Abstract
Inadvertent excision of lumps which turn out to be sarcomas is not uncommon.
Imaging has a limited role in detecting microscopic residual disease but can show the extent of the previous surgical field.
Standard treatment is wide re-excision, usually combined with radiotherapy.
Residual tumour is found in an average of 50% of reported cases.
The presence of residual disease is an adverse prognostic factor.
All lumps bigger than a golf ball should have a diagnosis prior to excision.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180060
Keywords: inadvertent excision, local recurrence, soft tissue sarcoma, unplanned excision, wide re-excision
Soft tissue sarcomas (STS) are rare, with a combined incidence of about 60/100,000 population a year. It is generally stated that there are 100 benign soft tissue tumours for every soft tissue sarcoma. As a result of this there is a low level of awareness of the possibility of a sarcoma entering the differential diagnosis in many situations. Delays in diagnosis of both bone and soft tissue sarcomas are common, and paralleling this is the high frequency of inadvertent excision (IE) of lesions which then turn out to be sarcomas. This was first described by Giuliano and Eilber in 19851 but has undoubtedly been commonplace for many years. It has acquired the eponym of a ‘whoops’ procedure as the surgeon (and the patient) are both surprised and maybe distressed when the pathologist reports that the lesion which they thought was benign, is in fact malignant!2 Almost always the surgeon will have ‘shelled out’ the lump and will usually confidently say that they ‘have got it all out’. In the past many of these patients then had no further treatment but there was a very high rate of local recurrence (up to 70%) and it is now far more common for these patients to be referred to a sarcoma unit for definitive management.2 In 1994, Gustafson et al showed that patients treated somewhere other than a tumour centre required significantly more operations than those treated primarily at a tumour centre, either to have a re-excision to get clear margins, or for management of local recurrence.3
The significance of an inadvertent excision has been assessed by numerous authors and this article aims to summate the body of knowledge there is on the subject, identify current best practice and suggest future possibilities for research and investigation.
Incidence
The actual incidence of inadvertent excisions (IE) is unknown but numerous reports on this topic have now been published. Whilst some papers report only on patients who have had an IE, others report on the incidence of this as a proportion of total referrals. These are summarized in Table 1 and show that the proportion of IEs ranges from 18% to 53% of all referrals with an average of 33%.
Table 1.
The main papers dealing with inadvertent excision of soft tissue sarcomas
| Author (Ref) | Year | Total number | Number with IE | % IE | Number reexcised | % with residual tumour | LR at 5 yrs after reexcise |
|---|---|---|---|---|---|---|---|
| Giuliano1 | 1985 | 90 | all | all | 90 | 49% | |
| Peabody39 | 1994 | 172 | 74 | 43% | 72 | 51% | |
| Zornig40 | 1995 | 189 | 67 | 35% | 67 | 45% | 7% |
| Goodlad41 | 1996 | 236 | 95 | 40% | 95 | 59% | |
| Noria15 | 1996 | 292 | 65 | 22% | 65 | 35% | 8% |
| Davis42 | 1997 | 239 | 239 | all | 186 | 8% | |
| Siebenrock43 | 2000 | 16 | all | 16 | 63% | 19% | |
| Lewis35 | 2000 | 1092 | 407 | 38% | 407 | 39% | 15% |
| Zagars27 | 2003 | 666 | all | all | 295 | 46% | 15% |
| Peiper44 | 2004 | 110 | all | all | 110 | 31% | 13% |
| Davies10 | 2004 | 111 | all | 111 | 57% | ||
| Wong20 | 2004 | 76 | 18 | 24% | 16 | 56% | |
| Rougraff45 | 2005 | 106 | 75 | all | 75 | 65% | 11% |
| Fiore46 | 2006 | 598 | 318 | 53% | |||
| Chandrasekar19 | 2008 | 2201 | 402 | 18% | 316 | 59% | 21% |
| Potter21 | 2008 | 203 | 64 | 32% | 64 | 72% | 34% |
| Morii47 | 2008 | 77 | all | 45 | 45% | 12% | |
| Rehders48 | 2009 | 143 | all | 139 | 31% | 12% | |
| Arai22 | 2010 | 191 | 63 | 33% | 63 | 8% | |
| Funovics18 | 2010 | 752 | 310 | 41% | |||
| Han17 | 2011 | 104 | all | 104 | 51% | 26% | |
| Zacherl49 | 2012 | 266 | 131 | 49% | |||
| Umer30 | 2013 | 135 | 51 | 38% | 4 | 14% | |
| Alamanda7 | 2013 | 400 | 147 | 37% | |||
| Arai32 | 2014 | 113 | all | 113 | 51% | 7% | |
| Morii31 | 2015 | 92 | 24 | 26% | 24 | 71% | |
| Koulaxouzidis50 | 2015 | 204 | 110 | 39% | 110 | 53% | 11% |
| Saeed24 | 2016 | 245 | 34 | 14% | 25 | 72% | 45% |
| Charoenlap36 | 2016 | 451 | 161 | 36% | 161 | 55% | 13% |
| Jones25 | 2016 | 44 | all | 44 | 39% | 5% | |
| Smolle34 | 2017 | 728 | 281 | 39% | |||
| Gingrich11 | 2017 | 76 | all | 64 | 70% | ||
| Nakamura51 | 2017 | 197 | all | 197 | 58% | 9% |
Note: The first author and year of publication are followed by columns listing: The total number of STS in the series; the number of these with IE and then the % of the whole series with IE. The next column is the number who underwent re-excision and the following column details the % found to have residual tumour. The final column gives the rate of LR reported at 5 years.
IE, inadvertent excision; LR, Local Recurrence
Cause
The reasons for which patients undergo IE are multiple but very often it is because the operating surgeon has not thought that the lump could be a sarcoma. If they had entertained this thought, then the patient would have been properly investigated first with imaging and usually biopsy. There are multiple guidelines now available defining the management pathway for a suspicious lump.4–6
Alamanda et al investigated whether IEs were more common the further the patient lived away from a sarcoma centre but found no evidence of this.7 They also found no difference based on insurance status. Kang et al noted that patients who had the primary IE at a tertiary centre tended to have a lower rate of residual tumour than those having the IE at a non-tertiary hospital and that they also ended up with a lower rate of local recurrence, but that there was no difference in survival.8
Size and depth
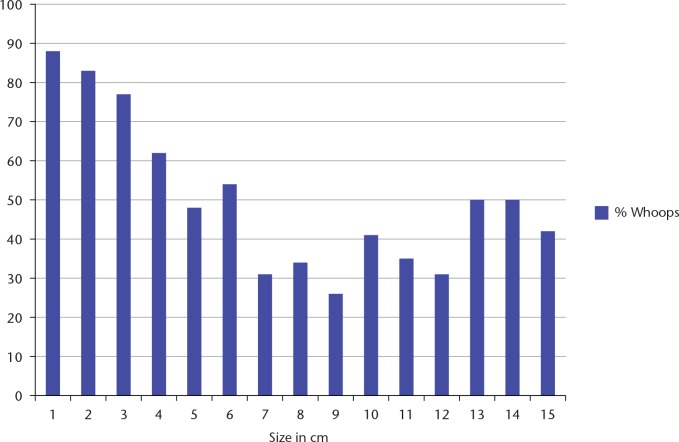
Current guidelines suggest that any lump which is either deep or bigger than 5 cm should be investigated prior to excision and thus it would be expected that most lumps submitted to IE would be small and subcutaneous.4–6,9 This is supported by several papers that have documented the size of the tumour that has been excised and the depth of the tumours. The average size of IE tumours varies from 3.2 to 8.9 cm with a mean of 5.7 cm, with most authors commenting that superficial tumours undergoing IE were smaller than deep tumours. In our own practice we have investigated the proportion of patients undergoing IE related to the actual size of the tumour. Fig. 1 shows the data for superficial (to the fascia) tumours showing a decreasing proportion of IEs as the tumours became bigger. However, it was still the case that 56% of all superficial STS presented to our unit following an IE. Those papers which have reported depth conclude that 55% of all patients with inadvertent excision had superficial tumours, this contrasts with the generally accepted incidence of superficial soft tissue sarcomas being about 25%.
Fig. 1.
The proportion of patients referred to our unit with an inadvertent excision based on the size of the original tumour (subcutaneous tumours only).
Changes with time
It is generally accepted that the better the education of the surgical fraternity, the lower the proportion of IEs that will be carried out. In the UK, national guidelines were produced in 2000 (and re-issued five-yearly thereafter) and sent to all GPs stating that any patient with a lump that was
bigger than 5 cm
increasing in size
deep to the fascia
painful
should be considered to be a sarcoma until proved otherwise and referred for investigation to a sarcoma unit.9 It was hoped that this would result in both earlier diagnosis of sarcomas and less IEs.
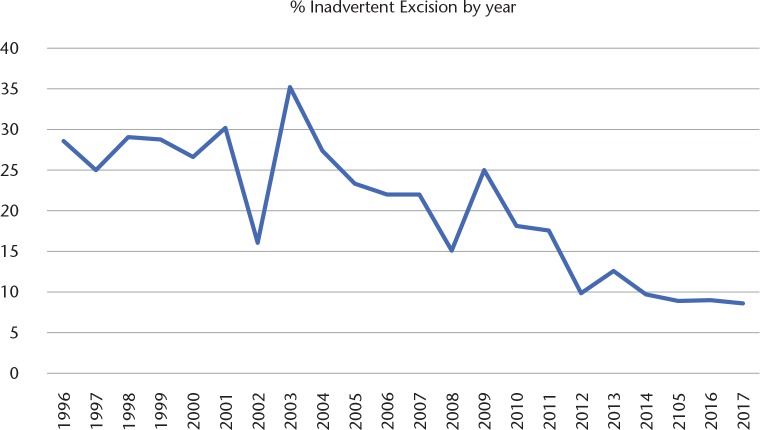
Data from our own unit show that the proportion of patients with an IE referred over the past 20 years has slowly decreased but is still around 10% of all referrals (Fig. 2).
Fig. 2.
Graph showing how the percentage of inadvertent excisions as a proportion of all new referrals has decreased over a 20-year period.
Imaging
When a patient has undergone an IE for sarcoma it is mandatory for them to be referred to a sarcoma centre for ongoing management. If pre-operative imaging has been performed, this should be reviewed as it will help greatly in establishing the extent of the primary tumour. Furthermore, an appreciation of the imaging appearances of the tumour at presentation is of value in the assessment of any potential residual tumour. Following IE, standard practice is to perform a new MRI to assess whether there is any residual tumour apparent. In the presence of post-surgical change after IE, visualizing areas within the surgical bed that show similar imaging appearances on MRI to the original tumour is highly suggestive of residual disease. Several papers have reported on the utility of this, comparing the radiologists’ reports with the eventual findings at re-excision. Davies et al’s paper in 2004 found that MRI had a poor negative predictive value (0.67) but a positive predictive value of 0.93 with sensitivity of 0.64 and specificity of 0.93, although these were all non-contrast enhanced scans.10 Gingrich et al in 2017 found that the overall accuracy of MRI was only 78% in predicting residual disease, which they described as ‘modest’ and Wang et al in 2018 also found that MRI was not that accurate but commented that extensive soft tissue oedema increased the likelihood of finding residual tumour.11,12
Whilst there are no studies showing the optimum imaging modality, MRI is widely considered the most useful (Fig. 3). Whilst ultrasound can certainly be used to detect residual disease, the surgical planning of re-excision is typically performed on MRI. The timing of the MRI is also important; carried out too early following the IE it will show post-operative oedema/haemorrhage, and carried out too late it may delay re-excision. The optimum timing is thus probably between 4 and 8 weeks following the IE. In general, this is the typical timeframe in which patients will be seen at a sarcoma centre following IE. This apparent delay in management is needed to allow review of the histology of the IE to confirm sarcoma and clinical review to explain the management plan.
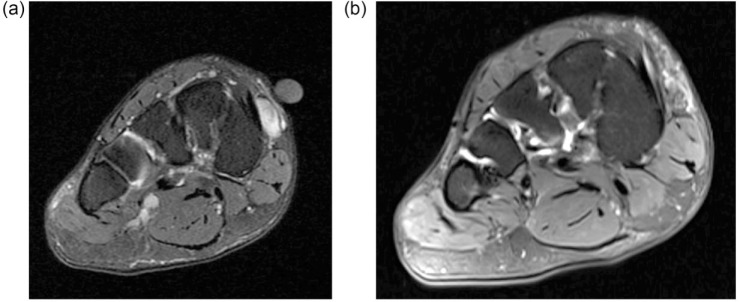
Fig. 3.
(a) Coronal fat suppressed image shows a 1 cm lesion in the medial mid foot. Inadvertent excision of this lesion was performed with a subsequent histological diagnosis of synovial sarcoma. (b) Coronal fat suppressed image at restaging shows some oedema in the superficial fat at the site of the previous synovial sarcoma and no definite macroscopic residual disease. Wide re-excision showed no residual tumour.
The aim of the MRI is not only to show the extent of any residual tumour but also to show the extent of the previous surgical excision. Clearly the patient should also have appropriate staging studies, usually a CT chest as a minimum, according to local or national guidelines. PET-CT is not used to try and detect residual disease as there will be high uptake from the wound in the post-operative period.
Pathology
It is essential that the previously excised lesion should be reviewed by an experienced pathologist. Studies have shown up to a 30% error in non-specialist reporting of soft tissue lesions thought to be sarcomas. The pathologist should also be able to get an idea of the margins of excision and the size of the lesion, which are often not reported in the initial pathology report from a non-specialist centre.
Numerous papers have commented on the types of tumour most likely to have residual disease left behind after IE. The most common tumours are DFSP (Dermato Fibro Sarcoma Protuberans), MPNST (Malignant Peripheral Nerve Sheath Tumour) and myxofibrosarcomas, followed by synovial sarcoma and undifferentiated pleomorphic sarcoma. The ones with the lowest risk of residual tumour are liposarcoma and leiomyosarcoma.
Margins
Most lumps that are inadvertently removed will be ‘shelled out’ resulting in either an intralesional or marginal margin (through the ‘reactive zone’) as described by Enneking.13 Although the operating surgeon will almost always say ‘I got it out completely’ this is usually false optimism with sarcomas. One of the problems with an inadvertent excision is that they are often carried out through inappropriate approaches (e.g. transverse incisions) and there may have been a drain placed which further extends the field of contamination.
It is essential that a true ‘wide’ excision should be achieved, but the actual extent of this is difficult to decide. Very often the post-excision MRI will show haemorrhage or oedema and the surgeon must make an experience-based decision as to how extensive any further surgical excision should be. It is also helpful to obtain comment from the pathologist on the appearance of the margins of the excised lesion and whether they show a diffuse infiltrating pattern as this may influence the width of re-excision. Lewis et al aimed to get 2 cm around the ‘residual’ tumour, whilst Sugiura and Arai suggested that 3 cm should be the target.35,14 Noria et al in 1996 highlighted the difficulty of achieving adequate margins when there is no residual tumour left to feel and give the surgeon the tactile sensation of how close he/she may be to the tumour.15 They also pointed out the surprisingly high rate of positive margins when residual disease was present, in 9 of 23 of their patients (39%), compared with their expected rate of 10% in primary excisions.
The principles therefore of managing a patient following an inadvertent excision are to firstly establish the diagnosis, grade, size, stage and location of the excised tumour and then to identify the likelihood of there being any residual tumour. Next the surgeon should evaluate whether those margins could be improved with further surgery and also, with the Multi Disciplinary Team, decide whether adjuvant or neoadjuvant therapy may have a role in managing the tumour.
In many situations, especially with superficial tumours, the mainstay of further treatment will be a wide re-excision, taking generous margins around the previous excision cavity and often removing the deep fascia as a relatively impermeable deep barrier. This may require plastic surgical reconstruction. For inadvertently excised deep tumours, obtaining wide margins at the time of re-excision may be more challenging and may involve sacrifice of deep structures. If, however, the re-excision might involve critical structures such as nerves or blood vessels then there may be a role for more aggressive adjuvant or neoadjuvant therapy. In this setting of a previous IE, obtaining a planned positive margin as described by Gerrand et al may be difficult.16 Amputation is sometimes needed either due to the nature of the excised tumour or due to its location.
Timing of re-excision
The optimum timing of re-excision has not been established. Han et al reported no difference in outcomes between patients undergoing early re-excision (before 32 days, the median in his series) and after 32 days.17 Funovics et al found that undergoing re-excision within 12 weeks of initial IE led to a better prognosis.18
Re-excision findings
There have been over 45 publications dealing with inadvertent excision of soft tissue sarcomas, with 2693 patients having a re-excision. Of those, 1350 (50%) were found to have residual tumour. The rates in different series varied, however, from 31% to 74% (Table 1). The risk of residual tumour was greatest with high-grade deep tumours and lowest with low-grade superficial tumours (Table 2).
Table 2.
Showing rates of residual tumour depending on depth and grade (ROH data)
| Total | High | Inter | Low | |
|---|---|---|---|---|
| Total | 60% | 72% | 55% | 49% |
| Deep | 68% | 78% | 59% | 63% |
| SC | 55% | 67% | 53% | 47% |
Note: SC, subcutaneous
Following re-excision there are likely to be four categories of situations:
No residual tumour found
Macro or microscopic residual tumour excised with clear margins
Macro or microscopic tumour excised with a marginal margin
Macro or microscopic residual disease excised but with an involved margin
In the first two situations, re-excision has been successful and the only decision is whether further adjuvant therapy is needed. In most cases of a high-grade tumour, radiotherapy should be considered, especially for larger or deep tumours, according to national guidelines.4–6 If residual disease is encountered and there are involved margins, then further excision should be considered as it should with a marginal margin.
Few publications have reported the rate and quality of the re-excision margins, but those which have (Table 1) have reported clear margins in 74–91% of cases.15,19
Reconstruction
Several authors have commented on the high rates of limb reconstruction needed after wide re-excision. The highest numbers of patients requiring plastic surgery, not surprisingly, come from units with very active plastic surgery support. Wong et al in 2004 reported that 89% of patients undergoing re-excision required plastic surgery reconstruction.20 These rates have not been matched by others but there is no doubt that patients undergoing re-excision have a greater number of operations and are more likely to need plastic surgery than those having primary resections.21,22
Radiotherapy
The use of radiotherapy varies from country to country although more recent guidelines have emphasized the importance of radiotherapy (RT) in decreasing the risk of Local Recurrence (LR) after sarcoma resection. In general, most guidelines will now stipulate that all high-grade sarcomas and all larger low-grade tumours should include radiotherapy as part of their treatment. There is thus no difference between patients with IEs and those treated primarily. There has, however, been an increasing trend for the use of neoadjuvant RT, especially in primary STS, following the results of the Canadian trial.23 This has been explored for patients who have had an IE and Jones et al reported that following pre-operative RT followed by excision they only had a 5% LR rate at 5 years, although they did report a peri-operative morbidity of 25%. This was supported in a small series by Saeed et al who found a 9% LR after pre-op RT and 33% with post-op RT combined with re-excision after IE.24,25
In 1998 Pollack et al reported on their experience of radiotherapy alone in treating patients who had undergone IE, finding a 25% rate of LR at 10 years which they found unacceptable.26 They also found that a dose of 50 Gy after IE was insufficient. Zagars et al in 2003 reported a similar outcome with 22% LR at 5 years in those just receiving radiotherapy after IE compared with 15% in those undergoing re-excision and RT (p = 0.03).27 This, however, contrasts with the experience of Kepka et al who identified 78 patients treated with RT alone after IE.28 They used a 66 Gy dose and reported a 12% LR rate at 5 years but with 10 major radiotherapy complications. Most of their patients were treated in the 1970s or 1980s since when they have also carried out re-excision.
Several papers have commented on how difficult it is to identify what benefit RT has after re-excision for IE as it is so heavily case selected with those cases at highest risk receiving RT whilst low-risk cases may not have it.19,21
Cost analysis
Alamanda et al carried out a detailed analysis of the financial burden of an IE, finding that the charges for re-excision surgery were approximately 1/3 greater than for a primary tumour and thus the total cost of an IE was almost double that of primary treatment at a specialist centre.29 Umer et al commented that from the patient’s point of view one operation was better than two as it meant less time in hospital and away from work.30
Function
It might be expected that with wide re-excision, patients with IE may end up with worse functional outcomes than those undergoing primary excision. Morii et al investigated this and found no difference in functional outcomes, probably because many of their patients had small superficial tumours.31 Interestingly they found that patients who underwent a re-excision had better emotional acceptance than those undergoing primary surgery.
Local recurrence
Clearly the aim of wide re-excision is to eventually obtain wide margins of excision to minimize the risk of local recurrence. Numerous authors have reported the rate of local recurrence after wide re-excision with results ranging from 5% to 45% at 5 years. The average, however, of the 2693 patients reported is 14% at 5 years. This figure is similar to figures from large series of primary excisions. Nearly all series, however, found that patients with residual tumour had a higher rate of local recurrence. Potter et al reported a 17% LR rate if the re-excision showed no residual tumour, rising to 38% if residual tumour was found and 64% if there was gross residual disease.21 Results from our own series demonstrate the effect of grade and residual tumour on LR.
High-grade / residual tumour / +ve margin = 63%
High-grade / residual tumour / -ve margin = 25%
High-grade / no residual tumour = 23%
Low-grade / residual tumour / +ve margin = 16%
Low-grade / residual tumour / -ve margin = 11%
Low-grade / no residual tumour = 2%
Arai et al found the converse, with an 8% LR rate in patients with IE compared to 15% LR in their patients treated with a planned excision.32 They attributed this to their aggressive policy of wide excision and use of flaps in 71% of their patients, which resulted in a very low use of RT (19% after planned excision and 13% after IE).
O’Donnell et al have taken the work of Gerrand further by looking at the Toronto Unit’s experience of local recurrence and confirming that with a planned positive margin their LR rate was 14.6% but for a tumour bed re-excision with a +ve margin it was 21.1%.33 They have commented on how the biology of the tumour (and its invasiveness) is reflected by the ability to obtain clear margins and in the eventual rate of LR as well as the cause-specific survival.
Disease-free survival
It is generally accepted that the main risk factors for survival in soft tissue sarcomas are grade, size and depth of the tumour as well as age of the patient. Numerous published papers have compared outcomes in patients having an inadvertent excision with those treated primarily, and the general consensus is that, compared to age and matched case controls, there is little difference overall as patients with IEs tend to have smaller and more superficial tumours which themselves have a better prognosis. Smolle et al confirmed this in a large recent study using propensity scores to show that IE did not seem to adversely affect prognosis.34
As long ago as 2000, however, Lewis et al reported that in their patients who had an IE and then had a wide re-excision, there were better rates of disease-free survival at 5 years in those undergoing re-resection even when factors such as age, grade, depth, histology and margins were taken into account.35 This was particularly marked for those with AJCC (American Joint Committee on Cancer) Stage 3 tumours (high-grade, deep, > 5 cm). This led to the conclusion that ‘two excisions are better than one’. This unexpected finding was not matched by improved local control rates in the re-excised group. It was postulated that this could be due to the removal of histologically undetectable microfoci of disease. Charoenlap et al also compared outcomes for patients with IE by stage versus those treated primarily and found that residual disease indicated a worse prognosis both in terms of LR, survival and also a higher eventual amputation rate (18% vs. 1.8%).36 There was very little difference in outcomes when patients were matched by stage. Most authors, however, have found no such improvement in survival, particularly when account is taken of the fact that most tumours undergoing IE are small and many are superficial. The general consensus is that the finding of residual disease is likely a hallmark of tumour aggressiveness which is also then linked to worse rates of both LR and disease-free survival.32
Outstanding issues
It seems clear from these papers that wide re-excision remains the standard therapy in most units around the world following IE. If the original excision can be proved (pathologically) to have obtained wide margins, or if further surgery is impossible without amputation then there may be a case for giving the appropriate adjuvant (radiotherapy) and careful follow up. In the majority of cases, however, where excision has been with a ‘shell out’, then repeat imaging, re-excision and radiotherapy are likely to provide the best outcome. The width of the re-excision remains debatable. The greater the margin attempted the more likely a flap will be needed and the morbidity of this needs taking into account, as do the effects of radiotherapy. Wider margins are, however, likely to improve local control. Impressive results of local control are now being reported from units routinely giving radiotherapy prior to re-excision and it may be that a randomized trial could be considered to compare this with standard post-operative radiotherapy. Undoubtedly, however, virtually all authors agree that avoiding IEs remains the ideal aim and this will involve continuing education of not only GPs but also surgeons. A good mantra is that ‘any lump bigger than a golf ball should have a diagnosis prior to excision’.37
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol 1985;3:1344–1348. [DOI] [PubMed] [Google Scholar]
- 2. Bhangu AA, Beard JA, Grimer RJ. Should soft tissue sarcomas be treated at a specialist centre? Sarcoma 2004;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gustafson P, Dreinhöfer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center: a comparison of quality of surgery in 375 patients. Acta Orthop Scand 1994;65:47–50. [DOI] [PubMed] [Google Scholar]
- 4. Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:536–563. [DOI] [PubMed] [Google Scholar]
- 6. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 7. Alamanda VK, Delisca GO, Archer KR, Song Y, Schwartz HS, Holt GE. Incomplete excisions of extremity soft tissue sarcomas are unaffected by insurance status or distance from a sarcoma center. J Surg Oncol 2013;108:477–480. [DOI] [PubMed] [Google Scholar]
- 8. Kang S, Han I, Lee SA, Cho HS, Kim HS. Unplanned excision of soft tissue sarcoma: the impact of the referring hospital. Surg Oncol 2013;22:e17–e22. [DOI] [PubMed] [Google Scholar]
- 9. NICE. Suspected cancer: recognition and referral. https://www.nice.org.uk/guidance/ng12 (date last accessed August 2018).
- 10. Davies AM, Mehr A, Parsonage S, Evans N, Grimer RJ, Pynsent PB. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol 2004;14:506–513. [DOI] [PubMed] [Google Scholar]
- 11. Gingrich AA, Elias A, Michael Lee CY, et al. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res 2017;208:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Pretell-Mazzini J, Kerr DA, et al. MRI findings associated with microscopic residual tumor following unplanned excision of soft tissue sarcomas in the extremities. Skeletal Radiol 2018;47:181–190. [DOI] [PubMed] [Google Scholar]
- 13. Enneking WF, Spanier SS, Malawer MM. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer 1981;47:1005–1022. [DOI] [PubMed] [Google Scholar]
- 14. Sugiura H, Takahashi M, Katagiri H, et al. Additional wide resection of malignant soft tissue tumors. Clin Orthop Relat Res 2002;394:201–210. [DOI] [PubMed] [Google Scholar]
- 15. Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am 1996;78:650–655. [DOI] [PubMed] [Google Scholar]
- 16. Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br 2001;83:1149–1155. [DOI] [PubMed] [Google Scholar]
- 17. Han I, Kang HG, Kang SC, Choi JR, Kim HS. Does delayed reexcision affect outcome after unplanned excision for soft tissue sarcoma? Clin Orthop Relat Res 2011;469:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Funovics PT, Vaselic S, Panotopoulos J, Kotz RI, Dominkus M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: a single institution experience with 682 patients. J Surg Oncol 2010;102:626–633. [DOI] [PubMed] [Google Scholar]
- 19. Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008;90:203–208. [DOI] [PubMed] [Google Scholar]
- 20. Wong CK, Lam YL, So YC, Ngan KC, Wong KY. Management of extremity soft tissue sarcoma after unplanned incomplete resection: experience of a regional musculoskeletal tumour centre. Hong Kong Med J 2004;10:117–122. [PubMed] [Google Scholar]
- 21. Potter BK, Adams SC, Pitcher JD, Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008;466:3093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arai E, Nishida Y, Tsukushi S, Wasa J, Ishiguro N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res 2010;468:3028–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235–2241. [DOI] [PubMed] [Google Scholar]
- 24. Saeed H, King DM, Johnstone CA, et al. Preoperative radiation therapy followed by reexcision may improve local control and progression-free survival in unplanned excisions of soft tissue sarcomas of the extremity and chest-wall. Int J Surg Oncol 2016;2016:5963167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones DA, Shideman C, Yuan J, Dusenbery K, Carlos Manivel J, Ogilvie C, Clohisy DR, Cheng EY, Shanley R, Chinsoo Cho L. Management of unplanned excision for soft-tissue sarcoma with preoperative radiotherapy followed by definitive resection. Am J Clin Oncol 2016;39:586–592. [DOI] [PubMed] [Google Scholar]
- 26. Pollack A, Zagars GK, Goswitz MS, Pollock RA, Feig BW, Pisters PW. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998;42:563–572. [DOI] [PubMed] [Google Scholar]
- 27. Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–2553. [DOI] [PubMed] [Google Scholar]
- 28. Kepka L, Suit HD, Goldberg SI, et al. Results of radiation therapy performed after unplanned surgery (without re-excision) for soft tissue sarcomas. J Surg Oncol 2005;92:39–45. [DOI] [PubMed] [Google Scholar]
- 29. Alamanda VK, Delisca GO, Mathis SL, et al. The financial burden of reexcising incompletely excised soft tissue sarcomas: a cost analysis. Ann Surg Oncol 2013;20:2808–2814. [DOI] [PubMed] [Google Scholar]
- 30. Umer HM, Umer M, Qadir I, Abbasi N, Masood N. Impact of unplanned excision on prognosis of patients with extremity soft tissue sarcoma. Sarcoma 2013;2013:498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morii T, Aoyagi T, Tajima T, Yoshiyama A, Ichimura S, Mochizuki K. Unplanned resection of a soft tissue sarcoma: clinical characteristics and impact on oncological and functional outcomes. J Orthop Sci 2015;20:373–379. [DOI] [PubMed] [Google Scholar]
- 32. Arai E, Sugiura H, Tsukushi S, et al. Residual tumor after unplanned excision reflects clinical aggressiveness for soft tissue sarcomas. Tumour Biol 2014;35:8043–8049. [DOI] [PubMed] [Google Scholar]
- 33. O’Donnell P, Griffin A, Eward W, et al. The effect of a positive surgical margin in soft tissue sarcoma. Cancer 2014;120:2866–2875. [DOI] [PubMed] [Google Scholar]
- 34. Smolle MA, Tunn PU, Goldenitsch E, et al. The prognostic impact of unplanned excisions in a cohort of 728 soft tissue sarcoma patients: a multicentre study. Ann Surg Oncol 2017;24:1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg 2000;231:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charoenlap C, Imanishi J, Tanaka T, et al. Outcomes of unplanned sarcoma excision: impact of residual disease. Cancer Med 2016;5:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nandra R, Forsberg J, Grimer R. If your lump is bigger than a golf ball and growing, think sarcoma. Eur J Surg Oncol 2015;41:1400–1405. [DOI] [PubMed] [Google Scholar]
- 38. Peabody TD, Monson D, Montag A, Schell MJ, Finn H, Simon MA. A comparison of the prognoses for deep and subcutaneous sarcomas of the extremities. J Bone Joint Surg Am 1994;76:1167–1173. [DOI] [PubMed] [Google Scholar]
- 39. Zornig C, Peiper M, Schröder S. Re-excision of soft tissue sarcoma after inadequate initial operation. Br J Surg 1995;82:278–279. [DOI] [PubMed] [Google Scholar]
- 40. Goodlad JR, Fletcher CD, Smith MA. Surgical resection of primary soft-tissue sarcoma: incidence of residual tumour in 95 patients needing re-excision after local resection. J Bone Joint Surg Br 1996;78:658–661. [PubMed] [Google Scholar]
- 41. Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997;66:81–87. [DOI] [PubMed] [Google Scholar]
- 42. Siebenrock KA, Hertel R, Ganz R. Unexpected resection of soft-tissue sarcoma: more mutilating surgery, higher local recurrence rates, and obscure prognosis as consequences of improper surgery. Arch Orthop Trauma Surg 2000;120:65–69. [PubMed] [Google Scholar]
- 43. Peiper M, Knoefel WT, Izbicki JR. The influence of residual tumor on local recurrence after unplanned resection of soft tissue sarcoma. Dtsch Med Wochenschr 2004;129:183–187. [DOI] [PubMed] [Google Scholar]
- 44. Rougraff BT, Davis K, Cudahy T. The impact of previous surgical manipulation of subcutaneous sarcoma on oncologic outcome. Clin Orthop Relat Res 2005;438:85–91. [DOI] [PubMed] [Google Scholar]
- 45. Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol 2006;13:110–117. [DOI] [PubMed] [Google Scholar]
- 46. Morii T, Yabe H, Morioka H, Anazawa U, Suzuki Y, Toyama Y. Clinical significance of additional wide resection for unplanned resection of high grade soft tissue sarcoma. Open Orthop J 2008;2:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rehders A, Stoecklein NH, Poremba C, Alexander A, Knoefel WT, Peiper M. Reexcision of soft tissue sarcoma: sufficient local control but increased rate of metastasis. World J Surg 2009;33:2599–2605. [DOI] [PubMed] [Google Scholar]
- 48. Zacherl M, Kastner N, Glehr M, et al. Influence of prereferral surgery in soft tissue sarcoma: 10 years’ experience in a single institution. Orthopedics 2012;35:e1214–e1220. [DOI] [PubMed] [Google Scholar]
- 49. Koulaxouzidis G, Schwarzkopf E, Bannasch H, Stark GB. Is revisional surgery mandatory when an unexpected sarcoma diagnosis is made following primary surgery? World J Surg Oncol 2015;13:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakamura T, Kawai A, Sudo A. Analysis of the patients with soft tissue sarcoma who received additional excision after unplanned excision: report from the Bone and Soft Tissue Tumor Registry in Japan. Jpn J Clin Oncol 2017;47:1055–1059. [DOI] [PubMed] [Google Scholar]