Abstract
Related substances in pharmaceutical formulations are associated with their safety, efficacy and stability. However, there is no overall study already published on the assessment of related substances in the Compound Ketoconazole and Clobetasol Propionate Cream. In this work, a reliable HPLC-TOF-MS qualitative method was developed for the analysis of related substances in this preparation with a quick and easy extraction procedure. Besides the active pharmaceutical ingredients, two compounds named ketoconazole impurity B′ optical isomer and ketoconazole impurity E were identified. Furthermore, a new HPLC method for qualitative and quantitative assessment on related substances and degradation products, which were found in the stability test, was established and validated. The single standard to determine multi-components method was applied in the quantitative analysis, which was an effective way for reducing cost and improving accuracy. This study can provide a creative idea for routine analysis of quality control of the Compound Ketoconazole and Clobetasol Propionate Cream.
Keywords: Compound Ketoconazole and Clobetasol Propionate Cream, Ketoconazole impurity B' optical isomer, Ketoconazole impurity E, Quality control, HPLC-TOF-MS, HPLC
1. Introduction
The inflamed skin conditions, such as eczema and dermatitis, are common in infants, adolescents and adults, which can be further complicated by bacterial infections [1]. Topical antifungals and corticosteroids are mainstays for the treatment of inflammatory skin conditions. Among them, ketoconazole has a broad spectrum of microbiologic activities against dermatophytes and yeasts as the first orally active azole antifungal agent [2], [3]. Clobetasol propionate, a highly potent drug of all the available corticosteroids, is widely used in the treatment of various skin disorders [4]. Hence, the topical pharmaceutical cream combining the active pharmaceutical ingredients (APIs) of ketoconazole and clobetasol propionate was produced. Fortunately, the Compound Ketoconazole and Clobetasol Propionate Cream brought out a good overall safety profile, allowing rapid clearance of disease symptoms during the initial treatment and providing an efficacious therapy for relapse prevention compared to ketoconazole monotherapy [5].
The impurity, even in small amounts, is important with respect to the safety, efficacy and stability of pharmaceutical products. Thus, increasing regulatory and pharmacopeia were focused on related substances and degradation products in drug substances and the finished pharmaceutical formulations [6]. The related substances described in pharmacopeias are consistent, five related substances for ketoconazole API named from A-E and thirteen related substances for clobetasol propionate API named from A-M have been listed in European Pharmacopeia (EP), United States Pharmacopeia (USP) and British Pharmacopoeia (BP). In the previous studies, there have been several research articles on the determination of ketoconazole and clobetasol propionate in plasma, serum, urine, as well as in pharmaceuticals-creams, shampoo and tablets [7], [8], [9], [10]. However, no pharmacopeia and few studies have been able to draw on any systematic research into quality control of related substances in the compound ketoconazole and clobetasol propionate pharmaceutical formulations. Based on the data obtained from preliminary studies, ketoconazole and minor related substances (i.e., B, C, D and E described in EP) contained in commercial preparations have been found to be strong activators of human Aryl hydrocarbon receptor [11], [12]. The related substances existing in ketoconazole may be in connection with skin chemoprevention and/or damage. Consequently, it is urgent to develop a simple, rapid and stability-indicating method for determination of related substances in the Compound Ketoconazole and Clobetasol Propionate Cream.
In general, the related substances of compound preparations are numerous and have a wider sources compared to single preparations. There may exist potential drug-drug interactions, and drug-excipients interactions, which makes the separation, qualitative and quantitative work more difficult. Mass spectrometry, especially the TOF-MS, has become the dominant qualitative tool for trace components like impurities in drug substances and products due to higher selectivity and sensitivity [13]. Quantitative methods for related substances in the pharmacopeia of different countries include the external standard method, the principal component self-control method with correction factors, and the principal component self-control method without correction factors [14]. Since it is difficult to obtain the impurity reference standards, the single standard to determine multi-components (SSDMC) quantitative method based on the relative response factors (RRFs) is the most efficient way to correct the differences in responses between APIs and their related substances [15].
In order to improve the quality control of related substances in this preparation efficiently, the whole analytical process was optimized from sample preparation to chromatographic separation. A sensitive and reliable HPLC-TOF-MS method was developed for the identification of related substances and an HPLC quantitative method using SSDMC was also developed and validated. The RRFs of related substances were determined for quantitative analysis. The present study provided new information and supporting data for the overall quality control of the Compound Ketoconazole and Clobetasol Propionate Cream, and the method developed in this study can be used as a model for the analysis of the related substances or for analyses with similar requirements.
2. Materials and methods
2.1. Reagents and chemicals
Cream formulation prototypes and placebos were provided by pharmaceutical factory (Guangdong, China). Reference standards of ketoconazole (purity of 99.4%), ketoconazole impurity B (K-B) (purity of 95.6%), ketoconazole impurity C (K-C) (purity of 98.0%), ketoconazole impurity D (K-D) (purity of 99.7%), ketoconazole impurity E (K-E) (purity of 99.4%), clobetasol Propionate (purity of 99.5%) were purchased from National Institutes for Food and Drug Control (Shenyang, China). Reference standards of clobetasol propionate impurity C (C-C) were obtained from TLC Pharmaceutical Standards Co., Ltd. (Moraiya, Ahmedabad), clobetasol propionate impurity J (C-J) was from Toronto Research Chemicals (Canada). HPLC-grade acetonitrile was supplied by Fisher Scientific (Fisher, Scientific, USA), ammonium hydroxide and ammonium acetate used as mobile phase additives were obtained from Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). High purity water was acquired from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China). All other chemicals and reagents of analytical grade were from Shandong Yu Wang Chemical Reagent Factory (Shandong, China).
2.2. Apparatus
2.2.1. HPLC conditions
The liquid chromatographic analysis was conducted on an Agilent 1260 HPLC system (Agilent, USA). The HPLC instrument consists of a binary pump, an online degasser, an auto plate-sampler, a thermostatically controlled column compartment and a variable wavelength detector. The chromatographic separation was achieved on an Agilent poroshell HPH-C8 (4.6 mm × 100 mm, 2.7 µm) (Agilent, USA) column with detection carried out at 239 nm, and the temperature of column and auto-sampler was maintained at 25 °C and 20 °C, respectively. Analysis was completed with a gradient elution of acetonitrile (A) and ammonium acetate (pH 7.5; 0.01 M) in purified water (B) and the gradient program is as follows: 25%–30% A at 0–7 min; 30%–34% A at 7–8 min; 34%–40% A at 8–25 min; 40%–48% A at 25–35 min; 48%–55% A at 35–42 min; 55% A at 42–47 min. The injected sample volume was 10 μL and the flow rate was 1.0 mL/min.
2.2.2. TOF-MS conditions
The HPLC-TOF-MS system was equipped with AB Sciex Triple TOF™ 5600 mass spectrometer and Agilent 1260 HPLC (quaternary pump) system. The TOF mass spectrometer was operated with an electrospray ionization source in the following conditions: The ion spray voltage was set at 5500 V for positive mode; the curtain gas, nebulizer gas and heater gas using nitrogen were set at 35, 55 and 55 psi, respectively at a source temperature of 550 °C; collision energy, and declustering potential were at 10 V and 100 V. Automatic MS/MS experiments were carried out as follows: Collision energy, declustering potential were at (45 ± 15) V and 100 V. The TOF-MS analysis worked using full scan mode and mass range was set at m/z 100–1000. Analyst software (version 1.5.2, AB Sciex, USA) and PeakView® (version 2.2.0, AB Sciex, USA) were used to control the equipment and for data acquisition and analysis. Prior to analysis of samples, the mass axis was calibrated.
2.3. Preparation of standard solutions
Each standard stock solution was prepared by dissolving accurately weighed amount of compounds in acetonitrile. The concentrations of K-B, K-C, K-D, C-C and C-J are 50.0 μg/mL and K-E is 250.0 μg/mL. The five concentration levels of working standard solutions were diluted to a series of appropriate concentrations with acetonitrile. The concentration of each compound in working standard solution was 0.2, 0.4, 1.0, 2.0 and 5.0 μg/mL of K-B, K-C, K-D and C-C, and 1.0, 2.0, 5.0, 10.0 and 25.0 μg/mL of K-E.
2.4. Preparation of sample solution
Samples were prepared by weighing approximately 2.0 g of cream into a 25 mL beaker. 10 mL of acetonitrile was added and stirred to disperse the cream, which was dissolved in 80 °C water-bath, and the content was transferred completely to a 25 mL volumetric flask. The solution was allowed to cool to room temperature before being made up to volume with acetonitrile. Then a portion was refrigerated centrifuged at 12,000 rpm for 10 min (4 °C), and then the supernatant was filtered through a 0.20 µm Captiva Econofltr PTFE filter (Agilent, USA) into a suitable HPLC vial.
2.5. Method validation
The HPLC method developed for qualitative and quantitative analysis of related substances was validated according to International Conference on Harmonization guidelines [16], [17] to demonstrate suitability for the intended purpose. System suitability, selectivity, accuracy, precision, linearity, range, limit of determination (LOD), limit of quantitation (LOQ) and robustness were evaluated.
System suitability parameters were measured so as to verify the system, method, and column performance. The selectivity of the method was studied by comparing the test sample solution, placebo solution and the forced degradation solution to evaluate the resolution of the analyte from other interfering components. To establish the linearity and range, at least five concentration levels for each analyte were diluted. Linearity was described by regression line equation and its correlation coefficient. The LOD and LOQ were defined as the concentration of the analyte with a signal-to-noise ratio of 3:1 and 10:1, respectively. The precision was a measure of reproducibility of the analytical method and determined by preparation and measurement of further six samples. Recovery experiments were carried out by the standard spiked technique; three different concentration levels (80%, 100%, and 120%) of impurity reference standards were spiked to the pre-analyzed placebo samples in triplicate. Robustness was a measure of the method's capacity to remain unaffected by small variations in flow rate (1.0 ± 0.2 mL/min), column oven temperature (25 ± 5 °C), the pH of mobile phase (7.5 ± 0.2) and the concentration of ammonium acetate (0.01 ± 0.005 M).
3. Results and discussion
3.1. Optimization of sample preparation
Sample preparation is an essential and difficult part of method development. Because the recovery of different constituents may be affected, especially when dealing with complex matrices, such as biphasic emulsions, the extraction of the drugs may be incomplete with low recoveries and reproducibility due to inappropriate sample preparation [18]. Furthermore, the cream matrix is harmful to the performance of columns. In the exercise of sample preparation, the extraction of target compounds and removal of cream matrix are two key steps. The water-bath in 80 °C (the extraction recovery of each analyte was in the range of 92.5%–109.2%) showed higher extraction recovery of target compounds than sonication (the extraction recovery was in the range of 82.5%–93.8%) and n-hexane-acetonitrile 1:1 liquid-liquid extraction (the extraction recovery was in the range of 75.90%–92.4%). Refrigerated centrifugation under 4 °C for 10 min could remove the cream matrix effectively and conveniently and extend the service life of chromatographic columns significantly compared to water-ice bath for 2 h. We abandoned the liquid-liquid extraction pre-treatment method due to the low extraction recovery, although it can remove the cream matrix more effectively. In this case, a quick and easy extraction procedure was developed to obtain a satisfactory extraction recovery and obviously prolong the service life of chromatographic columns. Moreover, this sample preparation step guaranteed the precision and acceptable recovery of the analytical procedure.
3.2. HPLC condition for separation of all constituents in test solution
In order to obtain better detection, the HPLC condition was optimized. A series of aqueous mobile phases with different pH values in combination with different organic modifiers were tested. Acetonitrile was chosen as the organic portion of the mobile phase because of low background noise and strong elution effect for target compounds to shorten analysis time, when compared with methanol. Blank acetonitrile did not interfere with the determination of related substances. Moreover, the addition of ammonium acetate in the aqueous portion (pH 7.5) of the mobile phase contributed to better symmetry of chromatographic peaks, because all compounds were in the undissociated form and were compatible with LC-MS. Since the C8 column has less hydrophobic properties than C18 column, whose bonding reactions with the cream matrix (long chain alkane) were weaker than the octadecyl silane phase for column packing. Two different packing materials, Agilent poroshell HPH and Agilent Zorbax SB, of analytical C8 columns were tested. However, a complete separation of K and ketoconazole impurity B′ enantiomer was not achieved in the latter case. In the meantime, the application of short column packed with 2.7 µm porous particles on conventional HPLC provided an excellent performance as a special UPLC system. As for column temperature, the resolution between C and C-J peaks was decreased with the slightly higher temperatures, so the ideal column temperature was 25 °C. Ultimately, the end-capped Agilent poroshell HPH-C8 (4.6 mm × 100 mm, 2.7 µm) column was applied for all components with a good peak shape and high resolution.
3.3. Characterization and identification of related substances in test solution
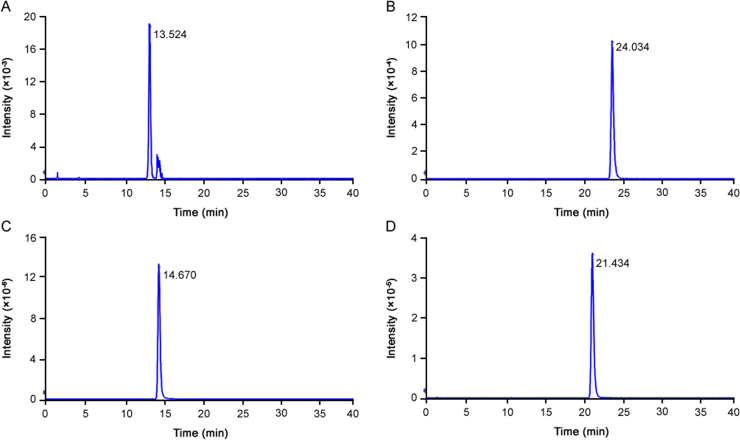
Compared to negative ion mode, positive ion mode showed higher sensitivity, and was therefore used for analysis. The accuracy for confirmation of elemental compositions was less than ±1 ppm mass error, showing a high mass accuracy. In total, four compounds (Table 1) were obtained and identified by comparing their MS fragmentation behaviors with reference standards and literature information. Peak 1, Peak 2, Peak 3 and Peak 4 were identified as K-B, K-E, ketoconazole and clobetasol propionate. The extracted ion chromatograms for the protonated molecules of these 4 components are shown in Fig. 1. The key fragmentation pathways along with the MS/MS spectrum are given in Figs. S1–S4, respectively.
Table 1.
Identification of chemical constituents of preparations by HPLC-TOF-MS.
| Peaka | tR(min) | Formula | [M+H]+m/z |
Fragment ions (RDB) | |||
|---|---|---|---|---|---|---|---|
| Theoretical (Da) | Measured (Da) | Error (ppm) | |||||
| 1 | 13.52 | C38H42Cl2N6O6 | 749.2619 | 748.2543 | 0.4 | 204.1250 [C12H16N2O]+ (5.5) | 255.0094 [C11H10Cl2N2O]+ (6.5) |
| 311.0349 [C14H16N2O2]+ (7.5) | 438.2249 [C24H30N4O4]+ (11.5) | ||||||
| 517.1399 [C25H26Cl2N4O4]+ (13.5) | 707.2520 [C36H39Cl2N6O5]+ (18.5) | ||||||
| 2 | 24.03 | C21H20Cl2N2O5S | 483.0545 | 482.0470 | 0.4 | 155.0157 [C7H10N2O2]+ (3.5) | 158.9765 [C6H6O3S]+ (5.5) |
| 255.0092 [C11H10Cl2N2O]+ (6.5) | 311.0356 [C14H14Cl2N2O2]+ (7.5) | ||||||
| 3 | 14.67 | C26H28Cl2N4O4 | 531.1562 | 530.1488 | 0.3 | 122.0750 [C6H11NO]+ (1.5) | 177.1007 [C10H14N2O]+ (4.5) |
| 219.1120 [C12H16N2O2]+ (5.5) | 255.0071 [C11H10Cl2N2O]+ (6.5) | ||||||
| 267.0078 [C12H13Cl2N2O]+ (6.5) | 421.1085 [C21H24Cl2N2O3]+ (9.5) | ||||||
| 489.1427 [C24H26Cl2N4O3]+ (12.5) | |||||||
| 4 | 21.43 | C25H32ClFO5 | 467.1997 | 466.1922 | 0.3 | 121.0643 [C8H10O]+ (3.5) | 147.0802 [C10H12O]+ (4.5) |
| 171.0798 [C11H13FO2]+ (4.5) | 221.0952 [C13H17FO2]+ (4.5) | ||||||
| 248.1190 [C12H19ClO3]+ (2.5) | 263.1424 [C16H21FO2]+ (5.5) | ||||||
| 278.1664 [C17H25FO2]+ (5.5) | 355.1460 [C22H31FO3]+ (7.5) | ||||||
The notion for analyte refers to Fig. 1. RDB is an abbreviation of ring double bond.
Fig. 1.
HPLC-TOF-MS extracted ion chromatograms in positive ion modes of (A) Peak 1, (B) Peak 2, (C) Peak 3, and (D) Peak 4.
The TOF-MS data showed that the molecular ions of Peak 1, Peak 2 and Peak 3 were 749.2619, 483.0545 and 531.1562, respectively. The same characteristic fragment ions at m/z 255 and 311 appeared in the MS/MS analysis, which were assigned to the ketal fragmentation originated from ketoconazole's skeleton. Due to the losses of neutral ketene (42 Da) fragment in MS/MS, fragment at m/z 707 was provided. Fragment at m/z 517 was derived via loss of 1-Acetyl-4-(4-hydroxyphenyl) piperazine (190 Da) from primary ions, a side chain of ketoconazole. Thus, Peak 1 was tentatively assigned as K-B. The fragment ion at m/z 158 was identified as phenylsulfonic acid. The fragment ion presented at m/z 155 was assigned to 1, 3-Dioxolane bonding to an imidazole group. Peak 2 was deduced preliminarily to K-E. Peak 3 was concluded as ketoconazole by the fragment ions at m/z 489, 420 and 255, which were in accordance with the literature data of ketoconazole [19], [20]. The molecular ion of Peak 4 was 467.1997. The fragments at m/z 278, 263, 248, 171, 147 were formed by typical fragmentation pattern of steroid structure and due to successive loss of CH3 (15 Da) fragment, which were compatible with the data of clobetasol propionate [10]. Thus, Peak 4 was characterized as clobetasol propionate.
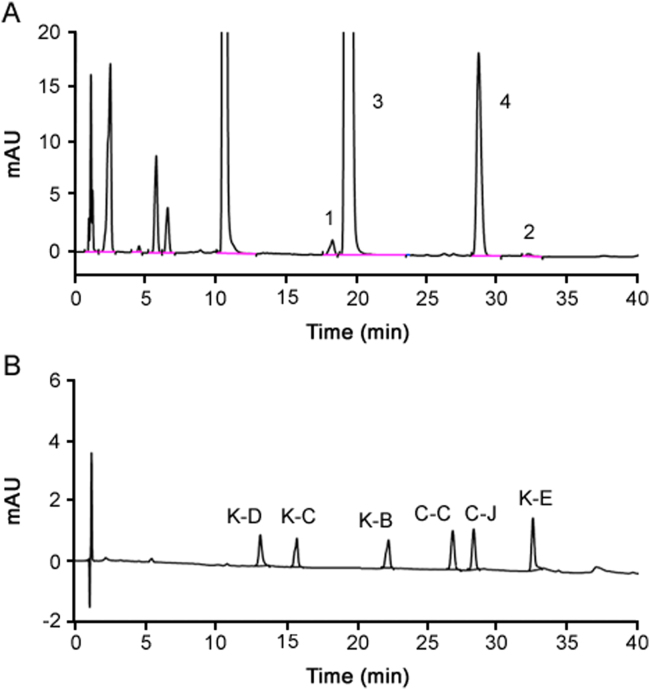
Compounds with the same fragmentation ions were just as attributed to the same structure rather than same molecule, because MS data were insufficient to identify isomers [21]. Then comparing the retention time and MS/MS fragments between the test sample and reference substances of impurities (K-B, K-C, K-D, K-E, C-C, C-J) under the same chromatographic conditions can verify the qualitative results. The results of the HPLC profiles are given in Fig. 2. Although Peak 1 was eluted earlier than the reference substance of K-B, indicating they are different compounds, the MS/MS result showed the same fragments ions for Peak 1 and K-B, which suggested these two impurities may be optical isomers. Peak 2 was identified as K-E.
Fig. 2.
The HPLC chromatogram of test sample of Compound Ketoconazole and Clobetasol Propionate Cream (A) and mixed reference substances (B). Peak assignment as follows: 1. Ketoconazole impurity B′ enantiomer; 2. Ketoconazole impurity E; 3. Ketoconazole; 4. Clobetasol propionate; K-B. Ketoconazole impurity B; K-C. Ketoconazole impurity C; K-D. Ketoconazole impurity D; K-E. Ketoconazole impurity E; C-C. Clobetasol propionate impurity C; and C-J. Clobetasol propionate impurity J. The unlabelled polar peaks in the same figure was assigned to excipients.
3.4. Revalidation of TOF-MS results
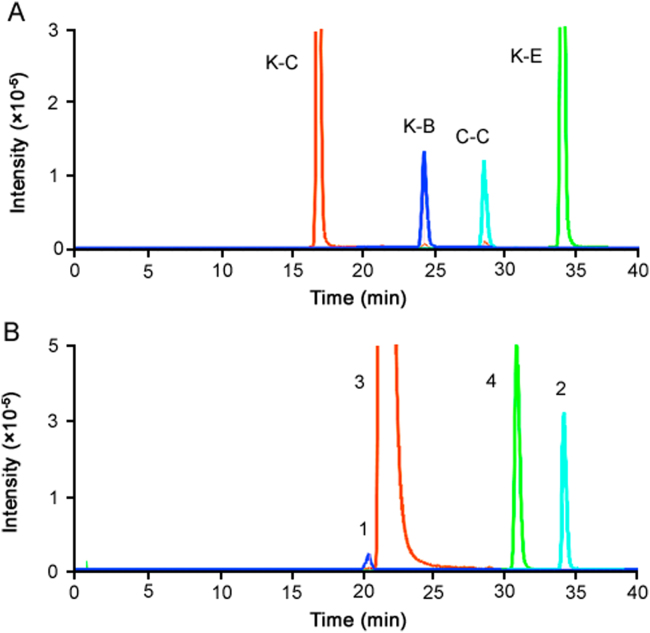
Since the structures of ketoconazole and clobetasol propionate contain multiple chiral centers, there may exist many optical isomers. Among them, ketoconazole and K-C, clobetasol propionate and C-C are optical isomers, respectively, according to the EP. Similarly, ketoconazole and K-C, clobetasol propionate and C-C were used to further illustrate the optical isomeric enantiomeric identity of K-B and Peak 1. It was difficult to distinguish the optical isomers only by their MS/MS spectra, but it was easier to identify them by comparing retention time with the reference standards. The HPLC-TOF-MS extracted ion chromatograms in positive ion modes of mixed reference substances and test sample of the Compound Ketoconazole and Clobetasol Propionate Cream are shown in Fig. 3. The analytical contents are summarized in Table 2. The results showed that ketoconazole and K-C, clobetasol propionate and C-C, K-B and Peak 1 were optical isomers with the same MS/MS fragments but different retention time. K-E and Peak 2 were the same substances with the same MS/MS fragments and same retention time. (The HPLC condition employed in all Tables and Figures was the same. The difference in retention time of the same compounds in Table 1, Table 2 and Fig. 1, Fig. 3 was due to the pipeline change of HPLC during the instrument maintenance. The retention time of the same compounds in Fig. 2 (HPLC method) and Fig. 3 (HPLC-TOF-MS method) was different because the former HPLC system was equipped with a quaternary pump and the latter equipped with a binary pump.)
Fig. 3.
HPLC-TOF-MS extracted ion chromatograms in positive ion modes of mixed reference substances (A) and test sample of Compound Ketoconazole and Clobetasol Propionate Cream (B). Peak assignment as follows: K-B. Ketoconazole impurity B; K-C. Ketoconazole impurity C; K-E. Ketoconazole impurity E; C-C. Clobetasol propionate impurity C; 1. Ketoconazole impurity B′ enantiomer; 2. Ketoconazole impurity E; 3. Ketoconazole; and 4. Clobetasol propionate.
Table 2.
The analytical contents of all constituents in mixed reference substances and test sample of the Compound Ketoconazole and Clobetasol Propionate Cream.
| Analytea | tR (min) | [M+H]+m/z |
|---|---|---|
| K-B | 24.84 | 749.2581 |
| K-C | 17.38 | 531.1533 |
| K-E | 34.57 | 483.0517 |
| C-C | 29.08 | 467.1971 |
| Peak 1 | 20.90 | 749.2586 |
| Peak 2 | 34.73 | 483.0521 |
| Peak 3 | 22.01 | 531.1532 |
| Peak 4 | 31.40 | 467.1971 |
The notion for analyte refers to Fig. 3. Peak assignment as follows: K-B. Ketoconazole impurity B; K-C. Ketoconazole impurity C; K-E. Ketoconazole impurity E; C-C. Clobetasol Propionate impurity C; 1. Ketoconazole impurity B′ enantiomer; 2. Ketoconazole impurity E; 3. Ketoconazole; and 4. Clobetasol propionate.
3.5. Impurities of quality control
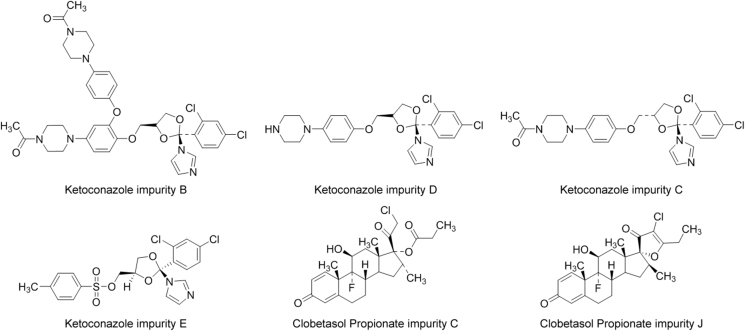
In addition to contribution from starting materials, related substances can also be formed by the potential reactions of drug-drug and drug-excipients in manufacturing processes and the degradation in drug formulations. In this work, two related substances were identified as the impurities from ketoconazole bulk by the HPLC-TOF-MS qualitative method in the finished drug products. The related substances existing in the Compound Ketoconazole and Clobetasol Propionate Cream were characterized as the degradation products of ketoconazole. The results were consistent with those of the previous HPLC study by the comparison of placebo preparation, ketoconazole placebo preparation and clobetasol propionate placebo preparation. Forced degradation study, accelerated test and long-term test are important to predict the drug's potential degradation products and to develop a stability indicating method [22]. Based on the previous HPLC results, four identified potential degradation products produced by the Compound Ketoconazole and Clobetasol Propionate Cream were K-C, K-D, C-C and C-J. Thus, we employed the above-mentioned related substances as quality control impurities of the Compound Ketoconazole and Clobetasol Propionate Cream. The structural formulas of these related substances are presented in Fig. 4.
Fig. 4.
Chemical structures of related substances for quality control of the preparation.
3.6. Validation of the HPLC method
In this study, system suitability, selectivity, accuracy, precision, linearity, range, LOQ, LOD and robustness were evaluated for the validation of the HPLC method. The system suitability test was determined by six replicate injections of the standard preparation. The RSDs of each compound's peak area and retention time were less than 1.0%, peak resolution was greater than 1.5, theoretical plate number was at least 3000 for each peak, and tailing factor was in the range of 0.9–1.1. The results indicated that the system was suitable for all peaks (Fig. S5). The outcome of selectivity demonstrated that all peaks were separated by baseline and there were no interferences. The method had the capacity to separate the degradation products of the APIs. The detailed methodology of validation for each method validation parameter is summarized in Table 3. The precision RSDs of each analyte was less than 5.0%. The mean percentage recoveries (n = 3) and % RSD were calculated. The accuracy of each analyte was 93.0%–111.0% (relative standard deviations (RSDs) = 2.1%–3.4%) at spiked 80%, 100% and 120%. There was no evidence to show that the results were affected by the studied parameters during robustness assays. Therefore, the recommended method could be considered reliable and robust.
Table 3.
The detailed methodology of validation for each method validation parameter.
| Analytes | Regression equationa | R2 | Linearity range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) | Precision (RSD%) | Mean recovery (%) | System suitability |
|
|---|---|---|---|---|---|---|---|---|---|
| R | n | ||||||||
| Ketoconazole | Y = 17.489X−0.0204 | 1.0000 | 0.20–5.00 | 0.06 | 0.20 | 0.3 | 97.0 | 9.1 | 29,921 |
| K-B | Y = 17.412X+0.2147 | 0.9999 | 0.19–4.80 | 0.06 | 0.20 | 0.7 | 99.1 | 5.2 | 29,486 |
| K-C | Y = 15.097X+0.1637 | 0.9999 | 0.20–4.89 | 0.06 | 0.20 | 0.7 | 100.8 | 6.7 | 21,647 |
| K-D | Y = 16.712X−0.0334 | 0.9998 | 0.20–5.00 | 0.06 | 0.20 | 3.9 | 93.8 | 49.6 | 20,341 |
| K-E | Y = 4.6743X+0.1424 | 0.9993 | 1.00–25.00 | 0.30 | 1.00 | 2.0 | 111.0 | 8.7 | 127,129 |
| Clobetasol propionate | Y = 20.381X+0.1007 | 0.9999 | 0.20–4.99 | 0.06 | 0.20 | 0.3 | 103.4 | 1.9 | 79,852 |
| C-C | Y = 20.698X+0.5393 | 0.9999 | 0.20–5.00 | 0.06 | 0.20 | 1.1 | 104.4 | 9.2 | 54,129 |
| C-J | Y = 20.509X+0.3345 | 0.9999 | 0.20–4.99 | 0.06 | 0.20 | 0.4 | 100.2 | 3.4 | 76,805 |
Y is the peak area and X is the concentration injected.
3.7. Calculation of RRF
The RRF is a popular analytical parameter frequently used in quantitative analysis. The HPLC method was subsequently applied to determine the RRFs of related substances. The RRFs were obtained by calculating the ratio of regression linear equation slope of APIs (ketoconazole and clobetasol propionate) and related substances with five concentration standard solutions, respectively [23]. Due to the absence of the reference standard of K-B optical isomer, we used K-B as a substitute. In order to observe the variations on RRFs, triplicate experiments were performed under the same analytical conditions, and the average value of RRF was calculated based on the results obtained in each experiment (Table 4). The average value of relative retention time is given in Table S1.
Table 4.
The relative response factors (RRFs) of related substances.
| Instrumentsa | K-B | K-C | K-D | K-E | C-C | C-J |
|---|---|---|---|---|---|---|
| Agilent 1260 (VWD) | 1.00 | 1.15 | 1.04 | 3.79 | 0.99 | 1.00 |
| Agilent 1100 (DAD) | 0.94 | 1.07 | 1.15 | 2.93 | 0.93 | 0.94 |
| Agilent 1100 (VWD) | 0.91 | 1.02 | 1.01 | 3.12 | 0.90 | 0.91 |
| RRFAVE | 0.95 | 1.08 | 1.07 | 3.28 | 0.94 | 0.95 |
Three different instruments were performed under the same analytical conditions. The employed HPLC method was developed on an Agilent poroshell HPH-C8 (4.6 mm × 100 mm, 2.7 µm) column with a gradient elution of acetonitrile (A) and ammonium acetate (pH 7.5; 0.01 M) in purified water (B). The HPLC conditions was as follows: 25%–30% A at 0–7 min; 30%–34% A at 7–8 min; 34%–40% A at 8–25 min; 40%–48% A at 25–35 min; 48%–55% A at 35–42 min; 55% A at 42–47 min.
3.8. Determination of impurities in 3 batches of samples
The shortage and high expense of reference standards have become the current bottleneck for quality control of related substances. In order to save reference standards in contrast with traditional external standard method (ESM), the developed quantitative method based on SSDMC was applied for the determination of related substances in the Compound Ketoconazole and Clobetasol Propionate Cream. The content of related substances obtained by SSDMC method was compared to the results obtained by ESM (Table 5). It was found that Prob > |t| values were greater than 0.05 in all cases indicating there are no remarkable differences between the methods of ESM and SSDMC.
Table 5.
Content of related substances obtained by SSDMC compared to ESM method.
| No. | K-B (%) |
K-C | K-D | K-E (%) |
C-C | C-J | ||
|---|---|---|---|---|---|---|---|---|
| SSDMCa | ESMb | SSDMCa | ESMb | |||||
| 20170410-1 | 0.13 | 0.14 | – | – | 0.07 | 0.07 | – | – |
| 0.14 | 0.15 | – | – | 0.06 | 0.07 | – | – | |
| 0.14 | 0.14 | – | – | 0.06 | 0.07 | – | – | |
| 20170410-2 | 0.14 | 0.14 | – | – | 0.05 | 0.06 | – | – |
| 0.12 | 0.13 | – | – | 0.07 | 0.08 | – | – | |
| 0.13 | 0.13 | – | – | 0.06 | 0.06 | – | – | |
| 20170410-3 | 0.15 | 0.15 | – | – | 0.05 | 0.06 | – | – |
| 0.15 | 0.15 | – | – | 0.05 | 0.05 | – | – | |
| 0.14 | 0.15 | – | – | 0.05 | 0.05 | – | – | |
“–”: not found.
SSDMC: the single standard to determine multi-components quantitative method.
ESM: the external standard method. These two different quantitative methods were performed under the same analytical conditions shown in Table 4.
4. Conclusions
In this paper, a systematic qualitative and quantitative method of related substances in the Compound Ketoconazole and Clobetasol Propionate Cream was successfully developed and validated. We defined K-B, K-C, K-D, K-E, C-C and C-J as the quality control impurities according to the results of HPLC-TOF-MS and HPLC experiments. Then we calculated the RRFs of these related substances for quantitative assessments. The content of related substances could be determined accurately and conveniently by the SSDMC method. We plan to transfer this developed method to other topical preparations with ketoconazole and clobetasol propionate. The present study provides new information and supporting data for the quality control and clinical application of the Compound Ketoconazole and Clobetasol Propionate Cream.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under the responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at https://doi:10.1016/j.jpha.2018.08.006.
Appendix A. Supplementary material
Supplementary material
References
- 1.Proksch E., Fölster-Holst R., Jensen J.M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006;43:159–169. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Heeres J., Backx L.J., Mostmans J.H. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J. Med. Chem. 1979;22:1003–1005. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 3.Nimura K., Niwano Y., Ishiduka S. Comparison of in vitro antifungal activities of topical antimycotics launched in 1990s in Japan. Int. J. Antimicrob. Ag. 2001;18:173–178. doi: 10.1016/s0924-8579(01)00365-x. [DOI] [PubMed] [Google Scholar]
- 4.Wiedersberg S., Leopold C.S., Guy R.H. Bioavailability and bioequivalence of topical glucocorticoids. Eur. J. Pharm. Biopharm. 2008;68:453–466. doi: 10.1016/j.ejpb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Ortonne J.P., Nikkels A.F., Reich K. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0.05% combined with ketoconazole shampoo 2%: a randomized, controlled study. Br. J. Dermatol. 2011;165:171–176. doi: 10.1111/j.1365-2133.2011.10269.x. [DOI] [PubMed] [Google Scholar]
- 6.Solank R. Impurity profiling of active pharmaceutical ingredients and finished drug products. Int. J. Drug. Res. Tech. 2012;2:7. [Google Scholar]
- 7.Wang K., Wu Y., Chi Z. A highly sensitive LC-MS/MS method for determination of ketoconazole in human plasma: application to a clinical study of the exposure to ketoconazole in patients after topical administration. J. Pharm. Biomed. Anal. 2016;128:504–509. doi: 10.1016/j.jpba.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Low A.S., Wangboonskul J. An HPLC assay for the determination of ketoconazole in common pharmaceutical preparations. Analyst. 1999;124:1589–1593. doi: 10.1039/a907400g. [DOI] [PubMed] [Google Scholar]
- 9.Gordien J.B., Pigneux A., Vigouroux S. Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 2009;50:932–938. doi: 10.1016/j.jpba.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Sparidans R.W., van Velsen S.G.A., de Roos M.P. Liquid chromatography-tandem mass spectrometric assay for clobetasol propionate in human serum from patients with atopic dermatitis. J. Chromatogr. B. 2010;878:2150–2154. doi: 10.1016/j.jchromb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak Z. Pivotal role of the aryl hydrocarbon receptor in modulations caused by benzo[a]pyrene and ketoconazole in the estrogenic responses induced by 17β-estradiol in male goldfish. Environ. Sci. Pollut. Res. Int. 2016;23:9247–9248. doi: 10.1007/s11356-016-6387-9. [DOI] [PubMed] [Google Scholar]
- 12.Grycová A., Dořičáková A., Dvořák Z. Impurities contained in antifungal drug ketoconazole are potent activators of human aryl hydrocarbon receptor. Toxicol. Lett. 2015;239:67–72. doi: 10.1016/j.toxlet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Narayanam M., Handa T., Sharma P. Critical practical aspects in the application of liquid chromatography–mass spectrometric studies for the characterization of impurities and degradation products. J. Pharm. Biomed. Anal. 2014;87:191–217. doi: 10.1016/j.jpba.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Liu S., Yao S., Zhang H. Determination of relative response factors of cefazolin Impurities by quantitative NMR. AAPS PharmSciTech. 2017;18:1895–1900. doi: 10.1208/s12249-016-0654-4. [DOI] [PubMed] [Google Scholar]
- 15.He Y., Li Q., Bi K.S. Simultaneous determination of six active components by a single standard to determine multicomponents combined with fingerprint analysis for the quality control of Rhizoma Chuanxiong. J. Sep. Sci. 2015;38:1090–1099. doi: 10.1002/jssc.201401280. [DOI] [PubMed] [Google Scholar]
- 16.ICH. Q2 (R1): Validation of Analytical Procedures: Text and Methodology, International Conference on Harmonization, Geneva, 2005.
- 17.ICH. Q1A (R2): Stability Testing of New Drug Substances and Products, International Conference on Harmonization, Geneva, 2000.
- 18.Galimany-Rovira F., Pérez-Lozano P., Suñé-Negre J.M. Development and validation of a new RP-HPLC method for the simultaneous determination of hydroquinone, kojic acid, octinoxate, avobenzone, BHA and BHT in skin-whitening cream. Anal. Methods. 2016;8:1170–1180. [Google Scholar]
- 19.Fitch W.L., Tran T., Young M. Revisiting the metabolism of ketoconazole using accurate mass. Drug. Metab. Lett. 2009;3:191–198. doi: 10.2174/187231209789352085. [DOI] [PubMed] [Google Scholar]
- 20.Mhaske R.A., Sahasrabudhe S. Identification of major degradation products of ketoconazole. Sci. Pharm. 2011;79:817–836. doi: 10.3797/scipharm.1107-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Li J., Wang C. Identification of impurities in macrolides by liquid chromatography–mass spectrometric detection and prediction of retention times of impurities by constructing quantitative structure–retention relationship (QSRR) J. Pharm. Biomed. Anal. 2017;145:262–272. doi: 10.1016/j.jpba.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 22.Blessy M., Patel R.D., Prajapati P.N. Development of forced degradation and stability indicating studies of drugs — a review. J. Pharm. Anal. 2014;4:159–165. doi: 10.1016/j.jpha.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou J.J., Wu W.Y., Da J. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J. Chromatogr. A. 2011;1218:5618–5627. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material