Abstract
Background
SEMA3B is known as an inhibitor of angiogenesis and cell proliferation. During carcinogenesis, the loss of SEMA3B function is observed, which results in the progression of neoplastic changes. The aim of this study was to evaluate the expression profile of SEMA3B in endometrial cancer (G1–G3) in comparison to the control group and to assess whether the observed changes in expression could become a molecular marker in endometrial cancer.
Material/Methods
The study group consisted of 45 patients diagnosed with endometrial cancer (G1, 17; G2, 15; G3, 13). The control group included 15 patients. SEMA3B expression was assessed using the immunohistochemical method. Statistical analysis was carried out using the Statistica 12 PL program (StatSoft, USA). It included the Kruskal-Wallis test and post hoc Dunn’s test (p<0.05).
Results
Statistically significant differences in the level of SEMA3B expression were observed between all analyzed groups. The expression pattern of SEMA3B was as follows: cancer cells G1>G2>G3; endothelial cells: G3>G1>G2; stromal cells: G2>G1>G3.
Conclusions
Analysis of the SEMA3B expression profile shows the complexity of neoplastic transformation, which confirms the different expression of SEMA3B in endometrial cancer cells and endothelial cells. The present results and data in the literature data suggest that SEMA3B expression indicates the progression of carcinogenesis in the context of endometrial cancer.
MeSH Keywords: Angiogenesis Inducing Agents, Apoptosis, Endometrial Neoplasms
Background
Semaphorins (SEMA) belong to a group of membrane or secretory proteins that influence proliferation, cancer cell migration, angiogenesis, and occurrence of drug resistance [1]. Among the semaphorin family, 8 subgroups can be distinguished, and the occurrence of 3–7 subgroups has been confirmed in vertebrates. The SEMA domain, common to all semaphorins, is key in signal transduction and is responsible for binding semaphorins to receptors [2,3]. Semaphorin 3B (SEMA3B) is a tumor growth suppressor. The biological activity of SEMA3B results from its interaction with neuropilin receptors (NRP1 and NRP2), which leads to reduced secretion of vascular endothelial growth factor and inhibition of formation of new blood vessel networks through which nutrients and oxygen are supplied to the tumor mass [4,5]. The abnormal expression pattern of SEMA3B has been reported in breast cancer [4], ovarian cancer [6], hepatocellular carcinoma [7], lung cancer [8], and endometrial cancer [9]. Recent studies indicate that SEMA3B plays an important role in osteoporosis, where its reduced level is observed. It is therefore suggested that SEMA3B is a promising new therapeutic target for osteoporosis caused by estrogen deficiency [10]. In stomach cancer, the relationship between SEMA3B level and prognosis, as well as the severity of clinical symptoms, has been reported [11].
Modern forms of diagnostics and therapy focus on molecular targets, enabling detection of abnormalities at an early stage, when the chances of successful therapy are greatest. It is also possible to quickly and early observe the loss of response to treatment before phenotypic changes occur. Thus, the chosen therapeutic strategy can be revised in a timely manner, which significantly increases the chances of inhibiting pathological processes [12–14]. Despite intensified studies of SEMA3B, based on the available literature, the relationship between SEMA3B expression and overall survival rate in patients with endometrial cancer has not yet been determined. Observations of Huang et al. on the expression of SEMA3B and overall survival rate (OSR) in patients with pancreatic cancer showed that the OSR was 19 months in patients with low levels of SEMA3B, while in patients with high SEMA3B expression, OSR was 41 months. The authors did not find a correlation between expression of SEMA3B and metastatic potential [15]. Tang et al. indicated that reduced SEMA3B expression in patients with esophageal cancer was associated with a higher OSR (41 months) compared to patients with normal levels of SEMA3B (19 months). Their results also suggest that as the degree of tumor cell differentiation decreases, the reduction in SEMA3B expression gradually increases [16].
The present study investigated the differences in SEMA3B expression depending on the degree of endometrial cancer differentiation (G1–G3) in comparison to controls. We also assessed the role of SEMA3B in endometrial cancer and angiogenesis as related to cancer processes.
Material and Methods
The study was approved by the Bioethics Committee of the Medical University of Silesia in Katowice (KNW/0022/KB/237/16).
The evaluation of SEMA3B expression at the protein level was carried out in the study group, consisting of 45 patients diagnosed with endometrial cancer (G1, 17; G2, 15; G3, 13), and in a control group of 15 patients without neoplastic changes who were sampled during routine gynecological examinations. Exclusion criteria for the study group were: non-endometrioid endometrial cancer, diagnosed endometriosis or adenomyosis, co-existing cervical cancer, BMI >40, and hormone replacement therapy within 24 months prior to surgery.
The analysis of SEMA3B level changes was performed based on the immunohistochemical reaction with rabbit polyclonal anti-SEMA3B antibody (Novus Biological). The Laboratory of Pathomorphology of Beskid Center of Oncology in Bielsko-Biała provided paraffin blocks from which slides were prepared. They were incubated in citrate buffer (pH 6, 30 min at 95°C) in a water bath to retrieve antigen, then treated with 3% (v/v) H2O2 in water for 10 min to block endogenous peroxidase activity. To block non-specific binding (30 min at room temperature), 1% BSA solution in PBS was used. The next step was incubation with anti-SEMA3B antibody in a humidified chamber (20 h at 4°C). The avidin-biotin complex (ABC) method was used according to the manufacturer’s instructions (Vectastain Elite ABC Kit, Vector Laboratories). Diaminobenzidine (DAB) was used to visualize the bound antibodies. The slides were stained with Gill’s hematoxylin, dehydrated, and coverslipped. Negative control was performed by replacing the primary antibody with rabbit IgG. An Eclipse E200 light microscope with DS-Fi1 digital camera (Nikon) was used to prepare photographic documentation (15 photos for each paraffin block, 200× magnification). To assess the optical density of a reaction product in fields where a positive reaction occurred, the NIS-AR (Nikon) program was used.
Statistical analysis was performed using the Statistica 12 PL program (StatSoft, USA). It was carried out at the statistical significance level p<0.05. The first step was to check the normality of the distribution of the obtained data with the Shapiro-Wilk test. On its basis, observing that the distribution of data does not meet the normal distribution assumptions (p>0.05), further statistical analysis was carried out using nonparametric methods. The results of changes in SEMA3B level in analyzed groups are presented as median (Me), lower quartile (Q1), and upper quartile (Q3). The Kruskal-Wallis test (nonparametric alternative to the analysis of variance) was performed to indicate statistically significant differences in SEMA3B expression between data in independent groups (C, G1–G3). To determine groups in which differences in expression were statistically significant, a post hoc Dunn’s test was performed.
Results
SEMA3B expression was observed in the membrane of uterine gland cells in samples from patients in the control group, but such expression was not observed in the endometrial cancer samples. On the other hand, within the tumor cells, SEMA3B expression was observed regardless of the degree of endometrial cancer differentiation (G1>G2>G3). We demonstrated that the optical density of the immunohistochemical reaction product of this protein in G1 cancer cells was similar to that in the membrane of uterine gland cells of the control group. Analyzing the level of SEMA3B in G2 endometrial cancer cells, we found that it was slightly lower than in grade 1 (about 85% of its level). In addition, we showed that the optical density of the reaction product of SEMA3B was the lowest in G3 samples, reaching 70% of that in the control group.
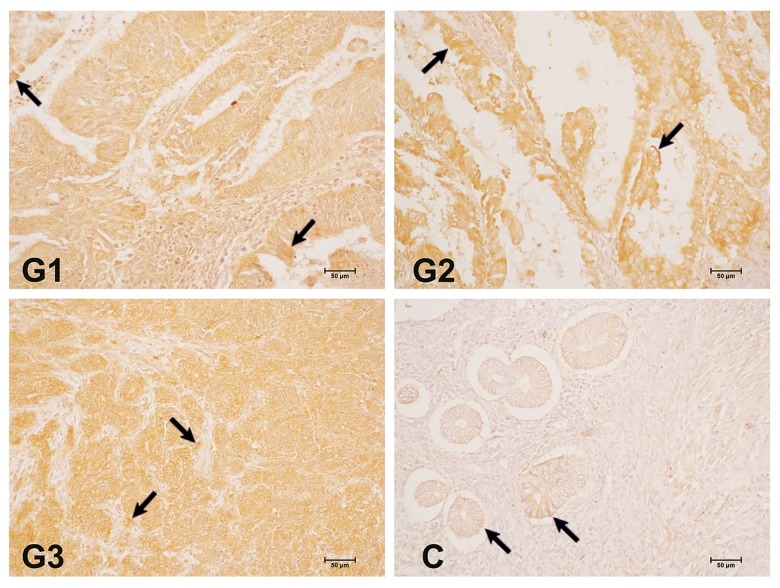
There were also changes in SEMA3B concentration in the vascular endothelium and stromal cells, both in control and G1–G3 endometrial cancer samples. In the case of the vascular endothelium, the dependence of SEMA3B expression was G3>G1>G2>C, and for the stromal cells the differences in protein level were G2>G1>G3>C. In grade 1, we observed that the optical density of the SEMA3B reaction product in endothelial cells was about 20% higher than in the control group. In grade 2, the expression of SEMA3B was similar to that observed in G1, whereas in G3 samples, the optical density was higher than in the other groups. The optical density of the SEMA3B reaction product in G1 stromal cells was 40% higher than in the control group and about 50% higher in G2 than in the control group. In contrast, in G3, the level of SEMA3B expression in stromal cells was at the level observed in G1 (Figure 1, Table 1). The arrows in Figure 1 show the places where the expression of SEMA3B was observed.
Figure 1.
Immunohistochemical localization of SEMA3B in different grades of endometrial cancer. C – control; G – grade of endometrial cancer. 200× magnification.
Table 1.
The optical density of the reaction product of SEMA3B in control and different grades of endometrial cancer.
| Localization | C | G1 | G2 | G3 | |
|---|---|---|---|---|---|
| The membrane of uterine gland cells | Me | 162.26 | |||
| Q1 | 151.77 | ||||
| Q3 | 172.22 | ||||
| Cancer cells | Me | 159.09 | 140.52b,d | 105.44c,e,f | |
| Q1 | 149.32 | 132.74 | 100.21 | ||
| Q3 | 170.37 | 149.67 | 111.78 | ||
| Vascular endothelium | Me | 117.89 | 141.79a | 140.54b | 154.77c,e,f |
| Q1 | 108.52 | 132.18 | 133.09 | 147.72 | |
| Q3 | 126.07 | 150.66 | 148.42 | 164.74 | |
| Stromal cells | Me | 131.33 | 179.38a | 198.82b,d | 177.67c,f |
| Q1 | 122.32 | 166.97 | 189.73 | 163.39 | |
| Q3 | 145.29 | 189.49 | 215.13 | 186.69 |
Symbols indicate statistically significant changes at p<0.05 between:
control and G1 endometrial cancer;
control and G2 endometrial cancer;
control and G3 endometrial cancer;
G1 and G2 endometrial cancer;
G1 and G3 endometrial cancer;
G2 and G3 endometrial cancer.
The exact values of SEMA3B expression (Me, Q1, Q3) are presented in Table 1 according to the structure in which its expression was observed, along with an indication of statistical significance of differences between the compared groups.
Discussion
The present study focused on the analysis of changes in the expression pattern of SEMA3B in 45 samples of endometrial cancer depending on the degree of its differentiation. The detection was based on immunohistochemical staining. In order to determine whether the observed changes in SEMA3B expression are characteristic of endometrial cancer, the level of this protein was also evaluated in the control group. This study is also a valuable addition to our previous observations. Analysis of the SEMA3B expression using microarrays showed the following direction and fold change in the transcriptional activity of this gene: G1 vs. C=+1.3; G2 vs. C=+1.50; G3 vs. C=+1.25. Regardless of the cancer grade, SEMA3B was overexpressed, but in the case of G3 endometrial cancer, the expression of the transcript begins to approach the level observed in grade 1 [17].
We found that SEMA3B was expressed in cancer cells, in stromal cells, and within the vascular endothelium. However, the expression profile of the analyzed protein in a given grade differed depending on the localization: G1>G2>G3 in tumor cells, G3>G1>G2 in vascular endothelium, and G2>G1>G3 in tumor stromal cells. In the stromal cells, the same direction of SEMA3B expression was observed as in the microarray analysis. Evaluation of the mRNA expression indicated that the lowest level of SEMA3B was in G3 endometrial cancer, as was also found for cancer cells and stromal cells. Combining analyses at the transcriptome and proteome levels allowed us to obtain an overall expression profile of the analyzed gene/protein.
Using SEMA3B in vitro, the proliferative capacity was reduced, while the process of programmed death of breast, lung, and ovarian cancer cells was induced [4,6,18]. Ferrira et al. analyzed changes in SEMA3B expression in samples of endometrial cancer in the proliferative and secretory phase of the menstrual cycle. They observed a relatively higher level of SEMA3B in biopsies obtained from patients in the proliferative phase of the cycle, although the differences were statistically insignificant [19]. In the present study, the lowest SEMA3B expression was noted in G3 endometrial cancer, which confirms its role as a tumor growth suppressor [1,4,20]. In grade 3, cancer cells lacking the regulatory role of SEMA3B undergo the most severe, uncontrolled proliferation. The observed level of expression may also result from the significant de-differentiation of endometrial cells in G3 [21], which may lead to the activation or inhibition of signaling pathways that show the opposite activity under physiological conditions. This indicates the complexity and pleiotropic nature of the processes associated with neoplastic transformation [22–24]. In relation to endometrial cancer, a close link is suggested between the activity of SEMA3B and VEGF165, which significantly reduced the pro-apoptotic and antimitotic effects of SEMA3B. SEMA3B acts as an inhibitor of VEGF165, an autocrine survival factor [4]. Nguyen et al. found a reduction in the level of SEMA3B and SEMA3F in endometrial cancer depending on its severity. With the increase in the aggressiveness of changes, the activity of these 2 semaphorins decreased [25]. These observations are consistent with ours. Osada et al. also demonstrated the association of the neoplastic process with SEMA3B expression. They observed a significantly lower expression of SEMA in stage IV carcinomas and concluded that the reduction of SEMA3B is an unfavorable prognostic marker [26]. This agrees with the study by Joseph et al., who indicated that the decrease in SEMA expression could be used as a molecular marker for the progression of neoplastic lesions. They also emphasize that gonadotropins and estrogens are involved in the control of angiogenesis and the metastatic potential of ovarian cancer with SEMA [27]. This is also important for our research because endometrial cancer is estrogen-dependent [28,29].
In the present study, the opposite situation was observed for SEMA3B concentration in the vascular endothelium than in tumor and stromal cells. The highest expression of SEMA3B was reported in G3 endometrial cancer. SEMA3B also plays a very important role in inhibiting angiogenesis [30]. Therefore, the changes in the level of SEMA3B in the vascular endothelium of tumor biopsies found in this work indicate that with the increase of endometrial cancer grade and cell de-differentiation, the processes of neovascularization are intensified [31–35]. Moreover, considering the pro-apoptotic role of the analyzed protein in the context of tumor angiogenesis, it is possible that there is a reduction in the percentage of normal vascular endothelial cells in endometrial cancer, with the simultaneous promotion of cell proliferation with accumulated mutations [4,5].
It should not be forgotten that SEMA3B affects the expression of interleukin 8 (IL-8), which helps in the influx of macrophages to the tumor microenvironment, promoting the metastatic activity of cancer. Elevated levels of IL-8 additionally suppress the anti-angiogenic properties of SEMA3B [36]. As a consequence, there is an uncontrollable, self-perpetuating process allowing further tumor growth and promoting its aggressiveness [37]. Therapy aimed at restoring the normal expression pattern of semaphorins, including SEMA3B, warrants further research [38].
Conclusions
The results of the present study, in comparison with the observations of other researchers, indicate that in, endometrial cancer, SEMA3B activity is deregulated compared to the physiological state. We found that with the increase of the endometrial cancer grade, the SEMA3B level within the tumor and stromal cells was reduced, whereas in the vascular endothelium it was elevated. This indicates a close link between metastasis and angiogenesis and emphasizes the complexity of processes and interactions occurring in carcinogenesis. Moreover, the SEMA3B expression assay is simple and imposes no additional burden on the patient. Our results and data reported in the literature suggest that SEMA3B expression indicates increased carcinogenesis in endometrial cancer. A better understanding of the various signaling mechanisms associated with the actions semaphorins is crucial for better understanding of carcinogenesis and endometrial cancer.
Footnotes
Source of support: The study was approved by the Bioethics Committee of the Medical University of Silesia in Katowice (KNW/0022/KB/237/16)
Conflict of interest
None.
References
- 1.Arbeille E, Reynaud F, Sanyas I, et al. Cerebrospinal fluid-derived Semaphorin 3B orients neuroepithelial cell divisions in the apicobasal axis. Nat Commun. 2015;6:6366. doi: 10.1038/ncomms7366. [DOI] [PubMed] [Google Scholar]
- 2.Ito D, Nojima S, Kumanogoh A. [The role of semaphorin family in immune systems]. Nihon Rinsho Meneki Gakkai Kaishi. 2014;37:1–10. doi: 10.2177/jsci.37.1. [In Japasene] [DOI] [PubMed] [Google Scholar]
- 3.Sabag AD, Smolkin T, Mumblat Y, et al. The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction. J Cell Sci. 2014;127:5240–52. doi: 10.1242/jcs.155960. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Rivera E, Ran S, Thorpe P, et al. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA. 2004;101:11432–37. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca FP, Bingle L, Santos-Silva AR, et al. Semaphorins and neuropilins expression in salivary gland tumors. J Oral Pathol Med. 2016;45:119–26. doi: 10.1111/jop.12341. [DOI] [PubMed] [Google Scholar]
- 6.Tse C, Xiang RH, Bracht T, et al. Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res. 2002;62:542–46. [PubMed] [Google Scholar]
- 7.Tischoff I, Markwarth A, Witzigmann H, et al. Allele loss and epigenetic inactivation of 3p21.3 in malignant liver tumors. Int J Cancer. 2005;115:684–89. doi: 10.1002/ijc.20944. [DOI] [PubMed] [Google Scholar]
- 8.Tomizawa Y, Sekido Y, Kondo M, et al. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci USA. 2001;98:13954–59. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attar R, Gasparri ML, Halim TA, et al. Legacy of vitamin D: Role of vitamin D in prevention of gynecological cancers. In: Farooqi A, Ismail M, editors. Molecular oncology: Underlying mechanisms and translational advancements. Springer; Cham: 2017. [Google Scholar]
- 10.Sang C, Zhang Y, Chen F, et al. Tumor necrosis factor alpha suppresses osteogenic differentiation of MSCs by inhibiting semaphorin 3B via Wnt/β-catenin signaling in estrogen-deficiency induced osteoporosis. Bone. 2016;84:78–87. doi: 10.1016/j.bone.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Zhuge X, Huang Z, et al. Analysis of SEMA3B methylation and expression patterns in gastric cancer tissue and cell lines. Oncol Rep. 2014;31:1211–18. doi: 10.3892/or.2014.2972. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal SN. The basic molecular biology of angiogenesis and its implication in anticancer therapeutics. Arch Int Surg. 2015;5:121–30. [Google Scholar]
- 13.Biankin VA, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526:361–70. doi: 10.1038/nature15819. [DOI] [PubMed] [Google Scholar]
- 14.Wcisło-Dziadecka D, Gola J, Grabarek B, et al. Effect of adalimumab on the expression of genes encoding TNF-α signal paths in skin fibroblasts in vitro. Postepy Dermatol Alergol. 2018;35:413–22. doi: 10.5114/ada.2018.77673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Cheng YY, Liao WC, et al. SOX4 transcriptionally regulates multiple SEMA3/plexin family members and promotes tumor growth in pancreatic cancer. PLoS One. 2002;7(12):e48637. doi: 10.1371/journal.pone.0048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang H, Wu Y, Liu M, et al. SEMA3B improves the survival of patients with esophageal squamous cell carcinoma by upregulating p53 and p21. Oncol Rep. 2016;36(2):900–8. doi: 10.3892/or.2016.4901. [DOI] [PubMed] [Google Scholar]
- 17.Opławski M, Michalski M, Witek A, et al. Identification of a gene expression profile associated with the regulation of angiogenesis in endometrial cancer. Mol Med Rep. 2017;16:2547–55. doi: 10.3892/mmr.2017.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahi P, Wang CY, Chou J, et al. GATA3 targets semaphorin 3B in mammary epithelial cells to suppress breast cancer progression and metastasis. Oncogene. 2017;36:5567–75. doi: 10.1038/onc.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira GD, Capp E, Jauckus J, et al. Expression of semaphorin class 3 is higher in the proliferative phase on the human endometrium. Arch Gynecol Obstet. 2018;297:1175–79. doi: 10.1007/s00404-018-4719-3. [DOI] [PubMed] [Google Scholar]
- 20.Bielenberg DR, Pettaway CA, Takashima S, et al. Neuropilins in neoplasms: Expression, regulation, and function. Exp Cell Res. 2006;312:584–93. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Bednarczyk M, Zmarzły N, Grabarek B, et al. Genes involved in the regulation of different types of autophagy and their participation in cancer pathogenesis. Oncotarget. 2018;9:34413–28. doi: 10.18632/oncotarget.26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorente J, Velandia C, Leal JA, et al. The interplay between autophagy and tumorigenesis: Exploiting autophagy as a means of anticancer therapy. Biol Rev Camb Philos Soc. 2018;93:152–65. doi: 10.1111/brv.12337. [DOI] [PubMed] [Google Scholar]
- 24.Mohlin S, Wigerup C, Jögi A, et al. Hypoxia, pseudohypoxia and cellular differentiation. Exp Cell Res. 2017;356:192–96. doi: 10.1016/j.yexcr.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen H, Ivanova VS, Kavandi L, et al. Progesterone and 1,25-dihydroxyvitamin D3 inhibit endometrial cancer cell growth by upregulating semaphorin 3B and semaphorin 3F. Mol Cancer Res. 2011;9:1479–92. doi: 10.1158/1541-7786.MCR-11-0213. [DOI] [PubMed] [Google Scholar]
- 26.Osada R, Horiuchi A, Kikuchi N, et al. Expression of semaphorins, vascular endothelial growth factor, and their common receptor neuropilins and allelic loss of semaphorin locus in epithelial ovarian neoplasms: Increased ratio of vascular endothelial growth factor to semaphorin is a poor prognostic factor in ovarian carcinomas. Hum Pathol. 2006;37:1414–25. doi: 10.1016/j.humpath.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Joseph D, Ho SM, Syed V. Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Mol Cancer Ther. 2010;9:499–509. doi: 10.1158/1535-7163.MCT-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahrenkamp JM, Yang CH, Rodriguez AC, et al. Clinical and genomic crosstalk between glucocorticoid receptor and estrogen receptor α in endometrial cancer. Cell Rep. 2018;22:2995–3005. doi: 10.1016/j.celrep.2018.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swasti Estrogen and progesterone receptors in endometrial cancer: Where are we today? Gynecol Obstet. 2018;8:e127. [Google Scholar]
- 30.Varshavsky A, Kessler O, Abramovitch S, et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008;68:6922–31. doi: 10.1158/0008-5472.CAN-07-5408. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto J, Hori M, Ichigo S, Tamaya T. Expression of basic fibroblast growth factor and its mRNA in uterine endometrial cancers. Invasion Metastasis. 1995;15(5–6):203–10. [PubMed] [Google Scholar]
- 32.Presta M. Sex hormones modulate the synthesis of basic fibroblast growth factor in human endometrial adenocarcinoma cells: Implications for the neovascularization of normal and neoplastic endometrium. J Cell Physiol. 1998;137(3):593–97. doi: 10.1002/jcp.1041370329. [DOI] [PubMed] [Google Scholar]
- 33.Chopra V, Ding TV, Hanningan EV. Serum levels of interleukins, growth factors and anglogenin in patients with endometrial cancer. J Cancer Res Clin Oncol. 1997;123(3):167–72. doi: 10.1007/BF01214669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribatti D, Finato N, Crivellato E, et al. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynaecol. 2005;193(6):1961–65. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 35.Chiu HC, Li CJ, Yiang GT, et al. Epithelial to mesenchymal transition and cell biology of molecular regulation in endometrial carcinogenesis. J Clin Med Res. 2019;8(4) doi: 10.3390/jcm8040439. pii: E439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolny C, Capparuccia L, Casazza A, et al. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med. 2008;205:1155–71. doi: 10.1084/jem.20072509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neufeld G, Mumblat Y, Smolkin T, et al. The semaphorins and their receptors as modulators of tumor progression. Drug Resist Updat. 2016;29:1–12. doi: 10.1016/j.drup.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Mishra R, Kumar D, Tomar D, et al. The potential of class 3 semaphorins as both targets and therapeutics in cancer. Expert Opin Ther Targets. 2015;19:427–42. doi: 10.1517/14728222.2014.986095. [DOI] [PubMed] [Google Scholar]