Abstract
In the Dutch Arthroplasty Register (LROI), the product and batch number of prosthetic components and cement are registered for traceability. Registration of the product number provides opportunities to extend the information about a specific prosthesis. All product numbers used from the beginning of the registration in 2007 were characterized to develop and maintain an implant library.
The Scientific Advisory Board developed a core-set that contains the most important characteristics needed to form an implant library. The final core-set contains the brand name, type, coating and material of the prosthesis. In total, 35 676 product numbers were classified, resulting in a complete implant library of all product numbers used in the LROI.
To improve quality of the data and increase convenience of registration, the LROI implemented barcode scanning for data entry into the database. In 2017, 82% of prosthetic components and cement stickers had a GS1 barcode. The remaining product stickers used HIBCC barcodes and custom-made barcodes.
With this implant library, implants can be grouped for analyses at group level, e.g. evaluation of the effect of a material of a prosthesis on survival of the implant. Apart from that, the implant library can be used for data quality control within the LROI database.
The implant library reduces the registration burden and increases accuracy of the database. Such a system will facilitate new designs (learning from the past) and thus improve implant quality and ultimately patient safety.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180063
Keywords: arthroplasty register, hip prosthesis, knee prosthesis, classification, implant library, barcodes
Introduction
The Dutch Arthroplasty Register (LROI) was initiated by the Netherlands Orthopaedic Association (NOV) in 2007 in order to evaluate outcome of arthroplasty procedures. For that purpose, patient, surgical procedure and implant characteristics of hip and knee arthroplasties in the Netherlands were registered.1 In 2014, the registrations of shoulder, elbow and ankle arthroplasties were added to the database, followed by wrist and finger arthroplasties in 2016. The primary goal of the LROI is to improve quality of orthopaedic care and to detect less optimal performing orthopaedic implants in an early phase. Second, its goal is to maintain patient safety by collecting patient- and implant-related data, which allows traceability of orthopaedic implants at a national level.1
Since 2007, > 710 000 arthroplasty procedures have been registered in the LROI.2 In 2017, almost 30 000 primary total hip, > 29 000 primary knee, 2900 primary shoulder, 116 primary ankle and 111 primary elbow arthroplasties performed in the Netherlands have been registered in the LROI.2
The REF number (product number) and LOT number (batch number) of each prosthetic component and cement are registered in the LROI. The product number identifies the prosthetic component while the batch number specifies production details. Registration of the product number provides opportunities to expand the information about a specific prosthesis that is provided within the register. Furthermore, the specific prosthesis information can be used to identify more generic implant characteristics across different implant types (e.g. hip, knee) which are (less) favourable to the outcome of these implants (i.e. implant survival).
The importance of a uniform implant library of orthopaedic implants is high for several reasons. First of all, implant names across countries, although manufactured by the same company, can be different. Thus, information on implant specific details are essential when comparing products. Second, the effect of certain implant characteristics across different implants (e.g. polished tapered hip stems, posterior stabilized knees etc.) can only be analysed with a clear-cut succinct definition of these implant characteristics. For that matter, product numbers of implant components and cement must be grouped by name, material, type etc. to analyse these groups. Several international initiatives have been introduced over the last years to develop one global uniform library on hip and knee prosthetic component characteristics.3,4 These projects show great promise to facilitate the process in the long term, but a short-term need for a generic orthopaedic implant product classification library has existed in the Netherlands since 2007.
The Medical Device Regulation (MDR) is the new European legislation to improve patient safety in the European Union for all medical devices. In the MDR, new regulations on medical devices were adopted and will become effective after a transitional period in May 2020. These new regulations on all medical devices, including joint prostheses, are aimed to ensure that all medical devices are safe and perform well. One important change following the MDR describes that manufacturers are obligated to place a Unique Device Identifier (UDI) on the medical devices, which leads to better traceability of devices in case of a recall.5 The European Commission designated Global Standards One (GS1) and Health Industry Business Communication Council (HIBCC) as the entities for the allocation of UDIs in accordance with the regulation.6 As a consequence of the new MDR, healthcare providers in the Netherlands are obligated to register the UDI of medical devices in the National Implant Registry (LIR) of the ministry of Health of the Dutch government from 1 January 2019 onwards, which guarantees traceability of all medical devices.
The aim of this study is to describe: 1) the development of a Dutch core set with specific implant characteristics and its implant library containing hip, knee, ankle, shoulder and elbow prosthetic component characteristics; and 2) the process of building and maintaining an implant library with the utilization of the barcode scanning to import prosthetic characteristics.
Defining, developing and maintaining an implant library
Data collection and storage
The LROI is a nationwide population-based register in which information on joint arthroplasties in the Netherlands, containing hip, knee, ankle, shoulder, elbow, wrist and finger prostheses, is collected.2 The LROI is initiated by the NOV, of which nearly all Dutch orthopaedic surgeons are members. The LROI is well supported by these members, resulting in a completeness of > 97% for primary total hip arthroplasties and 96% for primary knee arthroplasties, and a coverage of 100% of hospitals.7 The LROI is a foundation managed by the LROI board which is supported by the LROI office and receives advice from the Scientific Advisory Board.8
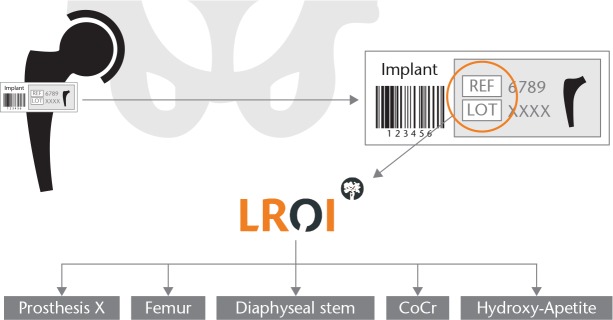
Patient and surgical characteristics as well as the product and batch number of the implant component and cement are registered by all orthopaedic departments and entered into a central online database. The product number identifies the characteristics of the orthopaedic implant/cement while the batch number is used to identify its specific production details (Fig. 1).
Fig. 1.
Example of characteristics recorded in the Dutch prosthesis implant library
LROI data can be entered directly into the database using the LROI webforms or by uploads from the electronic patient file of the healthcare provider. When the LROI webforms are used, the product numbers can be entered using a barcode scanner or by manual input. Barcode scanning is preferred as it prevents registration errors. The LROI register contains an implant library of all joint prostheses on the Dutch market; therefore, the database software ‘recognizes’ the product number and shows the brand name of the component and for which joint it is instructed to be used. When an entered product number does not correspond with the current target joint, the system displays a warning message indicating which product has been identified and for which joint it should be used. In case unknown product numbers are entered into the LROI database, the LROI office starts a verification procedure, which includes verifying the product sticker of the implanted prosthesis and/or contacting the manufacturer of the specific implant.
Defining an implant classification core set
Product numbers of orthopaedic prostheses provide extensive information. All characteristics of the prosthesis can be tracked using this number. Furthermore, additional prosthetic characteristics can be added to the LROI database using this product number, even when the prosthesis was placed many years ago. Thus, product numbers allow analyses at the highest granular level, even retrospective. To extract a determined set of characteristics from a product number, an implant classification core set containing the most important implant characteristics needs to be defined. For this purpose, the members of the Scientific Advisory Board of the LROI were consulted and they produced a (concept) core set during several Delphi meetings with clinical and engineering experts. Since product and batch numbers of the implant were entered into the LROI database from the beginning, the metrics for a generic comparison across implant types (e.g. hip, knee) were discussed during these consensus meetings. This core set was sent to the boards of the Dutch Hip and Knee Societies of the NOV for verification. Finally, implant manufacturers or distributors in the Netherlands were asked for feedback on this classification core set and agreed with its content.
In 2013, the core set for classification of product numbers of hip and knee prostheses was finalized. The variables in this final core set of hip and knee components included joint component, the brand name of the prosthesis, the manufacturer, the type, the material of the component, the material of the bearing surface, the coating of the surface where bone contact can be expected, the method of sterilization of the polyethylene and diameter of the head and bipolar head (for femoral head components) (Table 1). For bone cement, the name of bone cement, the manufacturer, type of cement, viscosity, whether the cement contains any antibiotics and use of a vacuum cement mixing system are collected (Table 2). In 2014, the process described above was repeated for shoulder, elbow and ankle components (since registration of these joints started in 2014). For shoulder, elbow and ankle components, the core set included joint component, the brand name of the components, the manufacturer, name of prosthesis, material and the method of sterilization of the polyethylene (Table 3).
Table 1.
Classification core set of hip and knee implants in the Netherlands
| Joint component | Brand name | Manufacturer | Type of component | Type of prosthesis | Material* | Material bearing surface* | Surface bone coating† | Method of sterilization PE‡ | Diameter head |
|---|---|---|---|---|---|---|---|---|---|
| Hip femoral stem | • Stem with fixed head • Stem with modular head • Other |
• Intramedullar • Metaphysar • Resurfacing |
Set 1 | - | Set 2 | - | - | ||
| Hip acetabulum | • Monoblock (complete cup) • Mobile backing |
• Cemented • Press-fit • Screw cup |
Set 1 | Set 1 | Set 2 | Set 3 | - | ||
| Hip liner | - | • Standard | Set 1 | Set 1 | - | Set 3 | - | ||
| Hip femoral head | - | • Conus • Other |
Set 1 | - | - | - | In mm | ||
| - | |||||||||
| Knee femoral stem | - | • PCL retaining • Posterior stabilized • Bicruciate retaining • Bicruciate sacrificing • Patellofemoral • Unicondylar • Semi-hinge/revision • Patellofemoral-uni-combination • Tumour prosthesis |
Set 1 | - | Set 2 | - | - | ||
| Knee tibial stem | - | • PCL retaining • Posterior stabilized • Bicruciate retaining • Unicondylar • Semi-hinge/revision • Patellofemoral-uni combination • Mobile bearing • Tumour prosthesis |
Set 1 | - | Set 2 | set 3 | - | ||
| Knee inlay | - | • Standard • Posterior stabilized • Unicondylar • Semi-hinge/revision • Mobile bearing |
Set 1 | Set 1 | - | Set 3 | - | ||
| Knee patella | • Monobloc patella • Metal backed patella |
• Fixed • Rotating patella • Patellofemoral |
Set 1 | Set 1 | Set 2 | Set 3 | - |
Set 1 material: stainless steel, cobalt chrome, titanium, ceramic, composite, titanium with hardened layer, PE standard, PE cross-linked, tantalum, oxidized zirconium
Set 2 bone surface structure: matt-coated, structure-coated, porous-coated, hydroxyapatite-coated, polished
Set 3 sterilization method PE: irradiation, ethylene-oxide, gas plasma sterilization
PE: polyethylene
Table 2.
Classification core set of bone cement in the Netherlands
| Product | Brand name |
Manufacturer | Type of cement | Viscosity | Antibiotics | Vacuum cement mixing system |
|---|---|---|---|---|---|---|
| Cement | • Primary procedures • Revision procedures • Both |
• Low • Medium • High |
• No • Gentamicin • Tobramycin • Gentamicin + clindamycin • Gentamicin + vancomycin • Erythromycin + colistin |
• Yes • No |
Table 3.
Classification core set of shoulder, elbow and ankle implants in the Netherlands
| Joint | Joint component | Brand name |
Manufacturer | Material* | Method of sterilization PE† |
|---|---|---|---|---|---|
| Ankle | • Tibial component • Talus component • Inlay component |
Set 1 | Set 3 | ||
| Shoulder | • Humeral stem • Humeral head • Humeral liner • Glenoid component cup • Glenoid component glenosphere • Humeral baseplate (metafyse) • Glenoid baseplate • Glenoid liner |
Set 1 | - | ||
| Elbow | • Humeral stem • Ulna stem • Radial head • Radial stem |
Set 1 | Set 3 |
Set 1 material: stainless steel, cobalt chrome, titanium, ceramic, composite, titanium with hardened layer, PE standard, PE cross-linked, tantalum, oxidized zirconium
Set 3 sterilisation method PE: irradiation, ethylene-oxide, gas plasma sterilization
PE: polyethylene
Development and updating of the implant library
All implant manufacturers or distributors cooperated to classify the product numbers of their products which had been registered since the start of the LROI in 2007. To achieve this, a list of product numbers to be classified was sent to the manufacturers/distributors followed by the finalized core set on implant characteristics. Hence, the manufacturers/distributors were asked to appoint the characteristics of the core set to their products. The Scientific Advisory Board and the LROI office ultimately validated the data before implementation of the characteristics in the LROI implant library. This was done by checking the product brochure of the implant components and bone cements and by using the knowledge and experience of the biomechanical engineer. After approval of the information, the final LROI implant library was linked to the LROI database.
In total, 32 500 different product numbers of hip and knee prosthetic components have been classified, resulting in an implant library containing all the hip and knee prosthetic components used in the Netherlands. In addition, 3000 product numbers of shoulder, elbow and ankle prostheses were classified according to a smaller classification core set of implant characteristics. A total of 170 product numbers were classified using the classification core set for bone cement (Table 4).
Table 4.
Number of product numbers included in the Dutch implant library and proportion of product numbers with GTIN
| Joint | Number of product numbers | Proportion of GTIN* (%) |
|---|---|---|
| Hip | 18 363 | 61 |
| Knee | 14 150 | 76 |
| Shoulder | 2134 | 81 |
| Ankle | 511 | 63 |
| Elbow | 350 | 85 |
| Cement | 168 | 46 |
| Total | 35 676 | 68 |
Based on all available product numbers per joint arthroplasty.
The implant library is maintained by the LROI office and updated continuously. This ensures consistent addition of new implant product numbers and a central point for contact and troubleshooting. Implant manufacturers announce the set of product numbers and classify the characteristics according to the classification of the LROI implant library for each new orthopaedic implant introduction onto the Dutch market. To safeguard valid implant entry into the LROI database, entry of unknown implant product numbers is signalled by both data entry specialists in hospitals as well as by the LROI office during monthly checks of the database. In this way, the implant library stays up-to-date and completeness is close to 100%.
Every year, new prosthetic components (or new implant characteristics to existing implants) are added to the implant library in order to guarantee up-to-date information. In 2017, the total number of new implant components added to the implant library was 1136, which includes all different sizes of 42 specific implants (e.g. stems, head, liners etc.). In 2017, for an overall 85 000 registrations, 25 implant entries were reported to the LROI office for missing data in the LROI implant library. The latter indicates that only a minute amount of the applied components cannot be recognized using the LROI implant library.
In the future, a core set for wrist and finger prostheses will be developed. Next to that, the core set for ankle, shoulder and elbow prostheses should be extended with more characteristics (i.e. to the same level as hip and knee).
Barcode scanning
Since the majority of orthopaedic implant manufacturers started using barcodes on component packages, and scanning of these barcodes reduces both registration burden as well as registration errors, the LROI started implementing barcode scanning for data entry into the LROI database in 2015. In the Netherlands, three types of barcodes are used on orthopaedic prosthetic components packaging: GS1; HIBCC; and manufacturers with custom barcodes.
GS1 barcodes on prosthetic component stickers are composed of at least a 14-digit Global Trade Item Number (GTIN), a six-digit expiry date and a batch number of varying length. Each GTIN number refers to a product number which enables it to be linked to product information using bar code scanning of GS1 barcodes. Apart from GS1 barcodes, HIBCC barcodes integrate product number and batch number in the barcode string. Logic applied within the LROI can extract both product and batch number from these barcodes and store it in the database. Besides this, only a small number of manufacturers work with custom barcode strings only containing product and batch number.
Scanning (and storage) of one-dimensional (1D; linear) and two-dimensional (2D; matrix) barcodes ensures that healthcare providers meet international guidelines (European Union Medical Device Regulation; MDR).5 MDR regulation imposes stricter requirements on the market authorization of medical devices. A UDI exists of Device Identifier (DI) of the product (e.g. GTIN or product number) and Production Identifier (PI) (e.g. expiration date, batch number and/or serial number).
All orthopaedic departments performing joint replacement operations in the Netherlands use the same barcode scanner provided by the LROI. This barcode scanner has been programmed to recognize all barcode structures on prosthetic components in the Netherlands. It extracts the required information and feeds that into the appropriate fields of the digital LROI registration form, which is part of the hospital’s electronic patient file.
The LROI developed a methodology to enable scanned barcodes to be added to the appropriate fields in the registration form, which improves accuracy in the data entry of product numbers. One challenge in this regard is the large variability of barcodes used for implant components, which complicates general applicability of generic import rules. It would be a large step forward when one uniform barcode system would be used in the Netherlands and Europe. Developments in this field are ongoing and we hope this will soon be fully implemented into practice. This would not only limit the intercomponent variability in codes that are being used, but also simplify the maintenance and development of the implant library.
After implementation of barcode scanning into the LROI database, 68% of all components could be identified using GTIN codes (Table 4). When considering only prosthesis used in 2017, this proportion was much higher: Of all registered components (including cement) in 2017, 82% had a GS1 barcode (Table 5). For knee, ankle, shoulder, elbow components and cement, > 95% of product stickers contain a GS1 barcode. For hip components, this was 67%. Of the 33% remaining hip components, 19% used HIBCC barcodes, 4% of barcodes contains only product and batch number and for 10%, it is unknown what kind of barcode is used (HIBCC, product and batch number barcodes, or presence of no barcode).
Table 5.
Number of prosthetic components and bone cement used in 2017 using GS1 barcodes
| Joint | Total number of components | Proportion scanned with GS1 barcode (%) |
|---|---|---|
| Hip | 128 796 | 67 |
| Knee | 95 349 | 95 |
| Ankle | 300 | 97 |
| Shoulder | 14 471 | 97 |
| Elbow | 268 | 98 |
| Bone Cement | 42 716 | 92 |
| Total | 281 900 | 82 |
To keep up with current developments, and to further facilitate data entry and limit the error-prone process of manual data entry, we are exploring the implementation of process batch-uploaded procedures using GTIN or UDI. Data entry of GTIN numbers will extract all necessary implant characteristics into the LROI database.
Implementation of the implant library
Using the LROI implant library reduces registration burden because there is no need to manually enter additional characteristics of implant components or bone cements. It also reduces typing errors since entering a specific product number alerts the LROI system to immediately show the data entry specialist the implant description and name of the prosthetic component or cement. Therefore, validation of the implant library is performed within the hospitals. If the implant library shows the incorrect name and description, hospital data entry specialists notify the LROI office, who will contact the manufacturer.
The LROI implant library and product number and batch number of all prosthetic components and cements resulted in the addition of new variables to the LROI database based on implant library data. It created the opportunity for researchers to assess how implant characteristics like type of articulation, femoral head size, stem shape or stem surface affect prosthesis survival. Prostheses can be grouped according to a specific generic characteristic present in several implants (e.g. cemented tapered polished hip stems or mobile-bearing HA-coated knee), so that these generic prosthesis characteristics can be evaluated. On top of that, the same could also be examined in specific patient groups (e.g. female patients aged < 45 years). Recent studies based on LROI data and its implant library demonstrate the value of this implant library in data analysis. Peters et al described lower mid-term revision rates for ceramic-on-highly cross-linked polyethylene, ceramic-on-ceramic and oxidized zirconium on (highly cross-linked) polyethylene total hip arthroplasties using data of this implant library.9 Another study focused on the femoral head size and surgical approach and the risk of revision due to dislocation after total hip arthroplasty,10 where femoral head size was retrieved from the implant library. A study on posterior stabilized versus cruciate retaining total knee prostheses showed worse performance of the posterior stabilized total knee arthroplasty.11 Many other studies are currently being performed in which data from the implant library are of crucial importance.
Apart from that, the implant library can be used for data quality control within the LROI database, for example in the case of ‘type of hip prosthesis’. Comparing the number of resurfacing hip prostheses registered in the LROI between 2007 and 2017, learned that 2598 hip resurfacing procedures were performed according to data entry variables. According to the actual product numbers, 2883 resurfacing hip arthroplasties were entered into the LROI database. This demonstrates that roughly 10% of resurfacing hip prosthesis are missed when only relying exclusively on a data entry variable. Integration of the implant library into the registration can detect these misclassified procedures and provide a means to improve data quality within a feedback quality control loop.
Registration of product number and batch number creates unique data registration and thus data quality. In addition, it makes traceability of these implants possible. In case of an emergency with a specific prosthetic component, this component can be traced based on these numbers using the LROI. The Dutch Ministry of Health, Welfare and Sports has denoted the LROI as a blueprint for the development of other registers of medical devices, in order to implement traceability of all medical devices used in the Netherlands. This demonstrates the applicability of the strategy and recognition as a template for other registers.
The importance of grouping implant data within national registries seems evident. Even more important is to have a uniform global classification system. The latter may have different levels of granularity, but exchange of coding between these different levels is a must, in order to improve innovation. A task force of representatives of the German Arthroplasty Registry (EPRD) published a universal, standardized implant database for product identification in 2015.12 That system is now adopted by the National Joint Registry of the UK, Wales, Northern Ireland & Isle of Man and recently a regional registry in Italy. A second initiative is done by the International Society of Arthroplasty Registers (ISAR).13 ISAR launched their International Prosthesis Library (IPL) in January 2019. It would be interesting to check the differences between the LROI Implant Library, EPRD and IPL. Because product numbers are registered in the LROI (i.e. the highest implant granularity), any international (future) implant library can be implemented.
In conclusion, a complete implant library containing characteristics of 32 500 orthopaedic implants used in the Netherlands was developed by the LROI, in close collaboration with manufacturers of orthopaedic implants and orthopaedic experts. The Dutch implant library covers a great deal of the global market on orthopaedic implants, with about 85 different hip implants and 85 different knee implants. The introduction of barcode scanning for orthopaedic implants improved and simplified registration of product numbers, which further enhances possibilities.
The necessity for a global implant library is undisputed with regard to analysis of generic implant design features across types of implants (e.g. hip, knee, shoulder) but also to improve checks on data quality within the LROI. The implant library reduces the registration burden and increases accuracy of the database. Such a system will facilitate new designs (learning from the past) and thus improve implant quality and ultimately patient safety.
Acknowledgments
For this paper, we would like to thank the former LROI commission, and especially Pieter Spierings, for their early work on the first version of the classification set.
We also would like to thank all the implant manufacturers in the Netherlands for classifying their own product numbers for the implant library. Without their help, the final version of the implant library would not have been achieved.
Footnotes
ICMJE Conflict of interest statement: NJJV reports payments from Invibio and Exactech for consultancy, outside the submitted work.
RGHHN reports payments for his responsibilities as Chairman of the Dutch Arthroplasty Board.
All other authors have nothing to declare.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. LROI. LROI Report 2014: Arthroplasty in the Picture. 2015. [Google Scholar]
- 2. LROI. www.lroi-report.nl (date last accessed 5 February 2019).
- 3. Endoprothesenregister Deutschland. www.eprd.de (date last accessed 7 August 2018).
- 4. International Consortium of Orthopaedic Registries. www.icor-initiative.org (date last accessed 23 February 2016).
- 5. European Union Medical Device Regulation. https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_en (date last accessed 30 August 2018).
- 6. European Commission. https://eur-lex.europa.eu/legal-content/NL/TXT/?uri=uriserv:OJ.L_.2017.117.01.0001.01.NLD&toc=OJ:L:2017:117:TOC (date last accessed 30 August 2018).
- 7. van Steenbergen LN, Denissen GA, Spooren A, et al. More than 95% completeness of reported procedures in the population-based Dutch Arthroplasty Register. Acta Orthop 2015;86:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LROI. www.lroi.nl (date last accessed at 8 February 2019).
- 9. Peters RM, Van Steenbergen LN, Stevens M, et al. The effect of bearing type on the outcome of total hip arthroplasty. Acta Orthop 2018;89:163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zijlstra WP, De Hartog B, Van Steenbergen LN, Scheurs BW, Nelissen RGHH. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty. Acta Orthop 2017;88:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spekenbrink-Spooren A, Van Steenbergen LN, Denissen GAW, et al. Higher mid-term revision rates of posterior stabilized compared with cruciate retaining total knee arthroplasties: 133,841 cemented arthroplasties for osteoarthritis in the Netherlands in 2007-2016. Acta Orthop 2018;89:640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blömer W, Steinbrück A, Schröder C, et al. A new universal, standardized implant database for product identification: a unique tool for arthroplasty registries. Arch Orthop Trauma Surg 2015;135:919-26. [DOI] [PubMed] [Google Scholar]
- 13. International Society of Arthroplasty Registers. www.isarhome.org (date last accessed 12 February 2019).