Abstract
Background and Aims:
Hemodynamic changes to pneumoperitoneum and postoperative pain can be detrimental in obese patients; we investigated whether intravenous (IV) or intraperitoneal (IP) magnesium sulphate (MgSo4) administration could attenuate the hemodynamic stress response to pneumoperitoneum and improve postoperative pain control after laparoscopic sleeve gastrectomy (LSG).
Material and Methods:
In total, 120 patients scheduled for LSG were randomized to either, control group (Group C, IV and IP saline), Group I (IV 30 mg/kg MgSo4), or Group P (IP 30 mg/kg MgSo4). Outcome variables were mean arterial pressure (MAP), heart rate (HR), postoperative pain score, total analgesic consumption, and incidence of adverse events.
Results:
MAP and HR were significantly lower in Groups I and P than that in control group. Pain score was better in Groups I and P than in control group up to 2 h postoperative (P = 0.023, 0.010, respectively); it was significantly lower in Group P than other two groups at 4 h postoperative (P <0.001). Significantly, reduced postoperative analgesic consumption with delayed onset to first analgesic request were observed in magnesium groups compared to control (P = 0.001, respectively). Moreover, onset to first analgesic request was longer in Group P than Group I (P = 0.001). No serious side effects were noticed.
Conclusion:
The IV and IP administration of MgSo4 significantly attenuated the circulatory response to pneumoperitoneum and reduced postoperative pain as well as opioid consumption as compared to controls in obese patients undergoing LSG with no serious adverse effects.
Keywords: Gastrectomy, injections, intraperitoneal, magnesium sulfate, obesity, pneumoperitoneum
Introduction
Laparoscopic sleeve gastrectomy (LSG) has gained wide popularity nowadays as one of the most commonly performed bariatric surgical procedures.[1] The laparoscopic approach being the conventional technique for performing bariatric surgeries including sleeve gastrectomy has the advantage of causing less postoperative pain, fewer complications as well as shorter duration of hospital stay than open laparotomy techniques.[2] However, the pneumoperitoneum created during laparoscopy can produce stress cardiovascular response with hemodynamic alterations leading to abrupt elevation of mean arterial pressure (MAP), mean pulmonary artery pressure, heart rate (HR), and systemic vascular resistance.[3] These changes may be potentially detrimental particularly in obese patients with altered cardiac morphology and function in relation to the degree of obesity.[4] Different pharmacological agents (α2 agonists,[5] beta-blockers,[6] and opioids[7]) are often used to attenuate the circulatory response caused by pneumoperitoneum.
Another major concern in this population is the management of postoperative pain. Opioids which represent the mainstay of acute pain relief might further contribute to the appearance of postoperative respiratory adverse events.[8] Various analgesic modalities, such as non-opioid systemic analgesics as well as regional anesthesia and non-opioid adjuvants, have been implemented either alone or combined in a multimodal approach to provide analgesia with opioid-sparing effect after bariatric surgery.[9,10]
Magnesium sulphate (MgSo4), N-Methyl-D-aspartate (NMDA) receptor antagonist, has been administered by different routes to attenuate pneumoperitoneum associated hemodynamic response and to reduce postoperative pain.[11,12]
We conducted this study to evaluate the efficacy of MgSo4 in attenuating cardiovascular stress response to pneumoperitoneum and its analgesic effects when administered using either intravenous (IV) route or intraperitoneal (IP) instillation in obese patients operated for LSG.
Material and Methods
This prospective randomized controlled double-blind study was conducted from January 2018 to May 2018. After approval of institutional ethics committee (31980/12/17) and registration in Pan African Clinical Trial Registry (PACTR201801002944228), a written informed consent was obtained, and 120 patients of either gender, aged 18–60 years old with a body mass index ≥35 kg/m2 and classified by American society of anesthesiologist as ASA I-II physical status were enrolled. All participants were scheduled for sleeve gastrectomy procedure by laparoscopic approach. Patients who had previous abdominal surgery or with hepatic or renal insufficiency, severe respiratory or cardiac disorders, pregnancy or lactation, heart block, allergy to any of the study drugs, hyper or hypomagnesaemia, on beta-blockers, calcium-channel blockers, sedatives or antipsychotics, were excluded from the study.
A pre-anesthetic check was performed including thorough history taking and complete physical examination. Pulmonary function tests, polysomnography, and baseline serum magnesium were requested and evaluated along with routine preoperative investigations, and low molecular weight heparin (enoxaparin 40 mg subcutaneous/12 h) was started for thrombotic prophylaxis and omitted 12 h before surgery to be continued postoperative.
Upon reaching the operating theater, an IV access was secured followed by premedication with midazolam 2 mg, ranitidine 50 mg as well as antiemetic prophylaxis in the form of ondansetron 4 mg and dexamethasone 8 mg. Standard monitors were attached to the patients including pulse oximetry, ECG, non-invasive arterial blood pressure, and capnography, and baseline hemodynamic parameters (HR and MAP) were recorded. In addition, elastic stockings were applied to all participants, and ringer's lactate infusion was started.
Randomization of the patients was performed using a computer-generated random sequence concealed in sealed opaque envelopes, and a blinded nurse randomly chose the envelope that determined the group of assignment. Patients were allocated into three groups (40 each) with 1:1:1 ratio to receive one of the following immediately after creation of pneumperitoneum and before surgical dissection.
Group C (control group): 100 ml 0.9% normal saline was infused over 10 min with IP instillation of 30 ml normal saline.
Group I (IV magnesium group): 100 ml of 30 mg/kg MgSo4 in 0.9% normal saline was infused over 10 min with IP instillation of 30 ml normal saline.
Group P (IP magnesium group): 100 ml 0.9% normal saline was infused over 10 min with IP instillation of 30 ml MgSo4 at a dose of 30 mg/kg.
A standardized anesthetic technique was accomplished using fentanyl 1 μg/kg, propofol 1-2 mg/kg for induction followed by cis-atracurium 0.15 mg/kg to facilitate endotracheal intubation. Maintenance of anesthesia was done by isoflurane 1–2% in an equal mixture of air, oxygen, and cis-atracurium 0.03 mg/kg as required. Patients were mechanically ventilated while keeping ETCO2 between 35–45 mm Hg. Intraoperative elevation of HR or MAP >20% of baseline was managed by administration of fentanyl 0.5 μg/kg, and the total dose of intraoperative rescue fentanyl was recorded, whereas bradycardia (HR <60 beat/min) and hypotension (MAP <20% of baseline) were treated by atropine 0.01 mg/kg and by fluid infusions and vasopressors respectively.
The intra-abdominal pressure was kept at 12–14 mmHg, and an identical surgical technique was used in all patients by the same experienced surgeon who also carried out the IP instillation of the study solutions below the diaphragm and around the surgical field through the ports of the laparoscope. All medications were prepared by an anesthesiologist not participating in the study, and data collection was done by another anesthesiologist unaware of group allocation.
After completion of the procedure, pneumoperitoneum was released and each of the laparoscopic port sites was infiltrated in all participants using 5 ml of 0.25% bupivacaine. Reversal of neuromuscular blockade was achieved using atropine 0.02 mg/kg and neostigmine 0.04 mg/kg followed by tracheal extubation, and the patients were moved to the post-anesthesia care unit (PACU) where they received oxygen supplementation using nasal cannula and regular analgesia with IV acetaminophen 1g/6 h and IV ketorolac 30 mg/8 h. Patients were kept under observation in the PACU till full recovery (modified Aldrete score ≥9), then they were transferred to the ward.
Our primary outcome variable was the change in HR and MAP. They were recorded at the following intervals: upon arrival to the operating room as a baseline (T0), 1 min after induction (T1), before pneumoperitoneum (T2), 5, 15, 30, and 60 min after pneumoperitoneum (T3, T4, T5, and T6, respectively), 5 min after release of pneumoperitoneum (T7), and upon arrival to the PACU (T8). The secondary outcomes were postoperative pain scores, analgesic consumption, and incidence of adverse events. Postoperative pain was assessed using 0–10 visual analogue score (VAS), where 0 represents no pain and 10 worst pain imaginable. Pain scores were recorded at 1, 2, 4, 8, 12, and 24 h postoperative. Patients with VAS ≥4 received rescue analgesia in the form of morphine 2 mg. Time to first postoperative rescue analgesic request, as well as total analgesic consumption in the first 24 h postoperatively were recorded. Other measured parameters included extubation time (time from the end of anesthesia till extubation) and recovery time (time elapsed since extubation till modified Aldrete score ≥9). In addition, blood samples for serum magnesium level were withdrawn the day before surgery (M0), 10 min after the IV bolus dose and IP instillation (M1), immediately before extubation (M2), and at 6 h postoperative (M3). The incidence of adverse events including postoperative nausea and vomiting (PONV), hypotension, bradycardia, and sedation ≥2 (assessed on a 4-point scale, where 0 = alert, 1 = quietly awake, 2 = asleep but easily aroused, and 3 = deep sleep) was recorded.
Statistical analysis
From the results of a previous study,[13] a sample size of 36 patients per group was required to detect a significant reduction of 10 mmHg in MAP as a primary outcome in magnesium groups relative to control group with a standard deviation of 14.9 mmHg at α error of 0.05 and power of study of 80%. Assuming a dropout rate of 10%, the sample size was increased up to 40 patients in each arm of study. Our secondary outcomes included pain scores and postoperative analgesia. The statistical software SPSS 16 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analysis. The Kolmogorov-Smirnov test was performed to check the assumption of normality. The parametric data were expressed as mean ± SD and analyzed utilizing One-way ANOVA with post hoc Tukey's HSD test. The categorical data were presented as patients' number or frequencies (%) and were analyzed using the Chi-square test. Within each group, the numerical data were compared using repeated measures analysis of variance, whereas the non-parametric data were analyzed using the Friedman test. The P value <0.05 was considered significant.
Results
In total, 133 subjects were scheduled. Nine patients did not meet our inclusion criteria and 4 patients declined to participate, so 120 patients were enrolled and randomly allocated into three groups (40 each).
The demographic data including age, gender, ASA physical status, BMI, and mean duration of surgery is shown in Table 1. Extubation and recovery times, as well as the incidence of complications did not differ significantly among the study groups [Table 1].
Table 1.
Demographic data and characteristics of patients in the studied groups
| Variable | Group C | Group I | Group P | P |
|---|---|---|---|---|
| Age (years) | 30.0±7.8 | 31.2±8.5 | 33.0±10.0 | 0.308 |
| Gender M/F | 29 (72.5%)/11 (27.5%) | 34 (85%)/6 (15%) | 32 (80%)/8 (20%) | 0.383 |
| ASA I/II | 27 (67.5%)/13 (32.5%) | 30 (75%)/10 (25%) | 25 (62.5%)/15 (37.5%) | 0.481 |
| BMI kg/m2 | 47.9±7.0 | 50.0±3.2 | 49.1±3.1 | 0.186 |
| Duration of surgery (min) | 139.5±23.5 | 129.3±16.6 | 131.3±20.7 | 0.063 |
| Extubation time (min) | 7.3±1.7 | 8.2±2.0 | 8.2±2.8 | 0.102 |
| Recovery time (min) | 19.7±9.3 | 23.2±7.4 | 22.7±4.5 | 0.076 |
| Hypotension | 2 (5%) | 3 (7.5%) | 1 (2.5%) | 0.591 |
| Bradycardia | 1 (2.5%) | 4 (10%) | 2 (5%) | 0.346 |
| PONV | 12 (30%) | 6 (15%) | 5 (12.5%) | 0.099 |
| Sedation ≥2 | 3 (7.5%) | 7 (17.5%) | 6 (15%) | 0.392 |
Data presented as mean±SD or patient's number (%)
Hemodynamic parameters were similar among the three groups at T0, T1, and T2. Significant lower values of MAP and HR from T3 to T8 were recorded in Group I and GroupPin comparison to control group. The MAP and HR were also significantly lower in Group I than GroupPat T3, T4, and T5 with lower values of both parameters at T6, T7, and T8 in Group I than GroupPbut without any statistical significance [Figure 1].
Figure 1.
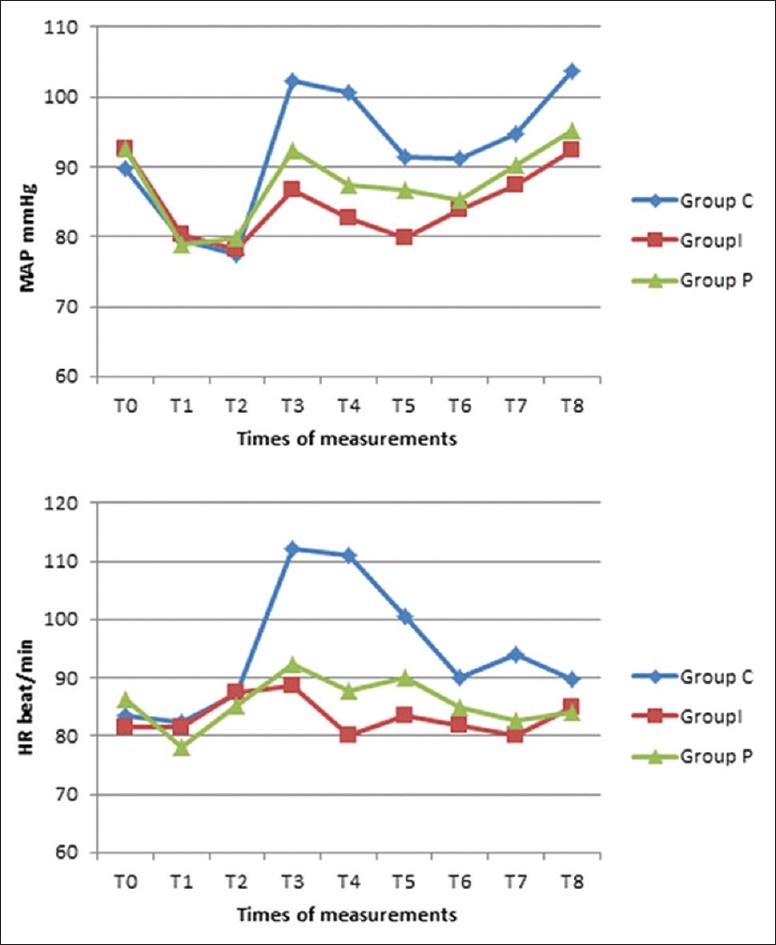
Mean arterial pressure and heart rate changes in the three groups. Data presented as mean ± SD
Referring to postoperative pain, pain scores were significantly higher in the control group than in both IV and IP magnesium groups at the first 2 h postoperative (P = 0.023, 0.010, respectively), whereas at 4 h, VAS score was better in GroupPthan in the other two groups (P <0.001), but with no significant differences between Groups C and I (P = 0.070). Postoperative pain scores did not differ significantly among the three groups at 8 h, 12 h, and 24 h postoperative (P = 0.194, 0.117, 0.193, respectively) [Figure 2].
Figure 2.
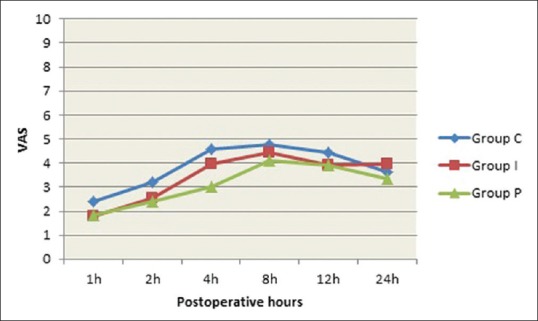
VAS score of the studied groups at all times of measurements during the first 24 postoperative hours. Data presented as mean ± SD
Analgesic consumption at both the intra- and postoperative periods was significantly lower in Groups I and P than in the control group. Although Group I had lower intraoperative analgesic requirement than GroupP, the total postoperative analgesic consumption showed no statistically significant difference between the two groups. In addition, time to first analgesic requirement was significantly longer in GroupPthan in Group C and Group I; it was longer in Group I than in the control group [Table 2].
Table 2.
Intra and postoperative analgesic consumption in the three groups
| Variable | Group C | Group I | Group P | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| Intraoperative fentanyl consumption(µg) | 83.3±21.9 | 43.7±11.2 | 55.5±19.3 | <0.001* | <0.001* | <0.001* | 0.001* |
| 24 h postoperative rescue analgesia (morphine) (mg) | 9.10±2.1 | 7.4±3.1 | 7.0±2.6 | 0.001* | 0.008* | <0.001* | 0.463 |
| Onset of first postoperative rescue analgesia (h) | 3.0±1.1 | 3.8±1.4 | 5.0±1.6 | <0.001* | 0.003* | <0.001* | 0.001* |
Data presented as mean±SD. P presented the comparison among the three groups. P1 presented the comparison between Group C and Group I. P2 presented the comparison between Group C and Group P. P3 presented the comparison between Group I and Group P. *Denoted statistically signficant difference (P<0.05)
Serum magnesium levels were comparable among the three groups at base line and at 6 h postoperative. However, 10 min after administration of the study medications, magnesium levels were significantly higher in Group I and GroupP than in Group C, and they were higher in Group I than in GroupP. However, immediately before extubation, GroupPhad higher levels of magnesium than the other two groups with comparable serum magnesium levels between Group C and Group I. Serum magnesium in the studied groups remained within the normal range at all times of measurements [Table 3].
Table 3.
Perioperative serum magnesium (mg/dl) in the studied groups
| Variable | Group C | Group I | Group P | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| M0 | 1.9±0.2 | 1.9±0.1 | 1.9±0.2 | 0.977 | |||
| M1 | 1.9±0.2 | 2.9±0.2 | 2.1±0.3 | <0.001* | <0.001* | 0.001* | <0.001* |
| M2 | 1.9±0.3 | 2.0±0.1 | 2.1±0.2 | <0.001* | 0.139 | <0.001* | <0.001* |
| M3 | 1.9±0.2 | 1.9±0.2 | 2.0±0.1 | 0.155 |
Data presented as mean±SD. P presented the comparison among the three groups. P1 presented the comparison between Group C and Group I. P2 presented the comparison between Group C and Group P. P3 presented the comparison between Group I and Group P. *Denoted statistically signficant difference (P<0.05)
Discussion
This controlled prospective randomized study proved that administering MgSO4 either by IV route or IP instillation had attenuated the hemodynamic stress response to pneumoperitoneum and reduced postoperative pain as well as consumption of postoperative opioids without increased incidence of side effects following sleeve gastrectomy.
Pneumoperitoneum causes an increase in systemic vascular resistance mediated mainly by vasopressin and catecholamines,[5] MgSO4 prevents the adrenal medullary release of catecholamines as well as their secretion from adrenergic nerve endings, and in addition, it has a direct effect on blood vessels producing vasodilatation with a subsequent reduction in blood pressure. Apart from that, it has also the ability to attenuate vasopressin–mediated vasoconstriction.[13]
The current study demonstrated the beneficial effects of IV and IP administration of MgSO4 in suppressing the unwanted hemodynamic changes produced by pneumoperitoneum as manifested by the lower values of HR and MAP in patients receiving magnesium than in controls. Our results were similar to those obtained by Paul et al.[14] who reported a decrease in HR and MAP during pneumoperitoneum after IV bolus dose of 30 mg/kg MgSO4. Furthermore, Jee et al.[12] concluded that IV 50 mg/kg magnesium effectively blunted the increase in blood pressure due to pneumoperitoneum in laparoscopic cholecystectomy. Ali et al.[11] administered 20 ml MgSO410% by IP instillation after creation of pneumoperitoneum in patients undergoing laparoscopic cholecystectomy, and they found that the use of magnesium was associated with attenuation of adverse hemodynamic stress response compared to control group. The decrease in hemodynamic parameters was more obvious in IV Group than IP MgSO4 Group after pneumoperitoneum, which might have been attributed to the immediate onset of action as well as the rapid rise of serum magnesium levels in IV MgSO4 group.
The IP route for administration of local anesthetics with or without adjuvants has proved efficacy in improving pain management in various laparoscopic surgeries including sleeve gastrectomy. This may be attributed to blocking of visceral pain conduction; in addition, absorption from the large peritoneal surface may also contribute to analgesia.[15,16,17]
Our results showed that patients receiving magnesium had better pain relief up to 2 h postoperative in IV Group and up to 4 h in IP Group with lower consumption of rescue analgesics than in control group. Safe management of postoperative pain in obese patients represents a unique challenge, and multimodal analgesia is usually implemented to decrease opioids requirements with their induced airway obstruction and respiratory depression particularly in those with obstructive sleep apnea.[10] The perioperative use of MgSO4 by various routes of administration in non-obese patients has shown to decrease postoperative pain as well as postoperative analgesic consumption.[18,19,20] However, evidence for its use in morbidly obese is extremely limited. A recent published study by Kizilcik et al.[21] reported better pain management with reduction in postoperative opioid requirements when MgSO4 was administered as an IV bolus of 30 mg/kg followed by infusion of 20 mg/kg/h for 24 h in patients undergoing sleeve gastrectomy. Moreover, IP instillation of MgSO4 alone or with local anesthetics during laparoscopic surgeries has been shown to be beneficial in enhancing the quality of postoperative analgesia as well as decreasing postoperative analgesic requirements.[17,20]
The analgesic properties of magnesium are mainly attributed to its N-Methyl-D-aspartate (NMDA) receptor antagonist effect, besides it regulates calcium influx into the cell. The peripheral distribution of glutamate receptors allowed the use of peripheral NMDA receptor antagonists such as MgSO4 to alleviate pain.[20] Although magnesium has mild sedative effects, it lowers intraoperative anesthetic requirements; this could be advantageous in reducing the residual anesthetic effects in obese. No serious magnesium related side effects were noticed in our study; the measured level of serum magnesium throughout the period of the study was less than 2 mmol/l (equivalent to 4.86 mg/dl), which was the level reported to produce minor side effects.[22] Serum magnesium was higher in Group I than in GroupPafter administration of study medications. IV magnesium has a rapid onset with an elimination half life of 30 min. However, the steady and more continual absorption of IP magnesium through the large peritoneal surface into the systemic circulation has contributed to the higher serum magnesium levels found in Group P than in Group I at the time of extubation with serum levels remained within the normal range in both circumstances.
The anesthetic and analgesic sparing effects of magnesium might have contributed to the decreased incidence of PONV in magnesium groups; another explanation is the antagonist effect of MgSO4 on NMDA receptors located in the common pathway of nausea and vomiting. However, no clear data regarding this effect is available.[17]
This study showed some limitations:First, we did not measure any of the blood markers of stress response during or after surgery to demonstrate the effect of magnesium on those indicators. Second, the dose of magnesium in our study was calculated depending on lean body weight (LBW). Because of the limited data on the use of magnesium in obese patients, we used LBW from reports, suggesting that it is the ideal weight scalar for drug administration in those patients as it is closely correlated with the cardiac output.[23]
Finally, we conclude that administration of MgSO430 mg/kg as IV or IP anesthesia adjunct effectively blunted the hemodynamic stress response to pneumoperitoneum and enhanced the quality of postoperative analgesia with no serious adverse events in obese patients undergoing LSG.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, et al. Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21:11804–14. doi: 10.3748/wjg.v21.i41.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Tovar J, Muñoz JL, Gonzalez J, Zubiaga L, García A, Jimenez M, et al. Postoperative pain after laparoscopic sleeve gastrectomy: Comparison of three analgesic schemes (isolated intravenous analgesia, epidural analgesia associated with intravenous analgesia and port-sites infiltration with bupivacaine associated with intravenous analgesia) Surg Endosc. 2017;31:231–6. doi: 10.1007/s00464-016-4961-3. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005;241:219–26. doi: 10.1097/01.sla.0000151791.93571.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpert MA, Omran J, Bostick BP. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep. 2016;5:424–34. doi: 10.1007/s13679-016-0235-6. [DOI] [PubMed] [Google Scholar]
- 5.Hazra R, Manjunatha S, Manuar M, Basu R, Chakraborty S. Comparison of the effects of intravenously administered dexmedetomidine with clonidine on hemodynamic responses during laparoscopic cholecystectomy. Anaesth Pain Intensive Care. 2014;18:25–30. [Google Scholar]
- 6.Koivusalo AM, Scheinin M, Tikkanen I, Yli-Suomu T, Ristkari S, Laakso J, et al. Effects of esmolol on haemodynamic response to CO2 pneumoperitoneum for laparoscopic surgery. Acta Anaesthesiol Scand. 1998;42:510–7. doi: 10.1111/j.1399-6576.1998.tb05159.x. [DOI] [PubMed] [Google Scholar]
- 7.Lentschener C, Axler O, Fernandez H, Megarbane B, Billard V, Fouqueray B, et al. Haemodynamic changes and vasopressin release are not consistently associated with carbon dioxide pneumoperitoneum in humans. Acta Anaesthesiol Scand. 2001;45:527–35. doi: 10.1034/j.1399-6576.2001.045005527.x. [DOI] [PubMed] [Google Scholar]
- 8.Budiansky AS, Margarson MP, Eipe N. Acute pain management in morbid obesity–an evidence based clinical update. Surg Obes Relat Dis. 2017;13:523–32. doi: 10.1016/j.soard.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Lam KK, Mui WL. Multimodal analgesia model to achieve low postoperative opioid requirement following bariatric surgery. Hong Kong Med J. 2016;22:428–34. doi: 10.12809/hkmj154769. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez A, Singh PM, Sinha AC. Postoperative analgesia in morbid obesity. Obes Surg. 2014;24:652–9. doi: 10.1007/s11695-014-1185-2. [DOI] [PubMed] [Google Scholar]
- 11.Ali RM, Rabie AH, Elshalakany NA, El Gindy TM. Effect of intraperitoneal magnesium sulfate on hemodynamic changes and its analgesic and antiemetic effect in laparoscopic cholecystectomy. Ain-Shams J Anaesthesiol. 2015;8:153–9. [Google Scholar]
- 12.Jee D, Lee D, Yun S, Lee C. Magnesium sulphate attenuates arterial pressure increase during laparoscopic cholecystectomy. Br J Anaesth. 2009;103:484–9. doi: 10.1093/bja/aep196. [DOI] [PubMed] [Google Scholar]
- 13.Dar S, Gupta D, Deopujari R, Gomes P. Effect of magnesium sulphate on attenuation of hemodynamic stress responses during laparoscopic abdominal surgeries. J Anesth Clin Res. 2015;6:590. [Google Scholar]
- 14.Paul S, Biswas P, Bhattacharjee DP, Sengupta J. Effects of magnesium sulfate on hemodynamic response to carbon dioxide pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. Anesth Essays Res. 2013;7:228–31. doi: 10.4103/0259-1162.118970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamdari NM, Bakhtiyari M, Gholizadeh B, Shariati C. Analgesic effect of intraperitoneal bupivacaine hydrochloride after laparoscopic sleeve gastrectomy: Arandomized clinical trial. J Gastrointest Surg. 2018;22:396–401. doi: 10.1007/s11605-017-3659-8. [DOI] [PubMed] [Google Scholar]
- 16.Badawy AM. Intraperitoneal analgesia to reduce pain after laparoscopic hysterectomy. Int J Reprod Contracept Obstet Gynecol. 2017;6:3235–40. [Google Scholar]
- 17.Yadava A, Rajput SK, Katiyar S, Jain RK. A comparison of intraperitoneal bupivacaine-tramadol with bupivacaine-magnesium sulphate for pain relief after laparoscopic cholecystectomy: A prospective, randomised study. Indian J Anaesth. 2016;60:757–62. doi: 10.4103/0019-5049.191696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht E, Kirkham K, Liu S, Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: A meta-analysis. Anaesthesia. 2013;68:79–90. doi: 10.1111/j.1365-2044.2012.07335.x. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira GS, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Survey Anesthesiol. 2016;60:166–7. doi: 10.1097/ALN.0b013e318297630d. [DOI] [PubMed] [Google Scholar]
- 20.Anand S, Bajwa SJS, Kapoor B, Jitendera M, Gupta H. Comparative evaluation of intraperitoneal bupivacaine, magnesium sulfate and their combination for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Niger J Surg Sci. 2014;24:42–8. [Google Scholar]
- 21.Kizilcik N, Koner O. Magnesium sulfate reduced opioid consumption in obese patients undergoing sleeve gastrectomy: Aprospective, randomized clinical trial. Obes Surg. 2018 doi: 10.1007/s11695-018-3243-7. doi:10.1007/s11695-018-3243-7. [DOI] [PubMed] [Google Scholar]
- 22.Hwang J-Y, Na H-S, Jeon Y-T, Ro Y-J, Kim C-S, Do S-H. IV infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth. 2009;104:89–93. doi: 10.1093/bja/aep334. [DOI] [PubMed] [Google Scholar]
- 23.Ingrande J, Lemmens H. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105:i16–23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]


