Abstract
Background
The apoptosis of corneal epithelial cells participates in the pathological processes of dry eye, which is expected to be a treatment target for dry eye. The aim of this study was to investigate the effects of vitamin A (VA) on apoptosis of corneal epithelial cells in a mouse model with dry eye induced by benzalkonium chloride (BAC).
Material/Methods
We randomly divided 60 male BALB/c mice aged 8–10 weeks into 3 groups: the blank control group, the dry eye+vehicle group, and the dry eye+drug group. On the 7th day after the dry eye model successfully induced, the mouse eyeballs removed, and the mouse corneal tissues were isolated. The expression levels of Bax and Bcl-2 in corneal tissues were detected via reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting. The apoptotic corneal epithelial cells were quantified using terminal deoxynucleotidyl transferase (TdT) deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining technique.
Results
VA suppressed the upregulation of the Bax gene at the mRNA and protein levels, and upregulated the expression of the Bcl-2 gene (P<0.05). TUNEL results revealed that the number of apoptotic epithelial cells in the dry eye group was 40 times larger as that in the blank control group. After the intervention of VA at an appropriate concentration, the number of apoptotic corneal epithelial cells was remarkably reduced to about 10 times that in the blank control group (P<0.05).
Conclusions
VA can inhibit upregulation of the expressions of Bax and Bcl-2 in the epithelial cells of mice with dry eye induced by BAC, so as to suppress the apoptosis of epithelial cells in mice with dry eye.
MeSH Keywords: Apoptosis, Epithelial Cells, Vitamin A
Background
Dry eye is also known as tear insufficiency syndrome, whose primary clinical manifestations include abnormalities in the tear quality and/or tear fluid dynamics. It can cause tear film instability and ocular surface damage, and ultimately triggers a train of eye discomfort symptoms [1]. Dry eye, one of the most common ocular surface diseases in China and the world at present, has a complex and unclear pathogenesis. Currently, the symptoms of eye discomfort caused by dry eye clinically are alleviated mainly by locally dropping artificial tears [2]. Although artificial tears can improve tear film stability, their effects are not ideal for moderate to severe dry eye [3]. Additionally, the frequent application of artificial tears is inconvenient for patients [4]. Patients with dry eye are often accompanied with a certain degree of apoptosis of corneal epithelial cells, infiltration of inflammatory cells, and even the tendency of epithelial cells transforming into small cells [5,6]. These pathological processes will ultimately lead to the apoptosis of corneal epithelial cells [7]. The inhibition of the apoptosis of corneal epithelial cells, therefore, is expected to be a treatment target for dry eye.
As one of the most important essential nutrients in mammals, vitamin A (VA) has a complex metabolic process, which involves a variety of derivatives. Cell experiments showed that when corneal epithelial cells were cultured, the addition of VA at a lower concentration than normal lead to pathological changes such as the hyperkeratinization of epithelial cells, increased cell layers, thickened epithelia, and structural disorders [8,9]. The above results indicate that VA exerts a crucial effect in the growth and differentiation of the keratoconjunctival epithelium. However, the effect of VA on dry eye is not clear yet, thus we hypothesize that VA may have a beneficial effect on dry eye. The present study therefore assessed the effects of VA on the expressions of B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) and Bcl-2 in epithelial cells of mice with dry eye triggered by benzalkonium chloride (BAC), thus providing a theoretical reference for the mechanism and treatment of dry eye. The BAC-induced dry eye model is a drug toxicity-induced dry eye model with ocular surface cell damage and inflammation. It is suitable for studying the pathological mechanism of dry eye and can be used to evaluate the therapeutic or protective effects of some new therapies or drugs on dry eye [10].
Material and Methods
Animal grouping and model establishment
A total of 60 (the sample size was calculated based on pre-experimental results, which was not shown) male BALB/c mice aged 8–10 weeks, weighing (25.13±2.31) g were randomly divided by random number table method into 3 groups: the blank control group (control group, n=20), the dry eye+vehicle group (BAC+vehicle group treated with blank microemulsion, n=20), and the dry eye+drug group (BAC+VA group, n=20). No statistically significant differences in basic data such as age and body weight of the 3 groups of mice were found, so they were comparable among groups (P>0.05). A model of dry eye was constructed by locally dropping BAC solution (0.25%, 5 μL) into eyes for 7 days (once in the morning and once in the evening) according to the reference [10], and the concentration and dose were adjusted according to the results of a pre-experiment. After the dry eye model was successfully induced, BAC was stopped and 5000 IU/g·ml (5 ul) VA microemulsion or vehicle was dropped into the eyes of mice in the BAC+VA and BAC+vehicle group. The VA microemulsion was prepared by Dain Biopharmaceutical (Shandong, China) every day before use and kept in the dark. On the 7th day after treatment, the mouse eyeballs were taken out and the mouse corneal tissues were isolated. All animal experiments were approved by the Animal Ethics Committee (No. 20170211).
Clinical parameters
Mice were clinically evaluated on the 7th day after dry eye induction. For measurement of tear volume, the test paper was reversed and hung over the skin. We closed the eyes and measured the length of the wetted filter paper after 1 min. For break-up time, the conjunctival sac was dripped with 1% liquid fluorescein. The mice were blinked manually to make the fluorescein evenly distributed on the surface of the eyes. After 3 blinks, the time from the last blink to the first black spot on the cornea was defined as the BUT. Each right eye was tested 3 times, and the final result was the average of 3 measurements.
Detection of expressions of apoptosis-associated genes Bax and Bcl-2 via reverse transcription-polymerase chain reaction (RT-PCR)
1) TRIzol method (Invitrogen, Carlsbad, CA, USA) was used to extract the total ribonucleic acid (RNA) in the cornea, and then an ultraviolet spectrophotometer was used to detect the concentration and purity of the extracted RNA. When the ratio of the absorbance at 260 nm to that at 280 (A260/A280) was 1.8–2.0, the RNA could be used. 2) The messenger RNA (mRNA) was synthesized into complementary deoxyribonucleic acid (cDNA) by reverse transcription and stored in a freezer at −80°C. 3) For the RT-PCR system, we used 2.5 μL 10×buffer, 2 μL cDNA, 0.25 μL forward primers (20 μmol/L), 0.25 μL reverse primers (20 μmol/L), 0.5 μL deoxy-ribonucleotide triphosphates (dNTPs) (10 mmol/L), 0.5 μL Taq enzyme (2×106 U/L), and 19 μL distillation-distillation H2O (ddH2O). The amplification systems for RT-PCR were identical (Table 1).
Table 1.
Primer sequences of various indicators in RT-PCR.
| Target gene | Primer sequence | |
|---|---|---|
| GAPDH | Forward | 5′-GACATGCCGCCTGGAGAAAC-3′ |
| Reverse | 5′-AGCCCAGGATGCCCTTTAGT-3′ | |
| Bax | Forward | 5′-TGCTGCCTTTTCTGTTCCTT-3′ |
| Reverse | 5′-AAGGTGCTGGGTAGGGAAGT-3′ | |
| Bcl-2 | Forward | 5′-GTCCACGAACCCGTAAGGT-3′ |
| Reverse | 5′-CATCTTTTCCCGATAGGTCCA-3′ |
Detection via Western blotting
The whole corneal tissues of each group of mice were thoroughly ground in lysis buffer followed by ultrasonic lysis. After the lysate was centrifuged, the supernatant was absorbed and dispensed into an Eppendorf tube, and the protein concentration was measured by bicinchoninic acid assay and ultraviolet spectrometry. The proteins of all samples were set to constant volumes. After dispensing, the proteins were stored in a freezer at −80°C. We loaded 10 μL total protein for sodium dodecyl sulphate-polyacrylamide gel electrophoresis, after which the protein in the gel was transferred onto a polyvinylidene fluoride membrane, and the primary antibodies, including BAX (1: 500, ab216494, Abcam, Boston, MA, USA), Bcl-2 (1: 500, ab182858, Abcam, Boston, MA, USA), and GAPDH (1: 1000, ab181602, Abcam, Boston, MA, USA), were added for incubation at 4°C overnight. After that, the goat anti-rabbit HRP conjugated antibody (1: 1000, ab181662, Abcam, Boston, MA, USA) was used for incubation for 1 h away from light. The protein bands were scanned and quantified using the Odyssey membrane scanner, and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was applied to correct the level of the protein to be tested.
Immunohistochemical staining
The epithelial tissue was embedded with paraffin and cut into 5-um sections. The sections were baked in an oven at 60°C for 30 min, followed by dewaxing (5 min for 3 times) with xylene, and dehydration with 100% ethanol, 95% ethanol, and 70% ethanol for 3 times. The activity of endogenous peroxidases was inhibited with hydrogen peroxide (3% in methanol), and then the tissues were blocked using sheep serum for 1 h. Antibodies against Bax (ab216494, Abcam, Boston, MA, USA) and Bcl-2 (ab182858, Abcam, Boston, MA, USA) were diluted at 1: 200 [phosphate-buffered saline (PBS)], incubated at 4°C overnight, and then washed with PBS 4 times on a shaker. After the addition of the secondary antibody, color development was carried out with diaminobenzidine (DAB). After the color development was completed, 6 samples were randomly selected from each group, and 5 fields of view were randomly selected from each sample. Finally, images were taken under an optical microscope (400×).
Terminal deoxynucleotidyl transferase (TdT) deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining
The cut epithelial tissue sections were baked in an oven at 60°C for 30 min, followed by dewaxing (5 min for 3 times) with xylene, and dehydration with 100% ethanol, 95% ethanol, and 70% ethanol 3 times. TUNEL staining was performed according to the manufacturer’s instructions (Roche, Basel, Switzerland). Briefly, the sections were incubated with protein kinase K for 0.5 h, and after washing with PBS, TdT and luciferase-labeled dUTP were added for 1 h of reaction at 37°C. Then, a specific antibody labeled with horseradish peroxidase was added for incubation again for 1 h (37°C) in an incubator. After that, DAB was used as a substrate for reaction at room temperature for 10 min. After the nucleus was stained with hematoxylin, photos were taken under an optical microscope, and counting was conducted.
Statistical analysis
All data were parametric and analyzed using Statistical Product and Service Solutions (SPSS) 22.0 (IBM, Armonk, NY, USA). Measurement data are expressed as mean ± standard deviation, and comparison between groups was done using one-way ANOVA test followed by the post hoc test (least significant difference). P<0.05 represented that the difference was statistically significant.
Results
Vitamin A can effectively inhibit the reduction of tear secretion and enhance tear film stability
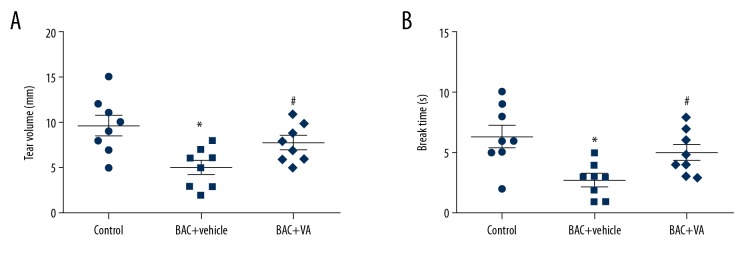
Measurement of tear volume showed that, compared with those in the control group, the tear volume and break-up time were significantly decreased in the BAC+vehicle group. After 5000 IU/g·mD VA microemulsion was locally dropped into eyes of mice with dry eye for 7 consecutive days, the tear volume and break-up time were significantly increased (Figure 1).
Figure 1.
(A, B) Vitamin A can effectively inhibit the reduction of tear secretion and enhance tear film stability. Control: blank control group. BAC+vehicle: dry eye group+vehicle group. BAC+VA: drug intervention group. * P<0.05 vs. the control group, and # P<0.05 vs. the BAC group. (n=8).
MRNA expression levels of the apoptosis-associated genes in the 3 groups of corneal epithelial cells
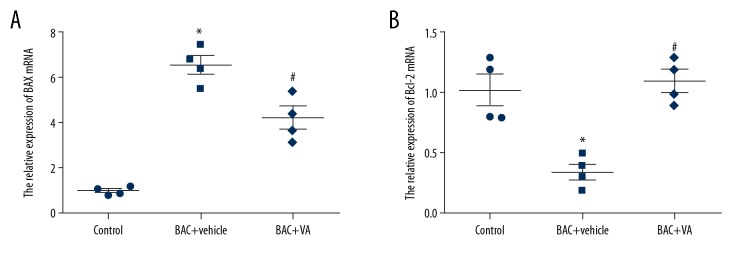
RT-PCR results revealed that, compared with those in the control group, the mRNA expression level of the pro-apoptotic gene Bax was remarkably downregulated, while that of the anti-apoptotic gene Bcl-2 was significantly upregulated in the BAC+vehicle group (P<0.05). After 5000 IU/g·mD VA microemulsion was locally dropped into eyes of mice with dry eye for 7 consecutive days, the transcriptional level of the pro-apoptotic gene Bax in mouse corneal epithelial cells was markedly reduced, while that of the anti-apoptotic gene Bcl-2 was evidently increased (P<0.05) (Figure 2). The above results indicate that VA intervention can obviously regulate the transcription of the apoptosis-associated genes in mouse corneal epithelial cells.
Figure 2.
(A, B) MRNA expression levels of apoptosis-associated genes Bax and Bcl-2 in corneal epithelial cells in different groups. Control: blank control group. BAC+vehicle: dry eye group+vehicle group. BAC+VA: drug intervention group. * P<0.05 vs. the control group, and # P<0.05 vs. the BAC group. (n=4).
Protein expression levels of the apoptosis-associated genes in the 3 groups of corneal epithelial cells
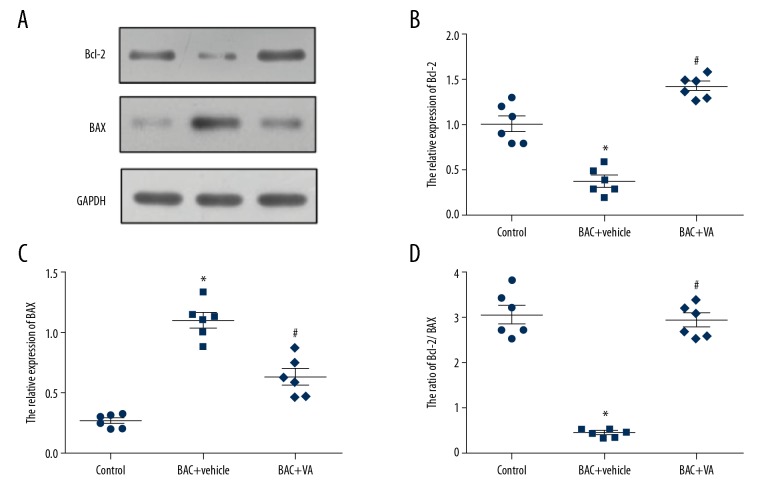
The results of Western blotting showed that, compared with those in the control group, the protein expression level of Bcl-2 was markedly inhibited and that of Bax was significantly activated in the BAC+vehicle group (P<0.05). However, after 5000 IU/g·mD VA microemulsion was used treat mice with dry eye for 7 consecutive days, the protein expression of Bax in mouse corneal epithelial cells was significantly inhibited by VA. In contrast, VA activated the protein expression of Bcl-2 (P<0.05) (Figure 3). The above results are consistent with the mRNA detection results, verifying that VA regulates the expressions of Bax and Bcl-2 at the transcriptional and translational levels.
Figure 3.
(A–D) Protein expression levels of Bcl-2 and Bax in 3 groups of corneal epithelial cells. Control: blank control group. BAC+vehicle: dry eye group+vehicle group. BAC+VA: drug intervention group. * P<0.05 vs. the control group, and # P<0.05 vs. the BAC group. (n=4).
Immunohistochemical staining results of the apoptosis-associated proteins in the 3 groups of corneal epithelial cells
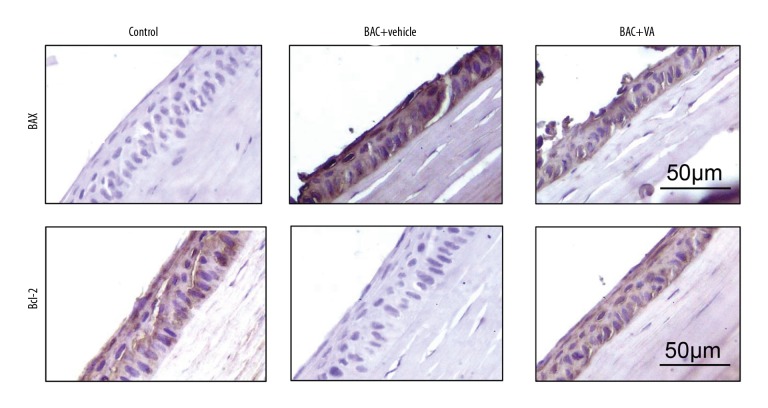
According to the immunohistochemical staining results and the positive cell quantification results (Figure 4, Table 2), the protein expression level of the pro-apoptotic gene Bax and the positive cells expressing Bax were significantly increased in the epithelial cells of the BAC+vehicle group. However, the protein expression level of Bcl-2 and the positive cells expressing Bcl-2 were evidently decreased (P<0.05). After the VA intervention, the positive results in the tissue were significantly reversed, which once again confirmed that VA regulated the apoptosis-related genes in epithelial cells of mice with dry eye.
Figure 4.
Immunohistochemical staining for the apoptosis-associated proteins Bax and Bcl-2 in the 3 groups of corneal epithelial cells. Control: blank control group. BAC+vehicle: dry eye group+vehicle group. BAC+VA: drug intervention group. Brown represents positive results. (n=6).
Table 2.
Comparisons of the numbers of positive cells expressing the apoptosis-associated genes Bax and Bcl-2 in the 3 groups of corneal epithelial cells (χ̄±s).
| Group | n | Bax | Bcl-2 |
|---|---|---|---|
| Control | 6 | 5.23±2.34 | 56.13±15.41 |
| BAC | 6 | 154.43±21.11 | 4.43±2.06 |
| BAC+VA | 6 | 45.34±12.36 | 44.57±13.12 |
| t | 11.234 | 12.311 | |
| p | 0.001 | 0.000 |
Control – blank control group; BAC – xerophthalmia group; BAC+VA – drug intervention group.
Comparisons of TUNEL results
To further investigate the effect of VA on the apoptosis of corneal epithelial cells in mice with dry eye, TUNEL staining was carried out for the obtained epithelial tissues to quantify the apoptotic epithelial cells. The results showed that there were 40 times more apoptotic epithelial cells in the BAC+vehicle group than in the control group. After treatment with VA at an appropriate concentration, the number of apoptotic corneal epithelial cells was remarkably reduced by 10-fold that in the control group (P<0.05) (Figure 5). The above results suggest that the VA inhibits the BAC-induced apoptosis of corneal tissue epithelial cells in mice with dry eye by regulating the expressions of the apoptosis-associated genes Bax and Bcl-2.
Figure 5.
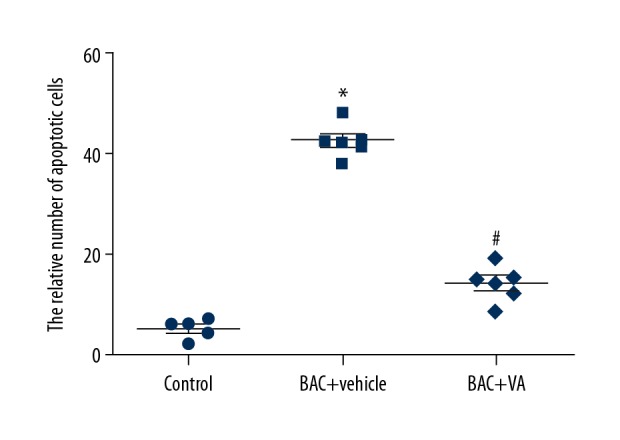
Comparisons of TUNEL results of the 3 groups of corneal epithelial cells. Control: blank control group. BAC+vehicle: dry eye group+vehicle group. BAC+VA: drug intervention group. * P<0.05 vs. the control group, and # P<0.05 vs. the BAC group. (n=6).
Discussion
Dry eye is a common ophthalmic disease that is easily overlooked clinically [11]. With the increased use of electronic screens, the incidence rate of dry eye is increasing steadily, as high as 16.9% in Europe and the USA and more than 20% in Asian populations [12]. Patients with dry eye are often accompanied with such symptoms of eye discomfort as long-term dry eye, pain, photophobia, blurred vision, and even visual impairment, which seriously influence quality of life [13]. Currently, the pathogenesis and treatment strategies of dry eye are still unclear, and certain controversies exist, so there is a lack of effective prevention and control of dry eye in clinical practice. In the present study, BAC was used to induce dry eye, which was treated by VA. We also assessed the expressions of apoptosis-associated genes in corneal epithelial cells and the apoptosis of these cells, so as to further assess the mechanism of action and therapeutic significance of VA in apoptosis of epithelial cells in mice with dry eye.
The corneas of patients with dry eye usually have a certain degree of apoptosis of corneal epithelial cells, ultimately leading to the apoptosis of these cells [5]. According to studies [14,15], in the normal proliferation, differentiation, and development processes of human corneal epithelial cells, it is necessary for VA to be involved. VA can clearly improve the ocular surface epithelial damage resulting from dry eye, eye trauma, and other diseases. The mechanism may involve prevention of squamatization of the keratoconjunctival epithelium by VA, leading to the dekeratinization of keratoconjunctival epithelium, which promotes differentiation of non-secretory epithelium into secretory epithelium, thus alleviating dry eye and other symptoms [16]. VA also promotes the synthesis of glycoproteins in corneal epithelial cells, with increased adenosine triphosphate expression and decreased adenosine monophosphate expression, suggesting that VA exerts a prominent effect in corneal energy metabolism [17].
Previous studies found that VA promotes the proliferation of alveolar wall cells, inhibits the apoptosis of them in rats with emphysema, and eventually impedes the occurrence and development of emphysema in rats [18]. VA can also inhibit the phosphorylation of protein kinase B and forkhead box O3, so as to stimulate the apoptosis of colon cancer cells [19]. These studies indicate that VA exerts a crucial effect in the process of apoptosis, and the regulatory effect of VA on the apoptosis may be completely opposite in different disease models. However, the role of VA in the apoptosis of epithelial cells in mice with dry eye is unclear. In this study, a mouse model of dry eye was established using BAC mice treated with VA at a standard dose. We found that VA obviously suppressed the expression of the pro-apoptotic gene Bax at mRNA and protein levels, and increased the expression of the apoptotic gene Bcl-2. TUNEL staining results confirmed the inhibitory effect of VA on the apoptosis of epithelial cells.
This study has certain limitations that must be considered. 1) We did not confirm our results by cell experiments. 2) The direct target of the vitamin anti-apoptotic effect was not investigated. 3) The initiation of apoptosis varies, so it is unclear whether the initiation of apoptosis in epithelial cells in mice with dry eye occurs via outside pathways (such as Fas or tumor necrosis factor pathways) or via an unknown mitochondrial pathway. These issues need to be further explored. Despite these deficiencies, we showed the importance of apoptosis of epithelial cells in dry eye and the therapeutic effect of VA intervention.
Conclusions
VA partially inhibits the apoptosis of epithelial cells in mice with experimental dry eye and it may become a promising drug for treatment of dry eye in the future.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Than J, Balal S, Wawrzynski J, et al. Fingerprick autologous blood: A novel treatment for dry eye syndrome. Eye (Lond) 2017;31:1655–63. doi: 10.1038/eye.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittpenn JR, Tseng SC, Sommer A. Detection of early xerophthalmia by impression cytology. Arch Ophthalmol. 1986;104:237–39. doi: 10.1001/archopht.1986.01050140091027. [DOI] [PubMed] [Google Scholar]
- 3.Barabino S, Labetoulle M, Rolando M, Messmer EM. Understanding symptoms and quality of life in patients with dry eye syndrome. Ocul Surf. 2016;14:365–76. doi: 10.1016/j.jtos.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100:745–49. doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Gelber-Schwalb TA, Addeo JV, Stern ME. Apoptosis in the lacrimal gland and conjunctiva of dry eye dogs. Adv Exp Med Biol. 1998;438:453–60. doi: 10.1007/978-1-4615-5359-5_63. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Sheng M, Li J, et al. Tear proteomic analysis of Sjogren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci Rep. 2014;4:5772. doi: 10.1038/srep05772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–8. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faustino JF, Ribeiro-Silva A, Dalto RF, et al. Vitamin A and the eye: An old tale for modern times. Arq Bras Oftalmol. 2016;79:56–61. doi: 10.5935/0004-2749.20160018. [DOI] [PubMed] [Google Scholar]
- 9.Khan KN, Carss K, Raymond FL, et al. Vitamin A deficiency due to bi-allelic mutation of RBP4: There’s more to it than meets the eye. Ophthalmic Genet. 2017;38:465–66. doi: 10.1080/13816810.2016.1227453. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Liu X, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–64. [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi A, Flamion B, Baudouin C. Keratitis in dry eye disease and topical ciclosporin A. Ocul Immunol Inflamm. 2017;25:577–86. doi: 10.1080/09273948.2016.1276933. [DOI] [PubMed] [Google Scholar]
- 12.Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl 1):3–47. doi: 10.1097/01.icu.0000512373.81749.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maulvi FA, Shaikh AA, Lakdawala DH, et al. Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: In vitro and in vivo evaluation. Acta Biomater. 2017;53:211–21. doi: 10.1016/j.actbio.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 14.Herminghaus P, Geerling G, Hartwig D, et al. [Epitheliotrophic capacity of serum and plasma eyedrops. Influence of centrifugation]. Ophthalmologe. 2004;101:998–1005. doi: 10.1007/s00347-003-0979-8. [in German] [DOI] [PubMed] [Google Scholar]
- 15.Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: Clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85:1188–97. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCusker MM, Durrani K, Payette MJ, Suchecki J. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin Dermatol. 2016;34:276–85. doi: 10.1016/j.clindermatol.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Ubels JL, Dennis MH, Rigatti BW, et al. Nuclear retinoic acid receptors in the lacrimal gland. Curr Eye Res. 1995;14:1055–62. doi: 10.3109/02713689508998530. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Molteni A, Latkovich P, et al. Vitamin A depletion induced by cigarette smoke is associated with the development of emphysema in rats. J Nutr. 2003;133:2629–34. doi: 10.1093/jn/133.8.2629. [DOI] [PubMed] [Google Scholar]
- 19.Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: A pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745–57. doi: 10.1007/s10552-010-9549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]