Abstract
In the United States, early detection methods have contributed to the reduction of overall breast cancer mortality but this pattern has not been observed uniformly across all racial groups. A vast body of research literature shows a set of health care, socio-economic, biological, physical, and behavioural factors influencing the mortality disparity. In this paper, we review the modelling frameworks, statistical tests, and databases used in understanding influential factors, and we discuss the factors documented in the modelling literature. Our findings suggest that disparities research relies on conventional modelling and statistical tools for quantitative analysis, and there exist opportunities to implement data-based modelling frameworks for (1) exploring mechanisms triggering disparities, (2) increasing the collection of behavioural data, and (3) monitoring factors associated with the mortality disparity across time.
Keywords: Breast cancer, racial disparities, statistical analysis, operations research
1. Introduction
In the United States (U.S.), breast cancer (BC) is the most common cause of death among Hispanic women and second most common cause of death among non-Hispanic white (NHW), African-American (AA), Asian/Pacific Islander (API), and American Indian/Alaska Native (AI/AN) women (CDC, n.d.). Every year, more than a billion dollars are spent in BC research (BCC, n.d.), and such investments have contributed to the steady decrease in BC deaths rates since 1989 (ACS, n.d.). Despite these advancements, BC mortality has decreased unequally across races (Aizer et al., 2014; Jatoi et al., 2005; Wheeler et al., 2013), which has prompted the Department of Health and Human Services and the American Cancer Society to set the elimination of disparities as a national priority goal (ACS, n.d.; DHH, n.d.).
The causes of racial disparities in BC mortality are complex and resulting from many factors, ranging from those that influence the human cellular and cancer makeup (biological), to those that characterise the social, economic, health, physical, cultural, and psychological standing of a BC patient. None of these factors seem to individually explain, but rather interact among with others to produce a disparity. For example, it has been observed that the receipt of radiation therapy significantly decreases the chances of BC mortality after lumpectomy. However, older patients living in non-urban areas are less likely to receive radiation therapy after lumpectomy, probably due to the absence of adequate health services (Martinez et al., 2012). For these older patients, their treatment, age, residential setting, and health services are interacting to create a distinct scenario for a possible disparity. Interventions on some of the modifiable factors (like better provision of transportation services or improvement of the non-urban health care infrastructure) may produce an effect in reducing the disparity. Hence, it is important to properly identify factors and interactions to build meaningful evidence towards effective interventions.
There is an extensive body of literature identifying factors and interactions that significantly contribute to racial disparities in BC mortality. There are also meta-analyses (Newman et al., 2002, 2006), and reviews of the literature (Danforth, 2013; Wheeler et al., 2013) that discuss the biological and non-biological factors influencing disparities in BC mortality between NHW and AAs. Meta-analyses concluded that AA ethnicity is an independent predictor of poor outcome for BC survival (Newman et al., 2002) even after adjusting for age, stage, and socio-economic status (Newman et al., 2006). Literature reviews discuss the reasons and hypothesise the relationships among the factors from a biological perspective (Danforth, 2013), or further examine the role of treatment and health services in the disparity (Wheeler et al., 2013). These reviews unanimously conclude that understanding the underlying mechanisms (i.e., which factor triggers the reaction of another factor) is key for creating effective and innovative policies and interventions (Danforth, 2013;Wheeler et al., 2013). These meta-analyses and reviews rely on data-based models to discuss and summarise their findings.
In this paper, our objective is to identify which tasks are yet to be accomplished for identification of factors and mechanisms influencing BC mortality disparities in the United States. To accomplish this, we (1) provide a summary of the significant factors found using data-based modelling tools, (2) conduct a review of the models and data sources that have been used to assess the factor effects, and (3) discuss potential research tasks and useful modelling tools. We conducted (1) to contextualise the interested reader in the issue of disparities in BC mortality, and to address the lack of summaries about ethnic groups different from AAs and NHWs. With activities (2) and (3), we are filling the gap of literature reviews in data-based modelling approaches and we provide understanding on the use of these approaches for identification of factors and mechanisms. In what follows, we describe the methods used to conduct the review of the literature (Section 2), report the results of the review (Section 3), provide a discussion about research opportunities (Section 4), and make concluding remarks (Section 5).
2. Methodology
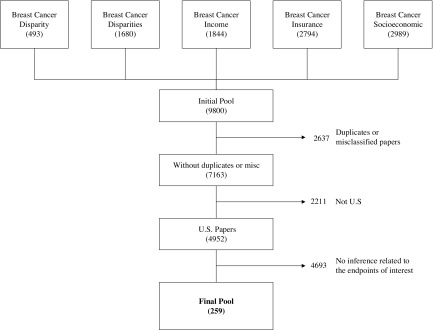
We conducted a search of research literature published from January 1990 to May 2016 in the MEDLINE and PubMed databases, by independently using each of the following Medical Subject Heading (MeSH) terms: “Breast cancer socioeconomic”, “Breast cancer insurance”, “Breast cancer disparity”, “Breast cancer disparities”, and “Breast cancer income”. The keywords used in separate searches were broad enough to yield a wide variety of studies with no restriction of targeted population and/or treatment, obtaining 9800 papers. Figure 1 shows the exclusion criteria used for obtaining the final pool of papers. Duplicates were excluded from the pool through the review of the title. Misclassified papers with titles unrelated to one or more of the MeSH terms were also excluded from the pool. The inclusion criteria were defined as studies performed in the U.S. testing the significance of factors influencing breast cancer disparities in mortality after diagnosis. Publications that did not meet these requirements were excluded from the initial pool through the revision of the title and the abstract. Two reviewers read the abstract and verbally discussed disagreements in the inclusion. Some papers were reviewed in their content if the title and the abstract were missing details concerning the inclusion criteria. This led to a final pool of 259 papers.
Figure 1.
Exclusion criteria.
A team of medical doctors and engineers was trained to collect relevant data from the papers by means of a computer-based questionnaire. After the training, papers were divided into groups and assigned to a single member of the team. The following information was collected from each paper: (1) Modelling approaches used to explore the influence of factors in BC mortality disparities, (2) Data-sets used in the models, (3) Factors significantly influencing mortality outcomes, and (4) Whether the effect of the factor was reducing or increasing BC-specific mortality (or all-cause mortality in the absence of evidence relating to BC mortality).
3. Results
3.1. Factors
In what follows, we discuss the factors found by the models to be associated with BC mortality. We have divided such factors into four categories: (1) Health care, (2) Resource deprivation, (3) Tumour stage and biology, (4) Comorbidities and lifestyle.
3.1.1. Health care
Screening mammography has been uniformly used across racial groups in the last 30 years but this behaviour has not reduced the racial disparity in BC mortality (Aizer et al., 2014; Van Ravesteyn et al., 2011; Wheeler et al., 2013). Once the cancer is detected, factors associated with health care personnel, settings, and treatments also influence the disparity.
With the objective of improving health services, factors including type of cancer centre attended (Bradley et al., 2003; Breslin et al., 2009; Onega et al., 2010; Roohan et al., 1998), specialised personnel assistance and availability (Chien et al., 2015; Fisher et al., 2013), and quality of care (Wagner et al., 2012) have been studied. Being assisted at a National Cancer Institute-designated centre seems to reduce the disparity in BC mortality between AAs and NHWs (Onega et al., 2010). In contrast, patients in hospitals with large minority populations, nursing homes, or long-term facilities have significantly worse survival chances than patients in other hospitals (Bradley et al., 2003; Breslin et al., 2009). For the whole U.S., worse mortality outcomes among AAs were obtained in health districts with worse access and quality of care (Wagner et al., 2012). Women with more than two primary care physician (PCP) visits during 2 years had lower odds of BC mortality compared to women with 1 or 0 PCP visits (Fisher et al., 2013). The mortality disparity is affected by the interaction between PCP availability, population density, the presence of Medicare beneficiaries, and socio-economic deprivation (Chien et al., 2015).
A wide variety of treatments have been observed for their associations with mortality disparity: bilateral and unilateral mastectomy, breast-conserving surgery (BCS), adjuvant therapy (i.e., radiotherapy, chemotherapy, and hormone therapy), and post-treatment surveillance mammography. In general, the literature concludes that the less timely or underused the health care, the more the likelihood of BC mortality (Hershman et al., 2006; McCarthy et al., 2006; Smith, Ziogas, & Anton, 2013; Wilson et al., 2007; Yood et al., 2008). For overall treatment courses, it seems that this association is more pronounced in AI/AN women (Wilson et al., 2007), in young AAs with public or no insurance, and in patients with low socio-economic status (SES) (Smith et al., 2013). For radiotherapy after BCS, underuse and treatment delays have been identified in the elderly (Celaya et al., 2006; Chagpar et al., 2008; Dragun et al., 2011; Hershman et al., 2008; Smith et al., 2010), in the poor/uninsured (Buchholz et al., 2006; Dragun et al., 2011; Foley et al., 2007; McCarthy et al., 2006), in AAs (Dragun et al., 2011; Hershman et al., 2008; Joslyn, 2002; Smith et al., 2010), and in rural/Appalachian patients for which the reach and quality of services are scarce (Dragun et al., 2011). For chemotherapy, delay of initiation and premature termination have been associated with AA (Fedewa et al., 2010; Hershman et al., 2005, 2006, 2009; Nurgalieva et al., 2013b), Hispanic, and Medicare patients (Nurgalieva et al., 2013b). In addition, post-diagnosis mammograms that are administered inconsistently have increased the number of undetected new or recurrent breast cancers in Hispanic patients (Smith-Gagen et al., 2013). With regard to hormone therapy, there is evidence that non-adherence or discontinuation is associated with higher all-cause mortality (Hershman et al., 2011), but we could not find evidence of mortality outcomes that were altered in interaction or combination with race or SES. One of the causes for non-adherence or discontinuation was the cost of the therapy. Women who took generic Aromatase Inhibitors (GAI) were less likely to discontinue therapy compared to brand-name Aromatase Inhibitors (BAI), as the median 30-day copayment was higher for BAI ($33.3) than for GAI ($9.04) (Hershman et al., 2014).
Other conclusions have been reached by comparing observed vs ideal or baseline treatments. The study in (Javid et al., 2014) concludes that a baseline treatment of surgery, adjuvant therapy, and post-treatment surveillance has been received by AI/AN women in lower proportions. The authors in (Kurian et al., 2014) demonstrate that bilateral mastectomy or BCS plus radiation yields lower mortality than unilateral mastectomy, but unilateral mastectomy has been observed more frequently in Filipina and Hispanic minorities than the other two options. Using BCS with radiation as a baseline, the authors in (DeRouen et al., 2013) demonstrate that AAs receiving either mastectomy or BCS without radiation are more likely to die of breast cancer.
3.1.2. Resource deprivation
Resource deprivation has been studied from multiple factors, including socio-economic status, income, employment status, insurance status, urban/rural household, educational level, poverty level, and proportion of minorities in a residential community. Higher BC mortality has been associated with lower SES (Feinglass et al., 2015; Schlichting et al., 2012), lower income level (Ansell et al., 1993; Cella et al., 1991; Komenaka et al., 2010; Grann et al., 2006; Whitman et al., 2012), lower salary (Kallan, 1997; O’Malley et al., 2003; Vinnakota and Lam, 2006), Medicare/Medicaid enrollment (Yu et al., 2014), rural household (Hall et al., 2004; Hershman et al., 2006; Singh et al., 2011; Tian et al., 2012), and living in areas of high poverty (Du et al., 2008; Grann et al., 2006; Niu et al., 2010; Schootman et al., 2008). Also, although there is considerable evidence that higher educational attainment improves the likelihood of BC survival (Albano et al., 2007; Cella et al., 1991; Grann et al., 2006; Herndon et al., 2013; Kim et al., 2005a; Sprague et al., 2011; Vinnakota & Lam, 2006), it seems that the opposite can also be true, as higher educational attainment can reduce BC survival in patients with more complex jobs and lifestyles (Kallan, 1997; Okunade & Karakus, 2003).
Several resource deprivation factors have jointly contributed to the BC mortality disparity. In a study that included BC and other cancer types, patients with lower income and lower education levels had greater chances to die of cancer (Cella et al., 1991). A more recent study concludes that areas with low educational attainment and employment, and a high proportion of people with low paying jobs (e.g. mining, construction, and transportation) are those with worse BC prognosis (Vinnakota & Lam, 2006).
Race and resource deprivation have been studied for their association with BC mortality. Using death files of the 25 largest cities in the U.S., it was concluded that median household income and the ratio of AA/NHW local presence explained the mortality disparity between AAs and NHWs (Whitman et al., 2012). Enrolment in Medicaid, Medicare, public, or no insurance also increases short-term mortality likelihood for AAs vs NHWs (Yu et al., 2014). In general, patients with no insurance or Medicaid have had higher overall mortality (Shi et al., 2015). Comparing BC death rates of women living in the Mississippi Delta region and women living elsewhere in the U.S., it was observed that white rural and black urban residents are more likely to have higher death rates than their counterparts in other regions (Hall et al., 2004). A more recent study concluded that SES was a stronger determinant for patients living in metropolitan areas despite race (AA or NHWs) (Singh et al., 2011). Among women with invasive breast cancer, being insured and having access to medical care does not eliminate the AA vs NHW survival disparity (Field et al., 2005).
3.1.3. Tumour stage and biology
Tumour stage and biology may interact with social and health systems factors to produce mortality disparities. Tumour stage describes tumour size and invasion (i.e., localised, regional, metastasis), while biology refers to cellular histology, tumour phenotype, and cellular mechanisms. Worse tumour stage and biology are usually associated with a higher mortality risk (Danforth, 2013; El-Tamer, 1999; Herndon et al., 2013; Hill et al., 2010; Maskarinec et al., 2011; Meng et al., 1997b; Simon & Severson, 1997; Wheeler et al., 2013; Yu et al., 2014). Compared to NHWs, AAs present a more aggressive histology (i.e. higher likelihood of the tumour spreading to regional and distant sites while still small), earlier onset, and a more advanced stage of presentation (Batina et al., 2013; Crowe et al., 2005; Danforth, 2013; Edwards et al., 1998; Simon & Severson, 1997; Wheeler et al., 2013; Yu et al., 2014). Hispanic women usually present at a later stage and with more aggressive forms of BC (Lopez et al., 2013). Asians present a more advanced disease than non-Hispanic white patients, but a less advanced disease than AA and Hispanic white patients (Yi et al., 2012). An exception among Asians are Japanese, who present a survival advantage over non-Hispanic white and other Asian subtypes in the localised and regional stages. Japanese survival advantage disappears in the metastatic stage (Yi et al., 2012).
Tumour phenotypes provide information about the mechanisms that stimulate tumour growth. Estrogen receptor (ER+) or progesterone receptor (PR+) tumours likely grow due to the action of the hormones, while tumours producing an excess of the human epidermal growth factor 2 (HER2+) protein use this mechanism to grow. Tumours can grow due to the action of more than one mechanism. Mortality has been found to be significantly worse in AA women with ER+/PR+ (Zhang et al., 2014) or with ER+/PR+/HER2- (where HER2- stands for tumours not influenced by HER2) (Sparano et al., 2011; Wright et al., 2012). More recently, there is specific evidence suggesting that AAs with ER+/PR+/HER2- cancers in stages 2 and 3 present a higher risk of death compared to NHWs (95% CI hazard ratios of 1.03–1.65 and 1.10–1.75, for stages 2 and 3, respectively) (Tao et al., 2015).
Compared to NHWs, ER- tumours and ER-/PR-/HER2- (i.e., tumours not influenced by any of the three mechanisms) are more common in AA women (Danforth, 2013; Sparano et al., 2011; Swede et al., 2011; Wright et al., 2012; Yu et al., 2014). Although some research suggests that the mortality disparity is independent of the ER-/PR-/HER2- status (Swede et al., 2011; Wright et al., 2012), some new evidence indicates that ER-/PR-/HER2- in stage 3 are at a higher death risk compared to NHWs (Tao et al., 2015). Such knowledge can inform policies aimed at identifying risk groups in need of closer follow-up for treatment.
Hispanic women with a family history of breast cancer are more likely to develop ER- cancer compared to NHWs (Lopez et al., 2013). However, links between the cancer phenotypes and mortality in Hispanics have not been found. In Asians, a recent study suggested that Japanese patients were more likely to be diagnosed with stage I disease and ER+ and PR+ tumours than patients in the other Asian groups (Yi et al., 2012). API patients had more ER-/PR- tumours and worse survival than NHW and other Asian subtypes. (Yi et al., 2012).
Cellular mechanisms are major drivers of BC. Cell proteins serve as regulators of cellular processes and their functions are encoded by genes that can be altered as a result of environmental stimuli. Well-known proteins and genes that influence BC are the BRCA (BReast CAncer susceptibility), which are expressed in the breast tissue and repair chromosomal damage in the cellular DNA. If BRCA genetic mutations occur and the proteins are expressed inappropriately, BC risk may increase. BRCA mutations seem to be prevalent across different ethnicities, and therefore genetic testing for these mutations is recommended for all women who have a family or personal history of BC (Hall et al., 2009).
Several proteins, associated genes, and gene alterations (i.e., single nucleotide polymorphisms, SNPs) influence the mortality risk per ethnicity. In AA women, increased mortality risk is influenced by the enhanced expression of proteins p16, p53, cyclin E, cyclin A, cyclin B, and the reduced expression of cyclin E and RASSF1A (Danforth, 2013; Wheeler et al., 2013). The role of these regulators in BC mortality is reviewed in Danforth 2013 (Danforth, 2013). Researchers from the “Breast Cancer Health Disparities Study (BCHDS)" have performed gene extraction in a large sample of Hispanic and (AI/AN) women confirmed with BC. The study analyses genes of cellular proteins that have been previously linked to breast cancer progression since the proteins participate in the execution of a variety of cellular processes, including cell proliferation, differentiation, migration, transcription regulation, development, apoptosis, extracellular matrix remodelling, inflammatory response, and angiogenesis. An example from the BCHDS is the genetic analysis of interleukin (IL) proteins, which have been associated with breast cancer as they control inflammatory and anti-inflammatory responses of the immune system. One SNP in the IL17A gene was significantly associated with breast cancer mortality and all-cause mortality among women with greater Native American ancestry. Two SNPs in the IL23R gene were significantly associated with breast cancer-specific mortality among women with lower Native American ancestry (Slattery et al., 2014a). From the 17 publications of the Breast Cancer Disparities Study included in this review (Boone et al., 2014a, 2015; Connor et al., 2013, 2014, 2016a, b; Pellatt et al., 2013, 2016; Slattery et al., 2013a, b, c, 2014a, b, c, d, 2015a, b), 10 found genetic variations in cellular proteins that were specifically associated with breast cancer mortality by racial genetic ancestry (Connor et al., 2013; Pellatt et al., 2013, 2016; Slattery et al., 2013a, b, c, 2014a, b, c, d) thus providing evidence of the significant differences in breast cancer development per genetic ancestry. Such analyses are yet to be replicated in patients with racial backgrounds different from AA and Hispanic/Native American.
3.1.4. Comorbidities and lifestyle
Comorbidities that worsen the BC mortality risk include low pre-treatment haematological variables (e.g., hemoglobin) (Wang et al., 2015), extreme presence or absence of body fat (Izano et al., 2014; Kwan et al., 2014; Maskarinec et al., 2011), obesity (Connor et al., 2016b), and diabetes (Du & Simon, 2005; Lopez et al., 2013; Slattery et al., 2014b). AAs (but not NHWs) with lower pre-treatment levels of haematological variables and higher red blood cell distribution width present an increased mortality risk (Wang et al., 2015). Asian Americans (but not AAs, Latinas, or NHWs) with high waist-to-hip ratio present a higher risk of breast cancer mortality. Underweight NHWs present a higher breast cancer mortality risk, and this association is not observed in AAs and Asian Americans (Kwan et al., 2014). Also, obesity at age 30 increased BC-specific mortality in NHWs but not in Hispanics (Connor et al., 2016b). There exists the need for further evidence about the effect of diabetes per racial group as the current studies are yet few and contradictory (Lopez et al., 2013). In general, AA patients diagnosed with triple negative breast cancer present higher comorbidities at diagnosis than NHWs (Swede et al., 2016).
Lifestyle factors that influence BC mortality include vitamin C intake, total caloric intake, aspirin, tobacco, alcohol consumption, body mass index (BMI), and marital status (Okunade & Karakus, 2003; Slattery et al., 2014b; Wu et al., 2013). We could find evidence of racial differences in BC mortality for tobacco, alcohol consumption, BMI, and marital status. In a study of AAs and white women, tobacco smoking was found a significant predictor of BC mortality only for the white women (Izano et al., 2014); also, among a pool of NHW, Hispanic, and AI/AN women, cigarette smoking and a larger BMI were associated with poorer survival among NHW women during pre-menopause. In the same pool, greater amount and long-term alcohol consumption were associated with poorer survival among Hispanic and AI/AN women (Slattery et al., 2014b). For marital status, it has been shown that married women are more likely to survive BC than non-married women (Gomez et al., 2010; Hershman et al., 2006; Meng et al., 1997a). Having the support and monitoring of a partner could help women receive treatments and adhere to them.
3.2. Models
Table 1 shows the models ranked by frequency of use. Modelling approaches have been used for the following purposes: (1) Bivariate statistical analyses, (2) Multivariate analyses, (3) Multilevel modelling, (4) Data mining, and (5) Simulation of disease progression.
Table 1.
Models used to analyse the effect of factors in racial mortality disparities ranked by frequency of use.
| Model type | References using the model | Total | % |
|---|---|---|---|
| Cox regression | Adams et al. (2012), Ademuyiwa et al. (2013), Ademuyiwa et al. (2015), Akinyemiju et al. (2013b), Aggarwal et al. (2015), Akinyemiju et al. (2016), Albain et al. (2009), Andres et al. (1996), Ansell et al. (1993), Ayanian et al. (1993), Balasubramanian et al. (2010), Barcenas et al. (2010), Berz et al. (2009), Beyer et al. (2016a), Beyer et al. (2016b), Bharat et al. (2009), Bleicher et al. (2016), Boone et al. (2014b), Boone et al. (2014a), Boone et al. (2015), Bradley et al. (2005), Braun et al. (2001), Breslin et al. (2009), Brooks et al. (2013), Byers et al. (2008), Camacho-Rivera et al. (2008), Castro-Echeverry et al. (2013), Chagpar et al. (2011), Chen et al. (2015), Cheng et al. (2015), Chu et al. (2010), Chuang et al. (2006), Clegg et al. (2002), Connor et al. (2013), Connor et al. (2014), Connor et al. (2016b), Darcy et al. (2015), Dawood et al. (2008), DeRouen et al. (2013), Deshpande et al. (2009), Dragun et al. (2011), Du and Simon (2005), Du et al. (2008), Du et al. (2011), Elmore et al. (2005), El-Tamer (1999), Feinglass et al. (2015), Fejerman et al. (2013), Field et al. (2005), Franzini et al. (1997), George et al. (2013), Giraldo-Jimenez et al. (2012), Gnerlich et al. (2009), Gomez et al. (2010), Grann et al. (2006), Grau et al. (2005), Greenwald et al. (1996), Haas et al. (2008), Haji-Jama et al. (2016), Hastert et al. (2014), Hernandez et al. (2015), Herndon et al. (2013), Hershman et al. (2005), Hershman et al. (2006), Hershman et al. (2009), Hill et al. (2010), Holmes et al. (2010), Howard et al. (1998), Hu et al. (2013), Iqbal et al. (2015), Izano et al. (2013), Izano et al. (2014), Javid et al. (2014), Joslyn (2002), Kallan (1997), Kaplan et al. (2015), Keegan et al. (2013), Keegan et al. (2015), Kim et al. (2005b), Kirsner et al. (2006), Kish et al. (2014), Komenaka et al. (2010), Koru-Sengul1 et al. (2016), Kroenke et al. (2014), Kurian et al. (2014), Kwan et al. (2014), Lee et al. (2014), Li et al. (2013), Lian et al. (2014), Liu et al. (2013), Ma et al. (2015), Maggard et al. (2003), Markossian and Hines (2012), Markossian et al. (2012), Martindale et al. (2014), Maskarinec et al. (2003), Maskarinec et al. (2011), McCarthy et al. (2007), Menashe et al. (2009), Meng et al. (1997a), Meng et al. (1997b), Ning et al. (2015), Niu et al. (2013), Nurgalieva et al. (2013c), Nurgalieva et al. (2013a), Nurgalieva et al. (2013b), O’Malley et al. (2003), Ohri et al. (2016), Ooi and Martinez (2011), Owusu et al. (2007), Parise and Caggiano (2015), Pellatt et al. (2016), Perkins et al. (1996), Potosky et al. (1997), Pruitt et al. (2015), Rajan et al. (2015), Richter et al. (2013), Roetzheim et al. (2008), Roohan et al. (1998), Roseland et al. (2015), Rueth et al. (2014), Rugo et al. (2013), Russell et al. (2011), Schlichting et al. (2012), Schonberg et al. (2010), Schinkel et al. (2014), Silber et al. (2013), Simon and Severson (1997), Simon and Severson (2006), Shariff-Marco et al. (2014), Shariff-Marco et al. (2015b), Shavers et al. (2003), Shi et al. (2013), Shi et al. (2015), Sineshaw et al. (2015), Slattery et al. (2014d), Slattery et al. (2013c), Slattery et al. (2014b), Slattery et al. (2014c), Smith et al. (2013), Smith-Gagen et al. (2013), Sposto et al. (2016), Sprague et al. (2011), Swede et al. (2011), Swede et al. (2016), Tabung et al. (2016), Tannenbaum et al. (2013), Tao et al. (2015), Tichy et al. (2015), Wang et al. (2015), Ward et al. (2008), Warner et al. (2015), Weaver et al. (2013), Wilson et al. (2007), Wolfson et al. (2015), Wray et al. (2013), Woodward et al. (2006), Wright et al. (2012), Wu et al. (2013), Yang et al. (2009), Yao et al. (2009), Yi et al. (2012), Zhu et al. (2014), Zeng et al. (2015) | 164 | 63.3 |
| Chi-square tests | Ademuyiwa et al. (2013),Ademuyiwa et al. (2015), Aggarwal et al. (2015), Akinyemiju et al. (2013b), Akinyemiju et al. (2015), Akinyemiju et al. (2016), Barcenas et al. (2010), Berz et al. (2009), Bharat et al. (2009), Bleicher et al. (2016), Boone et al. (2014b), Boone et al. (2014a), Boone et al. (2015), Braun et al. (2001), Breslin et al. (2009), Camacho-Rivera et al. (2008), Castro-Echeverry et al. (2013), Chang et al. (1998), Chen et al. (2015), Connor et al. (2013), Connor et al. (2014), Connor et al. (2016b), Connor et al. (2016a), Darcy et al. (2015), Du et al. (2008), Elmore et al. (2005), El-Tamer (1999), Feinglass et al. (2015), Fisher et al. (2013), Gnerlich et al. (2009), Gorey et al. (2013), Grau et al. (2005), Haji-Jama et al. (2016), Hassett et al. (2014), Herndon et al. (2013), Hernandez et al. (2015), Hershman et al. (2005), Hershman et al. (2006), Howard et al. (1998), Hu et al. (2013), Hunt et al. (2014), Iqbal et al. (2015), Izano et al. (2013), Izano et al. (2014), Javid et al. (2014), Joslyn (2002), Kaplan et al. (2015), Kim et al. (2005b), Kim et al. (2016), Kirsner et al. (2006), Klassen et al. (2015), Koru-Sengul1 et al. (2016), Kroenke et al. (2014), Lairson et al. (2015), Lee-Feldstein et al. (2001), Lian et al. (2014), Liu et al. (2013), Llanos et al. (2015), Ma et al. (2015), Mandelblatt et al. (2002), Markossian et al. (2012), Martindale et al. (2014), McDavid et al. (2003), Meng et al. (1997b), Mueller et al. (2015), Ning et al. (2015), Nurgalieva et al. (2013c), Nurgalieva et al. (2013a), Nurgalieva et al. (2013b), O’Malley et al. (2003), Owusu et al. (2007), Pellatt et al. (2013), Polednak (2002), Pruitt et al. (2015), Richter et al. (2013), Rizzo et al. (2015), Roetzheim et al. (2000), Roseland et al. (2015), Rueth et al. (2014), Russell et al. (2011), Samson et al. (2015), Shi et al. (2015), Slattery et al. (2014b), Slattery et al. (2014a), Slattery et al. (2015b), Short et al. (2010), Guadagnoli et al. (1997), Simon and Severson (2006), Sposto et al. (2016), Smith et al. (2013), Smith-Gagen et al. (2013), Sturtz et al. (2014), Swede et al. (2011), Swede et al. (2016), Tannenbaum et al. (2013), Tao et al. (2015), Toro et al. (2016), Trinh et al. (2015), Wang et al. (2015), Wilson et al. (2007), Woodward et al. (2006), Wray et al. (2013), Wright et al. (2012), Robin (2003), Yang et al. (2009), Yao et al. (2009), Yi et al. (2012), Zhang et al. (2015) | 108 | 41.7 |
| Kaplan-Meier survival curves | Ademuyiwa et al. (2013), Ademuyiwa et al. (2015), Aggarwal et al. (2015), Akinyemiju et al. (2016), Albain et al. (2009), Andres et al. (1996), Ansell et al. (1993), Ayanian et al. (1993), Balasubramanian et al. (2010), Barcenas et al. (2010), Beyer et al. (2016b), Biswas et al. (2015), Boone et al. (2014b), Bradley et al. (2003), Bradley et al. (2005), Breslin et al. (2009), Camacho-Rivera et al. (2008), Castro-Echeverry et al. (2013), Cella et al. (1991), Chagpar et al. (2011), Chang et al. (1998), Chen et al. (2015), Chu et al. (2010), Chuang et al. (2006), Clegg et al. (2002), Crowe et al. (2005), Darcy et al. (2015), Dawood et al. (2008), Dragun et al. (2011), Du and Simon (2005), Du et al. (2008), Du et al. (2011), Elmore et al. (2005), Feinglass et al. (2015), Fejerman et al. (2013), Franzini et al. (1997), Giraldo-Jimenez et al. (2012), Grann et al. (2006), Grau et al. (2005), Greenwald et al. (1996), Hernandez et al. (2015), Herndon et al. (2013), Hershman et al. (2005), Hershman et al. (2006), Hershman et al. (2008), Holmes et al. (2010), Hossain et al. (2008), Hu et al. (2013), Izano et al. (2013), Izano et al. (2014), Kaplan et al. (2015), Kim et al. (2005b), Kirsner et al. (2006), Kish et al. (2014), Komenaka et al. (2010), Koru-Sengul1 et al. (2016), Lairson et al. (2015), Liu et al. (2013), | 97 | 37.5 |
| Martindale et al. (2014), Maskarinec et al. (2003), Meng et al. (1997b), Ning et al. (2015), Niu et al. (2013), Nurgalieva et al. (2013c), Nurgalieva et al. (2013a), Ohri et al. (2016), O’Malley et al. (2003), Owusu et al. (2007), Parise and Caggiano (2015), Perkins et al. (1996), Potosky et al. (1997), Rizzo et al. (2015), Roohan et al. (1998), Roseland et al. (2015), Rueth et al. (2014), Rugo et al. (2013), Schlichting et al. (2012), Shavers et al. (2003), Shi et al. (2013), Shi et al. (2015), Sineshaw et al. (2015), Smith et al. (2013), Smith-Gagen et al. (2013), Swede et al. (2011), Tannenbaum et al. (2013), Tao et al. (2015), Tichy et al. (2015), Wang et al. (2015), Wolfson et al. (2015), Woodward et al. (2006), Wray et al. (2013), Wright et al. (2012), Yang et al. (2009), Yi et al. (2012), Schinkel et al. (2014), Zeng et al. (2015), Zhang et al. (2015) | |||
| Logistic regression | Akinyemiju et al. (2015), Akinyemiju et al. (2016), Berz et al. (2009), Bleicher et al. (2016), Boone et al. (2014a), Bradley et al. (2003), Bradley et al. (2002), Braun et al. (2004), Cella et al. (1991), Cheng et al. (2015), Connor et al. (2013), Connor et al. (2014), Connor et al. (2016a), Dragun et al. (2011), Du et al. (2011), Fisher et al. (2013), Gorey et al. (2013), Haas et al. (2008), Howard et al. (1998), Iqbal et al. (2015), Javid et al. (2014), Kim et al. (2005a), Kim et al. (2016), Klassen et al. (2015), Kurian et al. (2014), Lairson et al. (2015), Lee-Feldstein et al. (2000), Lee-Feldstein et al. (2001), Li et al. (2013), Llanos et al. (2015), Mandelblatt et al. (2002), Markossian and Hines (2012), Markossian et al. (2012), McCarthy et al. (2007), Onega et al. (2010), Ooi and Martinez (2011), Parikh et al. (2015), Pellatt et al. (2013), Polednak (2002), Potosky et al. (1997), Rajan et al. (2015), Richter et al. (2013), Roetzheim et al. (2000), Roetzheim et al. (2008), Rueth et al. (2014), Samson et al. (2015), Schonberg et al. (2010), Shavers et al. (2003), Short et al. (2010), Silber et al. (2013), Sineshaw et al. (2015), Guadagnoli et al. (1997), Slattery et al. (2014d), Slattery et al. (2013b), Slattery et al. (2014b), Slattery et al. (2014c), Slattery et al. (2015a), Slattery et al. (2015b), Sprague et al. (2011), Swede et al. (2011), Tian et al. (2011), Tian et al. (2012), Tichy et al. (2015), Wang et al. (2015), Warner et al. (2015), Wolfson et al. (2015), Wilson et al. (2007), Zhu et al. (2014), Zhang et al. (2015), | 69 | 26.6 |
| t-tests or z-tests | Ademuyiwa et al. (2015), Aggarwal et al. (2015), Akinyemiju et al. (2013b), Ansell et al. (1993), Barcenas et al. (2010), Berz et al. (2009), Bleicher et al. (2016), Boone et al. (2015), Camacho-Rivera et al. (2008), Chang et al. (1998), Chu et al. (2010), Connor et al. (2013), Connor et al. (2014), Connor et al. (2016b), Connor et al. (2016a), Darcy et al. (2015), El-Tamer (1999), Fejerman et al. (2013), Grau et al. (2005), Hassett et al. (2014), Herndon et al. (2013), Hershman et al. (2005), Hossain et al. (2008), Howard et al. (1998), Izano et al. (2013), Izano et al. (2014), Javid et al. (2014), Kim et al. (2016), Kirsner et al. (2006), Komenaka et al. (2010), Koru-Sengul1 et al. (2016), Levine et al. (2008), Ma et al. (2015), Markossian et al. (2012), Rajan et al. (2015), Rizzo et al. (2015), Rust et al. (2015), Short et al. (2010), Guadagnoli et al. (1997), Smith-Gagen et al. (2013), Sturtz et al. (2014), Swede et al. (2011), Swede et al. (2016), Wang et al. (2015), Whitman et al. (2011), Wray et al. (2013) | 46 | 17.7 |
| Log rank test | Ademuyiwa et al. (2013), Ademuyiwa et al. (2015), Aggarwal et al. (2015), Andres et al. (1996), Ayanian et al. (1993), Boone et al. (2014b), Breslin et al. (2009), Camacho-Rivera et al. (2008), Castro-Echeverry et al. (2013), Chagpar et al. (2011), Chang et al. (1998), Chen et al. (2015), Chuang et al. (2006), Dawood et al. (2008), Elmore et al. (2005), Feinglass et al. (2015), Franzini et al. (1997), Grau et al. (2005), Greenwald et al. (1996), Hernandez et al. (2015), Holmes et al. (2010), Hu et al. (2013), Iqbal et al. (2015), Kim et al. (2005b), Komenaka et al. (2010), Koru-Sengul1 et al. (2016), Niu et al. (2010), Niu et al. (2013), Nurgalieva et al. (2013a), Ohri et al. (2016), Osteen et al. (1994), Parise and Caggiano (2015), Roetzheim et al. (2000), Roseland et al. (2015), Rueth et al. (2014), Schinkel et al. (2014), Shi et al. (2013), Smith et al. (2013), Smith-Gagen et al. (2013), Sturtz et al. (2014), Tannenbaum et al. (2013), Wang et al. (2015), Woodward et al. (2006), Wray et al. (2013), Wright et al. (2012), Wang et al. (2015), Wolfson et al. (2015) | 47 | 18.1 |
| Incidence and mortality ratios | Albano et al. (2007), Baggett et al. (2015), Baquet et al. (2013), Chu et al. (2003), Chu et al. (2007), DeSantis et al. (2016), Haji-Jama et al. (2016), Hill et al. (2015), Levine et al. (2008), McCarthy et al. (2015), McDavid et al. (2003), Meliker et al. (2009), Menashe et al. (2009), Miller et al. (2008), Nurgalieva et al. (2013c), Samson et al. (2016), Simon and Severson (1997), Steenland et al. (2004), Tian et al. (2011), Tian et al. (2012), Whitman et al. (2012) | 21 | 8.1 |
| ANOVA | Adams et al. (2015), Akinyemiju et al. (2015), Akinyemiju et al. (2016), Braun et al. (2001), Darcy et al. (2015), Fejerman et al. (2013), Joslyn (2002), Koru-Sengul1 et al. (2016), Mandelblatt et al. (2002), Martindale et al. (2014), Mueller et al. (2015), Pruitt et al. (2015), Shavers et al. (2003), Sturtz et al. (2014), Toro et al. (2016), Trinh et al. (2015), Wray et al. (2013) | 17 | 6.6 |
| Linear regression | Akinyemiju et al. (2015), Belasco et al. (2014), Darcy et al. (2015), Edwards et al. (1998), Hassett et al. (2014), Hershman et al. (2006), Menashe et al. (2009), Mueller et al. (2015), Ohri et al. (2016), Okunade and Karakus (2003), Philips Jr et al. (2013), Rust et al. (2015), Sabik and Bradley (2013), Singh et al. (2011), Slattery et al. (2015a) | 15 | 5.8 |
| Bonferroni correction methods | Chu et al. (2003), Newman et al. (2006), Zeng et al. (2015), Boone et al. (2014a), Connor et al. (2014), Fisher et al. (2013), Pellatt et al. (2013), Pellatt et al. (2016), Slattery et al. (2013b), Slattery et al. (2013a), Slattery et al. (2013c), Slattery et al. (2014b), Slattery et al. (2014a), Slattery et al. (2015a), Slattery et al. (2015b) | 15 | 5.8 |
| Wald tests | Slattery et al. (2014d), Slattery et al. (2013b), Slattery et al. (2013a), Slattery et al. (2013c), Slattery et al. (2014b), Slattery et al. (2014a), Hernandez et al. (2015), Pellatt et al. (2016), Slattery et al. (2015a), Slattery et al. (2015b), Warner et al. (2015) | 11 | 4.3 |
| Fisher exact test | Biswas et al. (2015), Koru-Sengul1 et al. (2016), Izano et al. (2013), Izano et al. (2014), Silber et al. (2013), Smith et al. (2013), Sparano et al. (2011), Tichy et al. (2015), Wilson et al. (2007), Wright et al. (2012) | 10 | 3.9 |
| Wilcoxon rank sum test | Ademuyiwa et al. (2013), Ayanian et al. (1993), Hassett et al. (2014), Izano et al. (2013), Izano et al. (2014), Rueth et al. (2014), Silber et al. (2013), Slattery et al. (2015b), Tichy et al. (2015), Wilson et al. (2007) | 10 | 3.9 |
| Poisson regression | Akinyemiju et al. (2013a), Boscoe and Pradhan (2015), Jatoi et al. (2005), Menashe et al. (2009), Philips Jr et al. (2013), Steenland et al. (2004), Robin (2003) | 7 | 2.7 |
| Adaptive Rank Truncated Product | Slattery et al. (2014d), Slattery et al. (2013c), Slattery et al. (2014b), Slattery et al. (2014a), Slattery et al. (2015a), Slattery et al. (2015b), Pellatt et al. (2016) | 7 | 2.7 |
| Data Mining | Belasco et al. (2014), Boone et al. (2015), Darcy et al. (2015), Rajan et al. (2015), Tian et al. (2012), Toro et al. (2016), Vinnakota and Lam (2006) | 7 | 2.7 |
| Joinpoint regression | Akinyemiju et al. (2013a), Hall et al. (2004), Kurian et al. (2014), Ortiz et al. (2010), Whitman et al. (2011), Whitman et al. (2012) | 6 | 2.3 |
| Kruskal-Wallis | Chagpar et al. (2011), Lee-Feldstein et al. (2001), Liu et al. (2013), Mueller et al. (2015), Ning et al. (2015), Rueth et al. (2014) | 6 | 2.3 |
| Hierarchical linear modeling | Chien et al. (2015), Klassen et al. (2015), Schootman et al. (2008), Russell et al. (2011) | 4 | 1.6 |
| Mediation analysis | Russell et al. (2011), Schootman et al. (2008), Warner et al. (2015), Yu et al. (2014) | 4 | 1.6 |
| Geospatial analysis | Klassen et al. (2015), Mueller et al. (2015), Russell et al. (2011), Schootman et al. (2008) | 3 | 1.2 |
| Spearman correlation | Hassett et al. (2014), Mueller et al. (2015), Pruitt et al. (2015) | 3 | 1.2 |
| Simulation | Chang et al. (2012), Van Ravesteyn et al. (2011) | 2 | 0.8 |
| Kolmogorov-Smirnov tests | Izano et al. (2013), Izano et al. (2014) | 2 | 0.8 |
| Kriging | Chien et al. (2015), Silber et al. (2013) | 2 | 0.8 |
| Mantel-Haenszel | Owusu et al. (2007), Schonberg et al. (2010) | 2 | 0.8 |
| Spline functions | Levine et al. (2008), Menashe et al. (2009) | 2 | 0.8 |
| Likelihood ratio tests | Boone et al. (2015), Chagpar et al. (2011) | 2 | 0.8 |
| Propensity score adjustment | Bleicher et al. (2016), Lairson et al. (2015) | 2 | 0.8 |
| Competing risks regression | Bleicher et al. (2016), Trinh et al. (2015) | 2 | 0.8 |
| Geographically weighted regression models | Tian et al. (2011) | 1 | 0.4 |
| MCMC | Sprague et al. (2011) | 1 | 0.4 |
| F tests | Wagner et al. (2012) | 1 | 0.4 |
| Hardy-Weinberg statistics | Boone et al. (2014a) | 1 | 0.4 |
| McNemar test | Silber et al. (2013) | 1 | 0.4 |
| Prentice Wilcoxon test | Silber et al. (2013) | 1 | 0.4 |
| Linear trend test | Robin (2003) | 1 | 0.4 |
| Cochran-Armitage Trend test | Baggett et al. (2015) | 1 | 0.4 |
| Scheffe’s test | Joslyn (2002) | 1 | 0.4 |
| Hosmer-Lemeshow test | Wilson et al. (2007) | 1 | 0.4 |
| Smoothed hazard functions | Ademuyiwa et al. (2015) | 1 | 0.4 |
| Median test | Haji-Jama et al. (2016) | 1 | 0.4 |
| Age-period-cohort model | Masters et al. (2015) | 1 | 0.4 |
| Gray test | Ohri et al. (2016) | 1 | 0.4 |
| Probit regression | Pezzin et al. (2015) | 1 | 0.4 |
| Additive regression model with Bayesian priors | Chien et al. (2015) | 1 | 0.4 |
| Instrumental variable analysis | Pezzin et al. (2015) | 1 | 0.4 |
| Undescribed regression model | McDavid et al. (2003) | 1 | 0.4 |
3.2.1. Bivariate statistical analyses
Basic statistical tests are initially used to determine the univariate effect of a factor in a mortality outcome. Such analyses provide direction to interpret findings in multivariate models. Chi-square or Fisher’s exact tests are used to compare demographic and clinical characteristics across racial groups (Breslin et al., 2009; Izano et al., 2014); t, Kruskal–Wallis, Bivariate ANOVA, and Wilcoxon Rank Sum tests were used to determine whether the mean, median, or cumulative number of deaths can be significantly influenced by race (Adams et al., 2015; Ademuyiwa et al., 2015; Ning et al., 2015; Silber et al., 2013). Kaplan–Meier curves and Log-rank tests were calculated to understand the effect of a specific variable in the length of survival. For example, to compare the isolated effect of race in survival, a Kaplan–Meier curve can be fitted for each race group, and statistical differences between race groups can be obtained through the Log-rank test (Aggarwal et al., 2015). Single degree Wald tests were generally used to tests linear interactions between two factors (Pellatt et al., 2016). Adaptive Rank Truncated Products were always used to determine joint associations between genetic abnormalities and mortality (Slattery et al., 2014a). In our pool, Chi-square, Kaplan–Meier curves, t-tests, and Log-rank tests were the most frequently used (41.7, 37.5, 17.7, and 18.1%, respectively), followed by ANOVA (6.6%), Kruskal-Wallis (2.3%), Wald test (4.3%), Fisher’s exact test (3.9%), Wilcoxon Rank Sum test (3.9%), and Adaptive Rank Truncated Product (2.7%).
3.2.2. Multivariate analyses
Regression models have been consistently used to determine which factors (or variables) have a significant effect on the response. The response can be in the form of survival times as in Cox Regression models (Wu et al., 2013) or real/binary/count data as in Generalised Linear and Quantile regression models (Belasco et al., 2014). Cox regression is used by 63.3 % of the pooled literature, followed by Logistic (26.6%), Linear (5.8%), and Poisson (2.7%) regression models.
Time trend analyses were not very common in our pool, but they were usually conducted using Joinpoint regression, where the time is linearly associated with an outcome of interest (e.g., mortality rate) (Akinyemiju et al., 2013a). Several linear models are fitted to different trend segments, and statistical hypothesis testing is conducted to determine whether the slopes of each segment are significantly different. Joinpoint regression was used in 2.3% of our papers.
Few contributions were observed to identify spatial clusters of factors associated with mortality outcomes and to determine the significance of factors in the geographic distribution of mortality. Using Geographic Information Systems, data from factors including number and distance from medical providers (Russell et al., 2011), social-class and tobacco use (Klassen et al., 2015), and coal-mining exposure (Mueller et al., 2015) have been geographically linked to mortality in search of meaningful spatial clusters. Geographically weighted linear regression was used to determine whether joint racial disparities in late-stage diagnosis and mortality rates displayed a greater likelihood in adjacent census tracts compared to non-adjacent ones (Tian et al., 2011). Geospatial analysis and Geographically weighted linear regression were used in 1.2 and 0.4% of the papers in the pool, respectively.
Through multivariate regression models, researchers not only attempt to understand the association between independent and response variables, but they also attempt to understand associations among independent variables as well. A common way to understand associations among independent variables is by systematically controlling (i.e., blocking) the effect of one or more variables and observing the effect of the remaining variables on the response. Another way to understand associations is by incorporating interaction terms in the regression models. When, for example, a two-level interaction is included in a regression model, it explicitly tests the hypothesis that the effect of one independent variable on the response varies at different levels of another independent variable. With the previous approaches, it is possible to formulate hypotheses about variables interacting to moderate the response, but not about mechanisms under which variables exert direct and indirect effects on the response. One approach exploring simple mechanisms is called mediation analysis. Assume a binary response (e.g., dead or alive at the end of the third year of BC diagnosis), a categorical predictor (e.g., race), and a variable that mediates between the predictor and the response (e.g., insurance). In this case, the hypothesised scenario is that insurance exerts a direct effect on the response while race exerts an indirect effect in the response through insurance. In a mediation analysis, there can be two equations to be fitted with the data: one equation where the response is modelled as a function of race, and a second equation where the response is modelled as a function of both race and insurance. The effect of race on the response can be interpreted from its fitted coefficient in the first equation, and the effect of insurance on the response can be interpreted from its fitted coefficient in the second equation. From these coefficients, the proportion of change can be calculated in the response that could be attributed to the mediating variable. If the proportion of change is high, it can be concluded that the hypothesised mechanism is a plausible one. Formal mediation analyses were seldom observed in our pool (Russell et al., 2011; Schootman et al., 2008; Warner et al., 2015; Yu et al., 2014).
3.2.3. Multi-level modeling
Multi-level or hierarchical regression models are a set of nested models where a regression coefficient of a high-level hierarchy model is provided with its own regression model (Gelman, 2006). In our pool, multi-level models were built for residents nested in census-based regions (Klassen et al., 2015; Russell et al., 2011; Schootman et al., 2008). In this way, estimates of the response can better capture the variability within and across census-based regions (Gelman, 2006).
3.2.4. Data Mining models
Data mining models have been mostly used for data preprocessing and preliminary analysis. Principal Component Analysis was used for reducing the dimensionality of correlated socio-economic variables (Belasco et al., 2014; Boone et al., 2015; Rajan et al., 2015; Tian et al., 2012), and to evaluate patterns in principal components of gene expression variability (Toro et al., 2016). Clustering methods were used for observing ethnic clusters from genome data (Boone et al., 2015) and for imputation of missing data (Darcy et al., 2015). One association rule mining algorithm was applied to extract associations between the geographical distribution of cancer mortality rates and socio-economic variables (Vinnakota & Lam, 2006). One rule showed that, for example, high mortality rates were concentrated in areas where AA women were householders with underage children and no husband present.
3.2.5. Simulation models
Discrete event simulations have been used to understand factor effects in the mortality disparity (Chang et al., 2012; Van Ravesteyn et al., 2011). Simulations work by modelling individuals which may develop BC considering disease natural history parameters and intervention strategies. These models are useful to study disparities as it is possible to investigate the isolated effect of each factor by sequentially substituting parameters inherent to a racial group into the baseline white population model. Simulation has been useful to validate the fact that stage, phenotype, rate of tumor spread, and hormone therapy have a significant effect in the mortality disparity between AAs and NHWs (Van Ravesteyn et al., 2011). Simulation was also used to investigate the effect of obesity in the AAs-NHWs mortality disparity, concluding that the effect of obesity is marginal since it is a protective factor for patients 50 years, but it is a risk factor for patients 50 years (Chang et al., 2012).
3.3. Databases
Table 2 shows the data sources used in the identification of factors associated with BC mortality disparities. Local/state cancer registries and hospital databases were used in 48.6% of the papers, while 33.9% of the papers used the database from the Surveillance, Epidemiology, and End Results (SEER) Program. The SEER program is managed by the National Cancer Institute and collects data on cancer cases from several locations and sources across the U.S. Other cancer registry databases include the National Cancer Database (2.3%), and the National Program of Cancer Registries (0.4%). Further data analyses were conducted by linking cancer registry data to other demographic, economic, behavioural, and environmental data sources. In the United States, the most usual demographic and economic databases are Census (15.4%) and Medicare (4.3%). Behavioural databases included the Behavioural Risk Factors Surveillance Systems at the state level (1.2%), and the Consumer Expenditure Survey at the Bureau of Labor Statistics (U.S. Census). Environmental data sources included the U.S. Energy Information Administration (Mueller et al., 2015) and Daily Particulate Matter Monitoring (Hu et al., 2013). A portion of the papers (17.4%) used cancer registry data to identify patients for further data collection at the individual level. Individual data were collected in the form of interviews, retrospective chart reviews, observations, and DNA samples.
Table 2.
Databases used to analyse the effect of factors in racial mortality disparities ranked by frequency of use.
| Database | References using the model | Total | % |
|---|---|---|---|
| Local/state cancer registries and hospital databases | Adams et al. (2012), Aggarwal et al. (2015), Albain et al. (2009), Andres et al. (1996), Ansell et al. (1993), Akinyemiju et al. (2013a), Ayanian et al. (1993), Balasubramanian et al. (2010), Baggett et al. (2015), Baquet et al. (2013), Barcenas et al. (2010), Berz et al. (2009), Beyer et al. (2016a), Beyer et al. (2016b), Bharat et al. (2009), Biswas et al. (2015), Boone et al. (2015), Bradley et al. (2003), Bradley et al. (2005), Braun et al. (2001), Castro-Echeverry et al. (2013), Camacho-Rivera et al. (2008), Cella et al. (1991), Chagpar et al. (2011), Cheng et al. (2015), Chu et al. (2010), Connor et al. (2016b), Connor et al. (2016a), Crowe et al. (2005), DeRouen et al. (2013), Dragun et al. (2011), Du and Simon (2005), Elmore et al. (2005), El-Tamer (1999), Fejerman et al. (2013), Franzini et al. (1997), Giraldo-Jimenez et al. (2012), Gomez et al. (2010), Grau et al. (2005), Greenwald et al. (1996), Gorey et al. (2013), Haji-Jama et al. (2016), Herndon et al. (2013), Hershman et al. (2005), Hill et al. (2015), Hirschman et al. (2007), Howard et al. (1998), Hu et al. (2013), Izano et al. (2013), Izano et al. (2014), Jatoi et al. (2005), Kaplan et al. (2015), Keegan et al. (2013), Keegan et al. (2015), Kim et al. (2005b), Kim et al. (2016), Klassen et al. (2015), Komenaka et al. (2010), Koru-Sengul1 et al. (2016), Kroenke et al. (2014), Kurian et al. (2014), Lee et al. (2014), Lee-Feldstein et al. (2000), Lee-Feldstein et al. (2001), Lian et al. (2014), Llanos et al. (2015), Martindale et al. (2014), Maskarinec et al. (2003), Maskarinec et al. (2011), McDavid et al. (2003), Meliker et al. (2009), Meng et al. (1997a), Meng et al. (1997b), Mueller et al. (2015), Niu et al. (2010), Niu et al. (2013), Ohri et al. (2016), Ortiz et al. (2010), Owusu et al. (2007), Parikh et al. (2015), Parise and Caggiano (2013), Parise and Caggiano (2015), Pellatt et al. (2016), Perkins et al. (1996), Polednak (2002), Potosky et al. (1997), Pruitt et al. (2015), Rajan et al. (2015), Richter et al. (2013), Rizzo et al. (2015), Roetzheim et al. (2000), Roohan et al. (1998), Roseland et al. (2015), Russell et al. (2011), Sabik and Bradley (2013), Samson et al. (2015), Samson et al. (2016), Shariff-Marco et al. (2014), Shariff-Marco et al. (2015a), Shi et al. (2013), Simon and Severson (1997), Simon and Severson (2006), Slattery et al. (2015a), Slattery et al. (2015b), Smith et al. (2013), Sposto et al. (2016), Kwan et al. (2014), Wray et al. (2013), Wu et al. (2013), Sprague et al. (2011), Swede et al. (2011), Swede et al. (2016), Tannenbaum et al. (2013), Tian et al. (2011), Tian et al. (2010), Tian et al. (2012), Tichy et al. (2015), Wang et al. (2015), Wilson et al. (2007), Woodward et al. (2006), Wright et al. (2012), Tao et al. (2015), Yang et al. (2009), Zhang et al. (2015), Zhu et al. (2014), Wolfson et al. (2015) | 126 | 48.6 |
| SEER | Ademuyiwa et al. (2013), Ademuyiwa et al. (2015), Akinyemiju et al. (2013b), Akinyemiju et al. (2016), Ayanian et al. (1993), Bleicher et al. (2016), Boone et al. (2014b), Boscoe and Pradhan (2015), Bradley et al. (2002), Braun et al. (2004), Breslin et al. (2009), Brooks et al. (2013), Chang et al. (1998), Chen et al. (2015), Chien et al. (2015), Chu et al. (2003), Chuang et al. (2006), Clegg et al. (2002), Connor et al. (2016b), Dawood et al. (2008), DeSantis et al. (2016), Deshpande et al. (2009), Du and Simon (2005), Du et al. (2008), Du et al. (2011), Edwards et al. (1998), Field et al. (2005), Fisher et al. (2013), Franzini et al. (1997), George et al. (2013), Gnerlich et al. (2009), Gomez et al. (2010), Grann et al. (2006), Haas et al. (2008), Hassett et al. (2014), Hastert et al. (2014), Hernandez et al. (2015), Hershman et al. (2006), Hill et al. (2010), Holmes et al. (2010), Hossain et al. (2008), Hu et al. (2013), Iqbal et al. (2015), Jatoi et al. (2005), Javid et al. (2014), Joslyn (2002), Kaplan et al. (2015), Keegan et al. (2015), Kirsner et al. (2006), Kish et al. (2014), Lairson et al. (2015), Li et al. (2013), Liu et al. (2013), Maggard et al. (2003), Markossian and Hines (2012), McCarthy et al. (2007), Menashe et al. (2009), Meng et al. (1997a), Meng et al. (1997b), Miller et al. (2008), Mueller et al. (2015), Ning et al. (2015), Nurgalieva et al. (2013c), Nurgalieva et al. (2013a), Nurgalieva et al. (2013b), O’Malley et al. (2003), Onega et al. (2010), Ooi and Martinez (2011), Ortiz et al. (2010), Pellatt et al. (2016), Polednak (2002), Roetzheim et al. (2008), Schlichting et al. (2012), Schonberg et al. (2010), Schootman et al. (2008), Shavers et al. (2003), Silber et al. (2013), Simon and Severson (1997), Simon and Severson (2006), Smith-Gagen et al. (2013), Swede et al. (2011), Wagner et al. (2012), Robin (2003), Yi et al. (2012), Schinkel et al. (2014), Trinh et al. (2015), Zeng et al. (2015) | 87 | 33.9 |
| Census | Adams et al. (2015), Albano et al. (2007), Akinyemiju et al. (2013b), Cheng et al. (2015), Chien et al. (2015), Chu et al. (2007), Haji-Jama et al. (2016), Hastert et al. (2014), Hu et al. (2013), Hunt et al. (2014), Keegan et al. (2015), Kish et al. (2014), Markossian et al. (2012), McCarthy et al. (2015), Mueller et al. (2015), Okunade and Karakus (2003), Parise and Caggiano (2015), Pezzin et al. (2015), Philips Jr et al. (2013), Pruitt et al. (2015), Rajan et al. (2015), Roseland et al. (2015), Russell et al. (2011), Rust et al. (2015), Shariff-Marco et al. (2015b), Shariff-Marco et al. (2015a), Schlichting et al. (2012), Schootman et al. (2008), Simon and Severson (1997), Singh et al. (2011), Sprague et al. (2011), Steenland et al. (2004), Tannenbaum et al. (2013), Tao et al. (2015), Tian et al. (2011), Trinh et al. (2015), Vinnakota and Lam (2006), Wagner et al. (2012), Whitman et al. (2012), Robin (2003) | 42 | 15.4 |
| Collection of DNA, interview, chart, or observational data | Boone et al. (2014b), Boone et al. (2014a), Boone et al. (2015), Connor et al. (2013), Connor et al. (2014), Connor et al. (2016b), Connor et al. (2016a), Cheng et al. (2015), Gordon et al. (1992), George et al. (2013), Hastert et al. (2014), Hershman et al. (2009), Herndon et al. (2013), Izano et al. (2013), Izano et al. (2014), Kim et al. (2016), Kroenke et al. (2014), Kwan et al. (2014), Llanos et al. (2015), Ma et al. (2015), Pellatt et al. (2013), Pellatt et al. (2016), Pezzin et al. (2015), Rugo et al. (2013), Shariff-Marco et al. (2014), Shariff-Marco et al. (2015b), Shariff-Marco et al. (2015a), Slattery et al. (2014d), Slattery et al. (2013b), Slattery et al. (2013a), Slattery et al. (2014b), Slattery et al. (2014a), Slattery et al. (2015a), Slattery et al. (2015b), Sposto et al. (2016), Steenland et al. (2004), Sturtz et al. (2014), Swede et al. (2016), Tabung et al. (2016), Tichy et al. (2015), Toro et al. (2016), Wang et al. (2015), Ward et al. (2008), Whitman et al. (2011), Wu et al. (2013) | 45 | 17.4 |
| State and National Mortality databases | Albano et al. (2007), Baggett et al. (2015), Beyer et al. (2016a), Beyer et al. (2016b), Kim et al. (2005a), Masters et al. (2015), Pruitt et al. (2015), Rust et al. (2015), Singh et al. (2011), Tannenbaum et al. (2013), Tian et al. (2010), Tian et al. (2012), Wagner et al. (2012), Weaver et al. (2013) | 14 | 5.4 |
| Medicare | Aggarwal et al. (2015), Bleicher et al. (2016), Brooks et al. (2013), Hassett et al. (2014), Hershman et al. (2006), Kirsner et al. (2006), Nurgalieva et al. (2013b), Pezzin et al. (2015), Roetzheim et al. (2008), Schootman et al. (2008), Silber et al. (2013) | 11 | 4.3 |
| National Center for Health Statistics | Chu et al. (2007), Chien et al. (2015), Hall et al. (2004), Kallan (1997), Whitman et al. (2011), Hunt et al. (2014), McCarthy et al. (2015), Rust et al. (2015) | 8 | 3.1 |
| National Cancer Database (American College of surgeons) | Feinglass et al. (2015), Rueth et al. (2014), Shi et al. (2015), Sineshaw et al. (2015), Ward et al. (2008), Yao et al. (2009) | 6 | 2.3 |
| Centers for disease control and prevention | Jatoi et al. (2005), Levine et al. (2008), Okunade and Karakus (2003), Whitman et al. (2012) | 4 | 1.6 |
| Not specified/Not found | Belasco et al. (2014), Guadagnoli et al. (1997), Weber et al. (2014) | 3 | 1.2 |
| Medicaid | Aggarwal et al. (2015), Samson et al. (2015), Weaver et al. (2013) | 3 | 1.2 |
| National Cancer Institute | Adams et al. (2015), Hernandez et al. (2015), Samson et al. (2016) | 3 | 1.2 |
| State Behavioural Risk Factors Surveillance Systems | Mueller et al. (2015), Okunade and Karakus (2003), Samson et al. (2016) | 3 | 1.2 |
| Home Mortgage Disclosure Act data | Beyer et al. (2016a), Beyer et al. (2016b) | 2 | 0.8 |
| Health Resources and Services Administration | Adams et al. (2015), Akinyemiju et al. (2016) | 2 | 0.8 |
| National Establishment Time Series Database | Cheng et al. (2015), Shariff-Marco et al. (2015a) | 2 | 0.8 |
| California Department of Transportation | Cheng et al. (2015), Shariff-Marco et al. (2015a) | 2 | 0.8 |
| California neighbourhoods data systems | Cheng et al. (2015), Sposto et al. (2016) | 2 | 0.8 |
| Publicly available tumor gene expression datasets | Darcy et al. (2015) | 1 | 0.4 |
| U.S. Energy Information Administration | Mueller et al. (2015) | 1 | 0.4 |
| Medical Marketing Services | Russell et al. (2011) | 1 | 0.4 |
| American Hospital Association Survey | Pezzin et al. (2015) | 1 | 0.4 |
| Mammography machine data - US Food and drug administration | Pruitt et al. (2015) | 1 | 0.4 |
| Health Care Financing Administration | Mandelblatt et al. (2002) | 1 | 0.4 |
| HealthCore Integrated Research Database | Short et al. (2010) | 1 | 0.4 |
| National Institutes of Health | Sparano et al. (2011) | 1 | 0.4 |
| National Institute of Occupational Safety and Health | Steenland et al. (2004) | 1 | 0.4 |
| Social Security Denominator file | Silber et al. (2013) | 1 | 0.4 |
| Daily particulate matter monitoring data | Hu et al. (2013) | 1 | 0.4 |
| Department of Agriculture | Trinh et al. (2015) | 1 | 0.4 |
| National program of cancer registries | Byers et al. (2008) | 1 | 0.4 |
| National Comprehensive Cancer Network | Warner et al. (2015) | 1 | 0.4 |
| Health care Cost and Utilisation Project Nationwide Inpatient Sample | Akinyemiju et al. (2015) | 1 | 0.4 |
4. Research gaps and opportunities
4.1. Explore mechanisms triggering disparities using data-based models
A wide variety of systemic, social, and individual factors are determinants of BC mortality likelihood. Some of these factors influence the mortality likelihood in a different way, being ethnicity one of the major drivers of the association. In the U.S., BC mortality is different per racial background, being significantly different between AAs and NHWs, and between Hispanics and NHWs, where both AAs and Hispanics are highly disadvantaged. Such observation has motivated the challenge of eliminating the racial gap, not only for AAs and Hispanics but for all racial groups.
In this paper, we have discussed the influence of health care, resource deprivation, tumour stage and biology, physical status, behaviour, and lifestyle in BC mortality per race. Delayed or poor quality health care, high resource deprivation, poor physical status, and advanced tumour stage are usually stated as contributors to mortality disparities. Tumour phenotypes and cellular mechanisms, as well as lifestyle choices, are less intuitive in their influence in BC mortality, and their role in disparities is subject to ongoing research (Danforth, 2013; Lopez et al., 2013; Wheeler et al., 2013).
We have conducted a review of the data-based models that have been used to identify factors and explain mechanisms driving BC mortality disparities. In most papers, data are initially explored and aggregated using basic statistical analyses and data mining techniques such as Chi-square tests, Kaplan–Meier curves, Principal Component analysis, Clustering, and Association Rule Mining. Data are generally fed into multivariate regression models to determine single and interaction effects that are significantly associated with mortality outcomes. Mechanistic hypotheses are infrequently established by means of mediation analyses.
In general, there is considerable knowledge on the factors influencing mortality disparities. However, there is a gap in understanding the specific mechanisms where multiple factors interrelate to yield a mortality disparity.
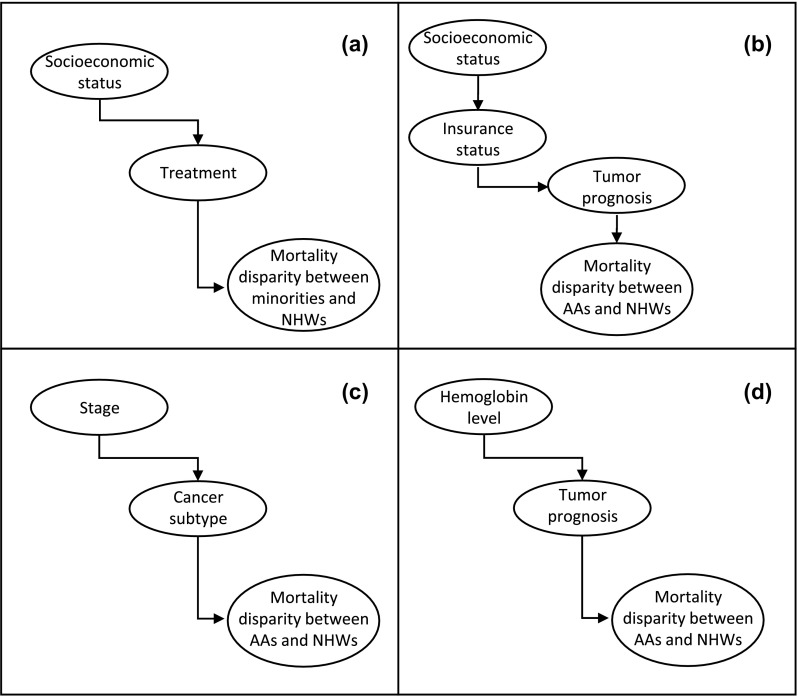
To understand this gap, consider four examples of mechanism that can be extracted from the literature. In the health care factors Section (3.1.1), we mentioned the results in (Kurian et al., 2014), where unilateral mastectomy patients from Filipina and Hispanic minorities were at a higher mortality risk than patients who underwent bilateral mastectomy or BCS plus radiation. The study also shows that unilateral mastectomy patients were less likely to live in high SES neighbourhoods and to attend National Cancer Center Hospitals; and more likely to hold public/Medicaid insurance and to be seen in hospitals targeted to the lower SES population. From these conclusions, the study discusses the possibility that patients with low SES characteristics seek unilateral mastectomy more often, exerting an indirect negative effect on the mortality (see Figure 2(a) for a raw depiction of the mechanism). In the Resource deprivation Section (3.1.2), the study in (Feinglass et al., 2015) concludes that race, insurance status, and stage at BC presentation reduce the effect of SES in all-cause mortality. A possible link among these factors is that SES contributes to lower quality or no insurance. A poor insurance status consequently increases the chances of a poor tumour prognosis (with the lack of resources for early tumour detection), and a poor tumour prognosis increases the AAs/NHWs mortality disparity (see Figure 2(b)). In the Tumour stage and biology Section (3.1.3), it is concluded that AAs with ER+/PR+/HER2- cancers in stages 2 and 3 present a higher risk of death compared to NHWs (Tao et al., 2015). An example mechanism is that the tumour stage is indirectly related to the mortality disparity through the cancer subtype (see Figure 2(c)), which may be possible if certain subtypes are more frequent in certain stages, and these subtypes increase in turn the mortality disparity. In the comorbidities and lifestyle Section (3.1.4), recent research provides concluding evidence to associate haematological variables with the higher survival advantage of NHW over AAs (Wang et al., 2015). An example of a possible mechanism might indicate that poorer health of AA patients at diagnosis, evidenced through low Haemoglobin levels, can contribute to a worse tumour prognosis, and a worse tumour prognosis increases the mortality disparity (see Figure 2(d)).
Figure 2.
Plausible mechanisms interconnecting factors influencing mortality disparities.
Notes: Factor names are inside the nodes, and the nodes are interconnected through arrows. The arrow direction indicates the direction of the influence from one factor to another.
The previous mechanisms were built using significant factors and hypothetical relationships that are presented in the discussion sections of the papers. But mechanisms like these are rarely modelled using existing data. Modelling mechanisms can be helpful to better quantify factor effects and target interventions on the factors with higher effects. In what follows, we present several frameworks for modelling mechanisms:
Bayesian networks. Mechanisms can be graphically represented using directed acyclic graphs (DAGs), where the factors are nodes and the influence of one factor in another is represented through an arrow pointing at the direction of the influenced factor. In its nonparametric form, DAGs can represent Bayesian Networks, where factors are interconnected through their probabilistic dependencies (e.g., the conditional dependence of factor B on A can be represented as a node A with an arrow pointing towards node B). The whole graph maps the joint probability distribution of all the factors. The advantage of Bayesian Networks is that factors effects can be easily interpreted through marginal probabilities computable from the network. Bayesian Networks have been previously used to predict cancer diagnosis and to characterise treatment recommendations (Soto et al., 2017; Waleska et al., 2015).
Structural equation models. Mechanisms can also be represented through path diagrams, where nodes represent either observed or latent variables, and arrows can be directed (indicating the orientation of the influence) or undirected (indicating correlations without orientation of the influence). Path diagrams are used to map structural equation models, where some assumptions are imposed to represent the influence of one factor in another (e.g., a node A with arc point towards node B represents , where represents the effect of A in B, homogeneous across the population under study (Aalen et al., 2012)). Structural equation models can be used to conduct mediation analyses that validate association patterns explaining the mortality outcomes. For example, mediation analysis has been helpful to validate that insurance types such us Medicare, Medicaid, public, or no insurance are covering AAs more frequently, and insurance types significantly explain BC mortality. Hence, there is a possible mechanism where the effect of race in BC mortality is mediated through effect of insurance type (Yu et al., 2014).
Computer simulations. In simulations, the tumour progression per patient is modelled subject to the effect of socio-demographic and treatment effectiveness parameters. Simulation parameters can be computed from multiple data sources, and hence data harmonisation procedures might not be needed. Simulations can generate scenarios that are very difficult to observe in reality (e.g., in a simulation with NHW parameters, some parameter values can be replaced by those of the AAs to observe the effect in mortality).
We believe that the previous modelling frameworks may benefit from the new information available on the associations between cellular proteins, associated genes, and gene alterations in the mortality disparity per ethnicity. It would be interesting to observe in which way external factors like lifestyle, resource deprivation, and health care interact with each other to influence (or be influenced by) cellular components. Exploration of these mechanisms is seldom found in the literature (Slattery et al., 2015b).
4.2. Monitor factors associated with the mortality disparity across time.
At present, Cox regression models are used to understand the multivariate effect of factors in the survival time. However, we were not able to find modelling frameworks that monitor factors associated with disparities across time. Monitoring might be used to continuously detect deviations from expected patterns that might be signalling a disparity. Consider the case of radiotherapy adherence. If a statistical control chart is implemented to detect cases in which radiotherapy was expected but it was never administered, it may be possible to conduct retrospective chart reviews on the fewer anomalous cases indicated by the control chart and the final mortality outcomes. Findings can be used for quality improvement efforts within the health care centres administering radiotherapy. In the United States, it might be possible to conduct statistical monitoring using data from SEER and hospital cancer registries.
4.3. Increase the collection of behavioural data
Lifestyle factors are significant contributors to the racial mortality disparity. To analyse lifestyle factors, registries were linked to additional data sources including interviews (Izano et al., 2014; Slattery et al., 2014b), census data, and retrospective chart reviews (Hershman et al., 2006; Meng et al., 1997a). We were not able to find studies that used lifestyle data gathered directly from local/state/national cancer registries. We believe this limitation might discourage further research for associations between racial mortality disparity and lifestyle. Cancer centres might benefit the research community by further customising their databases and data collection procedures to capture such relevant information.
5. Conclusion
We conclude that the literature is very consistent in identifying that delayed or poor-quality health care, high resource deprivation, poor physical status, and advanced tumour stage are associated with racial disparities in BC mortality. There is an increasing body of research investigating cellular mechanisms, and several genes have already been identified as contributors to BC mortality disparities in Hispanic and (AI/AN) women. We also found that the evidence about the association of lifestyle choices with BC mortality disparities is still weak, and might be improved by adapting electronic medical records to collect behavioural data. Stronger evidence is key for supporting policies aimed at promoting better population lifestyles.
We also discussed the need to improve factor characterisation by systematically exploring and evaluating mechanisms triggering racial mortality disparities. Most of the models already used have been suitable for understanding what factors are associated with a disparity, but are unsuitable for testing mechanisms aimed at determining the role of a factor in generating a disparity. Testing mechanisms can be accomplished by explicitly modelling factor relationships (e.g., Bayesian networks and structural equation models), and by evaluating scenarios for which data cannot be collected in reality (e.g., computer simulations). In addition, it may be important to further integrate novel genetic information into the mechanisms to be evaluated.
Finally, we observe that the use of systems that monitor factors across time might be helpful for the timely detection of patterns that can be hard to observe with aggregated data.
6. Limitations
Although our literature review is comprehensive, it is limited in the systematic procedures taken for paper search and screening. The search was conducted in the MEDLINE and not the EMBASE database. In addition, the number of papers for which there was disagreement in the inclusion criteria was not recorded.
We also note that the review is limited to the papers addressing racial disparities in BC mortality. Other endpoints, including racial disparities in BC incidence and treatment adherence, were not discussed in this paper and may be subject to future research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aalen O., Roysland K., Gran J., & Lederberger B. (2012). Causality, mediation and time: a dynamic viewpoint. Journal of the Royal Statistical Society: Series A ,175(4), 831–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S., Butler W., Fulton J., Heiney S., Williams E., Delage A., ... Hebert J.(2012). Racial disparities in breast cancer mortality in a multiethnic cohort in the southeast. Cancer, 118(10), 2693–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S., Choi S., Khang L., Campbell D., Friedman D., Eberth J., ... Hebert J. (2015). Decreased cancer mortality-to-incidence ratios with increased accessibility of federally qualified health centers. Journal of Community Health ,40, 633-–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademuyiwa F., Gao F., Hao L., Morgensztern D., Aft R., Ma C., & Ellis M.,(2015). US breast cancer mortality trends in young women according to race. Cancer ,121, 1469-–1476, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademuyiwa F., Groman A., Hong C., Miller A., Kumar S., ... Ambrosone C., (2013). Time-trends in survival in young women with breast cancer in a SEER population-based study. Breast Cancer Res Treat, 138, 241-–248 [DOI] [PubMed] [Google Scholar]
- Aggarwal H., Callahan C., Miller K., Wanzhu T.u. & Loehrer P. (2015). Are there differences in treatment and survival between poor, older black and white women with breast cancer? JAGS (The American Geriatrics Society), 63(10), 2008–2013. [DOI] [PubMed] [Google Scholar]
- Aizer A., Wilhite T., Chen M., Graham P., Choueiri M., Hoffman K., ... Nguyen P.(2014). Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer, 1532–1539. [DOI] [PubMed] [Google Scholar]
- Akinyemiju T., Moore J., Ojesina A., Waterbor J., & Altekruse S. (2016). Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast Cancer Res Treat, 157, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemiju T., Soliman A., Copeland G., Banerjee M., Schwartz K. & Merajver S. (2013a). Trends in breast cancer stage and mortality in Michigan (1992–2009) by race, socioeconomic status, and area healthcare resources. PLOS ONE, 8(4), e61879, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemiju T., Soliman A., Johnson N., Altekruse S., Welch K., Banerjee M., ... Merajver S. (2013b). Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. Journal of Cancer Epidemiology, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemiju T., Vin-Raviv N., Chavez-yenter D., Zhao X., & Budhwani H. (2015). Race/ehnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiology ,39, 745–751. [DOI] [PubMed] [Google Scholar]
- Albain K., Unger J., Crowley J., Coltman C. Jr, & Hershman D. (2009). Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. JNCI ,101(14), 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano J., Ward E., Jemal A., Anderson R., Cokkinides V., Murray T., ...Thun M. (2007). Cancer mortality in the United States by education level and race. J Natl Cancer Inst ,99(18), 1384–1394. [DOI] [PubMed] [Google Scholar]
- Andres T., Baron A., Wright R., & Marine W. (1996). Tracking community sentinel events: Breast cancer mortality and neighborhood risk for advanced-stage tumors in Denver. American Journal of Public Health ,86(5), 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell D., Whitman S., Lipton R., & Cooper R. (1993). Race, income, and survival from breast cancer at two public hospitals. Cancer ,72(10), 2974–2978. [DOI] [PubMed] [Google Scholar]
- Ayanian J., Kohler B., Abe T., & Epstein A. (1993). The relation between health insurance coverage and clinical outcomes among women with breast cancer. The New England Journal of Medicine ,329(5), 326–331. [DOI] [PubMed] [Google Scholar]
- Baggett T., Chang Y., Porneala B., Bharel M., Singer D., & Rigotti N. (2015). Disparities in cancer incidence, stage, and mortality at Boston Health Care for the homeless program. American journal of Preventive Medicine ,49(5), 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B., Demissie K., Crabtree B., Ohman Strickland P., Kohler B., & Rhoads G.(2010). Racial differences in adjuvant systemic therapy for early breast cancer among Medicaid beneficiaries. The Breast Journal ,16(2), 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet C., Mishra S., Commiskey P., Ellison G., & DeShields M. (2013). Breast cancer epidemiology in blacks and whites: Disparities in incidence, mortality, survival rates and histology. Journal of the National Medical Association ,100(5), 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas C., Wells J., Chong D., French J., Looney S., & Samuel T. (2010). Race as an independent risk factor for breast cancer survival: Breast cancer outcomes from the medical college of Georgia tumor registry. Clinical Breast Cancer ,10(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Batina N., Trentham-Dietz A., Gangnon R., Sprague B., Rosenberg M., Stout N., ... Alagoz O. (2013). Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat ,138(2), 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco E., Gong G., Pence B., & Wilkes E. (2014). The impact of rural health care accessibility on cancer related behaviors and outcomes. Applied Health Economics and Health Policy ,12(4), 461–470. [DOI] [PubMed] [Google Scholar]
- Berz J., Johnston K., Backus B., Doros G., Rose A., Pierre S., & Battaglia T. (2009). The influence of black race on treatment and mortality for early-stage breast cancer. Medical Care ,47(9), 986–-992. [DOI] [PubMed] [Google Scholar]
- Beyer K., Zhou Y., Matthews K., Bemanian A., Laud P., & Nattinger A. (2016a). New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for heath disparities research. Health and Place ,40, 34–43. [DOI] [PubMed] [Google Scholar]
- Beyer K., Zhou Y., Matthews K., Hoormann K., Bemanian A., Laud P., & Nattinger A. (2016b). Breast and colorectal cancer survival disparities in Southeastern Wisconsin. WMJ ,115(1), 17–21. [PubMed] [Google Scholar]
- Bharat A., Aft R., Gao F., & Margenthaler J. (2009). Patient and tumor characteristics associated with increased mortality in young women (= 40 years) with breast cancer. Journal of Surgical Oncology ,100, 248-–251. [DOI] [PubMed] [Google Scholar]
- Biswas T., Efird J., Prasad S., James S., Walker P., & Zagar T. (2015). Inflammatory TNBC breast cancer: Demography and clinical outcome in a large cohort of patients with TNBC. Clinical Breast Cancer ,16(3), 212–216. [DOI] [PubMed] [Google Scholar]
- Bleicher R., Ruth K., Sigurdson E., Daly J., Boraas M., Anderson P., & Egleston B. (2016). Breast conservation versus mastectomy for patients with T3 primary tumors (cm): A review of 5685 Medicare patients. Cancer ,122(6), 42-–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S., Baumgartner K., Baumgartner R., Connor A., John E., Guiliano A., ... Slattery M. (2015). Active and passive cigerette smoking and mortality among hispanic and non-hispanic white women diagnosed with invasive breast cancer. Annals of Epidemiology ,25, 824–-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S., Baumgartner K., Baumgartner R., Connor A., Pinkston C., Rai S., ... Slattery M. (2014a). Associations between CYP19A1 polymorphisms, Native American ancestry, and breast cancer risk and mortality: the Breast Cancer Health Disparities Study. Cancer Causes Control ,25, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S., Baumgartner K., Joste N., Pinkston C., Pinkston C., Yang D., & Baumgartner R. (2014b). The joint contribution of tumor phenotype and education to breast cancer survival disparity between hispanic and non-hispanic white women. Cancer Causes Control ,25, 273–-282. [DOI] [PubMed] [Google Scholar]
- Boscoe F., & Pradhan E. (2015). A Medicare-associated spike in U.S. cancer rates at age 65, 2000–2010. Public Health Reports ,130, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C., Gardiner J., Given C., & Roberts C. (2005). Cancer, medicaid enrollment, and survival disparities. Cancer ,103, 1712-–8. [DOI] [PubMed] [Google Scholar]
- Bradley C., Given C., & Roberts C. (2002). Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst ,94, 490–-496. [DOI] [PubMed] [Google Scholar]
- Bradley C., Given C., & Roberts C. (2003). Correlates of late stage breast cancer and death in a Medicaid-insured population. Journal of Health Care for the Poor and Underserved ,14(4), 503–-515. [DOI] [PubMed] [Google Scholar]
- Braun K., Fong M., Gotay C., Pagano I., & Chong C. (2001). Ethnicity and breast cancer in Hawaii: Increased survival but continued disparity. Ethnicity and Disease ,15, 453–-460. [PubMed] [Google Scholar]
- Braun K., Fong M., Gotay C., Pagano I., & Chong C. (2004). Ethnic differences in breast cancer in Hawaii: age, stage, hormone receptor status, and survival. Pacific Cancer and Health Studies ,11(2), 453–-460. [PubMed] [Google Scholar]
- Breslin J., Morris A., Gu N., Wong S., Finlayson E., Banerjee M., & Birkmeyer J. (2009). Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. Journal Of Clinical Oncology ,27, 3945–-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breast cancer statistics. (n.d.). Retrieved from http://www.cdc.gov/cancer/breast/statistics/
- Brooks G., Li L., Sharma D., Weeks J., Hassett M., Yabroff K., & Schrag D. (2013). Regional variation in spending and survival for older adults with advanced cancer. Journal of the National Cancer Institute ,105, 634-–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz T., Theriault R., & Niland J. (2006). The use of radiation as a component of breast conservation therapy in national comprehensive cancer network centers. Journal of Clinical Oncology ,24, 361–-369. [DOI] [PubMed] [Google Scholar]
- Byers T., Wolf H., Bauer K., Bolick S., Chen V., Finch J., ...Yin X. (2008). The impact of socioeconomic status on survival after cancer in the United States. Cancer ,113(3), 582-–591. [DOI] [PubMed] [Google Scholar]
- Camacho-Rivera M., Kalwar T., Sanmugarajah J., Shapira I., & Taioli E. (2008). Heterogeneity of breast cancer clinical characteristics and outcome in us black women-Effect of place of birth. The American Journal of Surgery ,195(6), 793-–798. [DOI] [PubMed] [Google Scholar]
- Castro-Echeverry E., Kao L., Robinson E., Silberfein E., Ko T. & Wray C. (2013). Relationship between documentation status and survival for medically underserved hispanic breast cancer patients. Journal of Surgical Research, 180(2), 284–289, [DOI] [PubMed] [Google Scholar]
- Cancer facts and figures. (n.d.). Retrieved from http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- Celaya M., Rees J., Gibson J., Riddle B., & Greenberg E. (2006). Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (united states). Cancer Causes Control ,17, 851–856. [DOI] [PubMed] [Google Scholar]
- Cella D., Orav J., Kornblith A., Holland J., Silberfarb P., Won K., ... Chahinian P. (1991). Socioeconomic status and cancer survival. Journal of Clinical Oncology ,9(8), 1500–-1509. [DOI] [PubMed] [Google Scholar]
- Chagpar A., Cruchter C., Cornwell L., & McMasters K. (2011). Primary tumor size, not race, determines outcomes in women with hormone-responsive breast cancer. Surgey ,150(4), 796–801. [DOI] [PubMed] [Google Scholar]
- Chagpar A., McMasters K., Scoggins C., Martin R., Thoene C., & Edwards M. (2008). The use of radiation therapy after breast-conserving surgery in hormonally treated breast cancer patients is dependent on patient age, geographic region, and surgeon specialty. The American Journal of Surgery ,195(6), 793–-798. [DOI] [PubMed] [Google Scholar]
- Chang S., Parker S., Pham T., Buzdar A., & Hursting S. (1998). Inflammatory breast carcinoma incidence and survival. Cancer ,82(12), 2366–-2372. [PubMed] [Google Scholar]
- Chang Y., Schechter C., van Ravesteyn N., Near A., Heijnsdijk E., Adams-Campbell L., ... Mandelblatt J. (2012). Collaborative modeling of the impact of obesity on race-specific breast cancer incidence and mortality. Breast Cancer Research and Treatment ,136, 823–-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I., Shariff-Marco S., Koo J., Monroe K., Yang J., John E., ... Keegan T. H. (2015). Contribution of the neighborhood environment and obesity to breast cancer survival: The California Breast Cancer Survivorship Consortium. AACR (American Association of Cancer Research), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Song C., Ouyang Q., Jiang Y., Ye F., Ma F., ... Shao Z. (2015). Differences in breast cancer characteristics and outcomes between caucasian and chinese women in the US. Oncotarget ,6(14), 12774–-12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C., Deshpande A., Jeffe D., & Schootman M. (2015). nfluence of primary care physician availability and socioeconomic deprivation on breast cancer from 1988 to 2008: A spatio-temporal analysis. I. PLoS One, 7(4), e35737, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S., Chen W., Hashibe M., Li G., & Zhang Z. (2006). Survival rates of invasive breast cancer among ethnic chinese women born in East Asia and the United States. Asian Pacific Journal of Cancer Prevention ,7, 221–-226. [PubMed] [Google Scholar]
- Chu Q., Burton G., Glass J., Smith M., & Li B. (2010). Impact of race and ethnicity on outcomes for estrogen receptor-negative breast cancers: Experience of an academic center with a charity hospital. Journal of the American College of Surgeons ,210, 585–-594. [DOI] [PubMed] [Google Scholar]
- Chu K., Lamar C., & Freeman H. (2003). Racial disparities in breast carcinoma survival rates separating factors that affect diagnosis from factors that affect treatment. Asian Pacific Journal of Cancer Prevention ,97, 2853–-60. [DOI] [PubMed] [Google Scholar]
- Chu K., Miller B., & Springfield S. (2007). Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. Journal of the National Medical Association ,99(10), 1092–-1104. [PMC free article] [PubMed] [Google Scholar]
- Clegg L., Li F., Hankey B., Chu K., & Edwards B. (2002). Cancer survival among us whites and minorities. Archives of Internal Medicine ,162, 1985–1993. [DOI] [PubMed] [Google Scholar]
- Connor A., Baumgartner R., Baumgartner K., Pinkston C., Boone S., John E., ... Slattery M. (2014). Associations between ALOX, COX, and CRP polymorphisms and preast cancer among Hispanic and non-Hispanic White Women: The Breast Cancer Health Disparities Study. Molecular Carcinogenesis ,54, 1541–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor A., Baumgartner K., Baumgartner R., Pinkston C., Boone S., John E., ... Slattery M. (2016a). Cigarette smoking and breast cancer risk in hispanic and non-hispanic white women: The Breast Cancer Health Disparities Study. Journal of Women’s Health ,25(3), 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor A., Baumgartner R., Baumgartner K., Pinkston C., John E., Torres G., ... Slattery M. (2013). Epidermal growth factor receptor (EGFR) polymorphisms and breast cancer among Hispanic and non-Hispanic White women: The Breast Cancer Health Disparities Study. International Journal of Molecular Epidemiology and Genetics ,4(4), 235–249. [PMC free article] [PubMed] [Google Scholar]
- Connor A., Visvanathan K., Baumgartner K., Baumgartner R., Boone S., ... Slattery M. (2016b). Ethnic differences in the relationships between diabetes, early age adiposity and mortality among breast cancer survivors: The Breast Cancer Health Disparities Study. Breast Cancer Research and Treatment ,157, 167–-178. [DOI] [PubMed] [Google Scholar]
- Crowe J., Patrick R., Rybicky L., Grundfest J., Kim S., & Lee K. (2005). Race is a fundamental prognostic indicator for 2325 Northeastern Ohio women with infiltrating breast cancer. The Breast Journal ,11(2), 235–249. [DOI] [PubMed] [Google Scholar]
- Danforth D. (2013). Disparities in breast cancer outcomes between Caucasian and African American women: A model for describing the relationship of biological and nonbiological factors. Breast Cancer Research, 15(3), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy M., Fleming J., Robinson W., Kirk E., Perou C., & Troester M. (2015). Race-associated biological differences among luminal A breast tumors. Breast Cancer Research and Treatment ,152, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood S., Broglio K., Gonzalez A., Buzdar A., Hortobagyi G., & Giordano S. (2008). Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. Journal of Clinical Oncology ,26(30), 4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRouen M., Gomez S., Press D., Tao L., Kurian A., & Keegan T. (2013). A population-based observational study of first-course treatment and survival for adolescent and young adult females with breast cancer. Journal of Adolescent and Adult Oncology ,2(3), 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis C., Siege R., Sauer A., Miller K., Fedewa S., Alcaraz A., & Jemal K. (2016). Cancer statistics for african americans, 2016: Progress and opportunities in reducing racial disparities. Cancer Statistics for African Americans ,66, 290–308. [DOI] [PubMed] [Google Scholar]
- Deshpande A., Jeffe D., Gnerlich J., Iqbal A., Thummalakunta A., & Margenthaler J. (2009). Racial disparities in breast cancer survival: An analysis by age and stage. Journal of Surgical Research ,153, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragun A., Huang B., Tucker T., & Spanos W. (2011). Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer. Cancer ,117, 2590–2598, [DOI] [PubMed] [Google Scholar]
- Disparities. (n.d.). Retrieved from https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities
- Du X., Fang S., & Meyer T. (2008). Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. American Journal of Clinical Oncology ,31(2), 125–132. [DOI] [PubMed] [Google Scholar]
- Du X., Lin C., Johnson N., & Altekruse S. (2011). Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival. Cancer ,117, 3242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., & Simon M. (2005). Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in Metropolitan Detroit. Breast Cancer Research and Treatment ,91, 243–248. [DOI] [PubMed] [Google Scholar]
- Edwards M., Gamel J., Vaughan W., & Wrightson W. (1998). Infiltrating ductal carcinoma of the breast: The survival impact of race. Journal of Clinical Oncology, 16(8), 2693–2699, [DOI] [PubMed] [Google Scholar]
- Elmore J., Nakano C., Linden H., Reisch L., Ayanian J., & Larson E. (2005). Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Medical Care ,43(2), 141–148. [DOI] [PubMed] [Google Scholar]
- El-Tamer M., amd Homel M., & Wait R. (1999). Is race a poor prognostic factor in breast cancer? Journal of the American College of Surgeons ,189(1), 41–-45. [DOI] [PubMed] [Google Scholar]
- Fedewa S., Ward E., Stewart A., & Edge S. (2010). Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: A national cohort study 2004–-2006. Journal of Clinical Oncology ,28(27), 4135–4141. [DOI] [PubMed] [Google Scholar]
- Feinglass J., Rydzewski N., & Yang A. (2015). The socioeconomic gradient in all-cause mortality for women with breast cancer: Findings from 19908 to 2006 national cancer data base with follow-up through 2011. Annals of Epidemiology ,25, 549–-555. [DOI] [PubMed] [Google Scholar]
- Fejerman L., Hu D., Huntsman S., John E., Stern M., Haiman C., ... Ziv E. (2013). Genetic ancestry and risk of mortality among U.S. latinas with breast cancer. Cancer ,73(24), 7243–7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T., Buist D., Doubeni C., Enger S., Fouayzi H., Hart G., ... Yao J. (2005). Disparities and survival among breast cancer patients. Journal of the National Cancer Institute ,35, 88–95. [DOI] [PubMed] [Google Scholar]
- Fisher K., Lee J., Ferrante J., McCarthy E., Gonzalez E., Chen R., ... Roetzheim R. (2013). The effects of primary care on breast cancer mortality and incidence among Medicare beneficiaries. Cancer ,1192964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K., Kimmick G., Camacho F., Levine E., Balkrishnan R., & Anderson R. (2007). Survival disadvantage among Medicaid insured breast cancer patients treated with breast conserving surgery without radiation therapy. Breast Cancer Research and Treatment ,101, 207–214. [DOI] [PubMed] [Google Scholar]
- Franzini L., Williams A., Franklin J., Singletary S., & Theriault R. (1997). Effects of race and socioeconomic status on survival of 1,332 black, hispanic, and white women with breast cancer. Annals of Surgical Oncology ,4(2), 111–118. [DOI] [PubMed] [Google Scholar]
- Gelman A. (2006). Multilevel (Hierarchical) modeling: What it can and cannot do. Technometrics ,48(3), 432–435. [Google Scholar]
- George S., Smith A., Alfano C., Bowles H., Irwin M., McTiernan A., ... Ballard-Barbash R. (2013). The association between television watching time and all-cause mortality after breast cancer. Journal of Cancer Survivorship ,7, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Jimenez M., Cabanillas F., Negron V., Echenique M., Mojica P., Santiago K., ... Carlo-Vargas V. (2012). Triple negative breast cancer: A retrospective study of hispanics residing in Puerto Rico. Puerto Rico Health Sciences Journal ,2, 45–-51. [PubMed] [Google Scholar]
- Gnerlich J., Deshpande A., Jeffe D., Sweet A., White N., & Margenthaler J. (2009). Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. Journal of the American College of Surgeons ,208, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S., Clarke C., Shema S., Chang E., Keegan T., & Glaser S. (2010). Disparities in breast cancer survival among asian women by ethnicity and immigrant status: A population-based study. American Journal of Public Health ,100(5), 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N., Crowe J., Brumberg D., & Berger N. (1992). Socioeconomic factors and race in breast cancer recurrence and survival. American Journal of Epidemiology ,135(6), 609–618. [DOI] [PubMed] [Google Scholar]
- Gorey K., Luginaah I., Holowaty E., Zou G., Hamm C., & Balagurusamy M. (2013). Mediation of the effects of living in extremely poor neighbourhoods by health insurance: Breast cancer care and survival in California, 1996 to 2011. International Journal for Equity in Health ,12(6), 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grann V., Troxel A., Zojwalla N., Hershman D., Glied S., & Jacobson J. (2006). Regional and racial disparities in breast cancer-specific mortality. Social Science and Medicine ,62, 337–-347. [DOI] [PubMed] [Google Scholar]
- Grau A., Ata A., Foster L., Ahmed N., Gorman D., Shyr Y., ... Pearson A. (2005). Effect of race on long-term survival of breast caner patients: Transinstitutional analysis from an inner city hospital and university medical center. The American Surgeon ,71(2), 164–170. [PubMed] [Google Scholar]
- Greenwald H., Polissar N., & Dayal H. (1996). Race, socioeconomic status, and survival in three female cancers. Ethnicity & Health ,1(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Guadagnoli E., Kaplan S., Silliman R., Troyan S., & Greenfield S. (1997). The impact of age, marital status, and physician-patient interactions on the care of older women with breast carcinoma. Cancer ,80, 1326-–1334. [PubMed] [Google Scholar]
- Haas J., Earle C., Orav J., Brawarsky P., Keohane M., Neville B., & Williams D. (2008). Racial segregation and disparities in breast cancer care and mortality. Cancer ,113(8), 2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji-Jama S., Gorey K., Luginaah I., Zou G., Hamm C. & Holowaty E. (2016). Disparities among minority women with breast cancer living in impoverished areas of California. Cancer Statistics for African Americans, 23(2), 157–162, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I., Jamison P., & Coughlin S. (2004). Breast and cervical cancer mortality in the Mississipi Delta, 1979–-1998. Southern Medical Journal ,97(3), 264–-272. [DOI] [PubMed] [Google Scholar]
- Hall M., Reid J., Burbidge L., Pruss D., Deffenbaugh A., Frye C., ... Noll W. (2009). BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer ,115(10), 2222–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett M., Neville B., & Weeks J. (2014). The relationship between quality, spending, and outcomes among women with breast cancer. Cancer ,113(8), 2166–2172. [DOI] [PubMed] [Google Scholar]
- Hastert T., Beresford S., Sheppard L., & White E. (2014). Disparities in cancer incidence and mortality by area-level socioeconomic status: A multilevel analysis. Journal of Epidemiology and Community Health, 1–9. [DOI] [PubMed] [Google Scholar]
- Hernandez B., Wilkens L., Marchand L., Horio D., Chong C., & Loo L. (2015). Differences in IGF-axis protein expression and survival among multiethnic breast cancer patients. Cancer Medicine ,4(3), 354–-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J., Kornblith A., Holland J., & Paskett E. (2013). Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psycho-Oncology ,22, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman D., Buono D., McBride R., Yann W., Joseph K., Grann V., & Jacobson J. (2008). Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst ,100(3), 199–206. [DOI] [PubMed] [Google Scholar]
- Hershman D., McBride R., Jacobson J., Lamerato L., Roberts K., Grann V., & Neugut A. (2005). Racial disparities in treatment and survival among women with early-stage breast cancer. Journal of Clinical Oncology ,23(27), 6639–6646. [DOI] [PubMed] [Google Scholar]
- Hershman D., Shao T., Kushi L., Buono D., Yann W., Fehrenbacher L., Kwan M., Gomez S., & Neugut A. (2011). Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Research and Treatment ,126, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman D., Tsui J., Meyer J., Glied S., Clarke G., Wright J., & Neugut A. (2014). The change from brand-name to generic Aromatase Inhibitors and hormone therapy adherence for early-stage breast cancer. Journal of the National Cancer Institute ,106(11), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman D., Unger J., Barlow W., Hutchins L., Martino S., Osborne K., Livingston R., & Albain K. (2009). Treatment quality and outcomes of African American versus White breast cancer patients: Retrospective analysis of southwest oncology studies S8814/S8897. Journal of Clinical Oncology ,27(13), 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman D., Wang X., McBride R., Jacobson J., Grann V., & Neugut A. (2006). Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Research and Treatment ,99, 313–321. [DOI] [PubMed] [Google Scholar]
- Hill D., Nibbe A., & Royce M. (2010). Method of detection and breast cancer survival disparities in hispanic women. Cancer Epidemiology, Biomarkers & Prevention ,19, 2453–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Watanabe S., Shostrom V., & Nsiah P. (2015). Breast cancer survival among african americans living in the Midwest: Disparities and recommendations to decrease mortality. Journal of National Black’s Nurses Association ,26(1), 8–14. [PubMed] [Google Scholar]
- Hirschman J., Whitman S., & Ansell D. (2007). The black:white disparity in breast cancer mortality: The example of Chicago. Cancer Causes Control ,18, 323–333. [DOI] [PubMed] [Google Scholar]
- Holmes L., Opara F., & Hossain J. (2010). A five-year breast cancer-specific survival disadvantage of african american women. African Journal of Reproductive Health ,14(3), 195–200. [PubMed] [Google Scholar]
- Hossain A., Sehbai A., Abraham R., & Abraham J. (2008). Cancer health disparities among indian and pakistani immigrants in the United States: A surveillance, epidemiology, and end results-based study. Cancer ,113, 1423–30. [DOI] [PubMed] [Google Scholar]
- How much money goes to breast cancer research? (n.d.). Retrieved from http://breastcancerconsortium.net/resources/beyond-awareness-workbook/background/research-dollars/
- Howard D., Penchansky R., & Brown M. (1998). Disaggregating the effects of race on breast cancer survival. Family Medicine ,30(3), 228–235. [PubMed] [Google Scholar]
- Hu H., Dailey A., Kan H., & Xu X. (2013). The effect of atmospheric particulate matter on survival of breast cancer among us females. Breast Cancer Research and Treatment ,139, 217–226. [DOI] [PubMed] [Google Scholar]
- Hunt B., Whitman S., & Hurlbert M. (2014). Increasing black:white disparities in breast cancer mortality in the 50 largest cities in United States. Cancer Epidemiology ,38(2), 118–123. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Ginsburg O., Rochon P., Sun P., & Narod S. (2015). Differences in breast cancer stage at diagnosis and cancer specific survival by race and ethnicity in the United States. JAMA, 313(2), 165–173, [DOI] [PubMed] [Google Scholar]
- Izano M., Satariano W., Hiatt R., & Braithwaite D. (2013). The impact of functional limitations on long-term outcomes among african-american and white women with breast cancer: A cohort study. Journal of Geriatric Oncology ,5, 266–275. [Google Scholar]
- Izano M., Satariano W., Tammemagi M., Ragland D., Moore D., Allen E., ... Braithwaitea D. (2014). Long-term outcomes among African-American and white women with breast cancer: What is the impact of comorbidity? Journal of Geriatric Oncology ,5, 266–275. [DOI] [PubMed] [Google Scholar]
- Jatoi I., Anderson W., Rao S., & Devesa S. (2005). Breast cancer trends among black and white women in the United States. Journal of Clinical Oncology ,23(31), 7836–7841. [DOI] [PubMed] [Google Scholar]
- Javid S., Varghese T., Morris A., Porter M., He H., Buchwald D., & Flum D.(2014). Guideline-concordant cancer care and survival among American Indian/Alaskan native patients. Cancer 120, 2183–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn S. (2002). Racial differences in treatment and survival from early-stage breast carcinoma. Cancer ,95(8), 1759–1766. [DOI] [PubMed] [Google Scholar]
- Kallan J. (1997). Effects of sociodemographic variables on adult mortality in the United States: Comparisons by sex, age, and cause of death. Social Biology, 94(1--2), 136–147 [DOI] [PubMed] [Google Scholar]
- Kaplan H., Malmgren J., Atwood M., & Calip G. (2015). Effect of treatment and mammography detection on breast cancer survival over time: 1990--2007. Cancer ,121, 2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan T., Kurian A., Gali K., Tao L., Lichtensztajn D., Hershman D., ... Gomez S. (2015). Racial/ethnic and socioeconomic differences in short-term breast cancer survival among women in an integrated health system. American Journal of Public Health, 105(5), 938–946, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan T., Press D., Tao L., DrRouen M., Kurian A., Clarke C., & Gomez S. (2013). Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Keegan et al. Breast Cancer Research, 15, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Eby E., & Piette J. (2005a). Is education associated with mortality for breast cancer and cardiovascular disease among black and white women? Gender Medicine ,2(1), 13-–18. [DOI] [PubMed] [Google Scholar]
- Kim S., Ferrante J., Won B., & Hameed M. (2005b). Barriers to adequate follow-up during adjuvant therapy may be improtant factors in the worse outcome for black women after breast cancer treatment. World Journal of Surgical Oncology ,6(26), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A., Lundgreen A., Wolff R., Fejerman L., John E., Torres-Meji G., ... Stern M. (2016). Red meat, poultry, and fish intake and breast cancer risk among hispanic and non-hispanic white women: The Breast Cancer Health Disparities Study. Cancer Causes Control ,27, 527–-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsner R., Ma F., Fleming L., Federman D., Trapido E., Duncan R., & Wilkinson J. (2006). The effect of Medicare health care delivery systems on survival for patients with breast and colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention ,15(4), 769–773. [DOI] [PubMed] [Google Scholar]
- Kish J., Yu M., Percy-Laurry A. & Altekruse S. (2014). Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. Journal of the National Cancer Institute Monographs ,2014(49), 236–243, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen A., Pankiewicz A., Hsieh S., Ward A., & Curriero F. (2015). The association of area-level social class and tobacco use with adverse breast cancer charecteristics among white and black women: Evidence from Maryland, 1992--2003. International Journal of Health Geographics, 14, 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenaka I., Martinez M., Pennington R., Hsu C., Clare S., Thompson P., ... Goulet R. (2010). Race and ethnicity and breast cancer outcomes in an underinsured population. Journal of the National Cancer Institute ,102, 1178–1187. [DOI] [PubMed] [Google Scholar]
- Koru-Sengul1 T., Santander A., Miao F., Sanchez L., Jorda M., Gluück S., ... Torroella-Kouri M. (2016). Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black latinas and caucasians. Breast Cancer Research and Treatment, 158, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke C., Sweeney C., Kwan M., Quesenberry C., Weltzien E., Habel L., Castillo A., ... Caan B. (2014). Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast Cancer Research and Treatment ,144, 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian A., Lichtensztajn D., Keegan T., Nelson D., Clarke C., & Gomez S. (2014). Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998--2011. JAMA ,312(9), 902-–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan M., John E., Caan B., Lee V., Bernstein L., Cheng I., Gomez S., ... Wu A. (2014). Obesity and mortality after breast cancer by race/ethnicity: The California Breast Cancer Survivorship Consortium. American Journal of Epidemiology ,179(1), 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairson D., Parikh R., Cormier J., Chan W., & Du X. (2015). Cost-effectiveness of chemotherapy for breast cancer and age effect in older women. Value in Health ,18, 1070–1078. [DOI] [PubMed] [Google Scholar]
- Lee-Feldstein A., Feldstein P., Buchmueller T., & Katterhagen G. (2000). The relationship of HMOs, health insurance, and delivery systems to breast cancer outcome. Medical Care ,38(7), 705–718. [DOI] [PubMed] [Google Scholar]
- Lee-Feldstein A., Feldstein P., Buchmueller T., & Katterhagen G. (2001). Breast cancer outcomes among older women. JGIM ,16, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Tannenbaum S., Koru-Sengul T., Miao F., Zhao W. & Byrne M. (2014). Native american race, use of the indian health service, and breast and lung cancer survival in Florida, 1996--2007. Preventing Chronic Disease, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R., Kilbourne B., Baltrus P., Williams-Brown S., Caplan L., Briggs N., ... Rust G. (2008). Black-white disparities in elderly breast cancer mortality before and after implementation of Medicare benefits for screening mammography. Journal of Health Care for the Poor and Underserved ,19, 103–134. [DOI] [PubMed] [Google Scholar]
- Lian M., Perez M., Liu Y., Schootman M., Frisse A., Foldes E., & Jeffe D. (2014). Neighborhood socioeconomic deprivation, tumor subtypes, and causes of death after non-metastatic invasive breast cancer diagnosis: A multilevel competing-risk analysis. Breast Cancer Research and Treatment ,147, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Malone K., & Daling J. (2013). Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of Internal Medicine ,163(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Liu P., Li X., Mittendorf E., Li J., Du X., He J., ... Yi M. (2013). Comparison of clinicopathologic features and survival in young American women aged 18--39 years in different ethnic groups with breast cancer. British Journal of Cancer ,109, 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos A., Chandwani S., Bandera E., Hirshfield K., Lin Y., Ambrosone C., & Demissie K. (2015). Associations between sociodemographic and clinicopathological factors and breast cancer subtypes in a population-based study. Cancer Causes Control ,26, 1737–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Agullo P., & Lakshmanaswamy R. (2013). Links between obesity, diabetes and ethnic disparities in breast cancer among hispanic populations. Obesity Reviews ,14, 679-–691. [DOI] [PubMed] [Google Scholar]
- Maggard M., O’Connell J., Lane K., Liu J., Etzioni D., & Ko C. (2003). Do young breast cancer patients have worse outcomes? Journal of Surgical Research ,113, 109–-113. [DOI] [PubMed] [Google Scholar]
- Ma H., Lu Y., Malone K., Marchbanks P., Deapen D., Spirtas R., ... Bernstein L.(2015). Mortality risk of black women and white women with invaisve breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer, 13, 225, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt J., Kerner J., Hadley J., Hwang Y., Eggert L., Johnson L., & Gold K. (2002). Variations in breast carcinoma treatment in older Medicare beneficiaries. Cancer ,95(7), 1401–-1414. [DOI] [PubMed] [Google Scholar]
- Markossian T., & Hines R. (2012). Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women and Health ,52(4), 317-–335. [DOI] [PubMed] [Google Scholar]
- Markossian T., Hines R., & Bayakly R. (2012). Disparities in late stage diagnosis, treatment, and breast cancer geographic and racial disparities in breast cancer-related outcomes in Georgia. Women and Health ,52(4), 317–-335. [DOI] [PubMed] [Google Scholar]
- Martindale S., Singh A., Wang H., Steinberg A., Homsi A., Zhang H., ... Pappas P. (2014). Racial disparities in survival and age-related outcome in postsurgery breast cancer patients in a New York City community hospital. Oncology, 1–-9. ISRN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S., Shah D., Tseng W., Canter R., & Bold R. (2012). Rural urban disparities in use of post-lumpectomy radiation. Medical Oncology ,29, 3250–-3257. [DOI] [PubMed] [Google Scholar]
- Maskarinec G., Pagano I., Lurie G., Bantum E., Gotay C., & Issell B. (2011). Factors affecting survival among women with breast cancer in Hawaii. Journal of women’s health ,20(2), 231–-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec G., Pagano I., Yamashiro G., & Issel B. (2003). Influences of ethnicity, treatment and comorbidity on breast cancer survival in Hawaii. Journal of Clinical Epidemiology ,56, 678–-685. [DOI] [PubMed] [Google Scholar]
- Masters R., Hummer R., Powers D., Beck A., Lin S., & Finch B. (2015). Long-term trends in adult mortality for U.S. blacks and whites: An examination of period- and cohort-based changes. Demography ,51, 2047-–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E., Ngo L., Chirikos T., Roetzheim R., Li D., Drews R., & Iezzoni L. (2007). Cancer stage at diagnosis and survival among persons with social security disability insurance on Medicare. Health Services Research ,42(2), 611-–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E., Ngo L., Roetzheim R., Chirikos T., Li D., Drews R., & Iezzoni L. (2006). Disparities in breast cancer treatment and survival for women with disabilities. Annals of Internal Medicine ,145(9), 637–-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A., Yang J., & Armstrong A. (2015). Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. American Journal of Public Health ,105(3), 446-–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDavid K., Tucker T., Sloggett A., & Coleman M. (2003). Cancer survival in Kentucky and health insurance coverage. Archives of Internal Medicine ,163, 2135-–2144. [DOI] [PubMed] [Google Scholar]
- Meliker J., Goovaerts P., Jacquez G., AvRuskin G., & Copeland G. (2009). Breast and prostate cancer survival in Michigan: Can geographic analyses assist in understanding racial disparities? Cancer ,115, 2212–-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe I., Anderson W., Jatoi I., & Rosenberg P. (2009). Underlying causes of the black-white racial disparity in breast cancer mortality: A population based analysis. Journal of the National Cancer Institute ,101, 993-–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Maskarinec G., & Lee J. (1997a). Ethnic differences and factors related to breast cancer survival in Hawaii. Journal of Clinical Epidemiology ,50(11), 1289–-1296. [DOI] [PubMed] [Google Scholar]
- Meng L., Maskarinec G., & Lee J. (1997b). Ethnicity and conditional breast cancer survival in Hawaii. Journal of Clinical Epidemiology ,50(11), 1289-–1296. [DOI] [PubMed] [Google Scholar]
- Miller B., Chu K., Hankey B., & Ries L. (2008). Cancer incidence and mortality patterns among specific asian and pacific islander populations in the U.S. Cancer Causes Control ,19, 227–-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller G., Clayton A., Zahnd W., Hollenbeck K., Barrow M., Jenkins W., & Ruez D. Jr (2015). Geospatial analysis of cancer risk and residential proximity to coal mines in Illinois. Ecotoxicology and Environmental Safety ,120, 155-–162. [DOI] [PubMed] [Google Scholar]
- Newman L., Griffith K., Jatoi I., Simon M., Crowe J., & Colditz G. (2006). Meta-analysis of survival in african american and white american patients with breast cancer: Ethnicity compared with socioeconomic status. Journal of Clinical Oncology ,24(9), 1342-–1349. [DOI] [PubMed] [Google Scholar]
- Newman L., Mason J., Cote D., Vin Y., Carolin K., Bouwman D., & Colditz G. (2002). African-american ethnicity, socioeconomic status, and breast cancer survival. Cancer ,94, 2844-–2854. [DOI] [PubMed] [Google Scholar]
- Ning J., Peng S., Ueno N., Xu Y., Shih Y., Karuturi M., ... Shen Y. (2015). Has racial difference in cause-specific death improved in older patients with late-stage breast cancer? Annals of Oncology ,26, 2161–-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Pawlish K., & Roche L. (2010). Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. Journal of Health Care for the Poor and Underserved ,21, 144-–160. [DOI] [PubMed] [Google Scholar]
- Niu X., Roche L., Pawlish K., & Henry K. (2013). Cancer survival disparities by health insurance status. Cancer Medicine ,2(3), 403-–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgalieva Z., Franzini L., Morgan R., Vernon S., & Du X. (2013a). Utilization of lymph node dissection, race/ethnicity, and breast cancer outcomes. The American Journal of Managed Care ,19(10), 1–-5. [PubMed] [Google Scholar]
- Nurgalieva Z., Franzini L., Morgan R., Vernon S., Liu C., & Du L. (2013b). Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Medical Oncology ,30(419), 1–-9. [DOI] [PubMed] [Google Scholar]
- Nurgalieva Z., Franzini L., Morgan R., Vernon S., Liu C., & Du X. (2013c). Surveillance mammography use after treatment of primary breast cancer and racial disparities in survival. Medical Oncology ,30(691), 1–-8. [DOI] [PubMed] [Google Scholar]
- Ohri N., Rapkin B., Guha C., Kalnicki S., & Garg M. (2016). Radiation therapy noncompliance and clinical outcomes in an urban academic center. International Journal of Radiation Oncology * Biology * Physics ,95(2), 563–-570. [DOI] [PubMed] [Google Scholar]
- Okunade A., & Karakus M. (2003). Mortality from breast carcinoma amoung US women: The role and implications of socio-economics, heterogeneous insurance, screening mammography, and geography. Health Care Management Science ,6, 237–-248. [DOI] [PubMed] [Google Scholar]
- O’Malley C., Le G., Glaser S., Shema S. & West D. (2003). Socioeconomic status and breast carcinoma survival in four racial/ethnic groups. Cancer, 97, 1303–1311, [DOI] [PubMed] [Google Scholar]
- Onega T., Duell E., Shi X., Demidenko E., & Goodman D. (2010). Race versus place of service inmortality among Medicare beneficiaries with cancer. Cancer ,116, 2698–-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi S., & Martinez M. (2011). Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Research and Treatment ,127, 729-–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A., Soto-Salgado M., Calo W., Nogueras G., Tortolero-Luna G., Hebl S., ... Suarez E. (2010). Disparities in breast cancer in Puerto Rico and among hispanics, non-hispanic whites, and non-hispanics blacks in the United States, 1992–2004. The Breast Journal ,16(6), 666-–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen R., Winchester D., Hussey D., Clive R., Friedman M., Cady B., ... Scotte R. L. (1994). Doggett. Insurance coverage of patients with breast cancer in the 1991 commission on cancer patient care evaluation study. Annals of Surgical Oncology ,1(6), 462–-467. [DOI] [PubMed] [Google Scholar]
- Owusu C., Lash T., & Silliman R. (2007). Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Research and Treatment ,102, 227-–236. [DOI] [PubMed] [Google Scholar]
- Parikh D., Chudasama R., Agarwal A., Rand A., Qureshi M., Ngo T., & Hirsch A. (2015). Race/ethnicity, primary language, and income are not demographic drivers of mortality in breast cancer patients at a diverse safety net academic medical center. International Journal of Breast Cancer ,2015, 1---6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise C., & Caggiano V. (2013). Disparities in race/ethnicity and socioeconomic status: Risk of mortality of breast cancer patients in the California Cancer Registry, 2000–2010. BMC Cancer, 13, 449, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise C., & Caggiano V. (2015). The influence of socioeconomic status on racial/ethnic disparities among the ER/PR/HER2 breast cancer subtypes. Journal of Cancer Epidemiology ,2016, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellatt A., Lundgreen A., Wolff R., Hines L., John E., & Slattery M. (2016). Energy homeostasis genes and survival after breast cancer diagnosis: The Breast Cancer Health Disparities Study. Cancer Causes Control ,27, 47–-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellatt A., Wolff R., John E., Torres G., Hines L., Baumgartner K., ... Slattery M. (2013). SEPP1 influences breast cancer risk among women with greater Native American Ancestry: The Breast Cancer Health Disparities Study. PLOS one, 8(11), e80554, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins P., Cooksley C., & Cox J. (1996). Breast cancer: Is ethnicity an independent prognostic factor for survival? Cancer ,78, 1241-–7. [DOI] [PubMed] [Google Scholar]
- Pezzin L., Laud P., Yen T., Neuner J., & Nattinger A. (2015). Reexamining the relationship of breast cancer hospital and surgical volume to mortality an instrumental variable analysis. Medical Care ,53(12), 1033–-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips Jr B., Belasco E., Markides K. & Gong G. (2013). Socioeconomic deprivation as a determinant of cancer mortality and the hispanic paradox in Texas, USA. International Journal for Equity in Health, 12(26), 26, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polednak A. (2002). Survival of breast cancer patients in Connecticut in relation to socioeconomic and health care access indicators. Journal of Urban Health: Bulletin of the New York Academy of Medicine ,79(2), 211-–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potosky A., Merrill R., Riley G., Taplin S., Barlow W., Fireman B., & Ballard-Barbash R. (1997). Breast cancer survival and treatment in health maintenance organization and fee-for-service settings. Journal of the National Cancer Institute ,89(22), 1683-–1691. [DOI] [PubMed] [Google Scholar]
- Pruitt S., Lee S., Tiro J., Xuan L., Ruiz J., & Inrig S. (2015). Residential racial segregation and mortality among black, white, and hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer ,151, 1845–-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Begley C., Highfield L., & Kim B. (2015). Survival benefits of treatment access among underserved breast cancer patients diagnosed through the Texas Breast and Cervical Cancer Services Program. Journal of Public Health Management and Practice ,21(5), 477–-486. [DOI] [PubMed] [Google Scholar]
- Richter N., Gorey K., Haji-Jama S. & Luginaah I. (2013). Care and survival of mexican american women with node negative breast cancer: Historical cohort evidence of health insurance and barrio advantages. Journal of Immigrant and Minority Health, 17, 652–659, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo J., Sherman W., & Arciero C. (2015). Racial disparity in survival from early breast cancer in the department of defense healthcare system. Journal of Surgical Oncology ,111, 819–-823. [DOI] [PubMed] [Google Scholar]
- Robin K. (2003). Yabroff and L. Gordis. Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case-fatality, and mortality? Social Science and Medicine ,57, 2265–-2279. [DOI] [PubMed] [Google Scholar]
- Roetzheim R., Chirikos T., Wells K., McCarthy E., Ngo L., Li D., ...Iezzoni L. (2008). Managed care and cancer outcomes for Medicare beneficiaries with disabilities. The American Journal of Managed Care ,14(5), 287–-296. [PMC free article] [PubMed] [Google Scholar]
- Roetzheim R., Gonzalez E., Ferrante J., Pal N., Durme D., & Krischer J. (2000). Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer ,89, 2202-–13. [DOI] [PubMed] [Google Scholar]
- Roohan P., Bickell N., Baptiste M., Therriault G., Ferrara E., & Siu A. (1998). Hospital volume differences and five-year survival from breast cancer. American Journal of Public Health ,88(3), 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseland M., Pressler M., Lamerato L., Krajenta R., Ruterbusch J., Booza J., ...Simon M. (2015). Racial differences in breast cancer survival in a large urban integrated health system. Cancer ,121, 3668-–75. [DOI] [PubMed] [Google Scholar]
- Rueth N., Lin H., Bedrosian I., Shaitelman S., Ueno N., Shen Y., ... Babiera G. (2014). Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: An analysis of treatment and survival trends from the national cancer database. Journal of Clinical Oncology, 32(19), 2018–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo H., Brufsky A., Yood M., Tripathy D., Kaufman P., ...Yardley D. (2013). Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Research and Treatment ,141, 461–-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E., Kramer M., Cooper H., Wilkins W., & Jacob K. (2011). Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. Journal of Urban Health ,88(6), 1117–-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust G., Zhang S., Malhotra K., Reese L., McRoy L., Baltrus P., ...Levine R. (2015). Paths to health equity: Local area variation in progress toward eliminating breast cancer mortality disparities, 1990–2009. Cancer ,121, 2765-–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabik L. & Bradley C. (2013). Differences in mortality for surgical cancer patients by insurance and hospital safety net status. Medical Care Research and Review ,70(1), 84-–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M., Adams S., Orekoya O. & Hebert J. (2015). Understanding the association of type 2 diabetes mellitus in breast cancer among african american and european american populations in South Carolina. Journal of Racial and Ethnic Health Disparities, ,3, 546–554, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M., Porter N., Hurley D., Adams S., & Eberth J. (2016). Disparities in breast cancer incidence, mortality, and quality of care among african american and european american women in South Carolina. Southern Medical Association ,109(1), 24–-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel J., Zahm S., Jatoi I., McGlynn K., Gallagher C., Schairer C., ...Zhu K. (2014). Racial/ethnic differences in breast cancer survival by inflammatory status and hormonal receptor status: An analysis of the Surveillance, Epidemiology, and End Results data. Cancer Causes Control ,25, 959-–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting J., Soliman A., Schairer C., Schottenfeld D., & Merajver S. (2012) Inflammatory and noninflammatory breast cancer survival by socioeconomic position in the Surveillance, Epidemiology, and End Results database, 1990–2008. Breast Cancer Research and Treatment, 134, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Marcantonio E., Li D., Silliman R., Ngo L. & McCarthy E. (2010). Breast cancer among the oldest old: Tumor characteristics, treatment choices, and survival. Journal of Clinical Oncology, 28(12), 2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schootman M., Jeffe D., Lian M., Gillanders W., & Aft R. (2008). The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: A geographic and multilevel analysis. American Journal of Epidemiology ,169(5), 554–-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff-Marco S., Gomez S., Sangaramoorthy M., Yang J., Koo J., Hertz A., ...Keegan T. (2015a). Impact of neighborhoods and body size on survival after breast cancer diagnosis. Health and Place ,36, 162–-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff-Marco S., Yang J., John E., Kurian A., Cheng I., Leung R., ...Gomez S. (2015b). Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer. Journal of Community Health ,40, 1287–-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff-Marco S., Yang J., John E., Sangaramoorthy M., Hertz A., Koo J., ...Gomez S. (2014). The influence of genetic ancestry and ethnicity on breast cancer survival associated with genetic variation in the TGF-b-signaling pathway: The Breast Cancer Health Disparities Study. Cancer Epidemiology, Biomarkers \ & Prevention ,23(5), 793-–811. [Google Scholar]
- Shavers D., Harlan L., & Stevens J. (2003). Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer ,97(1), 134–-147. [DOI] [PubMed] [Google Scholar]
- Shi R., Mills G., McLarty J., Burton G., Shi Z., & Glass J. (2013). Commercial insurance triples chances of breast cancer survival in a public hospital. The Breast Journal ,19(6), 664-–667. [DOI] [PubMed] [Google Scholar]
- Shi R., Taylor H., Mclarty J., Liu L., Mills G. & Burton G. (2015). Effect of payer status on breast cancer survival: a retrospective study. BMC Cancer, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short L., Fisher M., Wah P., Kelly M., Lawless G., White S., ...Brawley O. (2010). Disparities in medical care among commercially insured patients with newly diagnosed breast cancer. Cancer ,116, 193–-202. [DOI] [PubMed] [Google Scholar]
- Silber J., Rosenbaum P., Clark A., Giantonio B., Ross R., Teng Y., ...Fox K. (2013). Characteristics associated with differences in survival among black and white women with breast cancer. Japan Automobile Manufacturers Association ,310(4), 389–-397. [DOI] [PubMed] [Google Scholar]
- Simon M., & Severson R. (1997). Racial differences in breast cancer survival: The interaction of socioeconomic status and tumor biology. American Journal of Obstetrics \ & Gynecology, 176(6), s233–s239, [DOI] [PubMed] [Google Scholar]
- Simon M., & Severson R. (2006). Racial differences in breast cancer survival in the Detroit metropolitan area. Breast Cancer Research and Treatment ,97, 149–-155. [DOI] [PubMed] [Google Scholar]
- Sineshaw H., Freedman R., Ward E., Flanders W., & Jemal A. (2015). Black/white disparities in receipt of treatment and survival among men with early-stage breast cancer. American Society of Clinical Oncology , 33, 2337–2344, [DOI] [PubMed] [Google Scholar]
- Singh G., Williams S., Siahpush M. & Mulhollen A. (2011). Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. Journal of Cancer Epidemiology, Article ID 107497, 27. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Herrick J., Torres G., John E., Giuliano A., Hines L., ...Wolff R. (2014a). Genetic variants in interleukin genes are associated with breast cancer risk and survival in a genetically admixed population: the Breast Cancer Health Disparities Study. Carcinogenesis ,35(8), 1750–-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Hines L., Lundgreen A., Baumgartner K., Wolff R., Stern M., & John E. (2014b). Diet and lifestyle factors interact with MAPK genes to influence survival: the Breast Cancer Health Disparities Study. Cancer Causes Control ,25, 1211–-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., John E., Stern M., Herrick J., Lundgreen A., Giuliano A., ...Wolff R. (2013a). Associations with growth factor genes (FGF1, FGF2, PDGFB, FGFR2, NRG2, EGF, ERBB2) with breast cancer risk and survival: the Breast Cancer Health Disparities Study. Breast Cancer Research and Treatment ,140, 587–-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., John E., Torres G., Lundgreen A., Lewinger J., Stern M., ...Wolff R. (2013b). Angiogenesis genes, dietary oxidative balance and breast cancer risk and progression: The Breast Cancer Health Disparities Study. International Journal of Cancer ,134, 629–-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., John E., Torres G., Stern M., Lundgreen A., Hines L., ... Wolff R. (2013c). Matrix metalloproteinase genes are associated with breast cancer risk and survival: The Breast Cancer Health Disparities Study. PLOS one, 8(5), e63165, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Lundgreen A., Hines L., Torres G., Wolff R., Stern M., & John E. (2014c). Genetic variation in the JAK/STAT/SOCS signaling pathway influences breast cancer-specific mortality through interaction with cigarette smoking and use of aspirin/NSAIDs: the Breast Cancer Health Disparities Study. Breast Cancer Research and Treatment ,147, 145–-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Lundgreen A., Hines L., Wolff R., Torres-meija G., Baumgartner K., & John E. (2015a). Energy homeostasis genes and breast cancer risk: the influence of ancestry, body size, and menopausal status: The Breast Cancer Health Disparities Study. Cancer Epidemiology ,39, 1113-–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Lundgreen A., John E., Torres-Mejia G., Hines L., Giuliano A., ...Wolff R. (2015b). Mapk genes interact with diet and lifestyle factors to alter risk of breast cancer: The Breast Cancer Health Disparities Study. Nutrition and cancer ,67(2), 292-–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Lundgreen A., Stern M., Hines L., Wolff R., Giuliano A., ...John E. (2014d). The influence of genetic ancestry and ethnicity on breast cancer survival associated with genetic variation in the TGF-b-signaling pathway: The Breast Cancer Health Disparities Study. Cancer Causes Control ,25, 293–-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Gagen J., Carrillo E., Ang A., & Perez E. (2013). Practices that reduce the latina survival disparity after breast cancer. Journal of women’s health ,22(11), 938–-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., Shih Y., Xu Y., Giordano S., Smith B., Perkins G., ...Buchholz T. (2010). Racial disparities in the use of radiotherapy after breast-conserving surgery: A national Medicare study. Cancer ,116(3), 734–-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E., Ziogas A., & Anton H. (2013). Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA ,18(6), 516-–523. [DOI] [PubMed] [Google Scholar]
- Soto M., Prieto D., & Munene G. (2017). A bayesian network and heuristic approach for systematic characterization of radiotherapy receipt after breast-conservation surgery. BMC Medical Informatics and Decision Making ,17(1), 1-–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparano J., Wang M., Zhao F., Stearns V., Martino S., Ligibel J., ... Davidson N. (2011). Race and hormone receptor positive breast cancer outcomes in a randomized chemotherapy trial. Journal of the National Cancer Institute ,104, 406–-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sposto R., Keegan T., Vigen C., Kwan M., Bernstein L., John E., ... Wu A. (2016). The effect of patient and contextual characteristics on racial/ethnic disparity in breast cancer mortality. American Association for Cancer Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague B., Trentham-Dietz A., Gangnon R., Ramchandani R., Hampton J., Robert S., ... Newcomb P.(2011). Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer, 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Hu S., & Walker J. (2004). All-cause and cause-specific mortality by socioeconomic status among employed persons in 27 US states, 1984–1997. American Journal of Public Health ,94(6), 1037–-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtz L., Melley J., Mamula K., Shriver C., & Ellsworth R. (2014). Outcome disparities in african american women with triple negative breast cancer: A comparison of epidemiological and molecular factors between african american and caucasian women with triple negative breast cancer. Sturtz et al. BMC Cancer, 14(62), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swede H., Gregorio D., Tannenbaum S., Brockmeyer J., Ambrosone C., Wilson L., ... Runowicz C. (2011). Prevalence and prognostic role of triple-negative breast cancer by race: A surveillance study. Clinical Breast Cancer ,11(5), 332-–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swede H., Sarwar A., Magge A., Braithwaite D., Cook L., Gregorio D., ... Brockmeyer J. (2016). Mortality risk from comorbidities independent of triple-negative breast cancer status: NCI-SEER-based cohort analysis. Cancer Causes Control ,27, 627–-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabung F., Steck S., Liese A., Zhang J., Ma Y., Caan B., ... Hebert J. (2016). Association between dietary inflammatory potential and breast cancer incidence and death: Results from the women’s health initiative. British Journal of Cancer ,114, 1277–-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum S., Koru-Sengul T., Miao F., & Byrne M. (2013). Disparities in survival after female breast cancer diagnosis: A population-based study. Cancer Causes Control ,24, 1705–-1715. [DOI] [PubMed] [Google Scholar]
- Tao L., Gomez S., Keegan T., Kurian T., & Clarke C. (2015). Breast cancer mortality in african-american and non-hispanic white women by molecular subtype and stage at diagnosis: A population-based study. Cancer Epidemiology, Biomarkers and Prevention ,24, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N., Goovaerts P., Zhan B., Chow E., & Wilson J. (2012). Identifying risk factors for disparities in breast cancer mortality among african-american and hispanic women. Women’s Health Issues ,22(3), 267–-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N., Wilson G., & Zhan B. (2010). Female breast cancer mortality clusters within racial groups in the United States. Health and Place ,16, 209-–218. [DOI] [PubMed] [Google Scholar]
- Tian N., Wilson J., & Zhan B. (2011). Spatial association of racial/ethnic disparities between late-stage diagnosis and mortality for female breast cancer: Where to intervene? International Journal of Health Geographics ,10(24), 1–-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy J., Deal A., Anders C., Reeder-Hayes K., & Carey L. (2015). Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Research and Treatment ,150, 667-–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro A., Costantino N., Shriver C., Ellsworth D., & Ellsworth R. (2016). Effect of obesity on molecular characteristics of invasive breast tumors: Gene expression analysis in a large cohort of female patients. BMC Obesity ,3, 1–-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh Q., Nguyen P., Leow J., Dalela D., Chao G., Mahal B., ... Aizer A. (2015). Cancer-specific mortality of asian americans diagnosed with cancer: A nationwide population based assessment. Journal of the National Cancer Institute ,107(6), 1-–8. [DOI] [PubMed] [Google Scholar]
- Van Ravesteyn N., Schechter C., Near A., Heijnsdijk E., Stoto M., Draisma G., ... Mandelblatt J. (2011). Race-specific impact of natural history, mammography screening and adjuvant treatment on breast cancer mortality rates in the U.S. Cancer Epidemiology ,20(1), 112–-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnakota S., & Lam N. (2006). Socioeconomic inequality of cancer mortality in the United States: A spatial data mining approach. International Journal of Health Geographics, 5(9), 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Hurley D., Hebert J., McNamara C., Bayakly A. & Vena J. (2012). Cancer mortality-to-incidence ratios in Georgia. Cancer, 4032–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleska P., da Silva G., Pasquali G., Sasso C., Paul E., Modesto S., ... In\^{e}s M. (2015). Meta-analysis of the use of bayesian networks in breast cancer diagnosis. Cadernos de Sa\’{u}de P\’{u}blica, 31(1), 26–38. [DOI] [PubMed] [Google Scholar]
- Wang C., Civan J., Lai Y., Cristofanilli M., Hyslop T., Palazzo J., ... Yang H. (2015). Racial disparity in breast cancer survival: The impact of pre-treatment hematologic variables. Cancer Causes Control ,26, 45–-56. [DOI] [PubMed] [Google Scholar]
- Ward E., Halpern M., Schrag N., Cokkinides V., DeSantis C., Bandi P., ...Jemal A. (2008). Association of insurance with cancer care utilization and outcomes. CA: A Cancer Journal for Clinicians ,58, 9–-31. [DOI] [PubMed] [Google Scholar]
- Warner L., Tamimi M., Hughes R., Ottesen Y., Wong S., Edge R., ...Winer J. (2015). Weeks, and A. Partridge. Racial and ethnic differences in breast cancer survival: Mediating effect of tumor characteristics and sociodemographic and treatment factors. Journal of Clinical Oncology ,33(10), 2254–-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K., Camacho F., Hwang W., Anderson R., & Kimmick G. (2013). Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low income women. American Journal of Clinical Oncology ,36(2), 181–-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Kachare S., Vohra N., Fitzgerald T., & Wong J. (2014). Regional disparities in breast cancer outcomes and the process of care. The American Journal of Surgery ,80(7), 669–-674. [PubMed] [Google Scholar]
- Wheeler S., Reeder-Hayes K., & Carey L. (2013). Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. The Oncologist ,18, 986-–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman S., Ansell D., Orsi J., & Francois T. (2011). The racial disparity in breast cancer mortality. Journal of Community Health ,36(4), 588-–96. [DOI] [PubMed] [Google Scholar]
- Whitman S., Orsi J., & Hurlbert M. (2012). The racial disparity in breast cancer mortality in the 25 largest cities in the United States. Cancer Epidemiology ,36, 147–-131. [DOI] [PubMed] [Google Scholar]
- Wilson R., Adams M., Burhansstipanov L., Roubidoux M., Cobb N., Lynch C., & Edwards B. (2007). Disparities in breast cancer treatment among american indian, hispanic and non-hispanic white women enrolled in Medicare. Journal of Health Care for the Poor and Underserved ,18, 648–-664. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Sun C., Wyatt L., Hurria A., & Bhatia S. (2015). Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer ,121, 3885–-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward W., Huang E., McNeese M., Perkins G., Tucker S., Strom E., ... Buchholz T. (2006). African-american race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer ,107, 2662–-8. [DOI] [PubMed] [Google Scholar]
- Wray C., Phatak U., Robinson E., Wiatek R., Rieber A., Gonzalez A., ...Kao L. (2013). The effect of age on race-related breast cancer survival disparities. Annals of Surgical Oncology ,20, 2541–-2547. [DOI] [PubMed] [Google Scholar]
- Wright J., Reis I., Zhao W., Panoff J., Takita C., Sujoy V., ... Hurley J. (2012). Racial disparity in estrogen receptor positive breast cancer patients receiving trimodality therapy. The Breast, 276–283. [DOI] [PubMed] [Google Scholar]
- Wu A., Gomez S., Vigen C., Kwan M., Keegan T., Lu Y., ... Sposito R. (2013). The california breast cancer survivorship consortium (CBCSC): prognostic factors associated with racial/ethnic differences in breast cancer survival. Cancer Causes Control ,24, 1821–-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Cheung M., Hurley J., Byrne M., Huang Y., Zimmers T., & Koniaris L. (2009). A comprehensive evaluation of outcomes for inflammatory breast cancer. Breast Cancer Research and Treatment ,117, 631–-641. [DOI] [PubMed] [Google Scholar]
- Yao K., Winchester D., Czechura T., & Huo D. (2009). Contralateral prophylactic mastectomy and survival: Report from the national cancer data base, 1998–2002. Breast Cancer Research and Treatment ,117, 631-–641. [DOI] [PubMed] [Google Scholar]
- Yi M., Peijun L., Li X., Mittendorf E., He J., Ren Y., ... Hunt K. (2012). Comparative analysis of clinicopathologic features, treatment and survival of asian women with a breast cancer diagnosis residing in the United States. Cancer ,118, 4117–-4125. [DOI] [PubMed] [Google Scholar]
- Yood M., Owusu C., Buist D., Geiger A., Field T., Thwin S., ...Silliman R. (2008). Mortality impact of less-than-standard therapy in older breast cancer patients. Journal of the American College of Surgeons ,206(1), 66-–75. [DOI] [PubMed] [Google Scholar]
- Yu Q., Fan Y., & Wu X. (2014). General multiple mediation analysis with an application to explore racial disparities in breast cancer survival. Biometrics and Biostatistics ,17, 259-–269. [Google Scholar]
- Zeng C., Wen W., Morgans A., Pao W., Shu X., & Zheng W. (2015). Disparities by race, age, and sex in the improvement of survival for major cancers results from the national cancer institute surveillance, epidemiology, and end results (SEER) program in the united states, 1990 to 2010. JAMA Oncology ,1(1), 88–-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Franzini L., Chan W., Xu H., & Du X. (2015). Effect of health insurance on tumor stage, treatment and survival in large cohorts of patients with breast cancer and colorectal cancer. Journal of Health Care for the Poor and Underserved ,26, 1336–1358. [DOI] [PubMed] [Google Scholar]
- Zhang S., Ivy J., Wilson J., Diehl K., & Yankaskas B. (2014). Competing risks analysis in mortality estimation for breast cancer patients from independent risk groups. Healthcare Management Science ,5(2), 1–-9. [DOI] [PubMed] [Google Scholar]
- Zhu X., Decker V., Almodovar A., Litherland S., & Decker D. (2014). Ethnicity disparities of breast cancer within an insured population. The Breast Journal ,20(3), 331–332. [DOI] [PubMed] [Google Scholar]