ABSTRACT
Objective: To update the health economic evaluation of pirfenidone in the treatment of idiopathic pulmonary fibrosis (IPF) compared to all available alternatives strategies (Best supportive care – BSC and nintedanib), based on a cost-utility model previously validated by the CEESP’s (French Committee for Economic Evaluation) in 2014.
Methods: A standard Markov cohort model, adapted to French methodology guidelines, was used to simulate the therapeutic management and the course of IPF patients (including potential adverse events) using the collective perspective. Cost-effectiveness was evaluated regarding life years (LY); quality-adjusted life-years (QALY); average cumulative costs; the incremental cost-effectiveness ratio (ICER) expressed in cost per QALY gained. Data were retrieved from trials, meta-analysis, literature, health insurance and hospitalisation databases, and national tariffs.
Results: Over 15 years, total costs accumulated in the pirfenidone strategy were estimated at €99,477 per patient, €104,610 in nintedanib, and €14,177 in Best Supportive Care (BSC). The total number of QALYs accumulated equalled 5.20 (6.91 LYs), 4.52 (5.98 LYs), and 3.79 (4.98 LYs), respectively. Pirfenidone was estimated to be dominant over nintedanib with incremental costs of -€5,133 and 0.67 more QALYs accumulated. Incremental cost versus BSC was €85,300 and 1,404 QALY gained. The cost-effectiveness ratio was estimated at 60,738€/QALY when compared to BSC.
Conclusion: Pirfenidone is likely to be a cost–effective strategy compared to BSC and seems more efficient and less costly compared to nintedanib for the treatment of patients with IPF in France.
KEYWORDS: Pirfenidone, nintedanib, idiopathic pulmonary fibrosis, health-economic model, cost-effectiveness, France, CEESP, Esbriet, Ofev, cost-utility
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible, unpredictable, and ultimately fatal disease of unknown aetiology. IPF is characterised by progressive fibrosis of the interstitium of the lung, resulting in decreased lung volume and pulmonary insufficiency. Patients with IPF suffer from declining lung function, cough, shortage of sleep, fatigue, and acute episodes of rapid respiratory deterioration which ultimately lead to death. The outcome of IPF is fairly uniform, but the course of progression is variable from patient to patient. The course of disease includes periods of relative stability interspersed with episodes of stepwise deterioration in symptoms of breathlessness, cough, declining lung function, and acute episodes of rapid respiratory deterioration that may result in death. The classic clinical phenotype of IPF is one of slowly progressive decline in lung function and worsening dyspnoea leading to death within 2–5 years from diagnosis [1–5]. Complications associated with IPF place a significant burden on healthcare resources as patients often require prolonged hospitalisations. The incidence of IPF is rising meaning that healthcare resource utilisation and costs, which are already high, are likely to increase further in the future [6].
Due to the irreversible nature of IPF, the treatment goal should be to stabilise the disease if possible or at least to reduce the rate of progression [7]. Before the development of pirfenidone and nintedanib, there had been little therapeutic innovation in IPF treatment for several decades, and treatment options had provided very limited value in terms of either disease progression or physical performance. Pirfenidone (Esbriet®) is licensed for the treatment of patients with mild-to-moderate IPF. Licensing of pirfenidone was granted based on the evidence from the two multinational pivotal phase III trials, PIPF-004 and PIPF-006 (collectively known as the CAPACITY trials) as well as a third supportive phase III study, SP3, conducted in Japanese patients [8,9]. The confirmatory phase III trial PIPF-016 (ASCEND) was conducted on request of the USA Food and Drug Administration (FDA) with the results published in 2014 [10]. Nintedanib (Ofev®), another agent approved for the treatment of IPF, is an inhibitor of several tyrosine kinases involved in the development of pulmonary fibrosis, targeting in particular tyrosine kinases associated with the platelet-derived growth factor receptor, vascular endothelial growth factor receptor, and fibroblast growth factor receptor [11,12].
The cost-effectiveness analysis of pirfenidone was adpated to French methodology guidelines and submitted to the French Committee of Economic Evaluation and Public Health (Commission Évaluation Économique et de Santé Publique, CEESP) in 2014, which only considered the comparison of pirfenidone with the best supportive care (BSC, defined as a proactive approach to symptomatic treatment without IPF specific medicinal treatment, which may include oxygen therapy, pulmonary rehabilitation, opioids, anti-reflux therapy, the withdrawal of steroids or other immunosuppressive drugs, early detection of the terminal phase and cooperation with specialists in palliative care) as nintedanib was not on the market at the time of the evaluation [13]. The overall structure of the model was considered as acceptable by the CEESP. The main comments were related to the consideration of adverse events and related disutility and costs that was not explicit. The time horizon was also considered too long.
The aim of this analysis was to estimate the cost-effectiveness of pirfenidone in the treatment of IPF compared to all available strategies. The health economic model submitted to the CEESP for the evaluation of pirfenidone was updated incorporating their comments, and including nintedanib as a comparator.
Materials and methods
Model overview
A standard Markov cohort model, composed of four health states, was used to simulate the therapeutic management and the course of patients with IPF, including potential adverse events and complications. As recommended by the French High Authority for Health (Haute Autorité de Santé, HAS) guidelines, the analysis was conducted using the collective perspective [14]. The cost-effectiveness analysis was based on a 15-year time horizon with 3-month cycles. The cycle length was defined based on the interval of clinical trial’s visits (every 12 weeks in CAPACITY and every 13 weeks in ASCEND), following the data collection process, as well as on the French clinical guidelines for IPF recommending a specialist visits every 3–6 months [15]. Thus, the cycle length captured the minimum period from which a visit could occur. The initial timeframe in the model submitted to the CEESP was 33 years. However, the CEESP’s recommendation was to reduce the timeframe to 15 years due to a high degree of uncertainty related to data extrapolation over a long time horizon. A 15-year time horizon was considered in the base case analysis and a lifetime time horizon (33 years) was tested as a scenario analysis. The model was developed using Microsoft Excel 2010.
Model description
It was decided to adopt a health state structure similar to that used by Loveman et al. which included health states defined according to patients progression status (non-progressed vs progressed) [16]. Furthermore, as in Loveman’s structure, lung transplant (LT) was also included as a health state since, in clinical practice, patients who have progressed may receive a transplant, which can significantly affect costs and consequences in the ensuing years. The model structure including four mutually exclusive health states and transition probabilities is shown in Figure 1.
Figure 1.

Model structure.
Patient pathways
The progression-free state captured the proportion of patients at each point in time that had not experienced disease progression or died (also known as achieving progression-free survival). Progression-free survival (PFS) was defined based on the ASCEND trial criteria and was measured to the first occurrence of a confirmed ≥10% absolute decline in percent predicted forced vital capacity (FVC), a confirmed ≥50m decline in 6-minute walk teSt (6WMT) distance, or death [10]. These cut-offs were found clinically significant in the studies by du Bois et al. [17,18]
The progression state captured the proportion of patients that had experienced disease progression, but had not died or undergone lung transplant; i.e., patients who had experienced either of the following events: a confirmed ≥10% absolute decline in percent predicted FVC or a confirmed ≥50m decline in 6WMT distance. Pulmonary deterioration was considered irreversible (transition from ‘progressed’ back to ‘progression-free’ was impossible). Treatment was continued after the disease progression, which was expected to reduce the risk of death. Discontinuation rate was assumed to be the same before and after disease progression.
The lung transplant state captured the proportion of patients who had undergone lung transplantation. Patients could only enter the lung transplant state from the progressed disease state and if they were under the age of 65 years. This assumption was in line with the criteria patients must meet to be eligible for a lung transplant in clinical practice.
The death state captured the proportion of patients that had died at each point in time. This was an absorbing state in the model.
Adverse events
In the model submitted to the CEESP, adverse events were not integrated as explicit events but rather implicitly considered in the hospitalisation costs and utility losses. As it was considered an important limitation by the CEESP, leading to an underestimation of patients utility, the following adverse events were explicitly considered in the updated model: diarrhoea, serious gastro-intestinal (GI) perforation, serious cardiac event, photosensitivity and rash.
Population and treatment
As a base-case, patients affected by mild-to-moderate IPF in France were included (in line with the indication acknowledged by the French health technology assessment body: the HAS). Treatment alternatives considered in the model were pirfenidone, nintedanib, and BSC.
Outcomes
For each strategy, the following outcomes were evaluated: total life years (LY) and quality-adjusted life-years (QALY); average cumulative costs (treatment acquisition costs, disease management costs, exacerbation costs, adverse events costs, death costs, and total costs). Then, the incremental cost-effectiveness ratio (ICER), expressed as total costs per QALY gained, was computed comparing pirfenidone to its comparators, nintedanib and BSC.
Model input parameters
Model inputs were derived from clinical trials and published literature (Table 1).
Table 1.
Inputs for the reference analysis and ranges for the deterministic sensitivity analysis.
| Parameter | Base case value | Sensitivity analysis range (low | high values) | References | |
|---|---|---|---|---|
| Clinical inputs | ||||
| Progression | ||||
| PFS pirfenidone | Log-Normal (μ = 3.13; σ = 1.0569) | ASCEND/CAPACITY | ||
| PFS BSC | Log-Normal (μ = 2.77; σ = 1.0028) | ASCEND/CAPACITY | ||
| OR progression nintedanib vs pirfenidone* | 1.11 | 0.60 | 2.00 | Fleetwood, 2017 [30] |
| Overall survival | ||||
| OS pirfenidone | Weibull (α = 4.14; β = 0.844) | ASCEND/CAPACITY/RECAP | ||
| OS BSC | Weibull (α = 3.22; β = 0.1358) | Strand, 2014 [5] | ||
| OR overall mortality nintedanib vs pirfenidone | 1.39 | 0.62 | 3.13 | Fleetwood, 2017 [30] |
| % IPF-related mortality | ||||
| Pirfenidone | 53.1% | 37.4% | 68.5% | Pirfenidone NICE STA [28] ERG revised probabilities |
| BSC | 70.00% | 57.3% | 81.3% | |
| Nintedanib | 61.9% | 48.7% | 74.3% | |
| Exacerbation in progression-free | ||||
| OR pirfenidone vs BSC | 0.43 | 0.14 | 1.26 | Loveman, 2015b [19] |
| Probability BSC (per cycle) | 1.77% | 1.14% | 2.52% | Loveman, 2015a [16] |
| OR nintedanib | 0.5 | 0.31 | 0.79 | Loveman, 2015b [19] |
| Exacerbation in progression | ||||
| OR pirfenidone | 0.43 | 0.14 | 1.26 | Loveman, 2015b [19] |
| Probability BSC (per cycle) | 4.4% | 2.84% | 6.28% | Loveman, 2015a [16] |
| OR nintedanib | 0.5 | 0.31 | 0.79 | Loveman, 2015b [19] |
| Annual probability of lung transplant (<65 years) | 2.36% | 1.89% | 2.82% | INSEE, 2013 [20]; Agency for Biomedicine, 2014 [21]; Orphanet, 2014 [22] |
| Probability of death following lung transplant (per cycle) | 7.55% | 5.24% | 10.11% | Orphanet, 2014 [22] |
| Discontinuation | ||||
| Probability pirfenidone | Weibull (α = 1.10; β = 21.9464) | ASCEND/CAPACITY/RECAP | ||
| OR nintedanib vs pirfenidone | 0.962 | 0.541 | 1.724 | Fleetwood, 2017 [30] |
| Diarrhoea | ||||
| OR pirfenidone | 1.39 | 0.94 | 2.11 | Pirfenidone NICE STA [28] |
| Incidence BSC | 20.35% | 13.0% | 28.9% | ASCEND/CAPACITY |
| OR nintedanib | 7.32 | 4.82 | 11.13 | Pirfenidone NICE STA [28] |
| Serious GI perforation | ||||
| Incidence pirfenidone | 0% | - | - | Pirfenidone NICE STA [28] |
| Incidence BSC | 0% | - | - | Pirfenidone NICE STA [28] |
| Incidence nintedanib | 0.23% | 0.15% | 0.33% | Porte, 2018 [29] |
| Serious cardiac event | ||||
| OR pirfenidone | 1.36 | 0.54 | 3.46 | Pirfenidone NICE STA [28] |
| Incidence BSC | 4.49% | 2.9% | 6.4% | ASCEND/CAPACITY |
| OR nintedanib | 0.64 | 0.17 | 1.49 | Pirfenidone NICE STA [28] |
| Photosensitivity | ||||
| Incidence pirfenidone | 12.0% | 7.7% | 17.1% | Noble, 2011 [8] |
| Incidence BSC | 0% | - | - | Pirfenidone NICE STA [28] |
| Incidence nintedanib | 0% | - | - | Pirfenidone NICE STA [28] |
| Rash | ||||
| Incidence pirfenidone | 30.34% | 19.2% | 42.8% | ASCEND/CAPACITY |
| Incidence BSC | 0% | - | - | Pirfenidone NICE STA [28] |
| Incidence nintedanib | 0% | - | - | Pirfenidone NICE STA [28] |
| Costs | ||||
| Drug acquisition cost per day | ||||
| Pirfenidone | €65.49 | €64.44 | €66.54 | CNAM – BDM [35]; ASCEND/CAPACITY |
| Nintedanib | €74.46 | €59.57 | €89.35 | CNAM – BDM [35] |
| Average disease management costs per cycle 0–2 | CNAM – CCAM v.54, 2018 [36]; CNAM -TNB, 2018 [37]; CNAM -LPP, 2018 [38]; EcoSanté, 2014 [23]; DREES, 2012 [34]; INSEE, 2018 [32]; Clinical practice survey (in file); Expert opinion | |||
| Pirfenidone | €164.43 | |||
| BSC | €133.65 | |||
| Nintedanib | €164.43 | |||
| Average disease management costs per cycle 3+ | ||||
| Pirfenidone | €143.91 | |||
| BSC | €133.65 | |||
| Nintedanib | €143.91 | |||
| Oxygenotherapy (progressed only) | €265.73 | |||
| Cost of exacerbation | €3,468.66 | €2,774.93 | €4,162.39 | Cottin, 2017 [39]; DREES, 2012 [34]; HCAAM, 2013 [33]; INSEE, 2018 [32] |
| Cost of LT | €83,067.07 | €66,453.66 | €99,680.49 | Cottin, 2017 [39]; DREES, 2012 [34]; HCAAM, 2013 [33]; INSEE, 2018 [32] |
| Cost of follow-up after LT per cycle | €5,433.70 | €4,346.96 | €6,520.44 | CNAM ALD, 2009 [24]; DREES, 2012 [34]; HCAAM, 2013 [33]; INSEE, 2018 [32] |
| IPF related end of life costs | €7,725.68 | €6,180.55 | €9,270.82 | Cottin, 2017 [39]; DREES, 2012 [34]; HCAAM, 2013 [33]; INSEE, 2018 [32] |
| Diarrhoea cost | €586.58 | €469.27 | €703.90 | GHM 06M03T ATIH, 2018 [40]; ScanSante, 2017 [41] |
| Serious GI perforation cost | €2,391.61 | €1,913.29 | €2,869.93 | GHM 06M10 (level 1–4) + 06M11 (levels 1–4 + T) ATIH, 2018 [40]; ScanSante, 2017 [41]; DREES, 2012 [34]; HCAAM, 2013 [33]; |
| Serious cardiac event cost | €4,931.87 | €3,945.49 | €5,918.24 | Cottin, 2017 [39]; DREES, 2012 [34]; HCAAM, 2013 [33]; INSEE, 2018 [32] |
| Skin disorder cost | €556.29 | €445.03 | €667.54 | GHM 09M05T ATIH, 2018 [40]; ScanSante, 2017 [41]; DREES, 2012 [34]; HCAAM, 2013 [33]; |
| Rash cost | €556.29 | €445.03 | €667.54 | GHM 09M05T ATIH, 2018[40]; ScanSant, 2017 [41]; DREES, 2012 [34]; HCAAM, 2013 [33]; |
| Quality of Life | ||||
| Health state utilities | ||||
| Unprogressed IPF | 0.847 | 0.78 | 0.82 | Loveman, 2015a [16] |
| Progressed IPF | 0.782 | 0.71 | 0.77 | |
| Lung transplant | 0.700 | 0.66 | 0.73 | |
| Event disutilities | ||||
| Acute exacerbation | −0.20 | −0.13 | −0.28 | Loveman, 2015a [16] |
| Diarrhoea | −0.07 | −0.05 | −0.10 | |
| Serious GI perforation | −0.12 | −0.08 | −0.17 | |
| Serious cardiac event | −0.20 | −0.13 | −0.28 | |
| Skin disorder | −0.03 | −0.02 | −0.05 | |
| Rash | −0.03 | −0.02 | −0.04 | |
| Event durations | ||||
| Acute exacerbation | 30 | 24.00 | 36.00 | Pirfenidone NICE STA [28] |
| Diarrhoea | 15 | 12.00 | 18.00 | |
| Serious GI perforation | 90 | 72.00 | 108.00 | |
| Serious cardiac event | 90 | 72.00 | 108.00 | |
| Skin disorder | 15 | 12.00 | 18.00 | |
| Rash | 15 | 12.00 | 18.00 | |
*OR for nintedanid is based on the definition of progression considering only the criterion of confirmed ≥10% absolute decline in percent predicted FVC.
BSC, best supportive care; GI, gastrointestinal event; IPF, interstitial pulmonary fibrosis; LT, lung transplant; OR, odds ratio; PFS, progression-free survival.
Clinical efficacy data on pirfenidone and BSC were obtained from the randomized clinical trials (RCTs) including: ASCEND, CAPACITY-1, CAPACITY-2, and the extension study RECAP [8,10,25,26]. As patients from the BSC group received pirfenidone after the end of the CAPACITY study, overall survival (OS) data were retrieved from the American Strand registry, for BSC arm [5]. Parametric survival analyses were conducted to enable extrapolation of the clinical data to the modelled time horizon for BSC and pirfenidone. The following distributions were tested: exponential, Weibull, lognormal, and log-logistic. The selection of the best fitting distribution was made based on the Akaike information criterion (AIC), Bayesian information criterion (BIC), and visual inspection of the survival curves by clinical experts. The probability of a lung transplant for patients whose disease progressed was assumed to be equal for both treatment options since lung transplantations in ASCEND and CAPACITY studies occurred at similar rates in the pirfenidone (1.8%) and placebo (1.3%) groups [8,10]. For those who were eligible, the probability of receiving the lung transplant was based on French sources due to a significant heterogeneity observed in local data as well as in data from multinational studies. It was estimated that the quarterly probability of lung transplant in IPF patients under the age of 65 was 0.59%. Lung transplant survival was also considered. As no patient-level data were available to predict the survival of IPF patients following the lung transplant, the assumption was made based on data from the literature which suggested 5-year survival of 39% from which a cycle specific mortality rate of 4.6% was derived [27]. The probabilities of adverse events were mainly retrieved from the National Institute for Health and Care Excellence (NICE) Single Technology Appraisal (STA) of pirfenidone [28]. Weighted average incidences of diarrhoeas and cardiac events for BSC, and rash and photosensitivity for pirfenidone, were computed from the data of the RCTs included in the Network Meta-Analysis (NMA) conducted for the NICE STA of pirfenidone (i.e., ASCEND and CAPACITY) [8,10]. The incidence of gastrointestinal (GI) perforation for nintedanib was retrieved from the Porte et al. study [29]. Similarly to Loveman’s et al. study [16], we considered that adverse events occurred only in the first cycle, assuming that the patient and the practitioner would have found the right dosing during these three months or that the patient would have stopped the treatment. Acute exacerbation rates by progression-free and progression health states were collected from the study conducted by Loveman et al [16].
Odds ratios (OR) for nintedanib were collected from NMAs. Overall survival (OS) and progression-free survival (PFS) ORs were extracted from Fleetwood et al [30]. As in nintedanib trials the 6 minute walk test was not considered, PFS OR available was estimated considering only confirmed ≥10% absolute decline in percent predicted FVC as a criterion to define the progression. The OR related to the ‘Decrease in percent predicted FVC by ≥10%’ for nintedanib (OR vs. pirfenidone: 1.11) was therefore used to obtain an estimation of the progression-free survival. The underlying assumption is that the relative effect of nintedanib on the loss of at least 50m in 6MWT would be similar as in the 10% loss in FVC. ORs of adverse events and discontinuation were retrieved from the NICE STA of pirfenidone [28]. Probabilities of OS, PFS, and discontinuation were estimated at a reference point (time at OR measurement) from the ORs versus pirfenidone. Then, the scale parameters of the curves were calibrated, considering the same distribution and shape parameter as for pirfenidone, to get the curve passing by this reference point. Finally, the probabilities were estimated for each cycle based on these newly parameterised distributions.
Utilities
Utilities for progression-free, progressed, and after lung transplant were taken from Loveman’s et al. study [16]. The weighted average for the lung transplant, based on the time since transplant, was computed.
Disutility associated with adverse events considered in the CEM were taken from studies conducted by Ara et al [31]. and Porte et al [29]. Durations of events were based on the NICE STA of pirfenidone, yet a more realistic assumption for diarrhoea was considered (15 days instead of 3 months) [28].
Resource use and costs
A 2018 price year was used for all costs since this was the most recently published source of national costs. When necessary, costs were inflated based on index prices for health services and goods published by the French National Institute for Statistics (INSEE) [32]. Cost categories for inclusion within the model were chosen based on clinical practice and were in line with the collective perspective of the analysis. The complementary insurance cost and out-of-pocket costs were derived from costs collected from national sources [National Health Insurance (CNAM), Technical Agency for Information on Hospitalisations (ATIH)]. In compliance with the High Committee for the Future of the Health Insurance (HCAAM) observations, it was assumed that 86.5% of ambulatory care costs and 95% of hospitalisation costs were supported by the National Health Insurance [33]. According to the National Health Account (Comptes nationnaux de la santé), 37.5% of ambulatory care costs and 35.8% of hospitalisation costs are supported by the patients with the remaining costs being covered by complementary insurance [34]. The sum of these three sub-costs created the total collective perspective cost.
The following cost categories were considered for the economic evaluation: treatment acquisition costs, disease management costs, disease-related event costs, adverse event costs, lung transplant costs, and end of life (EoL) costs. Costs of transportation were included in disease management and LT costs.
There was no administration cost applied in the analysis since pirfenidone and nintedanib are delivered orally.
For treatment acquisition costs, costs of drugs and packaging were extracted from the drugs and tariffs database (BDMIT) of the National Health Insurance [35]. Pirfenidone is currently available in France as capsules form (267 mg) and as tablets (267 mg or 801 mg). These formulations have an equal cost per mg. An adjusted daily cost of pirfenidone was computed based on the average daily dose observed in the RCTs. Dose adjustments were conducted for patients experiencing adverse events during the RCTs. For nintedanib, the delivery form (150 mg soft capsules) did not allow for box optimisation, and dose adjustments were made with 100 mg soft capsules sold at the same price.
For the disease management costs, the same approach and resource use as in the HAS submission were applied, where resource uses were collected from a specialists survey and expert opinions [13]. They included: FVC test, CT scan, 6MWT, cardiac echography, diffusing capacity of the lung for carbon monoxide (DLCO) test, liver function test, oxygen therapy, pulmonologist and radiologist visits, and cost of transportation. Oxygen therapy was only considered for patients whose disease progressed. With the exception of liver tests conducted only in the treatment arms (pirfenidone and nintedanib), resource uses were assumed to be the same for all strategies. Unit costs were retrieved from official tariffs published by the National Health Insurance [36–38]. Costs per cycle were computed from resource uses and unit costs for progression-free and progressed health states.
Costs of exacerbation, lung transplant, and end of life were estimated by Cottin et al [39]. from a French real-world database of hospitalisation data (Programme de Médicalisation des Systèmes d’information, PMSI).
Cost of adverse events considered various types of clinical events. Costs of cardiac events were published by Cottin et al [39]. in their PMSI analysis. Other adverse event costs were retrieved from the 2018 tariffs by diagnosis-related groups (Groupe Homogène de Malades, GHM) published by the ATIH [40]. Average costs were computed based on the activities by sector (public or private establishments). 2017 activities by GHM were collected from ScanSanté [41]. Diarrhoeas, photosensitivity, and rash were generally controlled with a simple dose reduction during RCTs [42–44]. GHM for very short stays (≤1 day) were assumed to be a reasonable proxy of event costs. GI perforation cost was estimated from tariffs and activities for GHM corresponding to complicated and uncomplicated peptic ulcers.
Sensitivity analyses
Sensitivity analyses evaluated the impact of assumptions used in the model and variability surrounding model inputs. For deterministic sensitivity analyses, one variable or assumption was changed at a time. One-way sensitivity analyses were conducted on all model parameters associated with uncertainty (Table 1). Outcomes were computed using low and high values of model parameters. These values were estimated using credibility or confidence intervals, standard deviations of parameters, ±20% variation around parameter, or other fixed values. A probabilistic sensitivity analysis was also performed. Appropriate statistical distributions were assigned to input parameters. Values were drawn at random from statistical distributions for these variables and the process was iterated 10,000 times to provide distributions for ICERs. The cost-effectiveness acceptability curve (CEAC) and cost-effectiveness plane were generated from iterations’ results.
Scenario analyses
A scenario with a lifetime timeframe of 33 years (i.e., lifetime for the cohort aged 67 years on average at the simulation start), instead of 15 years, was conducted. In another scenario, nintedanib survival curves were estimated using BSC survival curves instead of pirfenidone survival curves. Two scenarios were conducted with alternative ORs: one with ORs used in Porte et al [29]. (OR vs BSC for PFS and OS) and a scenario where ORs that were not statistically significant were set to 1 (exacerbation, diarrhoea and serious cardiac event for pirfenidone; progression, overall mortality, serious cardiac event and discontinuation for nintedanib).
A scenario on treatment dose adjustment was also conducted, including the worst case without the adjusted dose of pirfenidone.
Results
Base case
Over a 15-year time horizon, total costs accumulated in the pirfenidone strategy were estimated at €99,477 per patient, €104,610 in nintedanib, and €14,177 in BSC. In the pirfenidone and nintedanib strategies, total costs were mostly driven by the drug acquisition costs: €83,842 and €90,067, respectively. The total number of QALYs accumulated equalled 5.20 in pirfenidone (6.91 LYs), 4.52 in nintedanib (5.98 LYs), and 3.79 in BSC (4.98 LYs). With negative incremental costs of -€5,133 and 0.67 additional QALYs accumulated, pirfenidone could be estimated to be dominant over nintedanib. The cost-effectiveness ratio was estimated at 60,738€/QALY when compared to BSC, with incremental cost of €80,060 and 1.4 QALY gained. Results of the base case analysis are presented Table 2.
Table 2.
Comparison between pirfenidone and nintedanib: base-case.
| Pirfenidone | Nintedanib | BSC | |
|---|---|---|---|
| Clinical outcomes (cumulative incidence) | |||
| Exacerbation | 86.2% | 70.9% | 78.5% |
| Diarrhoea | 26.1% | 64.3% | 20.1% |
| Serious gastrointestinal perforation | 0.0% | 0.2% | 0.0% |
| Serious cardiac event | 6.0% | 2.9% | 4.4% |
| Photosensitivity | 11.8% | 0.0% | 0.0% |
| Rash | 29.9% | 0.0% | 0.0% |
| Health outcomes | |||
| Total QALYs | 5.196 | 4.523 | 3.792 |
| Total Lys | 6.908 | 5.982 | 4.983 |
| Costs (€) | |||
| Treatment acquisition | 83,842 | 90,067 | 0 |
| Disease management | 8,187 | 6,864 | 5,794 |
| Disease related events | 2,360 | 2,001 | 2,296 |
| Adverse events | 433 | 396 | 144 |
| Lung transplant | 2,014 | 1,802 | 1,719 |
| Death | 2,641 | 3,479 | 4,224 |
| Total Costs (€) | 99,477 | 104,610 | 14,177 |
| Cost-effectiveness analysis | |||
| Incremental QALYs | 0.674 | 1.404 | |
| Incremental costs (€) | −5,133 | 85,300 | |
| Cost per QALY gained (ICER) | Dominant | 60,738 | |
ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life-year.
Sensitivity analyses
In the deterministic sensitivity analysis, the tornado diagram showing the impact of parameters on the ICER of pirfenidone vs nintedanib could not be drawn due to the dominant status of pirfenidone in the base case. Only five scenarios produced an ICER; in all other cases, pirfenidone was dominant over nintedanib. The main driver of the ICER was the OR of discontinuation for nintedanib, resulting in a maximum ICER observed in the deterministic sensitivity analysis (25,344€/QALY) when the OR for discontinuation of nintedanib was set at its highest value. The cost per day of nintedanib was the second main driver with an ICER of 19,124€/QALY when set to its lowest value. The OR of nintedanib for OS, produced an ICER in both extreme cases: 23,522€/QALY in the case of a lower OR and 7,979€/QALY with a higher OR. In the case of a lower probability of discontinuation of pirfenidone, the ICER was estimated to be 1,377€/QALY. The results of deterministic sensitivity analyses are displayed in Table 3.
Table 3.
Deterministic sensitivity analysis results.
| Parameter Description | Lower Bound (€) | Upper Bound (€) | Difference (€) |
|---|---|---|---|
| OR discontinuation – Nintedanib [0.54–1.72] | Dominant | 25,344 | 25,344 |
| Nintedanib cost per day (€) [59.57–89.35] | 19,124 | Dominant | 19,124 |
| Nintedanib OR of OS [0.37–1.26] | 23,522 | 7,979 | 15,543 |
| Proba. discontinuation – Pirfenidone [0.12–0.18] | 1,377 | Dominant | 1,377 |
OR, odds ratio; OS, overall survival.
In the probabilistic sensitivity analysis, pirfenidone was estimated to be dominant over nintedanib in 56.7% of the simulations and it was the cost-effective strategy in 96.8% of cases, when considering a willingness to pay of €50,000 per QALY. The cost-effectiveness acceptability curve for pirfenidone vs nintedanib is presented Figure 2.
Figure 2.
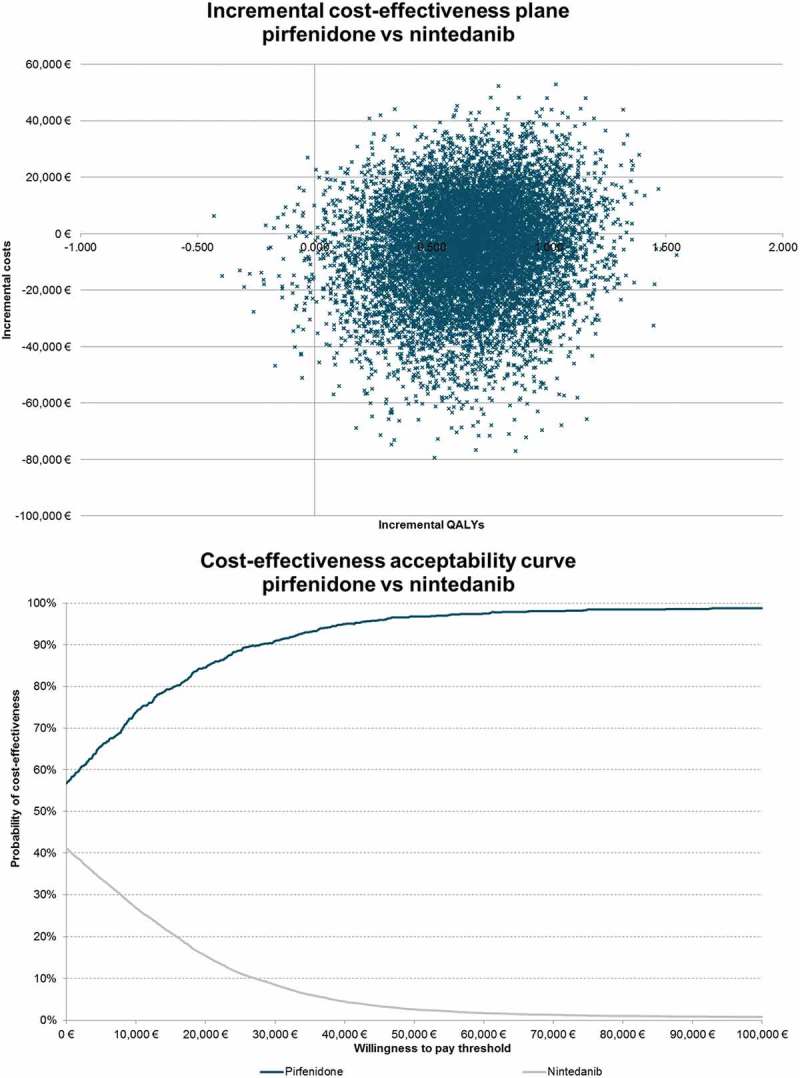
Incremental cost-effectiveness plane and acceptability curve – pirfenidone vs nintedanib.
Scenario analysis
Five scenarios were studied. In the first scenario, increasing the time horizon to 33 years did not change conclusions, as pirfenidone remained dominant over nintedanib. QALYs gained were slightly increased to 0.87 and cost savings were slightly reduced to -€4,300. In scenario 2, estimating nintedanib PFS and OS survival curves using BSC curves, instead of pirfenidone curves, led to reduced cost savings (-€3,837) and incremental QALYs (0.61); however, pirfenidone continued dominating nintedanib. In the third scenario, using the OR published by Porte et al [29]. led to an increase in the incremental costs up to €1,824, but had a low impact on the incremental effectiveness (0.62 QALYs gained). In this scenario, with a resulting ICER of 2,963€/QALY, pirfenidone was cost-effective compared to nintedanib. Scenario 4 was conducted with ORs that were not statistically significant set to 1, which increased cost savings to -€11,226 and reduced incremental effectiveness to 0.014 QALYs gained. In this fourth scenario, pirfenidone was dominant over nintedanib. In a fifth scenario, without a dose adjustment of pirfenidone and considering that patients will take the total dose (3 pills) every day, the ICER was estimated to be 10,025€/QALY, with incremental costs of €6,752, and incremental drug acquisition costs of €5,659.
Discussion
From a French collective perspective, this cost-effectiveness model suggests that pirfenidone is likely to be a dominant strategy (less costly and more effective) over nintedanib. When compared to BSC, pirfenidone was assessed with an ICER of 60,738€/QALY. The main drivers of the model were the OR of discontinuation of nintedanib and its daily cost. Sensitivity analysis showed that ICER varies between 1,377€/QALY and 25,344€/QALY, but pirfenidone remained dominant in most cases. The model was demonstrated as robust with a probability of 96.8% of pirfenidone to be cost-effective at a willingness to pay of 50,000€/QALY.
Even though the overall survival of the BSC arm was modelled on real-world data (Strand registry), a choice discussed in the 2014 CEESP appraisal, using efficacy real-world data could have given accuracy and modernity to this analysis. However, a real world study on patients suffering from IPF and treated with pirfenidone in France was performed and concluded that the observed effectiveness of pirfenidone was found similar to its efficacy demonstrated in the RCTs [5,13,45].
External validation of the cost-effectiveness model showed that results are coherent with previous findings of published cost-effectiveness models. In the previous submission to the HAS (2014) [13], the ICER was estimated to be 57,724 €/QALY vs BSC over a 33-year time horizon. Furthermore, this CEA reached the same conclusion as the Evidence Review Group’s alternative base case conducted for the NICE STA of nintedanib [46], i.e., the domination of pirfenidone over nintedanib. Moreover, simulated OS in pirfenidone and BSC arms were close to those from RCTs, registries, and previous submission results. In their cost-effectiveness analysis published in 2018, Porte et al [29]. compared nintedanib to pirfenidone and BSC in French settings. Whereas the estimated total costs of the BSC strategy at €15,448 over 30 years were really close to our estimation over the same time horizon (€14,672), the conclusion on the comparison of nintedanib and pirfenidone was the opposite as nintedanib was found to bringing greater benefits (3.34 vs 3.29 incremental QALYs). The difference in terms of assessed effectiveness observed between Porte et al [29]. and our study, leading to opposite conclusions, are mainly explained by the OS modelling. Where Porte et al [29]. used OR vs BSC for both treatments, our model used survival curves fitting observed data for pirfenidone and BSC, and applied the OR vs pirfenidone for nintedanib. Even if it could be confusing for the reader, this is not surprising that different data and methods led to different results. Indirect comparisons are necessary to conduct complete cost-effectiveness analyses although they bring uncertainty. This shows the interest of direct comparative studies in real life conditions which would reduce uncertainty around the cost-effectiveness assessment of both treatments. Nevertheless, a scenario where not statistically significant ORs were set to one led to the same conclusion as our base case.
As in Loveman et al [16]., our model considered lung transplant; pirfenidone reducing progression rate and improving overall survival, thus few more patients were eligible for LT; this led to a more costly period with a lower utility without any cost-effectiveness advantage for pirfenidone.
None of the previous CEAs assessing pirfenidone and nintedanib [16,29,47] considered pirfenidone dose adjustment, whereas it has been demonstrated that most of the photosensitivity events and diarrhoeas induced by pirfenidone could disappear after a dose reduction [42]. According to RCTs results, this would lead to an average number of 7, 88 pills per day (267mg dose), reducing the drug acquisition costs of pirfenidone considerably.
Porte et al [29]. reported a unit cost of photosensitivity rated at €4,722, yet this was an estimation of hospitalisation costs, whereas the rate considered for an adverse event was not limited to skin disorders with hospitalisation. Moreover, Costabel et al [42]. showed that none of skin and GI events led to hospitalisation in the RCTs. This led to an overestimation of adverse event costs in the Porte et al. analysis [29]. Furthermore, as reported by Costabel et al [42]., most of the skin disorders induced by pirfenidone could disappear by a dose reduction, generally conducted within the first three months of treatment. The same situation applied to serious GI events, where costs were estimated at €1,914.99 in Porte et al [29]., representing hospitalisation costs. Reported GI events were mainly serious diarrhoeas without hospitalisation, which can be controlled following recommendations on drug intake (intake during or after a meal) reducing the impact of those adverse events in real life [42].
The strength of our analysis lies in its improvement based on the consideration of the CEESP’s comments upon the initial submission. An additional advantage of our analysis, in comparison to previous ones, arises from including clinical recommendations on dose adjustments for adverse events management.
Nevertheless, in our model the progression state was not further split to fully capture the slow degradation of quality of life in this health state, and this can be considered as a limitation. As the progression state encompassed different levels of severity, the utility of patient should decrease over time in this state whereas an average utility value was considered for patients whose disease progressed in the model. As a result of this assumption, the patients considered in the progression state are very heterogeneous in term of utility level. In the literature, the utility level of patients needing a lung transplant was quite low (0.31 as estimated by Anyanwu et al [48].) whereas it reached 0.70 after the lung transplant. Those patients were pooled with all other patients whose disease progressed where they represented a small proportion. As a consequence, the average utility value assessed in this health state was higher than the utility level after a lung transplant.
Conclusion
Pirfenidone is likely to be a cost – effective strategy compared to BSC and seems more efficient and less costly compared to nintedanib for the treatment of patients with IPF in France. Additional researches on the direct comparative effectiveness of both treatments should be conducted in a near future.
Funding Statement
This work was supported by Roche and conducted by Creativ-Ceutical.
Authors contribution
EC, OC prepared and adapted the economic model. EC, OC prepare the first draft of the manuscript. MB, EC, OC validated the model structure and assumption. Data collection and results interpretation were performed by MB, EC, OC, RC and AP. VC validated data interpretation and medical content. All authors provided critical feedback on the manuscript and have approved the final version.
Disclosure statement
This work was supported by Roche and conducted by Creativ-Ceutical. VC declare consultancy/advisory roles for ROCHE SAS. VC also declares grant/research funding from Boehringer Ingelheim and Roche, and also a consultancy/advisory role for Actelion, Bayer, Boehringer Ingelheim, Biogen, Gilead, GSK, MSD, Novartis, Roche, Sanofi, Celgene, Promedior, and Galapagos NV.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- [1].Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–11. [DOI] [PubMed] [Google Scholar]
- [2].Ley B, Collard HR, King TE Jr.. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. [DOI] [PubMed] [Google Scholar]
- [3].Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66(6):462–467. [DOI] [PubMed] [Google Scholar]
- [4].Nunes H, Carton Z, Cottin V, et al. Preliminary results of the French national prospective cohort on IPF. Eur Respir J. 2011;38(Suppl 55):646. Available from: https://erj.ersjournals.com/content/38/Suppl_55/p646. [Google Scholar]
- [5].Strand MJ, Sprunger D, Cosgrove GP, et al. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest. 2014;146(3):775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee AS, Mira-Avendano I, Ryu JH, et al. The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med. 2014;108(7):955–967. [DOI] [PubMed] [Google Scholar]
- [7].Du Bois RM.Idiopathic pulmonary fibrosis: present understanding and future options. Eur Respir Rev. 2011;20(121):132–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. [DOI] [PubMed] [Google Scholar]
- [10].King TE Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. [DOI] [PubMed] [Google Scholar]
- [11].Richeldi L, Du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. [DOI] [PubMed] [Google Scholar]
- [12].NICE Nintedanib for treating idiopathic pulmonary fibrosis (review of TA379) -Committee papers [ID752]. London: NICE; 2015. [Google Scholar]
- [13].Haute Autorité de Santé (HAS) Avis d’efficience - ESBRIET® 267 mg (pirfénidone). Paris: Haute Autorité de santé; 2015. [Google Scholar]
- [14].Haute Autorité de Santé (HAS) Choix méthodologiques pour l’évaluation économique à la HAS 2011. HAS; Available fromhttps://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf [Google Scholar]
- [15].Cottin V, Crestani B, Valeyre D, et al. French recommendations for idiopathic pulmonary fibrosis: oriented toward practice. Rev Mal Respir. 2013;30(10):814–816. [DOI] [PubMed] [Google Scholar]
- [16].Loveman E, Copley VR, Colquitt J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Nat Inst Health Res. 2015; 19(20):i-xxiv, 1-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du Bois RM, Albera C, Bradford WZ, et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43(5):1421–1429. [DOI] [PubMed] [Google Scholar]
- [18].Du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–466. [DOI] [PubMed] [Google Scholar]
- [19].Loveman E, Copley VR, Scott DA, et al. Comparing new treatments for idiopathic pulmonary fibrosis–a network meta-analysis. BMC Pulm Med. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Institut National de la Statistique et des Etudes Economiques (INSEE) Bilan démographique 2013; 2013. Available from: https://www.insee.fr/fr/statistiques/12809562014
- [21].Agence de biomédecine Le rapport médical et scientifique de l’Agence de la biomédecine 2014. biomédecine Ad, editor Paris: Agence de biomédecine; 2014. [Google Scholar]
- [22].Orphanet Prevalence of rare diseases: bibliographic data. 2014. Available from: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. [Google Scholar]
- [23].CNAMTS; ID. Eco-Santé, 2014. Irdes, Drees, Cnamts. Available from: http://www.ecosante.fr/index2.php?base=DEPA&langh=FRA&langs=FRA
- [24].Caisse Nationale d’Assurance Maladie (CNAM) Coût des ALD en 2009; 2009. Available from: https://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/affection-de-longue-duree-ald/archives/cout-des-ald-en-2009.php
- [25].Costabel U, Albera C, Bradford WZ, et al. Analysis of lung function and survival in RECAP: an open-label extension study of pirfenidone in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(3):198–205. [PubMed] [Google Scholar]
- [26].Kreuter M. Pirfenidone: an update on clinical trial data and insights from everyday practice. Eur Respir Rev. 2014;23(131):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126(2):469–475. [DOI] [PubMed] [Google Scholar]
- [28].NICE Pirfenidone for treating idiopathic pulmonary fibrosis (review of TA282) - Committee papers [ID837]. USA: NICE; 2016. [Google Scholar]
- [29].Porte F, Cottin V, Catella L, et al. Health economic evaluation in idiopathic pulmonary fibrosis in France. Curr Med Res Opin. 2018March9;2018:1–10. [DOI] [PubMed] [Google Scholar]
- [30].Fleetwood K, McCool R, Glanville J, et al. Systematic review and network meta-analysis of idiopathic pulmonary fibrosis treatments. J Manag Care Spec Pharm. 2017;2017(23(3–b)):S5–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ara RBJ. Using health state utility values from the general population to approximate baselines in decision analytic models when conditionspecific data are not available. Value Health. 2011;2011(14):539–545. [DOI] [PubMed] [Google Scholar]
- [32].Institut National de la Statistique et des Etudes Economiques (INSEE) Indice des prix à la consommation - Base 2015 - Ensemble des ménages - France - Services de santé. INSEE; 2018. [cited 2018 August] Available from: https://www.insee.fr/fr/statistiques/serie/001763845
- [33].Haut Conseil pour l’avenir de l’Assurance Maladie (HCAAM) Rapport annuel du HCAAM. Paris, France: HCAAM; 2013. [Google Scholar]
- [34].Direction de la recherche des études de l’évaluation et des statistiques (DREES) Comptes nationaux de la santé (CNS). Paris, France: Ministère des Solidarités et de la Santé; 2012. [Google Scholar]
- [35].Caisse Nationale d’Assurance Maladie (CNAM) Base des médicaments et informations tarifaires (BDM_IT); 2018. [cited 2018 July] Available from: http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php?p_site=AMELI
- [36].Caisse Nationale d’Assurance Maladie (CNAM) Classification commune des actes medicaux (CCAM) v54; 2018. [cited 2018 July] Available from: https://www.ameli.fr/accueil-de-la-ccam/telechargement/index.php
- [37].Caisse Nationale d’Assurance Maladie (CNAM) Table nationale de codage de biologie (TNB); 2018. [cited 2018 July] Available from: http://www.codage.ext.cnamts.fr/codif/nabm/index_presentation.php?p_site=AMELI
- [38].Caisse Nationale d’Assurance Maladie (CNAM) Liste des produits et des prestations (LPP); 2018. [cited 2018 July] Available from: http://www.codage.ext.cnamts.fr/codif/tips/index_presentation.php?p_site=AMELI
- [39].Cottin V, Schmidt A, Catella L, et al. Burden of idiopathic pulmonary fibrosis progression: a 5-year longitudinal follow-up study. PLoS One. 2017;12(1):e0166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Agence Technique de l’Information sur l’Hospitalisation (ATIH) Tarifs MCO et HAD; 2018. [cited 2018 July] Available from: https://www.atih.sante.fr/tarifs-mco-et-had
- [41].ScanSanté Activité MCO par GHM ou racine; 2017. [cited 2018 July] Available from: https://www.scansante.fr/applications/statistiques-activite-MCO-par-GHM
- [42].Costabel U, Bendstrup E, Cottin V, et al. Pirfenidone in idiopathic pulmonary fibrosis: expert panel discussion on the management of drug-related adverse events. Adv Ther. 2014;31(4):375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hughes G, Toellner H, Morris H, et al. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med. 2016;5(9):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brunnemer E, Walscher J, Tenenbaum S, et al. Real-world experience with nintedanib in patients with idiopathic pulmonary fibrosis. Respiration. 2018;95(5):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jouneau S, Gamez AS, Traclet J, et al. A 2-year observational study in patients suffering from idiopathic pulmonary fibrosis and treated with pirfenidone: a French ancillary study of PASSPORT. Respiration. 2019;1–10. [DOI] [PubMed] [Google Scholar]
- [46].NICE Nintedanib for treating idiopathic pulmonary fibrosis NICE. London, UK: 2015. [Google Scholar]
- [47].Rinciog CWM, Chang S, Maher TM 5, et al. Diamantopoulos A. A cost-effectiveness analysis of nintedanib in idiopathic pulmonary fibrosis in the UK. Pharmacoeconomics. 2017;35(4):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Anyanwu ACMA, Rogers CA, Murday AJ. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax. 2001;56:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


