Abstract
Background and Aims:
Infratentorial neurosurgical procedures are considered high risk for the development of postoperative pulmonary complications (POPCs), prolonging hospital stay of patients with substantial morbidity and mortality.
Material and Methods:
Patients between the ages of 18 and 65 years, who underwent elective surgery for posterior fossa tumors over a period of two years, were reviewed. Data including American Society of Anesthesiologists physical status; comorbidities like hypertension, diabetes mellitus and hypothyroidism, history of smoking, obstructive sleep apnea, respiratory symptoms, lower cranial nerve (LCN) palsy; intraoperative complications such as hemodynamic alterations suggestive of brain stem or cranial nerve handling, tight brain as informed by the operating neurosurgeon, blood loss, and transfusion; and postoperative duration of mechanical ventilation, tracheostomy, POPCs, length of ICU and hospital stay, general condition of the patient at discharge, and cause of in-hospital mortality were collected. POPC was defined as the presence of atelectasis, tracheobronchitis, pneumonia, bronchospasm, respiratory failure, reintubation, or weaning failure.
Results:
Case files of 288 patients fulfilling the study criteria were analyzed; POPCs were observed in 35 patients (12.1%). On multivariate analysis, postoperative blood transfusion, LCN palsy, prolonged ICU stay, and tracheostomy were found to be independent predictors of POPC.
Conclusions:
The incidence of POPC was 12.1% following infratentorial tumor surgery. The predictors for the occurrence of POPCs were postoperative blood transfusion, LCN palsy, prolonged ICU stay, and tracheostomy.
Keywords: Neurosurgery, posterior fossa tumor, postoperative pulmonary complications
Introduction
Postoperative pulmonary complication (POPC) is defined as a clinically relevant and identifiable pulmonary alteration that adversely affects patient outcome postoperatively.[1] This definition lacks precision in diagnosing POPC. Therefore, there is a wide disparity in reported incidence of POPC in general surgical patients.[2] Most studies describe POPCs as the presence of atelectasis, pneumonia, pulmonary edema, exacerbation of underlying chronic lung disease, or respiratory failure in postsurgical patients.[3,4] Neurosurgical procedures are recognized as high risk for the occurrence of POPCs.[5] One plausible explanation for the increased risk is decrease in lung volumes and arterial blood gas tensions with concomitant change in respiratory pattern that occurs after craniotomy.[6] Patients undergoing posterior fossa surgery are at an even higher risk of developing POPCs because of the interplay of other mechanisms like mechanical obstruction, central respiratory dysfunction, and neuromuscular dysfunction.[7] Obstructive sleep apnea has been reported in association with posterior fossa lesions.[8] The risk factors for POPCs in this group of patients have not been widely studied. Hence, the aim of this study was to find the incidence of POPCs, associated risk factors and predictors of POPCs in adult patients who underwent craniotomy for posterior fossa tumors. The impact of POPCs on length of stay (LOS) and neurological outcome were also analyzed.
Material and Methods
After approval from the Institutional Ethics Committee, this retrospective study was conducted in adult patients (18–65 years), who underwent elective craniotomy for posterior fossa tumors, over a period of 2 years. Patients on preoperative mechanical ventilation and those who underwent emergent surgery were excluded. Relevant information was collected from the medical records of the patients. The preoperative data included age, sex, weight, American Society of Anesthesiologists (ASA) physical status, history of smoking, obstructive sleep apnea (OSA)/snoring, comorbid illnesses, type and location of posterior fossa tumor, preoperative respiratory status, signs of lower cranial nerve (LCN) involvement (impaired gag reflex was taken as the surrogate), level of consciousness and reports of laboratory investigations. Findings of preoperative x-ray chest and CT/MRI scan with respect to size of the lesion, presence of hydrocephalus, and brainstem compression were noted. The Glasgow Coma Scale score less than E4V5M6 was considered as “lower level of consciousness.” Intraoperative data included the details of anesthesia technique and surgery, surgical position, duration of surgery and anesthesia, estimated blood loss, IV fluid infused, volume of blood and blood component transfused, indication for postoperative ventilation, use of methylprednisolone, and any other complications. Transfusion exceeding 10 units of packed red blood cells (PRBCs) over a period of 24 hours or transfusion of at least four units of PRBC within 1 hour was considered as massive blood transfusion. Postoperative data included POPCs, need for reintubation, additional surgical interventions (re-exploration/ventricular diversion), LCN dysfunction, use of nasogastric tube (NGT), blood and blood component transfusion, duration of mechanical ventilation, tracheostomy, level of consciousness, and LOS in ICU and hospital. Patient's condition at the time of discharge from the hospital was used to assign Glasgow Outcome Score (GOS). Neurological outcome was defined as “good” if GOS was 4–5 and “poor” if it was 1–3.
All the patients were categorized into two groups based on the occurrence of POPCs: Group P, with POPCs and Group A, without POPC. POPC was defined as the first recorded event out of the following: atelectasis, bronchospasm, pneumothorax, pneumonia, tracheobronchitis, weaning failure/reintubation, and acute respiratory distress syndrome (ARDS). Atelectasis was defined as documented lobar collapse on chest radiograph. A diagnosis of pneumonia was made if pulmonary infiltrates were present on the chest x-ray along with two documented signs among purulent tracheobronchial secretions, elevation of body temperature (above 38.3°C), and abnormal leucocyte count (<4,000 or >12,000/mm3).[9] A documented increase in the quantity or change of the color or purulent aspect of tracheobronchial secretion with normal chest radiograph was defined as tracheobronchitis. Bronchospasm was said to be present for any recorded episode of wheezing associated with acute respiratory symptoms and relieved by bronchodilators. ARDS/acute respiratory failure was considered if mechanical ventilation was initiated in view of respiratory distress with acutely deficient exchange of gases on arterial blood gas analysis. Rate and indication for prolonged mechanical ventilation (defined as continuation of postoperative ventilation beyond 48 hours) was also recorded. Weaning failure was defined as need for re-intubation within 24 hours of extubation.
Statistical analysis
Stata 11.2 (College Stations, TX, USA) was used for statistical analysis. Data are presented as number (%)/mean ± SD/median (range), as appropriate. For univariate analysis, we used Chi-square test for 2 × 2 and 3 × 2 tables and Fisher's exact test (when one or more expected frequencies were <5) for categorical variables and Wilcoxon rank–sum (Mann–Whitney) test to compare nonparametric variables. Multivariate logistic regression analysis was undertaken to identify risk factors and independent predictors for the occurrence of POPC. The results were reported as odds ratio (OR) (95% confidence interval [CI]). A P value <0.05 was considered as statistically significant.
Results
The medical records of 288 patients who underwent elective craniotomy for posterior fossa tumors were analyzed [Figure 1]. Their demographic details are given in Table 1. Overall, there were 19 smokers (6.6%) and 2 (0.7%) of them had OSA. Eight patients (2.8%) had preoperative respiratory abnormality viz. chronic obstructive pulmonary disease (COPD) (2), old healed pulmonary tuberculosis (4), and recently treated (within 2 weeks) lower respiratory tract infection (2). Six patients (2.1%) had an abnormal preoperative chest x-ray. Eighty-nine patients (30.9%) had pre-existing LCN involvement and 13 (4.5%) had low level of consciousness. Majority of the patients (69.4%) had cerebello-pontine lesions; vestibular schwannoma was the commonest [Figure 2]. The mean tumor size was 45.3 ± 31.9 mm (range: 2.5–188.5 mm). Most patients (65.3%) underwent retromastoid suboccipital craniotomy in park-bench position. The mean duration of surgery and anesthesia were 349.8 ± 124.9 and 437.6 ± 130.3 minutes, respectively. The average volume of IV fluid infused was 4987.9 ± 1721.8 ml and the blood loss was 945.8 ± 790 mL. Intraoperatively, 141 patients (51%) received allogenic transfusion; 20 of these patients (6.9%) required massive transfusion.
Figure 1.
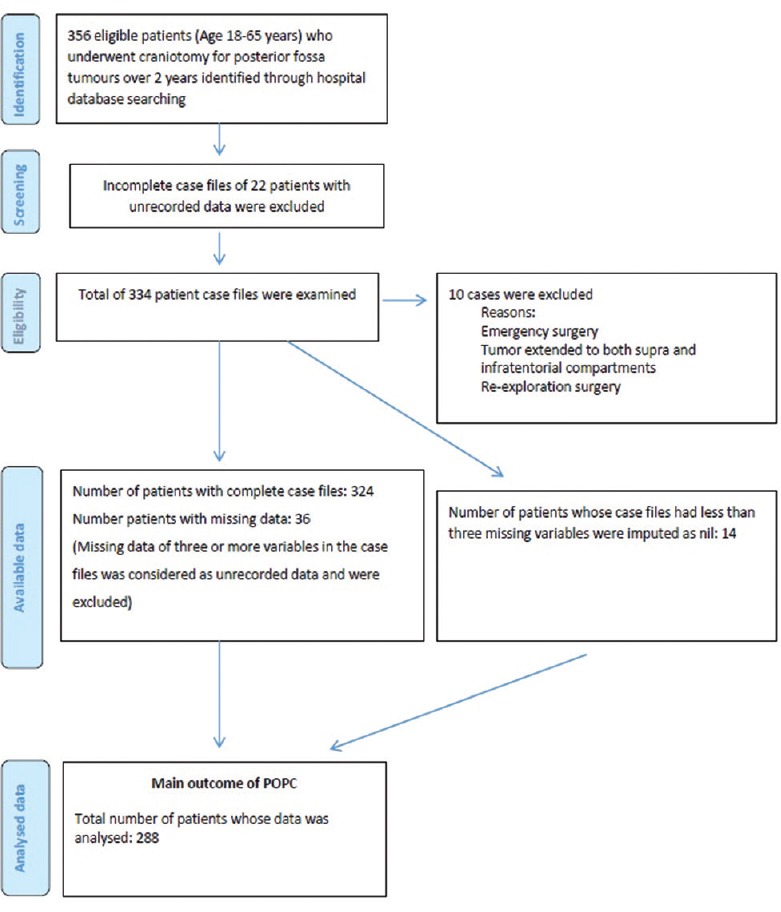
PRISMA diagram of case control study
Table 1.
Demographic characteristics of the patients†
| Parameter | Values |
|---|---|
| Age (years)* | 36.7±12.6 |
| Sex | |
| Male | 138 (47.9) |
| Female | 150 (52.1) |
| Weight (kg)* | 56.1±11.6 |
| ASA physical status | |
| I | 192 (66.7) |
| II | 85 (29.5) |
| III | 11 (3.8) |
†Data given as n (%) of patients unless specified, *Data are given as mean±SD. ASA=American Society of Anesthesiologists, SD=Standard deviation
Figure 2.
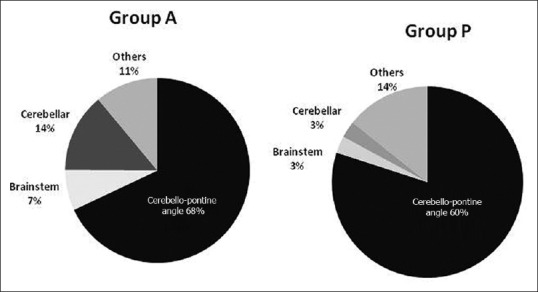
Distribution of patients based on tumor location in the two groups
In 192 patients (66.7%), neuromuscular blockade was not reversed at the conclusion of surgery and mechanical ventilation was continued because of preoperative LCN involvement (13.2%), intraoperative brainstem handling (11.5%), prolonged surgery (5.5%), brain bulge (5.2%), massive blood loss (4.2%), delayed arousal (4.2%), and preoperative low level of consciousness (LLC) (3.1%). Among the 66.7% patients requiring postoperative ventilation, 58 patients (20.1%) were ventilated due to concerns of residual tumor and hemostasis. The duration of mechanical ventilation was 85.3 ± 216 hours. Prolonged ventilation was required in 18 patients (6.2%). Twenty-four patients (8.3%) underwent tracheostomy, 8 each for persistent low level of consciousness, postoperative LCN involvement, and weaning failure. Postoperatively, 51 patients (17.7%) required blood transfusion during first 48 hours. NGT was inserted for enteral feeding in 127 patients (44.1%). The ICU and hospital stay were 4.3 ± 6.1 and 21.6 ± 15.5 days, respectively.
In this study, the rate of occurrence of POPC was 12.1% (35 patients) the details of which are given in Table 2. Bronchospasm was not documented in any of these patients. The patients with (Group P) and without POPC (Group A) were found to be comparable in terms of age, gender, body weight, sensorium, smoking, respiratory abnormality, laboratory parameters, tumor pathology, and surgical approach. More patients with POPCs had ASA physical status Grade III (P = 0.001) and pre-existing LCN involvement (P = 0.04). Intraoperatively, patients with POPC received higher volume of IV fluids. The blood loss was more (P < 0.001) and amount of blood transfused during surgery was significantly higher (P = 0.02). More patients in Group P underwent tracheostomy, NGT placement and blood transfusion (P < 0.001). The duration of mechanical ventilation was longer in these patients (P < 0.001). Patients in Group P had significantly longer ICU and hospital stay [Table 3]. Moreover, the outcome in these patients was skewed toward poor outcome (P < 0.001) with more patients (34.3%) with POPCs having GOS of 1-3 [Table 3]. Twelve patients (4.2%) died of various reasons, including sepsis (7), ARDS (3), and malignant intracranial hypertension (3).
Table 2.
Incidence of different postoperative pulmonary complications†
| Pulmonary complication | Frequency |
|---|---|
| Absent | 253 (87.9) |
| Atelectasis | 9 (3.1) |
| Pneumonia | 6 (2.1) |
| Tracheobronchitis | 6 (2.1) |
| Weaning failure/re-intubation | 9 (3.1) |
| Pneumothorax | 2 (0.7) |
| ARDS/respiratory failure | 3 (1.0) |
†Data given as n (%) of patients. ARDS=Acute respiratory distress syndrome
Table 3.
Outcome in the two groups of patients
| Parameter | Group P (n=35) | Group A (n=253) | P |
|---|---|---|---|
| ICU stay (days)† | 14.8±12 | 2.9±2.4 | <0.001 |
| Hospital stay (days)† | 41.8±31.2 | 18.8±8.9 | <0.001 |
| Neurologic outcome* | |||
| Good | 23 (65.7) | 244 (96.4) | <0.001 |
| Poor | 12 (34.3) | 9 (3.6) | |
| Mortality* | 12 (4.2) | 3 (1.04) | <0.001 |
†Data given as mean±SD, *Data given as n (%) of patients. Group A=Absent POPC, Group P=POPC present, POPC=Postoperative pulmonary complication, SD=Standard deviation, ICU=Intensive care unit
The risk factors associated with POPCs on univariate analysis are given in Table 4. On multivariate logistic regression analysis, postoperative blood transfusion, and tracheostomy were found to be independent predictors for the occurrence of POPCs. Postoperative LCN palsy and prolonged ICU stay demonstrated weak correlation with the POPCs [Table 5].
Table 4.
Univariate analysis of relative risk with various parameters for postoperative pulmonary complications†
| Parameters | Group P (n=35) | Group A (n=253) | OR | 95% CI | P |
|---|---|---|---|---|---|
| Preoperative LCN involvement | |||||
| No | 19 (54.3) | 180 (71.2) | 1.0 | 0.04 | |
| Yes | 16 (45.7) | 73 (28.8) | 2.1 | 1-4.2 | |
| ASA physical status grades | |||||
| I | 18 (51.4) | 174 (68.8) | 1.0 | - | - |
| II | 12 (34.3) | 73 (28.8) | 1.59 | 0.7-3.5 | 0.24 |
| III | 5 (14.3) | 6 (2.4) | 8.0 | 2.2-29 | 0.001 |
| Blood loss (mL) | |||||
| ≤500 | 9 (25.7) | 110 (43.5) | 1.0 | - | - |
| 501-1,000 | 5 (14.3) | 73 (28.8) | 0.8 | 0.2-2.5 | 0.76 |
| 1,001-1,400 | 6 (17.1) | 29 (11.5) | 2.5 | 0.8-7.7 | 0.10 |
| >1,400 | 15 (42.9) | 41 (16.2) | 4.5 | 1.8-11 | 0.001 |
| Massive transfusion | |||||
| No | 28 (80) | 240 (94.8) | 1.0 | - | 0.003 |
| Yes | 7 (20) | 13 (5.2) | 4.6 | 1.7-12.5 | |
| Duration of surgery (min) | |||||
| ≤300 | 6 (17.1) | 116 (45.8) | 1.0 | - | 0.002 |
| >300 | 29 (82.9) | 137 (54.2) | 4.1 | 1.6-10.2 | |
| Postoperative LLC | |||||
| No | 26 (74.3) | 244 (96.4) | 1.0 | - | 0.000 |
| Yes | 9 (25.7) | 9 (3.6) | 9.4 | 3.4-25.7 | |
| Postoperative LCN involvement | |||||
| No | 21 (60) | 233 (92.1) | 1.0 | - | 0.000 |
| Yes | 14 (40) | 20 (7.9) | 7.7 | 3.4-17.5 | |
| Postoperative NGT insertion | |||||
| No | 3 (8.6) | 158 (62.4) | 1.0 | - | 0.000 |
| Yes | 32 (91.4) | 95 (37.6) | 17.7 | 5.3-59.5 | |
| Postoperative blood transfusion | |||||
| No | 11 (31.4) | 226 (89.3) | 1.0 | - | 0.000 |
| Yes | 24 (68.6) | 27 (10.7) | 23.2 | 8-66.9 | |
| Re-exploration | |||||
| No | 31 (88.6) | 247 (97.6) | 1.0 | - | 0.001 |
| Yes | 4 (11.4) | 6 (2.4) | 2.7 | 1.5-4.9 | |
| Tracheostomy | |||||
| No | 13 (37.1) | 251 (99.2) | 1.0 | - | 0.000 |
| Yes | 22 (62.9) | 2 (0.8) | 212.3 | 45-1001.8 | |
| Mechanical ventilation (h) | |||||
| ≤48 | 23 (65.7) | 247 (97.6) | 1.0 | - | 0.000 |
| >48 | 12 (34.3) | 6 (2.4) | 4.6 | 2.7-7.9 | |
| ICU stay (days) | |||||
| ≤3 | 4 (11.4) | 187 (73.9) | 1.0 | - | 0.000 |
| >3 | 31 (88.6) | 66 (26.1) | 21.9 | 7.4-64.5 |
†Data shown as n (%). LCN=Lower cranial nerve, LLC=Low level of consciousness, NGT=Nasogastric tube, ASA=American Society of Anesthesiologists, OR=Odds ratio, CI=Confidence interval
Table 5.
Multivariate regression analysis of risk factors for postoperative pulmonary complications†
| Parameters | OR | 95% CI |
|---|---|---|
| Postoperative LCN palsy | 5.2 | 0.7-36.5† |
| Postoperative blood transfusion | 5.1 | 1.1-23.5 |
| Prolonged ICU stay | 3.8 | 0.9-16.4 |
| Need for tracheostomy | 110.9 | 7.3-1670† |
LCN=Lower cranial nerve, ICU=Intensive care unit, OR=Odds ratio, CI=Confidence interval
Discussion
Surgery for infratentorial lesions are plagued by myriad cardiopulmonary alterations placing these patients at high risk for the development of POPCs. In this study, we found that 12.1% patients who underwent craniotomy for various posterior fossa tumors developed POPCs. The commonest complications were weaning failure/reintubation and atelectasis in 3.1% patients each. Other notable complications were pneumonia and tracheobronchitis, each occurring in 2.1% patients, whereas ARDS and pneumothorax occurred in 1.4% and 0.7% patients, respectively. There was no bronchospasm found in this study. However, all the 18 patients, who required prolonged mechanical ventilation, received regular bronchodilator nebulization as institutional protocol in the ICU. Therefore, to assume that bronchospasm events did not occur in these patients is largely speculative in the light of retrospective nature of the study. It is highly likely that bronchospasm was underreported. Early reports of respiratory complications in patients undergoing elective neurosurgical procedures include respiratory depression, reintubation, bronchospasm, laryngospasm, and upper airway obstruction occurring in 2.8% patients.[10] Sogame reviewed 236 neurosurgical patients prospectively and reported an occurrence of POPCs in 58 patients (24.6%) due to tracheobronchitis (32), pneumonia (20), bronchospasm (19), and atelectasis (5).[11] The higher incidence of POPCs in their study may be because of inclusion of patients undergoing aneurysm surgery (36.4%), who are known to have higher incidence of cardiopulmonary dysfunction, and are usually operated on an urgent basis.
In a retrospective analysis of 1699 patients undergoing intracranial tumor resection, Flexman found that 3.8% of supratentorial procedures and 6.6% of infratentorial procedures had a composite primary outcome (reintubation within 30 days, failure to wean from mechanical ventilation within 48 hours and death within 30 days postoperatively).[12] Although the authors have reported a composite outcome, we observed similar rate of prolonged ventilation in 6.2% of our patients. Our findings are comparable to another study conducted at our center on 931 craniotomy patients, where an incidence of 14.3% of early (within 72 hours) respiratory complications, including reintubation in 7% patients was observed.[13]
In our study, on analyzing the demographic and perioperative variables of the patients, the following associations were observed.
Patient-related factors
Although patients with posterior fossa tumors more commonly present with long tract signs, cerebellar signs, or seventh/eighth cranial nerve dysfunction, there may be associated LCN involvement. LCN dysfunction presents as dysphonia, dysarthria, swallowing problems, depressed gag reflex, and palatal palsy with reduced sensation over the laryngo-pharynx, increasing the susceptibility of these patients for pulmonary aspiration. Moreover, preexisting LCN palsy may delay extubation and further predispose patients to pulmonary infections.[14] An impaired gag reflex was observed in 30.9% of patients. Abnormal gag reflex has been advocated as well as criticized as a surrogate clinical marker of pulmonary aspiration.[15] In this study, however, association of preoperative impaired gag reflex with POPCs was observed, indicating LCN involvement.
Another factor that has consistently found strong association with postoperative complications is the ASA physical status. The American College of Physicians suggests ASA physical status II or higher as a significant risk factor for POPCs in patients undergoing non-cardiothoracic surgery.[5] In this study, ASA class III patients were found to have a higher predilection for the development of POPCs (OR = 8, P = 0.001).
Surgery-related factors
Perioperative bleeding is a well-documented risk factor for postoperative infections and mortality. In neurosurgery, intraoperative blood loss as low as 350 mL has been found to be an independent predictor of postoperative complications in elderly patients.[16] In our study, however, intraoperative blood loss >1400 mL was found to be a significant risk for POPCs (OR = 4.5), probably because of a relatively younger patient population. Patients who developed POPC received more blood transfusion. Patients who received massive intraoperative blood transfusion were more likely to develop POPCs (OR = 4.6). Multivariate analysis demonstrated postoperative blood transfusion as a strong predictor of POPCs [OR = 5.1; CI (1.1, 23.5)]. This is probably because of transfusion-related immunomodulation (TRIM). TRIM has been found to increase perioperative and nosocomial infections, LOS, and in-hospital mortality.[17]
Alteration in pulmonary function with anesthesia and surgery can be exaggerated by intraoperative fluid overload. In this study, there was a significant difference in the amount of administered crystalloids and colloids in patients with and without POPCs. In a meta-analysis on perioperative fluid therapy, liberal fluid administration was found to be associated with risk of pneumonia, pulmonary edema, and a longer hospital stay compared with restrictive use.[18] On this backdrop, we analyzed the occurrence of POPC with respect to restrictive and liberal regimes (<3,000, 3,000–5,000, >5,000 mL) to investigate any possible association with POPCs. Although patients in POPC group received more IV fluids, no significant association was observed between liberal/restrictive regime with the occurrence of POPCs. The simultaneous use of mannitol in these patients with consequent increased urine output could have confounded the results.
Postoperative pulmonary atelectasis has been seen in 90% of patients receiving general anesthesia.[19] General anesthesia, along with positive pressure ventilation, has been found to exacerbate pulmonary conditions such as pneumonia and COPD. This hypothesis was studied in 47 patients undergoing craniotomy under anesthesia.[6] The investigators concluded that patients who underwent elective craniotomy may present in the postoperative period with altered respiratory mechanics. In this study, a longer duration of anesthesia was found to translate into higher rate of POPCs (P < 0.001).
Postoperative factors
Although pre-existing LCN dysfunction may be compensated, this may take several days and new postoperative acute disruption of LCNs may add to the risk of acute pulmonary aspiration. In a retrospective review of 102 cases of acoustic neuroma, 9 patients developed postoperative bulbar palsy, 5 of whom developed pulmonary complications.[20] Similarly, a strong association of postoperative LCN dysfunction and POPC was observed in this study, although, on multivariate analysis, it appeared as a weak independent predictor of POPCs.
Low level of consciousness (LLC) during the postoperative period may occur because of surgical complications, such as hydrocephalus, posterior fossa edema, operative site hematoma, brainstem infarct, and meningitis. Altered sensorium depresses airway reflexes with impaired cough reflex and pooling of secretions promoting micro/macroaspiration. Our findings are consistent with earlier studies where postoperative LLC was found to be an independent predictor of POPCs in neurosurgical patients.[11,12]
Prolonged mechanical ventilation is recognized as a harbinger of POPCs by increasing the likelihood for ventilator-associated pneumonia (VAP). It was a significant risk factor for POPCs in our study [OR, 4.6; CI (2.7, 7.9)]. These findings are congruous to a prospective observational study of 317 craniotomy patients, where 9.5% of patients who needed prolonged ventilation subsequently developed POPC (OR 4.3).[21]
Placement of NGT in the perioperative period is another risk factor for POPCs by facilitating regurgitation and microaspiration of gastric contents.[2,3,4] We also observed higher rate of POPC with its use. Nonetheless, majority of these patients had developed postoperative LCN dysfunction.
Tracheostomy was found to be another independent risk factor for development of POPCs. Many studies implicate tracheostomy as a risk factor for nosocomial pneumonia.[22] However, few other studies have maintained that tracheostomy is independently associated with reduced risk of VAP by facilitating nursing care, reducing requirement of sedatives, allowing enteral nutrition and facilitating early weaning from mechanical ventilation.[23] Furthermore, studies on timing of tracheostomy have implicated late tracheostomy as a cause for nosocomial pneumonia.[24] In our study, most patients were tracheostomized early (4-8 days) with six (25%) patients tracheostomized late (>10 days). No difference was observed with respect to the time of tracheostomy and occurrence of POPCs. This is a retrospective study and a temporal cause–effect relationship cannot be established.
Pulmonary dysfunction in the postoperative period is a major burden in terms of morbidity, mortality, prolonged hospital stay, and increased cost of care.[25] In our study, patients with POPCs had significantly longer ICU and hospital stay. In addition, ICU stay >3 days was found to be independently associated with POPCs. These findings are in tandem with other studies in neurosurgical patients.[11,12,26]
The retrospective nature of our study is a limitation because some data on various perioperative variables were missing. Numerous studies have additionally reported age, BMI, smoking, preoperative respiratory symptoms, OSA, COPD, and abnormal chest radiograph as predictors for POPCs in non-neurosurgical procedures.[11] This association could not be established in our study probably because the study population consisted of adult patients between 18 and 65 years, mainly of ASA physical status I and II undergoing elective surgeries.
Conclusions
The incidence of POPC was 12.1% following elective craniotomy for infratentorial tumor surgery. The most important predictors of POPCs were postoperative blood transfusion, LCN palsy, prolonged ICU stay, and tracheostomy. Patients who developed POPC have significantly longer ICU and hospital stay, and increased mortality. Nevertheless, large prospective studies with patients from all age groups and undergoing surgery on elective as well as emergency basis may shed more light on factors influencing the occurrence of POPCs, so that strategies for improvement of overall patient outcome can be devised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.O'Donohue WJ., Jr Postoperative pulmonary complications. When are preventive and therapeutic measures necessary? Postgrad Med. 1992;91:167. doi: 10.1080/00325481.1992.11701233. [DOI] [PubMed] [Google Scholar]
- 2.Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: A systematic review of blinded studies. Am J Med. 2002;112:219–25. doi: 10.1016/s0002-9343(01)01082-8. [DOI] [PubMed] [Google Scholar]
- 3.Hall JC, Tarala RA, Hall JL, Mander J. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest. 1991;99:923–7. doi: 10.1378/chest.99.4.923. [DOI] [PubMed] [Google Scholar]
- 4.Smetana GW, Lawrence VA, Cornell JE American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: A guideline from the American college of physicians. Ann Intern Med. 2006;144:575–80. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 6.Franceschini J, Sogame LC, Gazzotti MR, Vidotto MC, Jardim JR. Pulmonary function and thoraco-abdominal configuration after elective craniotomy. Neurosurg Q. 2008;18:22–7. [Google Scholar]
- 7.Howard R, Mahoney A, Thurlow AC. Respiratory obstruction after posterior fossa surgery. Anaesthesia. 1990;45:222–4. doi: 10.1111/j.1365-2044.1990.tb14689.x. [DOI] [PubMed] [Google Scholar]
- 8.Guleria R, Madan K. Pulmonary complications in neurosurgical patients. Indian J Neurosurg. 2012;1:175–80. [Google Scholar]
- 9.Raymer JM, Flynn LM, Martin RF. Massive transfusion of blood in the surgical patient. Surg Clin North Am. 2012;92:221–34. doi: 10.1016/j.suc.2012.01.008. vii. [DOI] [PubMed] [Google Scholar]
- 10.Manninen PH, Raman SK, Boyle K, el-Beheiry H. Early postoperative complications following neurosurgical procedures. Can J Anaesth. 1999;46:7–14. doi: 10.1007/BF03012507. [DOI] [PubMed] [Google Scholar]
- 11.Sogame LC, Vidotto MC, Jardim JR, Faresin SM. Incidence and risk factors for postoperative pulmonary complications in elective intracranial surgery. J Neurosurg. 2008;109:222–7. doi: 10.3171/JNS/2008/109/8/0222. [DOI] [PubMed] [Google Scholar]
- 12.Flexman AM, Merriman B, Griesdale DE, Mayson K, Choi PT, Ryerson CJ, et al. Infratentorial neurosurgery is an independent risk factor for respiratory failure and death in patients undergoing intracranial tumor resection. J Neurosurg Anesthesiol. 2014;26:198–204. doi: 10.1097/ANA.0b013e3182a43ed8. [DOI] [PubMed] [Google Scholar]
- 13.Bharati SJ, Pandia MP, Rath GP, Bithal PK, Dash HH. Respiratory complications in the early post-operative period following elective craniotomies. J Neuroanaesth Crit Care. 2015;2:114–20. [Google Scholar]
- 14.Cai YH, Zeng HY, Shi ZH, Shen J, Lei YN, Chen BY, et al. Factors influencing delayed extubation after infratentorial craniotomy for tumour resection: A prospective cohort study of 800 patients in a Chinese neurosurgical centre. J Int Med Res. 2013;41:208–17. doi: 10.1177/0300060513475964. [DOI] [PubMed] [Google Scholar]
- 15.Horner J, Brazer SR, Massey EW. Aspiration in bilateral stroke patients: A validation study. Neurology. 1993;43:430–3. doi: 10.1212/wnl.43.2.430. [DOI] [PubMed] [Google Scholar]
- 16.Asano K, Nakano T, Takeda T, Ohkuma H. Risk factors for postoperative systemic complications in elderly patients with brain tumors. Clinical article. J Neurosurg. 2009;111:258–64. doi: 10.3171/2008.10.17669. [DOI] [PubMed] [Google Scholar]
- 17.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: A stratified meta-analysis. Anesth Analg. 2012;114:640–51. doi: 10.1213/ANE.0b013e318240d6eb. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003;91:61–72. doi: 10.1093/bja/aeg085. [DOI] [PubMed] [Google Scholar]
- 20.Duane DT, Howard SJ, Kraayenbrink M. Incidence and predictors of bulbar palsy after surgery for acoustic neuroma. J Neurosurg Anesthesiol. 1997;9:263–8. doi: 10.1097/00008506-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Vidotto MC, Sogame LC, Gazzotti MR, Prandini M, Jardim JR. Implications of extubation failure and prolonged mechanical ventilation in the postoperative period following elective intracranial surgery. Braz J Med Biol Res. 2011;44:1291–8. doi: 10.1590/s0100-879x2011007500146. [DOI] [PubMed] [Google Scholar]
- 22.Georges H, Leroy O, Guery B, Alfandari S, Beaucaire G. Predisposing factors for nosocomial pneumonia in patients receiving mechanical ventilation and requiring tracheotomy. Chest. 2000;118:767–74. doi: 10.1378/chest.118.3.767. [DOI] [PubMed] [Google Scholar]
- 23.Nseir S, Di Pompeo C, Jozefowicz E, Cavestri B, Brisson H, Nyunga M, et al. Relationship between tracheotomy and ventilator-associated pneumonia: A case control study. Eur Respir J. 2007;30:314–20. doi: 10.1183/09031936.06.00024906. [DOI] [PubMed] [Google Scholar]
- 24.Szakmany T, Russell P. Impact of early tracheostomy on the ICU patient: A meta analysis. Crit Care Med. 2013;41:A94–5. [Google Scholar]
- 25.Marda M, Pandia MP, Rath GP, Bithal PK, Dash HH. Post-operative pulmonary complications in patients undergoing transoral odontoidectomy and posterior fixation for craniovertebral junction anomalies. J Anaesthesiol Clin Pharmacol. 2013;29:200–4. doi: 10.4103/0970-9185.111720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabaté S, Mazo V, Canet J. Predicting postoperative pulmonary complications: Implications for outcomes and costs. Curr Opin Anaesthesiol. 2014;27:201–9. doi: 10.1097/ACO.0000000000000045. [DOI] [PubMed] [Google Scholar]


