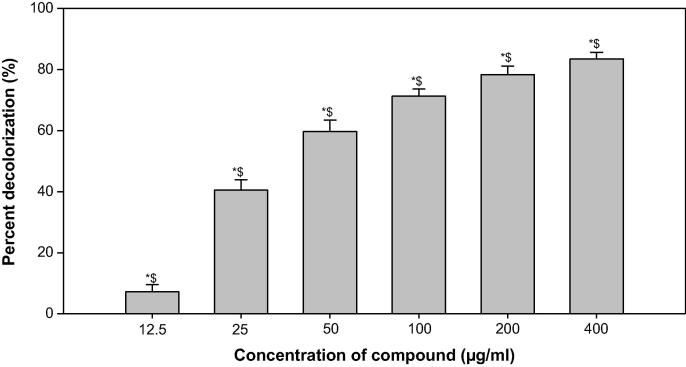
DPPH-radical scavenging assay showed a significant antioxidant activity of ONP toward ONP-PE at a dose dependent manner by 78.32% & 83.48% and 50.19 & 64.18 at 200 and 400 µg\mL respectively when compare to ascorbic acid and BHT, Table 2 & Fig. 6 and 7, respectively.
Table 2.
Free radical scavenging activity of ONP-PE&ONP by DPPH method.
| Name of the samples | % de-colorization by using DPPH method | |||||
|---|---|---|---|---|---|---|
| Concentrations of plant samples (µg/mL) | ||||||
| 12.5 | 25 | 50 | 100 | 200 | 400 | |
| ONP | 7.25 ± 2.32 | 40.54 ± 3.31 | 59.70 ± 3.68 | 71.3 ± 2.29 | 78.32 ± 2.80 | 83.48 ± 2.14 |
| ONP PE | 4.7 ± 0.99 | 27.63 ± 2.04 | 35.36 ± 3.23 | 41.92 ± 2.7 | 50.19 ± 3.46 | 64.18 ± 4.73 |
Fig. 6.
Free radical scavenging activity of ONP PE by DPPH method. The above data are the mean of three replicates; * shows significant with Ascorbic acid; $ shows significant with Butylated hydroxyl toluene (BHT).
Fig. 7.
Free radical scavenging activity of ONP by DPPH method. The above data are the mean of three replicates; * shows significant with Ascorbic acid; $ shows significant with Butylated hydroxyl toluene (BHT).
3.4. Violacein inhibition (VI) assay
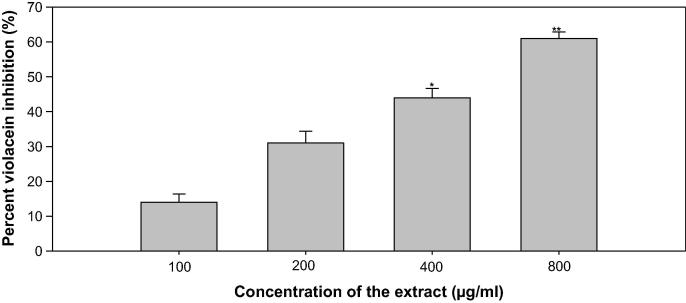
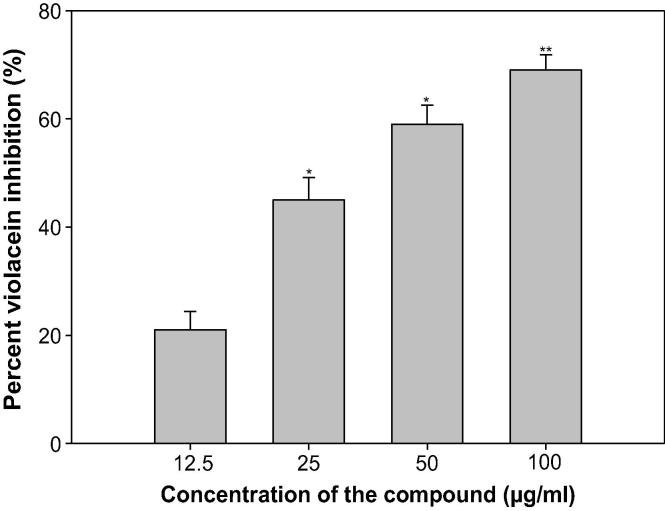
Violacein production (VP), a purple pigment in C. violaceum is a regulated process of QS, and its induction is organized by CviIR-dependent QS system. In this current study, ONP-PE inhibited production of violacein in wild-type C. violaceum CVO26 strain in a dose-dependent pattern and the growth of the bacteria not affected. Maximum significant reduction of 45% (p ≤ 0.05) & 60% (p ≤ 0.005) was noticed at 400 & 800 µg/mL respectively, whilst at lower concentrations (100 and 200 µg/mL) 14&31% decrease in VP respectively were recorded, Fig. 8. Moreover, maximum significant reduction of 69% was recorded at 100 µg/mL, while at lower concentrations (12.5, 25, and 50 µg/mL) 21–59% decrease in VP was noticed in ONP, Fig. 9. These concentration dependent actions of ONP-PE & ONP on VP are in alignment with the different reports (Zahin et al., 2010, Sybiya Vasantha Packiavathy et al., 2012).
Fig. 8.
Quantitative assessment of violacein inhibition in CVO26 by sub-MICs of ONP PE. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05, **, significance at p ≤ 0.005.
Fig. 9.
Quantitative assessment of violacein inhibition in CVO26 by sub-MICs of ONP. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05, **, significance at p ≤ 0.005.
3.5. ONP-PE effects on VFs of P. aeruginosa PAO1
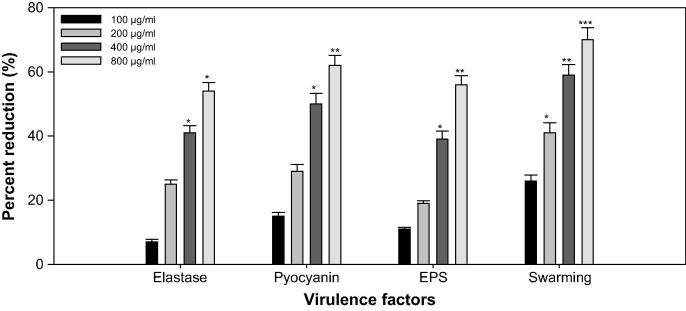
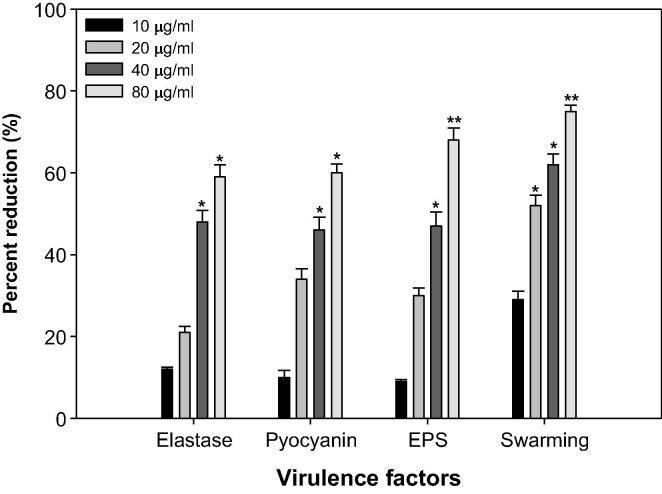
Profiteering P. aeruginosa (human pathogen) integrates AHL dependent signaling with 4-Quinolone dependent QS (Diggle et al., 2006). So, the rhl, las, and pqs QS systems of P. aeruginosa control the production of multi- extracellular VFs such as elastase, pyocyanin, swarming motility, and exopolysaccharide; EPS (Latifi et al., 1995, Winson et al., 1998, de Kievit and Iglewski, 2000, Williams, 2007). Sub-inhibitory concentrations effects of ONP-PE& ONP at different VFs of P. aeruginosa PAO1 is recorded in Figs. 10 and 11.
Fig. 10.
Effect of sub-MICs of ONP PE on inhibition of quorum sensing regulated virulence factors in P. aeruginosa PAO1. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05, **, significance at p ≤ 0.005, ***, significance at p ≤ 0.001.
Fig. 11.
Effect of sub-MICs of ONP on inhibition of quorum sensing regulated virulence factors in P. aeruginosa PAO1. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05, **, significance at p ≤ 0.005.
The activity of LasB elastase was significant decreased and noticed in the culture supernatant of PAO1 handled with ONP-PE &ONP sub-MICs. A significant inhibition was noticed by 41% & 57% when PAO1was cultured with ONP-PE at 400 & 800 µg/mL (p ≤ 0.05) concentrations of the pet.ether fraction. A minimum of 48% inhibition was observed when PAO1was cultured with ONP at a concentration of 40 µg/mL (p ≤ 0.05) and maximum of 59% inhibition was observed at 80 µg/mL (p ≤ 0.05) concentration of the compound. Elastase enzyme promotes the invasiveness of the pathogen by decomposing the structural components of the infected tissue (Kharazmi, 1989). In this current study, the ONP-PE & ONP showed concentration-dependent inhibition of elastase in PAO1, as recorded in Fig. 10 and 11. This data is in agreement with the report of Musthafa et al. (2010), who determined significant inhibition of LasB activity by many plants. Many reports suggest that steroidal rich fractions of plants exerts substantial inhibitory effect against LasB in PA01. In addition to, flavanones (Vandeputte et al., 2011), Sclerocarya birrea bark extract (Sarkar et al., 2014) and Trigonella foenum-graceum seed extract (Husain et al., 2015a) have been shown to inhibit elastase activity to appreciable levels. Production of blue colored pyocyanin is controlled by QS (Williams, 2007).
PP and phenazine-1-carboxylic acid (PCA) cause impairs neutrophil-mediated host defenses and neutrophil apoptosis (Fothergill et al., 2007). ONP-PE &ONP at sub-lethal concentrations exhibited appreciable decrease in the pyocyanin production (PP) by PAO1. The maximum reduction of 62% in PP was recorded at highest tested concentration 800 µg/mL, (p ≤ 0.005) followed by 49% at 400 µg/mL concentration, (p ≤ 0.05), Fig. 10. The maximum reduction of 60% in PP was recorded at highest tested concentration 80 µg/mL, (p ≤ 0.05) followed by 45% at 40 µg/mL concentration, (p ≤ 0.05), Fig. 11. Current results are in alignment with the results of recent reports wherein Krishnan et al. (2012), and Gala et al. (2016) demonstrated that fractions of S. aromaticum and Tinospora cordifolia from bud and stem respectively reduced the PP significantly.
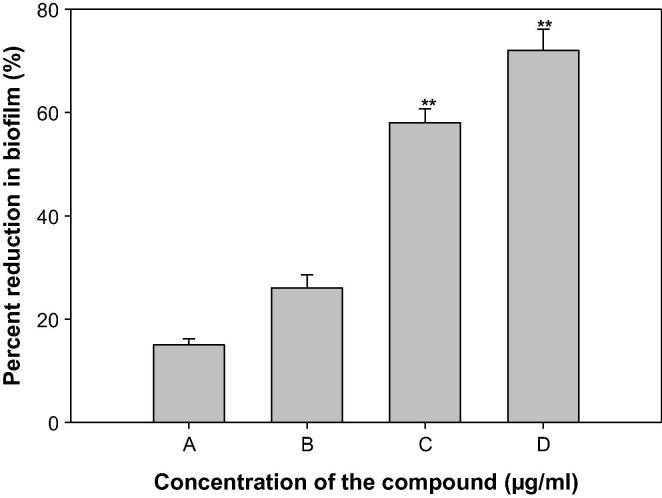
SM and EPS, by P. aeruginosa plays a vital role in the initiation, maturation, and maintenance of the biofilm architecture (Pratt and Kolter, 1998, Hentzer et al., 2003). Therefore, any interference with the SM and EPS is bound to affect the BFs by the pathogen. In the present study, treatment of PAO1 with sub-MICs of ONP-PE showed significantly reduced EPS, the compound (400 & 800 µg/mL) demonstrated inhibition in EPS to the level of 39 (p ≤ 0.05) & 58% (p ≤ 0.005) respectively. Similarly, SM of PAO1 was also significantly impaired the extract concentrations 200, 400 & 800 µg/mL, that demonstrated inhibition in SM to the level of 40% (p ≤ 0.05), 59% (p ≤ 0.005) & 71% (p ≤ 0.001) respectively after treatment with test concentrations of ONP-PE (Fig. 9). Furthermore, treatment of PAO1 with sub-MICs of ONP showed significantly reduced EPS, the compound (40 & 80 µg/mL) demonstrated inhibition in EPS to the level of 48 (p ≤ 0.05) & 69% (p ≤ 0.005) respectively. Similarly, SM of PAO1 was also significantly impaired the compound concentration 20, 40 & 80 µg/mL, that demonstrated inhibition in SM to the level of 50 (p ≤ 0.05), 61 (p ≤ 0.05) & 75% (p ≤ 0.005) respectively after treatment with test concentrations of ONP (Fig. 11). This statistically significant reduction of motility and exopolymeric material is reported with Trigonella foenum-graceum seed extract (Husain et al., 2015a). Biofilm is a drug resistant complex aggregation of microorganisms and is a key factor in the pathogenesis of P. aeruginosa (Caraher et al., 2007).
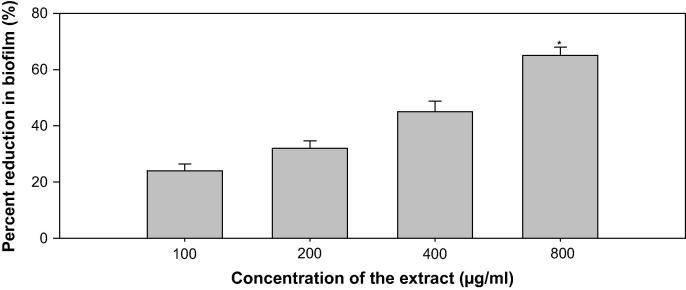
In a biofilm adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPS). Biofilms are the cause of severe persistent infection and BFs is considered as one of the potential drug targets to combat drug-resistant chronic infections (Hall-Stoodley et al., 2004, Wu et al., 2015). The ONP-PE showed 22, 30, 42 and 57% (p ≤ 0.05), decrease in the BFs ability of PAO1 at 100, 200, 400, and 800 µg/mL of extract concentrations, respectively, Fig. 12. The ONP showed 18, 25, 58 (p ≤ 0.005), and 73% (p ≤ 0.005), decrease in the BFs ability of PAO1 at 10, 20, 40, and 80 µg/mL of compound concentration, respectively, Fig. 13. Our findings are confirmed from previous studies on biofilm inhibition in PAO1, by Trigonella foenum graceum (Husain et al., 2015a), Mangifera indica (Husain et al., 2017), polyphenolic fraction of South Florida plants (Adonizio et al., 2008), Lagerstroemia speciosa (Singh et al., 2012), Rosa rugosa (Zhang et al., 2014), and Sclerocarya birrea (Sarkar et al., 2014),
Fig. 12.
Effect of sub-MICs of ONP PE on biofilm formation in P. aeruginosa PAO1. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05.
Fig. 13.
Effect of sub-MICs of ONP on biofilm formation in P. aeruginosa PAO1. All of the data are presented as mean ± SD. *, significance at p ≤ 0.05, **, significance at p ≤ 0.005.
3.6. Molecular docking (MD) and binding affinity
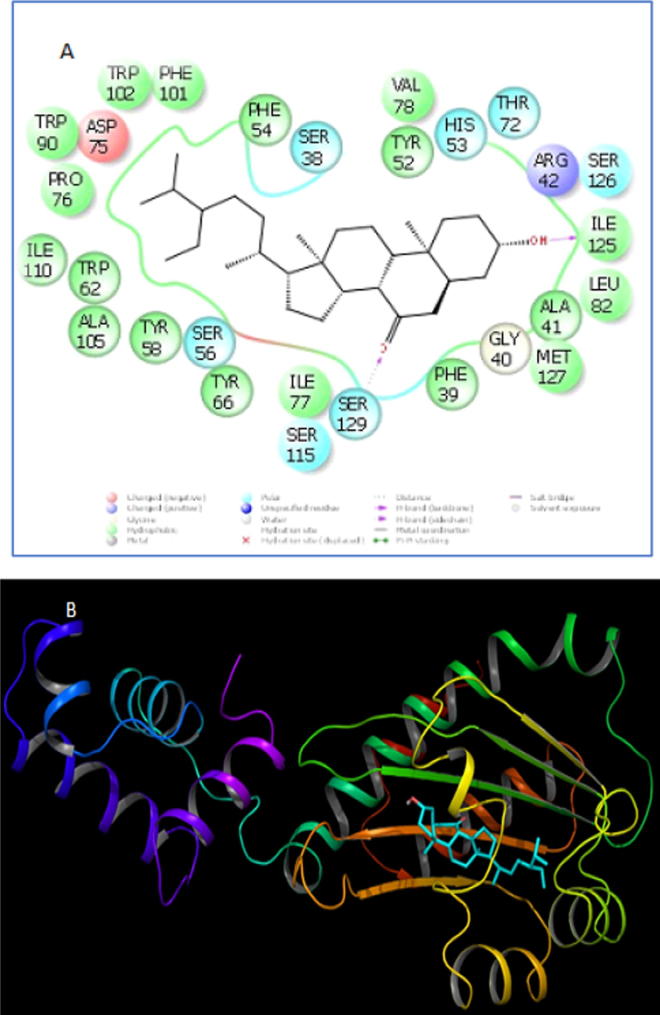
The amino acid residues of receptor 3SZT interacting with ligand are shown in Fig. 14A. Moreover, the overall ribbon structure is also shown in Fig. 14B. In the receptor-ligand complex, 28 amino acid residues of receptor interact with ligand. Ser-129 and Ile-125 displayed hydrogen bonding interaction with the ligand. The docking and glide score for the ligand is −10.93 Kcal/mol. Moreover, the calculated binding affinity was observed to be −160.33 Kcal/mol.
Fig. 14.
(A) Amino-acid residues in the binding pocket of 3SZT involved in interactions with ligand. (B) Overall ribbon form representation of docking complex of 3SZT with ligand.
Findings of the MD with ONP were confirmed in vitro using the CVO26 and PAO1 test strains. MIC of ONP was found to be 200 and 100 against CVO26 and PAO1, respectively. At the tested sub-MICs (12.5–100 µg/mL), ONP demonstrated statistically significant inhibition of violacein pigment ranging from 21 to 59% when compared to untreated control group (Fig. 9). IC50 value was found to be 30.98 µg/mL. ONP was further estimated for its anti-virulence properties in PAO1 and dose-dependent reduction in all the studied of VFs was observed. Test compound (ONP) decreased the elastase activity (10–59%), PP (9–60%), EPS (8–69%) and SM (29–75%) at sub-inhibitory concentrations ranging from 10 to 80 µg/mL (Fig. 11). BFs by PAO1 was also impaired by 18, 25, 58, and 73% at 10, 20, 40, and 80 µg/mL concentrations, respectively (Fig. 13). In other study, flavonoid without impacting the growth of PAO1, possess significantly inhibited (P < 0.05) BFs and production of VFs including PP, protease and elastase at sub-lethal doses (Ouyang et al., 2016). Moreover, the toxicity of this compound has been investigated, with the 7-Keto-(5-6-dihydro)-β-Sitosterol being the most toxic to HepG2, U937, and Caco2 cell lines in a similar way to their corresponding cholesterol counterparts, Ryan et al.
4. Conclusion
Allium cepa L, (pet.ether fraction), is used in this study to focus on its QS and BI properties against pathogenic bacteria. This study shows that ONP-PE&ONP might inhibit the production of QS mediated VFs in C. violaceum, and P. aeruginosa. Moreover, the used of sub-MICs of ONP-PE&ONP possess a significant inhibition of the QS-mediated BFs, SM, and EPS formation in these pathogens. Thus, these results suggest that ONP-PE and its new bioactive derived compound ONP might possess potential anti-infective properties, and might confirmed to be an effective anti-QS and anti-biofilm agent toward pathogens.
Conflict of interest
The authors declare that they did not have any conflict of interest
Footnotes
Peer review under responsibility of King Saud University.
References
- Adonizio A., Kong K.F., Mathee K. Inhibition of quorum-sensingcontrolled virulence factor production in Pseudomonas aeruginosa by south Florida plant extracts. Antimicrob. Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yousef H.M., Ahmed A.F., Al-Shabib N.A., Laeeq S., Khan R.A., Rehman M.T., Alsalme A., Al-Ajmi M.F., Khan M.S., Husain F.M. Onion peel ethylacetate fraction and its derived constituent quercetin 4′-O-β-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbial. 2017 doi: 10.3389/fmicb.2017.01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yousef H.M., Amina M. Essential oil of Coffee arabica L. Husks: a brilliant source of antimicrobial and antioxidant Agents. Biomed. Res. 2018;29(1):174–180. [Google Scholar]
- Blosser R.S., Gray K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods. 2000;40:47–55. doi: 10.1016/s0167-7012(99)00136-0. [DOI] [PubMed] [Google Scholar]
- Caraher E., Reynolds G., Murphy P., McClean S., Callaghan M. Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:213–221. doi: 10.1007/s10096-007-0256-x. [DOI] [PubMed] [Google Scholar]
- CLSI . CLSI; Wayne, PA: 2004. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, Approved Guideline. CLSI document M44-A. [Google Scholar]
- de Kievit T.R., Iglewski B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle S., Cornelis P., Williams P., Camara M. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int. J. Med. Microbiol. 2006;296:83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Gala V.C., John N.R., Bhagwat A.M., Datar A.G., Kharkar P.S., Desai K.B. Attenuation of quorum sensing-regulated behaviour by Tinospora cordifolia extract & identification of its active constituents. Indian J. Med. Res. 2016;144:92–103. doi: 10.4103/0971-5916.193295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi M.A., Yonamine M., Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol.: Vasc. Syst. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- Fothergill J.L., Panagea S., Hart C.A., Walshaw M.J., Pitt T.L., Winstanley C. Widespread pyocyanin overproduction among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 2007;7:45. doi: 10.1186/1471-2180-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C., Greenberg E.P. Listening in on bacteria: acyl homoserine lactone signalling. Nat. Rev. Mol Cell. Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- Hong K.W., Koh C.L., Sam C.K., Yin W.F., Chan K.G. Quorum quenching revisited-From signal decays to signaling confusion. Sensors (Basel) 2012;12:4661–4696. doi: 10.3390/s120404661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain F.M., Ahmad I. Doxycycline interferes with quorum sensing-mediated VFs and biofilm formation in gram-negative bacteria. World J. Microbiol. Biotechnol. 2013;29:949–957. doi: 10.1007/s11274-013-1252-1. [DOI] [PubMed] [Google Scholar]
- Husain F.M., Ahmad I., Al-thubiani A.S., Abulreesh H.H., AlHazza I.M., Aqil F. Leaf extracts of Mangifera indica L. inhibit quorum sensing regulated production of VFs and biofilm in test bacteria. Front. Microbiol. 2017;8:727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain F.M., Ahmad I., Khan M.S., Ahmad E., Tahseen Q., Khan M.S. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram negative bacteria. Front. Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain F.M., Ahmad I., Khan M.S., Al-Shabib N. Trigonella foenum-graceum (Seed) extract interferes with quorum sensing regulated traits and biofilm formation in the strains of Pseudomonas aeruginosa and Aeromonas hydrophila. Evid. Based Complement. Alternat. Med. 2015;879540 doi: 10.1155/2015/879540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A. Interactions of Pseudomonas aeruginosa proteases with the cells of the immune system. Antibiot. Chemother. 1989;42:42–49. doi: 10.1159/000417602. [DOI] [PubMed] [Google Scholar]
- Krishnan T., Yin W.F., Chan K.G. Inhibition of quorum sensing controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors. 2012;12:4016–4030. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A., Winson M.K., Foglino M., Bycroft B.W., Stewart G.S.A.B., Lazdunski A. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- McLean R.J., Pierson L.S., Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods. 2004;58:351–360. doi: 10.1016/j.mimet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Mohamed G.A., Ibrahim S.R.M., Shaaban M.I.A., Ross S.A. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia. 2014;98:215–221. doi: 10.1016/j.fitote.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Musthafa K.S., Ravi A.V., Annapoorani A., Sybiya Vasantha Packiavathy I.A., Pandian S.K. Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy. 2010;56:333–339. doi: 10.1159/000320185. [DOI] [PubMed] [Google Scholar]
- O’Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Ouyang J., Sun F., Feng W., Sun Y., Qiu X., Xiong L. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and VFs in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016;120:966–974. doi: 10.1111/jam.13073. [DOI] [PubMed] [Google Scholar]
- Pratt L.A., Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Ryan E., Chopra J., McCarthy F., Maguire A.R., O'Brien N.M. Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol oxidation products with their corresponding cholesterol oxidation products. Br J Nutr. 2005;94:443–451. doi: 10.1079/bjn20051500. [DOI] [PubMed] [Google Scholar]
- Sarkar R., Chaudhary S.K., Sharma A., Yadav K.K., Nema N.K., Sekhoacha M. Anti-biofilm activity of Marula – a study with the standardized bark extract. J. Ethnopharmacol. 2014;154:170–175. doi: 10.1016/j.jep.2014.03.067. [DOI] [PubMed] [Google Scholar]
- Sheikh I.A. Stereo selectivity and potential endocrine disrupting activity of Bis-(2-ethylhexyl) phthalate (DEHP) against human progesterone receptor: a computational perspective. J. Appl. Toxicol. 2016;36(5):741–747. doi: 10.1002/jat.3302. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Singh H.B., Singh A., Singh B.R., Mishra A., Nautiyal C.S. Lagerstroemia speciosa fruit extract modulates quorum sensing controlled virulence factor production and biofilm formation in Pseudomonas aeruginosa. Microbiology. 2012;158:529–538. doi: 10.1099/mic.0.052985-0. [DOI] [PubMed] [Google Scholar]
- Sybiya Vasantha Packiavathy I.A., Agilandeswari P., Musthafa K.S., Pandian S.K., Ravi A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against gram negative bacterial pathogens. Food Res. Int. 2012;45:85–92. [Google Scholar]
- Vandeputte O.M., Kiendrebeogo M., Rasamiravaka T., Stévigny C., Duez P., Rajaonson S. The flavanone naringenin reduces the production of quorum sensing-controlled VFs in Pseudomonas aeruginosa PAO1. Microbiology. 2011;157:2120–2132. doi: 10.1099/mic.0.049338-0. [DOI] [PubMed] [Google Scholar]
- Wallace R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutri Soc. 2004;63 doi: 10.1079/pns2004393. 621-629. 27. [DOI] [PubMed] [Google Scholar]
- Williams P. Quorum sensing, communication and cross kingdom signaling in the bacterial world. Microbiology. 2007;153:3923–3928. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- Winson M.K., Swift S., Fish L., Throup J.P., Jorgensen F., Chhabra S.R. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- Wu H., Moser C., Wang H.Z., Hoiby N., Song Z.H. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015;7:1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahin M., Hasan S., Aqil F., Khan M.S.A., Husain F.M., Ahmad I. Screening of certain medicinal plants from India for their anti-quorum sensing activity. Indian J. Exp. Biol. 2010;48:1219–1224. [PubMed] [Google Scholar]
- Zhang L.H., Dong Y.H. Quorum sensing and signal interference: diverse implications. Mol. Microbiol. 2004;53:15631567. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- Zhang J.M., Rui X., Wang L., Dong M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control. 2014;42:125–131. [Google Scholar]