ABSTRACT
Objectives: The aim of the study was to characterize the immediate and delayed effects of non-coherent blue-light treatment on the composition and viability of an in vitro biofilm composed of anaerobic multispecies, as well as the mechanisms involved.
Methods: A multispecies biofilm was constructed of Streptococcus sanguinis, Actinomyces naeslundii, Porphyromonas gingivalis and Fusobacterium nucleatum, test groups were exposed to blue light. The multispecies biofilm was explored with a newly developed method based on flow cytometry and confocal microscopy. The involvement of the paracrine pathway in the phototoxic mechanism was investigated by a crossover of the supernatants between mono-species P. gingivalis and F. nucleatum biofilms.
Results: Blue light led to a reduction of about 50% in the viable pathogenic bacteria P. gingivalis and F. nucleatum, vs that in the non-exposed biofilm. Biofilm thickness was also reduced by 50%. The phototoxic effect of blue light on mono-species biofilm was observed in P. gingivalis, whereas F. nucleatum biofilm was unaffected. A lethal effect was obtained when the supernatant of P. gingivalis biofilm previously exposed to blue light was added to the F. nucleatum biofilm. The effect was circumvented by the addition of reactive oxygen species (ROS) scavengers to the supernatant.
Conclusion: Blue-light has an impact on the bacterial composition and viability of the multispecies biofilm. The phototoxic effect of blue light on P. gingivalis in biofilm was induced directly and on F. nucleatum via ROS mediators of the paracrine pathway. This phenomenon may lead to a novel approach for ‘replacement therapy,’ resulting in a less periodonto-pathogenic biofilm.
KEYWORDS: Polymicrobial biofilm, blue light, periodontal disease
The bacteria in the oral cavity are organized in multispecies biofilms [1], which contain about 500–700 taxa at the species level and is the cause of several infectious oral diseases, such as caries and periodontal disease [2,3]. It is well known that bacteria within the biofilm exhibit characteristics different from those under planktonic conditions, due to the expression of specific genes [4]. The bacteria within the biofilm also display resistance to antimicrobial agents, physical forces, nutrient deprivation, pH changes, oxygen radicals and the immune system [5]. The concept of polymicrobial synergy among members of the biofilm is well documented, and experiments consistently show higher pathogenicity of mixed infection compared with monospecies infection [6–8]. This is also attributable to inter-taxa paracrine communication, such as quorum sensing, which facilitates bacterial virulence and survival [9]. Periodontal disease is an infectious, destructive disease characterized by chronic inflammation that eventually leads to tooth loss [10,11]. Previous reports showed that there is a correlation between load of Gram-negative anaerobic bacteria in the sub-gingival sulcus and disease severity [12]. Of those, Porphyromonas gingivalis and Fusobacterium nucleatum are known keystone pathogens of periodontal diseases, and are often identified together in the subgingival biofilm of periodontal lesions [13–19]. Gram-positive bacteria implicated in periodontitis include oral streptococci of the Mitis group and Actinomyces naeslundii. The streptococci once considered strict commensals in the oral cavity [6, 24] do not cause significant pathological changes [6,20,21]. However, mixed infections (for example, with P. gingivalis) may result in increased disease severity [6]. These findings contributed to the polymicrobial synergy and dysbiosis hypothesis [6,22], according to which the synergistic polymicrobial community is the cause of periodontitis. Within this community, specific constituents or combinations of functional genes fulfill distinct roles that converge to shape and stabilize a dysbiotic microbiota, which perturbs host homeostasis [6,23].
For almost 100 years the gold-standard treatment for periodontitis has been mechanical subgingival debridement (previously termed scaling and root planning) [24]. However, mechanical debridement does not always lead to major clinical improvement which sometimes can be ameliorated considerably by the adjunctive use of antibiotics [24]. Yet, antibiotics have side effects, and when overused may contribute to antibiotic resistance [20,21,25–27]. An alternative antimicrobial approach to the treatment of periodontitis is lethal photosensitization or antimicrobial photodynamic therapy (aPDT). Visible light, augmented by an exogenous photosensitizer (light-absorbing dye molecules), has been found to be effective as a non-specific treatment against a number of microbial species [28–33], and for inactivation of oral biofilm [33,34]. However, the use of dyes is associated with two main problems: the inability of the dye to diffuse throughout the biofilm and the compromised esthetic results owing to staining of the oral tissues. Despite the development of stain-free Phenalen-1-one photosensitizers (PS), such agents have not been approved for clinical use [21,34].
Having endogenous photosensitizers, black-pigmented bacteria (such as P. gingivalis) do not require the addition of dyes for the phototoxic effect [35–38]. Moreover, we previously showed that blue light had a phototoxic effect not only on the periodontal pathogen P. gingivalis but also on F. nucleatum, both with an impact greater than that on streptococcal species [39]. This selective phototoxic effect on the anaerobic bacteria is probably due to the photochemical mechanism mediated by reactive oxygen species (ROS) formation [40]. Therefore, in the absence of enzymes capable of scavenging ROS, anaerobic bacteria become more sensitive to oxygen when exposed to blue light [40].
Although various studies have demonstrated the selective inhibitory effect of blue light on periodonto-pathogens grown in biofilms in vitro and in vivo [11,35,41], the phototoxic mechanism of the anaerobic bacteria when in biofilm has been barely explored. As these bacteria are commonly adjacent to each other due to co-aggregation [42,43], we assumed that paracrine signaling may occur between P. gingivalis and F. nucleatum. Previous investigations of the effect of blue light on Streptoccocus mutans biofilm suggested a delayed bacterial death phenomenon, evident 6 h after the biofilm was exposed to light [44,45]. Thus, the objectives of the present study were to characterize the immediate and delayed effects of blue-light treatment on the composition and viability of an in vitro anaerobic multi-species biofilm model and to evaluate the possible contribution of bacterial interaction through a paracrine pathway to the phototoxic mechanism.
Materials and methods
Bacteria
F. nucleatum PK1594, P. gingivalis ATCC 33277, S. sanguinis NC02863 and A. naeslundii 17233 were grown in Wilkins-Chagren broth (Oxoid, Basingstoke, Hampshire, UK), and incubated at 37°C for 24 h under anaerobic conditions (N2 85%, H2 5%, CO2 10%). S. sanguinis and A. naeslundii were transferred to Wilkins broth enriched with 2% sucrose (Sigma, Rehovot, Israel) and cultured under anaerobic conditions for an additional 24 h. F. nucleatum and P. gingivalis were transferred to Wilkins broth and incubated for an additional 24 h under anaerobic conditions. The bacteria were then centrifuged (4,000 rpm, 15 min) and suspended in gingival crevicular fluid (GCF)-simulating medium [46] (60% RPMI medium, 40% donor horse serum (Biological Industries, Beit Ha’emek, Israel)) enriched with 5 µg/mL hemin (Sigma) and 0.5 µg/mL menadione (Sigma). The bacterial suspensions of S. sanguinis, A. naeslundii and P. gingivalis were adjusted spectrophotometrically to 109 cells/mL, and that of F. nucleatum was adjusted to 108 cells/mL [47–50].
Labeling of specific bacteria in biofilm
To focus on the interaction between specific bacteria within the multi-species biofilm and to examine the effect of blue light on the composition and the viability of each bacterial strain in the biofilm, a novel method was developed that entailed fluorescent labeling of the bacteria and flow cytometry. The assay is based on Fluorescein isothiocyanate (FITC) labeling of a particular bacterial species before its incorporation in the biofilm. Then, after light treatment and fluorescent staining for dead bacteria and dissociation of the mature biofilm into a single bacterium suspension, it is analyzed with flow cytometry (for assay and calibration details, see Polak et al. [51]). When specified, before incorporation in the biofilm, P. gingivalis or F. nucleatum was stained with FITC by incubating the bacteria for 20 min at room temperature in FITC buffer (1 mg fluorescein isothiocyanate (Sigma Rehovot, Israel) in 500 µl 0.5 M sodium carbonate buffer, pH 9, diluted to a total volume of 10 ml in PBS). Excess stain was removed by three washes with PBS. A previous study confirmed that FITC as a dye does not act as a PS, by the similar results obtained using FITC in FACS assay analysis and CFU counts for bacterial viability of light treated and untreated samples in planktonic suspensions [52].
Multispecies biofilm
Human saliva (Helsinki board approval HMO052511) diluted 1:4 in double distilled water (DDW) [53] was inoculated onto hydroxyapatite (HA) disks (Clarkson Chromatography Products, South Williamsport, PA) and incubated for 30 min at 37°C. The disks were washed with PBS, a suspension of S. sanguinis and A. naeslundii (1:1 ratio in a total volume of 1,000 µl GCF simulating medium) was inoculated, and they were incubated for 24 h at 37°C under anaerobic conditions. The discs with the newly formed biofilm were then washed with PBS, a suspension of P. gingivalis and F. nucleatum (1:1 ratio in a total volume of 1,000 µl GCF simulating medium) was inoculated, and they were incubated for an additional 48 h at 37°C under anaerobic conditions. The mature biofilms were washed and reconstituted in PBS (400 µl/disk).
Monospecies biofilm
Hydroxyapatite discs (Clarkson Chromatography Products) were placed in 24-well plates and inoculated with human saliva (Helsinki board approval HMO052511) diluted 1:4 with DDW [53] for 30 min at 37°C. The disks were washed with PBS, each of the four bacterial suspensions was inoculated separately onto the discs (i.e. one strain/disc in 1000 µl GCF simulating medium total volume), and the discs were incubated for 48 h at 37°C under anaerobic conditions. The mature biofilm was washed and reconstituted in PBS (400 µl/disk).
Light exposure
A halogen lamp (Belleglass HP, Kerr Inc, Orange, CA) was used for exposure to blue light (wavelength 400–500 nm). The light probe (diameter, 14 mm) was set at a distance of 10 mm from the biofilm sample, resulting in light exposure with a power density of 1.2 W/cm2, measured by a power meter (Ophir, Jerusalem, Israel). There was a distance of two wells in the 24-well plate between samples exposed to the light. After the biofilm supernatants were removed, the HA discs were washed, and each biofilm sample was reconstituted in PBS (400 μl in each well of a 24-well plate), and exposed to light for 2 min, equivalent to a fluence of 146 J/cm2. Fluence was calculated according to the following formula:
Fluence (J/cm2) = Power Density (W/cm2)×Irradiation Time (sec)
Flow cytometry (FACS analysis)
Immediately or 6 h after exposure to blue light, the biofilms were washed with PBS, and stained with 1 µg propidium iodide (Sigma) in 600 µL FACS buffer (0.5 M α-lactose monohydrate (Sigma) in PBS) at room temperature for identification of dead cells. The biofilm was then mechanically dissociated by scraping from the HA, filtered through a cell strainer (70 µm), and analyzed with Accuri C6 flow cytometry (BD Biosciences, San Jose, CA), as described in detail by Polak et al. [51].
Fluorescence staining and confocal scanning laser microscopy
A confocal scanning fluorescence microscope (Olympus FV300, Tokyo, Japan) with a x 10 lens was used to visualize the distribution of live and dead bacteria throughout the biofilm. The live bacteria were observed after SYTO9 staining (LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Eugene, OR)) and bacteria with a compromised membrane were seen after staining in the dark at room temperature for 25–30 min with a propidium iodide (PI) solution (1.0 mg PI/mL (Sigma)). Scans through the biofilm were made at 5 µm intervals. An Olympus fluoview ver.3.1 viewer (Olympus, Tokyo, Japan) was used for analysis and image processing.
Paracrine mechanism
To explore the involvement of the light-induced paracrine pathway, an experimental design was established based on blue-light exposure of mono-species (P. gingivalis or F. nucleatum) biofilm, and crossover of the supernatant between these biofilms. In addition, the involvement of two main factors – proteases and ROS – that may mediate intercellular effects by a paracrine mechanism following biofilm exposure to blue light was explored. Thus, the supernatants were treated with protease inhibitors or ROS scavengers (vitamin C and catalase) before adding them to the other bacterial biofilm. Scavengers in PBS at final concentrations of 20 U/mL vitamin C or 30 mM catalase were added to the biofilm before light exposure.
Data analyses
All the experiments were performed in triplicate and repeated at least three times. The data were analyzed with a statistical software package (SigmaStat, Jandel Scientific, San Rafael, CA). One-way-repeated measure analysis of variance (RM ANOVA) was applied to test the significance of the differences between the treated groups. If the results were significant, intergroup differences were tested for significance according to the Student’s t-test and the Bonferroni correction for multiple testing.
Results
Immediate effect of blue light on multispecies biofilm
Mature, 3-days-old, multispecies biofilms were analyzed with FACS immediately after exposure to light (test group). The results, although not statistically significant, showed that the control multispecies biofilms contained a total (all species included) 60% viable bacteria (Figure 1(a)), whereas the immediate effect of light exposure led to a total 50% viable bacteria (Figure 1(a), test group).
Figure 1.
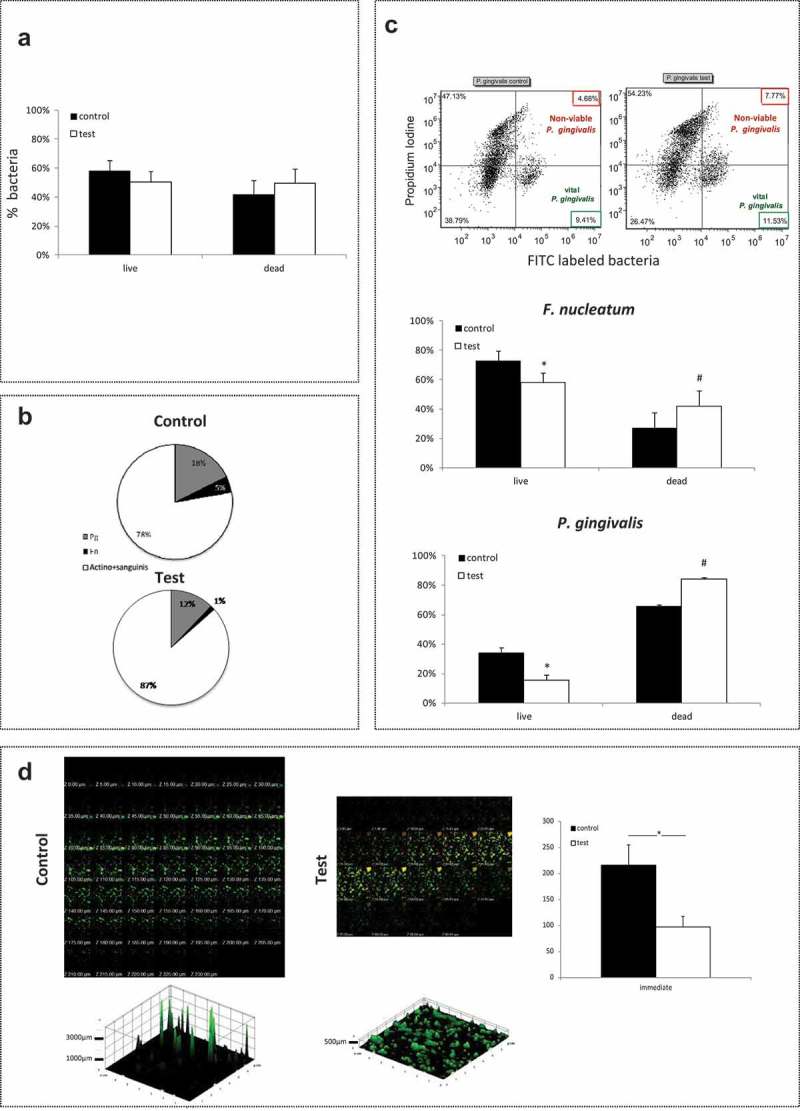
Immediate effect of blue-light exposure on multispecies biofilm Multispecies biofilms was exposed to blue light (test) and compared with non-exposed biofilm (control). P. gingivalis (pg) or F. nucleatum (fn) was stained with FITC. All the bacteria were stained with the PI marker for dead bacteria. (a) – Percent live bacteria in exposed vs. non-exposed biofilm. (b) – Breakdown of the percentage of viable pathogenic bacteria (pg and fn) in exposed vs. non-exposed biofilm. (c) – Representative dot plots of P. gingivalis with FITC and PI staining according to flow cytometry analysis (left – control, right – test), presented as the mean percentages of viable and non-viable bacteria in exposed vs. non-exposed biofilm for P. gingivalis and F. nucleatum. (d) – Confocal analysis of exposed (right) vs. non-exposed (left) biofilms stained for live/dead, and measurement of biofilm thickness. The results are expressed as the mean ± SD.
* and # significantly different from other groups (live or dead).
However, exposure to blue light had a clear impact on the composition of the viable bacteria within the biofilm. The relative number of non-pathogenic viable bacteria (S. sanguinis and A. naeslundii) increased from 78% in the non-exposed biofilm to 87% in the light-exposed biofilm, whereas the relative amount of pathogenic viable bacteria (P. gingivalis and F. nucleatum) dropped from 23% to 13%, with a reduction in the viability of P. gingivalis from 18% to 12% and in that of F. nucleatum from 5% to 1% of the total viable bacteria (Figure 1(b)). All the differences were statistically significant (p < 0.05). In addition, the viability percentage calculated for each of the pathogenic bacteria within the biofilm showed a statistically significant reduction immediately after exposure to light, from ≈ 35% to less than 15% for F. nucleatum, and from ≈ 80% to ≈ 60% for P. gingivalis (Figure 1(c)). Moreover, the biofilm thickness was reduced significantly (p < 0.05) from 220 µm to 100 µm (Figure 1(d)). The confocal images revealed that after exposure to blue light the biofilm contained more dead bacteria than the non-exposed biofilm. This effect was seen in all layers of the biofilm (Figure 1(d)).
Delayed effect of blue light on multispecies biofilm
Next, the delayed effect was examined after further (6 h) biofilm incubation following light exposure. The multispecies biofilms contained a total of 60% live bacteria (all strains) in both the test and control groups (Figure 2(a)). The shift towards a less pathogenic composition of the biofilm continued 6 h after light exposure, with 9% pathogenic bacteria in the exposed biofilm vs 18% in the non-exposed biofilm, i.e. a reduction of about 50% for each of the pathogenic bacteria (Figure 2(b)). These differences were statistically significant. In addition, the percent viability of each of the pathogenic bacteria within the biofilm showed a statistically significant reduction in the viability of F. nucleatum 6 h after exposure to light, from ≈ 60% to ≈ 40%. There was no noticeable difference in the viability of P. gingivalis (Figure 2(c)).
Figure 2.
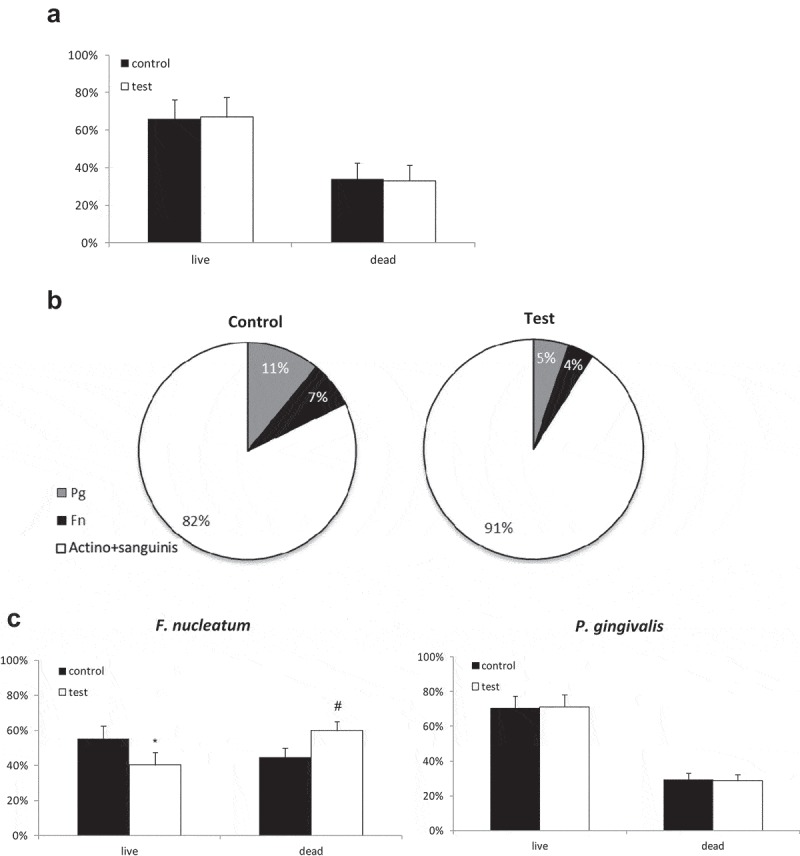
Persistent effect of blue-light exposure on multispecies biofilm. Multispecies biofilm was exposed to blue light and compared with non-exposed biofilm. The biofilm was further incubated for an additional 6 h after exposure. P. gingivalis (pg) or F. nucleatum (fn) were stained with FITC. All bacteria were stained with the PI marker for dead bacteria. (a) – Percentage of total live and dead bacteria in exposed vs. non-exposed biofilm. (b) – Breakdown of the percentage of viable pathogenic bacteria (pg and fn) in exposed vs. non-exposed biofilm. (c) – Mean percentages of viable and nonviable pg or fn in exposed vs. non-exposed biofilm. The results are expressed as the mean ± SD. * and # indicate a significant difference from the other groups (live or dead).
Blue light effect on mono-species biofilm
To elucidate the effect of blue light on the multi-species biofilm, its effect on mono-species biofilm formed by each of the four bacteria that composed the multi-species biofilm was examined. Only P. gingivalis biofilm exhibited susceptibility to blue light, with a statistically significant reduction in viability from 95% to 60% (Figure 3). Blue light did not affect the other monospecies biofilms (F. nucleatum, S. sanguinis and A. naeslundii) under the experimental conditions of this study (Figure 3).
Figure 3.
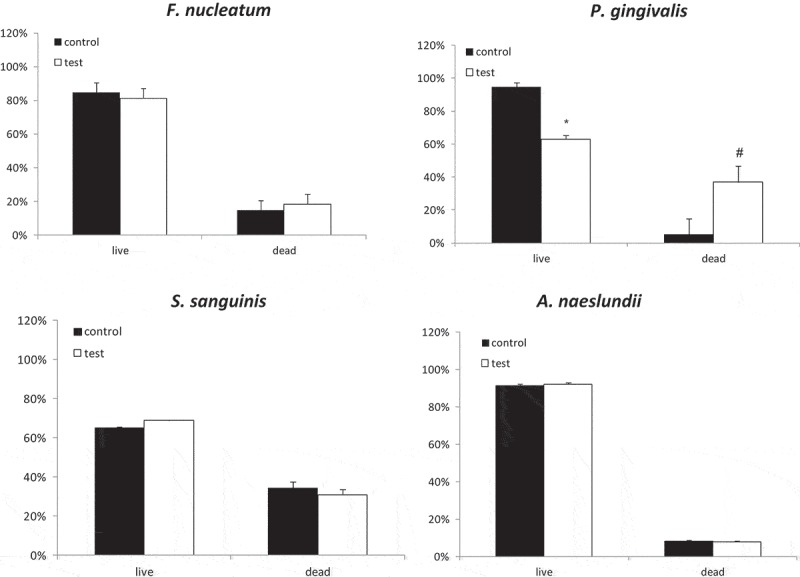
Blue light effect on single species biofilm Mono-species biofilm was exposed to blue light (test) and compared with non-exposed biofilm (control). All biofilms were stained with the PI marker for dead bacteria. The results are expressed as the mean ± SD. * and # = significant difference from the other groups.
Light-induced paracrine killing mechanism of bacteria
To explore the possibility of a light-induced paracrine pathway, an experimental design was established based on blue-light exposure of mono-species (P. gingivalis or F. nucleatum) biofilm, and crossover of the supernatant between the two biofilms (exposed biofilm supernatant exchange with exposed biofilm). Crossover of the non-exposed biofilm supernatants did not change the viability of F. nucleatum or P. gingivalis (compare control groups in Figures 4(a) and 3). Light-exposed P. gingivalis biofilm introduced into the supernatant of exposed F. nucleatum biofilm showed an effect similar to that seen in the previous experiments (reduction of live P. gingivalis – Figure 4(a), P. gingivalis test group vs that in Figure 3). Light-exposed F. nucleatum biofilm introduced into the supernatant of exposed P. gingivalis biofilm showed an increased bactericidal effect compared with that seen in the previous mono-specious experiment (Figure 4(a), F. nucleatum test group vs that in Figure 3). However, the bactericidal effect was similar to that observed in the multispecies biofilm experiments (Figure 4(a), F. nucleatum test group, compared with that in Figure 1(c)). These differences were statistically significant (p < 0.05).
Figure 4.
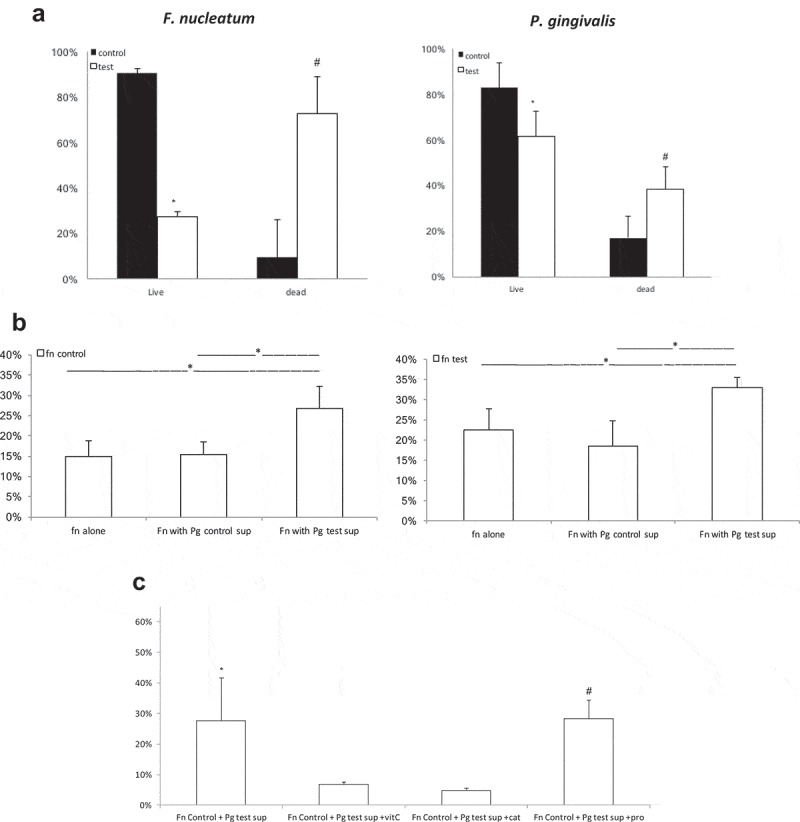
Supernatant paracrine effect of P. gingivalis and F. nucleatum monospecies biofilms after exposure to blue light. (a) – P. gingivalis and F. nucleatum biofilms exposed to blue light (test group) or non-exposed (control group) after a supernatant exchange of the two biofilms. (b) – F. nucleatum biofilm, non-exposed and exposed to blue light, after the addition of supernatants of P. gingivalis biofilm (exposed or non-exposed) and without supernatant (control). (c) – F. nucleatum biofilm non-exposed to light, with the addition of supernatant of exposed P. gingivalis biofilm, alone and with ROS scavengers (vitamin C or catalase) or protease inhibitor. The results are expressed as the mean ± SD. * and # = significant difference from the other groups.
These results show that the bactericidal effect of blue light on F. nucleatum biofilm is mediated by a factor present in the P. gingivalis supernatant. To determine whether the exposure of F. nucleatum to blue light has any impact, an experiment was performed in which non-exposed F. nucleatum mono-species biofilm (Figure 4(b), F. nucleatum control groups) was compared with light-exposed F. nucleatum biofilm (Figure 4(b), F. nucleatum test groups). F. nucleatum biofilm was introduced into the supernatant of P. gingivalis biofilm previously exposed to blue light (as well as to the supernatant of non-exposed P. gingivalis biofilm) and was compared with F. nucleatum biofilm with its own supernatant. The paracrine bactericidal effect was observed only when the supernatant of P. gingivalis biofilm previously exposed to blue light was used, regardless of F. nucleatum biofilm exposure to blue light (Figure 4(b)).
Two main factors may mediate the destructive interaction between P. gingivalis supernatant (following exposure to blue light) and F. nucleatum biofilm – (a) robust expression of P. gingivalis proteases, which may induce bacterial lysis, (b) expression of reactive oxygen species by P. gingivalis following exposure to blue light, which affects F. nucleatum viability. To determine the source of this interaction, the P. gingivalis supernatant was treated with protease inhibitors or scavenger molecules (vitamin C or catalase) before the crossover with F. nucleatum biofilm. The results showed that in the presence of ROS scavengers the P. gingivalis supernatant had a reduced bactericidal effect on the F. nucleatum biofilm, whereas supernatant with or without the addition of protease inhibitor had a similar bactericidal effect on the F. nucleatum biofilm (Figure 4(c)).
Discussion
The current study shows that blue light has an impact on the bacterial composition and viability of the multispecies biofilm. The phototoxic effect of blue light on P. gingivalis in biofilm was induced directly and on F. nucleatum via ROS mediators of the paracrine pathway. This phenomenon may lead to a novel approach for ‘replacement therapy,’ resulting in a less periodonto-pathogenic biofilm.
The present investigation aimed to examine not only the immediate but also the late effect of blue light on the composition of viable bacteria in the multi-species biofilm. As the simulation of the complex structure of the oral biofilm was beyond the scope of this study, an in vitro model simulating a periodontitis-associated dysbiotic biofilm, based on keystone pathogens of periodontal diseases, was established. The biofilm was grown on surfaces similar to those of the tooth (HA) and in a medium simulating the natural environment of the periodontal pocket [46]. Although real-time PCR (qPCR) is a widely used technique in quantitative analysis of multi-species samples, its main limitation is its inability to discriminate between live and dead cells. This problem was overcome by the use of a unique and reproducible assay developed to quantify dynamic changes in the viability of specific species in the biofilm with the aid of fluorescent staining and flow cytometry. Similarly, Cieplik et al. [21] assessed the damage of cytoplasmic membranes of three different bacteria in the biofilm. However that study was performed on a non-fluorescent side scatter FACS scale, which measures granularity and not always can be used to differentiate between different bacteria. The novelty of the present study is the ability to clearly differentiate fluorescently between the target pathogen and the other bacteria in a multispecies biofilm by using a flow cytometry setting.
This study demonstrates the selective bactericidal effect of blue light on the growth of P. gingivalis and F. nucleatum. Light exposure (at a fluence of 146 J/cm2) led to a ≈50% reduction in the relative number of viable pathogenic bacteria. Since this change in biofilm composition involves keystone pathogens, makes it highly significant in microbiome dysbiosis processes. This relative change in the bacterial composition of the biofilm was maintained for at least 6 h after exposure. In addition, blue light led to a reduction in total biofilm thickness by ≈ 50%, indicating an additional anti-biofilm effect of the light, which could be due to its direct or indirect continuous effect on bacterial interaction. Previous studies showed the long-term effect of light on the previously exposed Streptococcus mutans in biofilm, which could be due to the delayed death phenomenon of bacteria and/or as a result of changes in gene expression [44,45]. Thus, other mechanisms, such as co-aggregation, metabolic communication and quorum sensing [54], aside from the direct phototoxic effect of the light on the keystone periodonto-pathogens, may be involved and continue to affect the whole biofilm. These results correlate for the most part with those of Fontana et al. who showed a 50% reduction in bacterial growth on blood agar following blue light exposure, with a reduction in the percentage of the periodonto-pathogenic bacteria in the biofilm [11]. The phenomenon of selective reduction by phototargeting of human periodontal pathogens, such as black-pigmented species and F. nucleatum, was also demonstrated in vivo by twice daily application of blue light to oral plaque over a period of 4 days [41].
In the present in vitro model, as the composition and conditions of biofilm growth and exposure to light were controlled, it was possible to compare the effect of blue light on the multispecies biofilm with that on a specific mono-species biofilm of each bacterial strain within the biofilm, under similar conditions. Blue light affected only P. gingivalis biofilm, without any significant impact on F. nucleatum biofilm, although the exposure affected both bacteria when in a multispecies biofilm. These findings do not correlate completely with our previous results showing the high susceptibility to blue light of the two strains, when exposed in the planktonic state or when on agar plates [39]. This could be explained by the differences in F. nucleatum susceptibility to light when in the planktonic state, on agar or as a biofilm. Indeed, the above results were in agreement with those of Song et al. who found that after exposure to blue light, a similar reduction in P. gingivalis viability was observed in both the planktonic and biofilm states, whereas in F. nucleatum this was seen only in planktonic bacteria [35]. Another study suggested that F. nucleatum plays a protective role against ROS, atmospheric O2 and H2O2 within the oral biofilm, owing to protein defense systems [52], which may account, as previously demonstrated for the enhanced tolerance of F. nucleatum to blue light when in mono-species biofilm.
On the other hand, it was well demonstrated that, compared with streptococcal species, anaerobic bacteria, such as P. gingivalis and F. nucleatum, were more susceptible to blue light under aerobic conditions, owing to their greater sensitivity to oxidative stress [40]. The mechanism by which black-pigmented oral bacteria such as P. gingivalis were sensitized by blue light was attributed to their accumulation of iron protoporphyrin IX (PpIX), as endogenous photosynthesizer [35,55]. However, another study showed that although the amounts of endogenous porphyrin produced by Prevotella species were greater than those produced by Fusobacterium species, both genera exhibited a similar susceptibility to blue light [56]. Thus, it appears that photo-targeting of other endogenous chromophores, such as cytochromes and flavins, may contribute to the increased phototoxicity of fusobacteria [40], as well as to that of other periodontal pathogens, such as Aggregatibacter actinomycetemcomitans [57]. As F. nucleatum and P. gingivalis co-aggregate [58] and are probably located close to each other within the biofilm, we postulated that ROS production and/or the enzymatic content of P. gingivalis dead cells may affect the viability of F. nucleatum when in multi-species biofilm. Indeed, the results show that F. nucleatum in biofilm was not affected directly by blue light. However, the supernatant of P. gingivalis previously exposed to blue light had an impact on viability, and this effect was found to be mediated by oxidative species. Therefore, it is reasonable to assume that the phototoxic effect of blue light on F. nucleatum when in multi-species biofilm may not be a direct effect but mainly an indirect effect mediated by ROS and resulting in the reaction of P. gingivalis to the light.
The present study confirms the notion that the anaerobic pathogens P. gingivalis and F. nucleatum are more susceptible to the oxidative stress resulting from the blue-light effect on bacteria or via ROS produced by neighboring affected bacteria, than the commensal aerobic bacteria within the biofilm. Moreover, the exposure of biofilm to blue light induced a shift, maintained for at least 6 h, towards a less pathogenic composition as well as total biofilm reduction. The investigation demonstrates that blue light may serve as a potential tool for facilitating a sustained shift from a dysbiotic periodontal biofilm to a symbiotic biofilm. However, as the conclusions obtained here are derived from in vitro studies, their relevance to the processes occurring in vivo and the appropriate clinical protocol with the precise conditions of the light therapy should be further investigated. Yet, it appears that the blue-light technique might be applicable in periodontal disease, and may represent a novel approach to ‘replacement therapy’ in other biofilm-associated diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Zijnge V, van Leeuwen MB, Degener JE, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5(2):e9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guggenheim B, Gmür R, Galicia JC, et al. In vitro modeling of host-parasite interactions: the ‘subgingival’ biofilm challenge of primary human epithelial cells. BMC Microbiol. 2009;9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thompson H, Rybalka A, Moazzez R, et al. In vitro culture of previously uncultured oral bacterial phylotypes. Appl Environ Microbiol. 2015;81(24):8307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker C, Sedlacek MJ.. An in vitro biofilm model of subgingival plaque. Oral Microbiol Immunol. 2007;22(3):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shaddox LM, Alfant B, Tobler J, et al. Perpetuation of subgingival biofilms in an in vitro model. Mol Oral Microbiol. 2010;25(1):81–87. [DOI] [PubMed] [Google Scholar]
- [6].Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ebersole JL, Dawson D, Emecen-Huja P, et al. The periodontal war: microbes and immunity. Periodontol 2000. 2017;75(1):52–115. [DOI] [PubMed] [Google Scholar]
- [9].Mukherjee S, Bassler BK. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol. 2019;17(6):371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84(4 Suppl):S1–7. [DOI] [PubMed] [Google Scholar]
- [11].Fontana CR, Song X, Polymeri A, et al. The effect of blue light on periodontal biofilm growth in vitro. Lasers Med Sci. 2015;30(8):2077–2086. [DOI] [PubMed] [Google Scholar]
- [12].Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. [DOI] [PubMed] [Google Scholar]
- [13].Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. [DOI] [PubMed] [Google Scholar]
- [15].Holt SC, Bramanti TE. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2(2):177–281. [DOI] [PubMed] [Google Scholar]
- [16].Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(4 Suppl):322–331. [DOI] [PubMed] [Google Scholar]
- [17].Bradshaw DJ, Marsh PD, Watson GK, et al. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998;66(10):4729–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. [DOI] [PubMed] [Google Scholar]
- [19].Weiss EI, Shaniztki B, Dotan M. Attachment of Fusobacterium nucleatum PK1594 to mammalian cells and its coaggregation with periodontopathogenic bacteria are mediated by the same galactose-binding adhesin. Oral Microbiol Immunol. 2000;15(6):371–377. [DOI] [PubMed] [Google Scholar]
- [20].Olsvik B, Tenover FC. Tetracycline resistance in periodontal pathogens. Clin Infect Dis. 1993;16(Suppl 4):S310–313. [DOI] [PubMed] [Google Scholar]
- [21].Cieplik F, Steinwachs VS, Muehler D, et al. Phenalen-1-one-mediated antimicrobial photodynamic therapy: antimicrobial efficacy in a periodontal biofilm model and flow cytometric evaluation of cytoplasmic membrane damage. Front Microbiol. 2018;9:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44(2):328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feres M, Figueiredo LC, Soares GM, et al. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67(1):131–186. [DOI] [PubMed] [Google Scholar]
- [25].Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. Lancet. 2005;365(9455):253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feres M, Haffajee AD, Goncalves C, et al. Systemic doxycycline administration in the treatment of periodontal infections (II). Effect on antibiotic resistance of subgingival species. J Clin Periodontol. 1999;26(12):784–792. [DOI] [PubMed] [Google Scholar]
- [27].Durao P, Balbontin R, Gordo I. Evolutionary mechanisms shaping the maintenance of antibiotic resistance. Trends Microbiol. 2018;26(8):677–691. [DOI] [PubMed] [Google Scholar]
- [28].Okamoto H, Iwase T, Morioka T. Dye-mediated bactericidal effect of He-Ne laser irradiation on oral microorganisms. Lasers Surg Med. 1992;12(4):450–458. [DOI] [PubMed] [Google Scholar]
- [29].Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J Appl Bacteriol. 1993;75(4):299–306. [DOI] [PubMed] [Google Scholar]
- [30].Wood S, Nattress B, Kirkham J, et al. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J Photochem Photobiol B. 1999;50(1):1–7. [DOI] [PubMed] [Google Scholar]
- [31].O’Neill JF, Hope CK, Wilson M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg Med. 2002;31(2):86–90. [DOI] [PubMed] [Google Scholar]
- [32].Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47(3):932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pummer A, Knüttel H, Hiller K-A, et al. Antimicrobial efficacy of irradiation with visible light on oral bacteria in vitro: a systematic review. Future Med Chem. 2017;9(13):1557–1574. [DOI] [PubMed] [Google Scholar]
- [34].Cieplik F, Yang C-L, Ge M-Y, et al. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;5:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Song HH, Lee J-K, Um H-S, et al. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J Periodontal Implant Sci. 2013;43(2):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Duerden BI. Pigment production by Bacteroides species with reference to sub-classification. J Med Microbiol. 1975;8(1):113–125. [DOI] [PubMed] [Google Scholar]
- [37].Reid JS, Beeley JA, MacFarlane TW. A study of the pigment produced by Bacteroides melaninogenicus. J Dent Res. 1976;55(6):1130. [DOI] [PubMed] [Google Scholar]
- [38].Shah HN, Bonnett R, Mateen B, et al. The porphyrin pigmentation of subspecies of Bacteroides melaninogenicus. Biochem J. 1979;180(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feuerstein O, Persman N, Weiss EI. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochem Photobiol. 2004;80(3):412–415. [DOI] [PubMed] [Google Scholar]
- [40].Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 2005;81(5):1186–1189. [DOI] [PubMed] [Google Scholar]
- [41].Soukos NS, Stultz J, Abernethy AD, et al. Phototargeting human periodontal pathogens in vivo. Lasers Med Sci. 2015;30(3):943–952. [DOI] [PubMed] [Google Scholar]
- [42].Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009;191(22):6804–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rickard AH, Gilbert P, High NJ, et al. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11(2):94–100. [DOI] [PubMed] [Google Scholar]
- [44].Steinberg D, Moreinos D, Featherstone J, et al. Genetic and physiological effects of noncoherent visible light combined with hydrogen peroxide on Streptococcus mutans in biofilm. Antimicrob Agents Chemother. 2008;52(7):2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chebath-Taub D, Steinberg D, Featherstone JD, et al. Influence of blue light on Streptococcus mutans re-organization in biofilm. J Photochem Photobiol B. 2012;116:75–78. [DOI] [PubMed] [Google Scholar]
- [46].Hope CK, Wilson M. Biofilm structure and cell vitality in a laboratory model of subgingival plaque. J Microbiol Methods. 2006;66(3):390–398. [DOI] [PubMed] [Google Scholar]
- [47].Kolenbrander PE, Andersen RN. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989;57(10):3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Genco CA, Cutler CW, Kapczynski D, et al. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59(4):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miller CH, Palenik CJ, Stamper KE. Factors affecting the aggregation of Actinomyces naeslundii during growth and in washed cell suspensions. Infect Immun. 1978;21(3):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Herzberg MC, Erickson PR, Kane PK, et al. Platelet-interactive products of Streptococcus sanguis protoplasts. Infect Immun. 1990;58(12):4117–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Polak D, Shany-Kdoshim S, Zaydel L, et al. High-resolution novel method for tracking bacteria in a multi-species biofilm. Arch Microbiol. 2019;201(2):259–266. [DOI] [PubMed] [Google Scholar]
- [52].Steeves CH, Potrykus J, Barnett DA, et al. Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum. Proteomics. 2011;11(10):2027–2037. [DOI] [PubMed] [Google Scholar]
- [53].Rickard AH, Campagna SR, Kolenbrander PE. Autoinducer-2 is produced in saliva-fed flow conditions relevant to natural oral biofilms. J Appl Microbiol. 2008;105(6):2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hojo K, Nagaoka S, Ohshima T, et al. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88(11):982–990. [DOI] [PubMed] [Google Scholar]
- [55].Smalley JW,Silver J, Marsh PJ, Birss AJ. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J. 1998;331(Pt 3):681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Soukos NS, Som S, Abernethy AD, et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49(4):1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cieplik F, Späth A, Leibl C, et al. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Invest. 2014;18(7):1763–1769. [DOI] [PubMed] [Google Scholar]
- [58].Polak D, Shapira L, Weiss EI, et al. The role of coaggregation between Porphyromonas gingivalis and Fusobacterium nucleatum on the host response to mixed infection. J Clin Periodontol. 2012;39(7):617–625. [DOI] [PubMed] [Google Scholar]


