Abstract
Background and Aims:
Inadvertent perioperative hypothermia defined as the perioperative core temperature of <36°C is a common problem in day-to-day anesthesia practice. It is not clear from the literature whether prewarming, that is, initiation of convective warming of the patient at a time point prior to induction of anesthesia is superior or comparable to cowarming, that is, initiation of convective warming simultaneously with induction of anesthesia. We conducted this study to find whether cowarming is as good as prewarming in preventing the occurrence of intraoperative hypothermia.
Material and Methods:
Sixty-two adult patients undergoing major abdominal surgery under general anesthesia were randomized to receive either prewarming for 60 min at 40° C or cowarming using the Level 1® Equator ® body warmer. All patients who were prewarmed also received cowarming during induction of anesthesia. In both the groups, convective warming was continued during intraoperative period. Incidence of intraoperative hypothermia, core, and peripheral body temperatures were compared between the two groups.
Results:
Among 27 patients in each group who completed the study core temperature decreased to <35° C toward the end of surgery in 17 patients in group prewarming [mean (SD) 34.59 (1.17° C)] and 18 patients in group cowarming [mean (SD) 34.31 (1.34° C)]. The incidence of intraoperative hypothermia and the core temperature at the end of surgery were comparable (P = 0.42).
Conclusion:
Cowarming is as effective as prewarming to prevent intraoperative hypothermia.
Keywords: Body, equipment, hypothermia, temperature, warming devices
Introduction
Inadvertent perioperative hypothermia, defined as perioperative core temperature of <36°C is a common problem in day-to-day anesthesia practice with an incidence of 50%–70%.[1] Perioperative hypothermia has a negative influence on surgical outcome and postoperative course (decreased metabolic rate, decreased cardiac output, metabolic acidosis, prolongation of muscle relaxants, altered clotting functions, increased incidence of postoperative infection, postoperative shivering leading to increased oxygen consumption, norepinephrine release, and myocardial ischemia), even increasing the length of hospital stay and the cost of the treatment. Most of the temperature loss during general anesthesia occurs during the initial phase following induction of anesthesia due to redistribution of body heat. Anesthetic-induced vasodilation allows core heat to flow peripherally, warming the peripheries, but at the expense of core body temperature.[1] This redistribution can be prevented by several techniques. Prewarming of the patient before induction of anesthesia is one such technique, which by increasing the peripheral tissue temperature reduces core to peripheral temperature gradient. It is not clear from the literature whether prewarming, that is, initiation of convective warming of the patient at a time point prior to induction of anesthesia is superior or comparable to cowarming, that is, initiation of convective warming simultaneously with induction of anesthesia. We conducted this study to find whether cowarming is as effective as prewarming in preventing or reducing the incidence of intraoperative hypothermia by comparing core body temperature (primary outcome measure) and core to peripheral temperature gradient (secondary outcome measure) between the two groups.
Material and Methods
This prospective randomized study commenced after obtaining approval from the Institutional Ethics Committee. Sixty-two patients were included in this study [Figure 1]. The inclusion criteria were American Society of Anesthesiologists (ASA) physical status 1 and 2 adult patients of either gender, aged 18–65 years, with body mass index of 18.5–25 kg/m2 undergoing elective laparotomy under general anesthesia with expected duration of >2 h, for example, open cholecystectomy, transabdominal hysterectomy, gastrectomy, colorectal and abdominal procedures, etc. Following were the exclusion criteria:
Figure 1.
CONSORT flow chart
Patients suffering from endocrine disorders like thyroid disease, dysautonomia, Cushing syndrome, diabetes mellitus with autonomic neuropathy
Patients affected with peripheral vascular disease like Raynaud's syndrome
Critically ill or hemodynamically unstable patients who may require massive rapid intravenous fluid resuscitation
Febrile patients.
Patients were evaluated on the day prior to surgery. Written informed consent was obtained from the patients. Fasting and premedication were as advised by the concerned anesthesiologist. Patients were randomized into two groups: group prewarming and group cowarming. Consecutive patients meeting the inclusion criteria were enrolled. The first patient was randomized to group prewarming. The next patient scheduled for the same surgical procedure was randomized to the alternate group. This sequence of randomization was continued for the subsequent patients to match the surgical procedures between the two groups. For example, if a patient is posted for distal gastrectomy that patient goes to the prewarming group, the next distal gastrectomy case would be allocated to the cowarming group. If in between, any other case like hemicolectomy is scheduled that would go to prewarming group and subsequent hemicolectomy case would go to the cowarming group. This method of randomization was followed to match the type of surgeries between the two groups. In group prewarming, patients were prewarmed for 1 hour before the induction of anesthesia in the preoperative holding area using convective body warmer (Level 1® Equator®, Smith Medical ASD, USA) and full body warming blanket covered all the body parts except the face. Patients were prewarmed with temperature output of the warming unit set to 40°C for 60 min and warming continued during induction of anesthesia and the surgery. In group cowarming, full body (except the face) convective warming was started as soon as the patient was shifted to the operating room (convective body warmer set to an output of 40°C). Standard monitoring was established, intravenous access (IV) was secured, and anesthesia was induced following a standard protocol. During surgery, the warming blanket was placed over the patient's lower limbs and was connected to the warming unit. Soon after the surgical incision, the output temperature was increased to 44°C in both the groups. The anesthesiologist could reduce the output temperature of the warmer while monitoring the body temperature intraoperatively, only if required. Such intervention was recorded.
In both the groups, convective warming was continued (utilizing available body surface except face) during epidural access, vascular access, and urinary bladder catheterization. Warming was continued in the intraoperative period. In group prewarming, if there was a delay of >15 minutes from the completion time of prewarming to shifting the patient to operating room, these patients were excluded from the study.
During the conduct of general anesthesia, total fresh gas flow was 6 L/minute till tracheal intubation, 3 L/minute for first 15 minutes following intubation, and 2 L/minute until the end of anesthesia. Heat and moisture exchanger was used in all the cases, fluid warmers were used only to warm packed red blood cells, when transfused. The epidural catheter, if any, was activated with only opioid and local anesthetic was not used until the end of the surgery. The severity of hypothermia was graded based on core body temperature as follows: normothermia – 36°C–38°C, mild hypothermia – 32.2°C–35°C, moderate hypothermia – 28°C–32.2°C, and severe hypothermia – <28°C.
The baseline peripheral (thumb) temperature was recorded before starting the warming (IV line was present on contralateral upper limb) with skin probe (Datex Ohmeda 16560, 400 series). After induction of anesthesia, nasopharyngeal temperature probe (Datex Ohmeda 16561, 400 series) was inserted to a length of tragus to philtrum to measure core body temperature.
To compare the proportion of hypothermia in both groups, we anticipated 95% of the cowarming group to have hypothermia and 60% of the prewarming group to have hypothermia based on our pilot study. To detect a difference of 25% in the proportion of hypothermia, we needed 27 patients in each group with 80% power and 5% level of significance. Statistical analysis was done using SPSS version 20 for windows in consultation with the Department of Medical Statistics, Manipal University. Independent sample t-test, Mann–Whitney U-test and Fisher's exact test were applied as considered appropriate to interpret the results. P value <0.05 was considered as statistically significant.
Results
Period of study was between October 2015 and April 2017. A total of 62 patients were randomized to either prewarming or cowarming group [Figure 1]. Patient characteristics are given in Table 1. Factors which could confound the results like operating room temperature, duration of surgery, infused fluid volumes, total blood loss during the procedure, and intraoperative blood transfusion were analyzed and compared. These were comparable. The anesthesiologist did not reduce the output temperature of the warmer in any of the cases. The core temperature and core to peripheral temperature gradient were comparable at the time of induction of anesthesia (base line) and at the end of the surgery between the two groups [Table 2]. In the first 2 h following anesthetic induction, the rate of decrease in the core temperature was found to be higher among the patients in group cowarming than group prewarming, but not statistically significant [Table 3]. The incidence of hypothermia and its severity at any point of time during the surgery is illustrated in Figures 2 and 3 and found to be comparable between the two groups. The incidence of postoperative shivering was 11.1% in group prewarming and 14.8% in group cowarming (P = 0.68). Among 27 patients in group prewarming, 4 patients were uncomfortable during prewarming due to the warm air.
Table 1.
Patient characteristics
| Group prewarming | Group cowarming | |
|---|---|---|
| Age (years) | 56.0 (8.6) | 49.9 (10.2) |
| Gender (male/female) | 13/14 | 12/15 |
| ASAPS (1/2) | 11/16 | 13/14 |
| BMI (kg/m2) | 22.3 (1.9) | 21.5 (1.9) |
Data are mean (SD) for age and BMI, absolute numbers for gender, ASAPS. SD=Standard deviation, BMI=Body mass index, ASAPS=American Society of Anesthesiologists Physical Status
Table 2.
Comparison of body temperature between the groups
| Group prewarming | Group cowarming | P | |
|---|---|---|---|
| Core temperature (°C) base line | 36.1 (0.8) | 36 (0.58) | 0.6 |
| Core temperature (°C) at end of surgery | 34.6 (1.2) | 34.3 (1.3) | 0.4 |
| Peripheral temperature (°C) base line | 32.9 (2.4) | 32.1 (2.8) | 0.6 |
| Peripheral temperature (°C) at end of surgery | 33.0 (2.4) | 32.1 (2.8) | 0.6 |
| Core to peripheral temperature gradient (°C) base line | 3.4 (1.7, 4.5) | 3.5 (1.8, 5.1) | 1 |
| Core to peripheral temperature gradient (°C) at end of surgery | 2.8 (0.9, 6.4) | 2.9 (0.6, 6.2) | 0.7 |
Data are mean (SD) for core body temperature and peripheral temperature. Core to peripheral temperature gradient is expressed as median (interquartile range). SD=Standard deviation
Table 3.
Rate of decrease in core temperature in the first 2 h postinduction
| Rate of drop in core temperature (°C/h) | Group prewarming | Group cowarming |
|---|---|---|
| First hour | 0.8 (0.5, 1.2) | 0.9 (0.6, 1.5) |
| Second hour | 1.2 (0.7, 1.7) | 1.3 (0.8, 1.6) |
Data are median (interquartile range)
Figure 2.

Incidence and severity of hypothermia in group prewarming at any point of surgery
Figure 3.
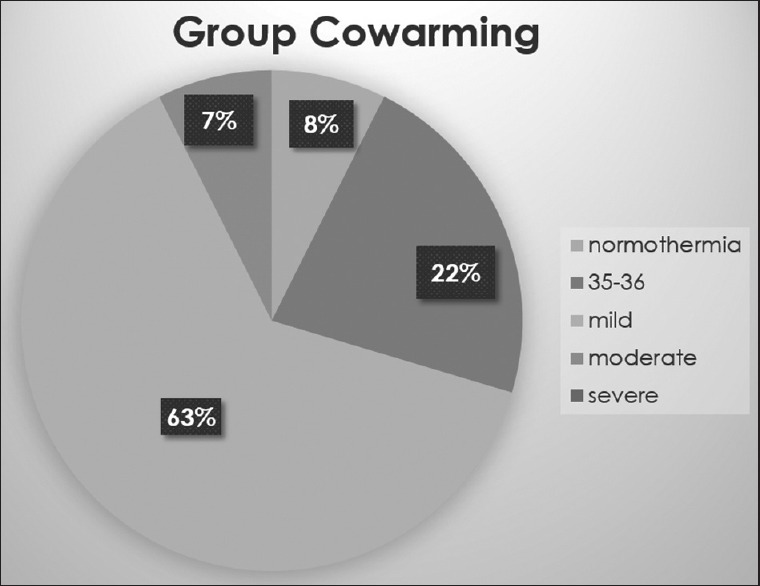
Incidence and severity of hypothermia in group cowarming at any point of surgery
Discussion
In this study, cowarming is found to be as effective as prewarming to prevent intraoperative hypothermia during major abdominal surgeries. Prewarming the patient for 1 hour before induction of anesthesia did not confer any added advantage compared with cowarming begun with the induction of anesthesia.
We chose a prewarming time duration of 1 hour before starting anesthesia based on the previous studies.[2,3,4,5] However, different time durations of prewarming have been used in various studies on prewarming ranging from 30 minutes to 2 hours.[6,7,8,9] Some studies have shown that even 15 minutes of prewarming is sufficient to prevent hypothermia.[10,11] Interestingly, it has also been found that even intraoperative warming, in the absence of prewarming, is sufficient to prevent hypothermia.[12,13] So, it is not clear from literature whether prewarming has any additional benefits when compared with intraoperative warming alone. From the mechanism of intraoperative heat loss under general anesthesia, it makes sense to start convective warming at least with the induction of anesthesia. We have called this technique as cowarming.
In both the groups, convective warming was continued in the intraoperative period. Patients belonging to group prewarming were also cowarmed (continued convective warming during induction of anesthesia). The possibility of temperature loss following anesthetic induction secondary to other procedures like vascular access, epidural access, and catheterization was minimized in both the groups by continued warming during these procedures. Confounding factors, like ambient room temperature, IV fluids, transfusion, and type of surgery, were either standardized or analyzed to eliminate their influence on the study findings.
Previous studies showed that after 1 hour of anesthesia the decrease in core temperature was less in patients prewarmed compared with control patients (no warming intraoperatively).[2,7,10] In our study after 1 hour of anesthesia though the rate of decrease of core temperature was less in group prewarming than cowarming, it was not clinically significant (<0.2°C; P = 0.3, independent sample t-test). Most of the studies have shown that prewarming is effective in preventing hypothermia during short-duration surgeries, and even in these studies, patients were not warmed by convective warmer intraoperatively.[2,6,7,8,9] Melling et al. found no additional advantage of prewarming compared with intraoperative warming.[14]
The concept of cowarming has not been evaluated so far. Our results with respect to prewarming concur with earlier studies supporting its role in prevention of hypothermia. Though Vanni et al. initiated warming 5 minutes after induction of anesthesia in the intraoperative warming group the results with respect to incidence of shivering and normothermia were comparable with the combined prewarming and intraoperative warming group. However, this study comprised of only 10 patients in each group.[3,14]
Not many studies have commented on the variations in the peripheral temperature and core to peripheral temperature gradient; they were more interested in the core temperature alone. Our study noted these variations and supported the mechanism by which prewarming and cowarming work.
Including only abdominal surgeries is one of the limitations of this study. The results may or may not be applicable to surgeries involving thorax, spine, or major orthopedic surgery. It must be remembered that patients who were prewarmed were also cowarmed during induction of anesthesia, which might not be done always practically.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sessler DI. Temperature regulation and monitoring. In: Miller RD, editor. Miller's Anesthesia. 8th ed. Philadelphia: Churchill Livingstone; 2010. pp. 1622–46. [Google Scholar]
- 2.Camus Y, Delva E, Sessler DI, Lienhart A. Pre-induction skin-surface warming minimizes intraoperative core hypothermia. J Clin Anesth. 1995;7:384–8. doi: 10.1016/0952-8180(95)00051-i. [DOI] [PubMed] [Google Scholar]
- 3.Vanni SM, Braz JR, Módolo NS, Amorim RB, Rodrigues GR., Jr Preoperative combined with intraoperative skin-surface warming avoids hypothermia caused by general anesthesia and surgery. J Clin Anesth. 2003;15:119–25. doi: 10.1016/s0952-8180(02)00512-3. [DOI] [PubMed] [Google Scholar]
- 4.Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101:627–31. doi: 10.1093/bja/aen272. [DOI] [PubMed] [Google Scholar]
- 5.Sessler DI, Schroeder M, Merrifield B, Matsukawa T, Cheng C. Optimal duration and temperature of prewarming. Anesthesiology. 1995;82:674–81. doi: 10.1097/00000542-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Glosten B, Hynson J, Sessler DI, McGuire J. Preanesthetic skin-surface warming reduces redistribution hypothermia caused by epidural block. Anesth Analg. 1993;77:488–93. doi: 10.1213/00000539-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology. 1993;79:214–8. doi: 10.1097/00000542-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of preinduction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology. 1993;79:219–28. doi: 10.1097/00000542-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bock M, Müller J, Bach A, Böhrer H, Martin E, Motsch J, et al. Effects of preinduction and intraoperative warming during major laparotomy. Br J Anaesth. 1998;80:159–63. doi: 10.1093/bja/80.2.159. [DOI] [PubMed] [Google Scholar]
- 10.Horn EP, Schroeder F, Gottschalk A, Sessler DI, Hiltmeyer N, Standl T, et al. Active warming during cesarean delivery. Anesth Analg. 2002;94:409–14. doi: 10.1097/00000539-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Chung SH, Lee BS, Yang HJ, Kweon KS, Kim HH, Song J, et al. Effect of preoperative warming during cesarean section under spinal anesthesia. Korean J Anesthesiol. 2012;62:454–60. doi: 10.4097/kjae.2012.62.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fossum S, Hays J, Henson MM. A comparison study on the effects of prewarming patients in the outpatient surgery setting. J Perianesth Nurs. 2001;16:187–94. doi: 10.1053/jpan.2001.24039. [DOI] [PubMed] [Google Scholar]
- 13.Joo Y, Kim HJ, Kim JT, Kim HS, Lee SC, Kim CS, et al. Effect of active warming on shivering during spinal anesthesia. Korean J Anesthesiol. 2009;57:176–80. doi: 10.4097/kjae.2009.57.2.176. [DOI] [PubMed] [Google Scholar]
- 14.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: A randomised controlled trial. Lancet. 2001;358:876–80. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]



