Abstract
Background: B-cell leukemia/lymphoma-2 (BCL-2) protein is an important part of apoptotic pathway, which is overexpressed in chronic lymphocytic leukemia cells, non-Hodgkin lymphoma cells, and myeloma cells. Venetoclax (ABT-199/GDC-0199) is a highly selective bioavailable inhibitor of BCL-2 protein, which is more effective and less valid against BCL-xL in BCL2-dependent leukemia and lymphoma cell.
Method: We searched PubMed database using retrieval keyword venetoclax, ABT-199, or GDC-0199 and investigated the data of the involved articles. Considering variability in different studies, the overall response rate was collected to assess the efficacy. Meanwhile, adverse events (AEs) were summarized, and event rates were calculated to assess the safety.
Results: When patients were treated with venetoclax monotherapy, the most common AEs were nausea, diarrhea, neutropenia, fatigue and thrombocytopenia, and neutropenia. In addition, thrombocytopenia, anemia, febrile neutropenia, and leukopenia appeared more common and are considered as the severe AEs (defined as grade ≥ 3 AEs). Although the occurrence of thrombocytopenia was relatively high, it was not the most severe type. When compared with patients treated with venetoclax and other drugs, nausea, diarrhea, and thrombocytopenia were still the most common AEs occurrence in the patients of all the grade. The overall event rate was 73%, which is quite satisfactory.
Conclusion: Venetoclax is a mild and efficient drug in treating advanced hematological malignancy, which can be specially fit for the chronic lymphocytic leukemia patients with del[17p], and AEs are well tolerable. Few tumor lysis syndrome occurred after taking the drugs. The AEs aroused by venetoclax, and the combination is well tolerable. Therefore, the use of venetoclax monotherapy or its combination with other therapies is desirable in hematological malignancy.
Keywords: venetoclax, Bcl-2 inhibitor, hematological malignancy, deletion of chromosome 17p, chronic lymphocytic leukemia
Introduction
B-cell leukemia/lymphoma-2 (BCL2) protein is considered as a key pathogenesis, which is overexpressed in chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and myeloma (ML) cells (Cimmino et al., 2005; Souers et al., 2013; Touzeau et al., 2014) as an important regulator of apoptotic pathway. Moreover, BCL2 protein is closely related to chemoresistance in hematological tumors (Marschitz et al., 2000). CLL is a prevalent adult blood tumor, whose prognosis can be predicted by the quantitation of BCL protein (Robertson et al., 1996; Cimmino et al., 2005), while BCL2 prevents cells from apoptosis by insulating proapoptotic stimuli instead of promoting proliferation of cells itself (Borner, 2003; Willis et al., 2003).
BH3 mimetics, a novel agent, initiates apoptosis by releasing proapoptotic proteins after it binds and suppresses antiapoptotic proteins, which can prevent the biology of BCL-2 protein family (Certo et al., 2006). BH3-mimetic drug is an emerging antineoplastic agent via simulating cellular antiapoptotic pathway, whose function is also shared by BCL2 protein (Oltersdorf et al., 2005; van Delft et al., 2006; Green and Walczak 2013). Navitoclax (ABT-737) is the first BH3-mimetic inhibitor of BCL2, which has been assessed in clinical trials and reported to have promising effect in relapsed CLL patients and observed to have positive responses in 35% of these patients (Roberts et al., 2012; Rudin et al., 2012). After its reported favorable tolerance against small cell lung cancer and lymphoma, the significant decrease of blood platelet counting in mice treated with navitoclax was also observed (Oltersdorf et al., 2005). The dose-dependent drug-related thrombocytopenia limits the advanced use of the navitoclax, despite the rapid recovery of platelet count after the primary treatment (Mason et al., 2007).
Venetoclax (ABT-199/GDC-0199) is another highly selective, orally bioavailable inhibitor of B-cell leukemia/lymphoma-2 protein, which is even more efficient than navitoclax and less valid against BCL-xL in BCL2-dependent leukemia and lymphoma (Souers et al., 2013). Compared with navitoclax, venetoclax poses less impact on platelets both in vivo and in vitro (Souers et al., 2013). Venetoclax prevents growth of primary CLL cells and a part of NHL cell lines in vitro. Meanwhile, it also manifested a promising antitumor efficacy in vivo (Souers et al., 2013). These unique characteristics provide insight in the treatment of leukemia and NHL. Patients treated with venetoclax plus rituximab maintained a high overall response rate (ORR) of 86% and possessed acceptable adverse events (AEs) in patients with CLL or small lymphocytic lymphoma (SLL) (Seymour et al., 2017). Subsequently, venetoclax was applied solely in relapsed or refractory (R/R) CLL patients, which also achieved a high ORR with acceptable toxicity (Stilgenbauer et al., 2016). Patients diagnosed as R/R NHL were tolerated with venetoclax monotherapy within a safety dose (Davids et al., 2017). AEs are inevitable, although the drug is tolerant. The M12-175 study is the first study in which R/R CLL or NHL patients received incremental dose of venetoclax. With gradual dose escalation, the risk of tumor lysis syndrome (TLS) increased (Roberts et al., 2016). Gradually, venetoclax combined with other drugs was also applied on multiple myeloma (MM) (Touzeau et al., 2014) and acute myelocytic leukemia (AML) (Konopleva et al., 2016; Wei et al., 2019). In this study, we analyzed these clinical trials to assess the safety and efficacy of venetoclax.
Methods
Study Design, Search Strategy, and Study Selection
Our research followed the guidance of the preferred reporting items for systematic reviews and meta-analysis statement. And the research question was structured according to the population, intervention, comparison, outcome format rules—population: hematological tumor adult patients; intervention: treated with BCL-2 inhibitor venetoclax (ABT-199 or GDC-0199); comparison: with/without control (patients treated with other drugs); outcomes: AEs after using the drug or the survival condition after using the drug, such as ORR, overall survival (OS), and progression free survival (PFS). A relevant literature search was performed on PubMed to identify relevant studies (up to May 2019). The following key words and their combinations were used without using any automatic filters: “venetoclax or ABT-199 or GDC0199.” In addition, the references of selected studies were also screened for additional relevant studies.
Inclusion Criteria and Excluded Criteria
The eligibility criteria in the study were as follows: 1) clinical trials in any phase of venetoclax with the survival of the hematological malignancy patients or AEs reported in articles; 2) the patients enrolled in the trials who were suffering from hematological tumor were confirmed by pathology, regardless of any treatment before; 3) full data of the safety or efficacy were available in the articles; 4) full texts could be found; 5) the drug was applied on human; 6) patients’ age was over 18 years. Articles were excluded when no raw data were provided or data were duplicated.
Data Extraction
Extracted data were as follows: 1) the fundamental information of studies: the first author name, ClinicalTrials.gov number, the phase of the articles, the year the articles were published, number of participating patients, cancer type, follow-up time, and age; 2) the characteristics of primary AEs were mentioned in at least two articles or in basic research articles. They are divided into AEs of all grades and grade ≥ 3 AEs; 3) Survival indicators, such as PFS, OS, or ORR of the patients.
Statistical Analysis
The Comprehensive Meta-Analysis program 2 (Biostat, Englewood, NJ) was used to carry out the analysis of the survival and AEs data. We calculated the proportion and derived 95% confidence interval (CI) of major AEs (involving both all grade and grade ≥ 3) in included studies. When the two-sided P values were less than 0.10, they were regarded as significant. If I 2 ≥ 50%, it was considered as random-effects model in the analysis or else a fixed-effects model was applied.
Quality of Studies
The systematic bias of the involved randomized controlled trial articles was evaluated by using the Cochrane’s risk of bias tool (Review Manager 5.3). The non-randomized trials were evaluated by the methodological index for non-randomized studies (Slim et al., 2003). These items are scored 0, 1, or 2. Score 0 demonstrates that the studies did not report this part; score 2 demonstrated the studies reported and were adequate. Score 1 demonstrates reported but not adequate. The global ideal score being 16 for studies that were non-comparative studies. Two researches scored each trial for the risk of bias independently. All the disagreement was solved by discussion.
Results
Study Selection and Characteristics
Seven hundred twenty possibly related articles were identified through searching in PubMed in May 2019. After carefully screening the titles and the offered abstracts, 41 articles were further investigated. Finally, 18 articles were involved in the research. Venetoclax monotherapy was reported in seven articles. The treatment used in the other 11 articles was venetoclax plus other therapies (Moreau et al., 2017; Seymour et al., 2017; Aldoss et al., 2018; Cramer et al., 2018; de Vos et al., 2018; DiNardo et al., 2018; Rogers et al., 2018; Flinn et al., 2019; Kater et al., 2019; Wei et al., 2019; Zelenetz et al., 2019). The details of the selecting process were shown in Figure 1 . The basic information of the articles is listed in Table 1 . Venetoclax and decitabine (group a), venetoclax and azacytidine (group b), or venetoclax, combined with decitabine and posaconazole (group c), were used by the patients in the study of DiNardo CD (DiNardo et al., 2018). Therefore, the involved patients were analyzed in three groups during the data analysis.
Figure 1.
Flow diagram of the literature search and trial selection process.
Table 1.
Basic information.
| Author | ClinicalTrials.gov number | Phase | Year | Number of patients | Study design | Cancer type | Follow-up | Age | MINORS score |
|---|---|---|---|---|---|---|---|---|---|
| Shaji Kumar | NCT01794520 | 1 | 2018 | 66 | Single-arm | Relapsed or refractory t(11;14) multiple myeloma | Ongoing | 63 years (31–79) | 16 |
| Matthew S. Davids | NCT01328626 | 1 | 2017 | 106 | Single-arm | Relapsed or refractory non-Hodgkin lymphoma (mantle cell lymphoma (MCL; n = 28), follicular lymphoma (FL; n = 29), diffuse large B-cell lymphoma (DLBCL; n = 34), DLBCL arising from chronic lymphocytic leukemia (Richter transformation; n = 7), Waldenstro¨m macroglobulinemia (n = 4), and marginal zone lymphoma (n = 3)) | 5.3 months (0.2–46) | 66 years (25–86) | 13 |
| Steven Coutre | NCT02141282 | 2 | 2018 | 36 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 14 months (1–29) | 68 years (56–85) | 16 |
| Jeffrey A. Jones | NCT02141282 | 2 | 2017 | 91 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 14 months (8–18) | 66 years (28–81) | 16 |
| Stephan Stilgenbauer | NCT01889186 | 2 | 2016 | 107 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 12.1 months (10.1–14.2) | 67 years (37–85) | 16 |
| Andrew W. Roberts | NCT01328626 | 1 | 2016 | 116 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 17 months (1–44) | 66 years (36–86) | 16 |
| Marina Konopleva | NCT01994837 | 2 | 2016 | 32 | Single-arm | Relapsed or refractory acute myelogenous leukemia | Not given | 71 years (19–84) | 16 |
| Philippe Moreau | NCT01794507 | 1b | 2018 | 66 | Single-arm | Relapsed or refractory multiple myeloma | 5.9 months (0.3–29) | 63 years (31–79) | 16 |
| Courtney D. DiNardo | NCT02203773 | 1b | 2018 | 57 | Single-arm | Untreated acute myeloid leukemia | 12.4 months (8.3–15.8) | 75 years (71–80) | 16 |
| Prof John F. Seymour | NCT01682616 | 1b | 2018 | 49 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 28 months (19–32) | 68 years (50–88) | 16 |
| Andrew H. Wei | NCT02287233 | 1b/2 | 2019 | 82 | Single-arm | Untreated acute myeloid leukemia | 4.2 months (0.2–29) | 74 years (63–90) | 16 |
| Andrew D. Zelenetz | NCT02055820 | 1b/2 | 2019 | 56 | Single-arm | Non-Hodgkin lymphoma | 22 months (11.4–36.4) Treatment duration |
arm A 60.3 years (37–79); arm B 61.1 years (24–76) |
14 |
| Kerry A. Rogers | NCT02427451 | 1b | 2018 | 12 | Single-arm | Relapsed or refractory chronic lymphocytic leukemia | 24.4 months (no mention) | 57 years (42–70) | 15 |
| Sven de Vos | NCT01594229 | 1b | 2018 | 60 | Single-arm | Relapsed or refractory non-Hodgkin lymphoma | 7.4 months (0.1–55.1) | 62 years (29–90) | 15 |
| Ibrahim Aldoss | No mention | Respective analyze | 2018 | 33 | Single-arm | Relapsed or refractory acute myeloid leukemia | 6.5 months (0.8–12.4) | 62 years (19–81) | 11 |
| Ian W. Flinn | NCT01685892 | 1b | 2019 | 77 | Single-arm | Chronic lymphocytic leukemia | 29.3 months (3–55) in R/R patients; 26.7 months (16–39) in 1L patients |
61 years (42–80) in R/R patients; 63 in 1L patients (47–73) | 16 |
| Paula Cramer | NCT02401503 | 2 | 2018 | 66 | Single-arm | Chronic lymphocytic leukemia | 16 months (15–18) | 59 years (54–67) | 16 |
| Arnon P. Kater | NCT02005471 | 3 | 2018 | 194 | Randomized study | Relapsed or refractory chronic lymphocytic leukemia | 9.9 months (1.4–22.5) | No mention | RCT |
The study of Andrew W. Roberts and Matthew S. Davids share the same ClinicalTrials.gov number, but the patients reported in the studies were different. Andrew W. Roberts reported the relapsed or refractory chronic lymphocytic leukemia patients. Matthew S. Davids reported the relapsed or refractory non-Hodgkin lymphoma patients. In Andrew D. Zelenetz’s study, arm A is treated with venetoclax combined with R-CHOP, arm B is treated with venetoclax combined with G-CHOP. RCT, randomized controlled trial.
Safety
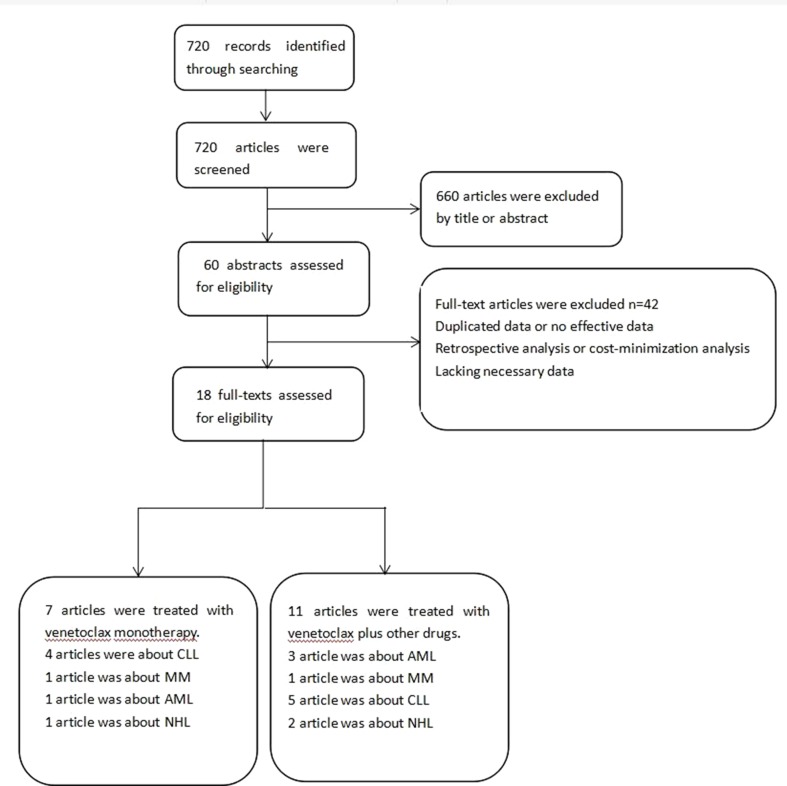
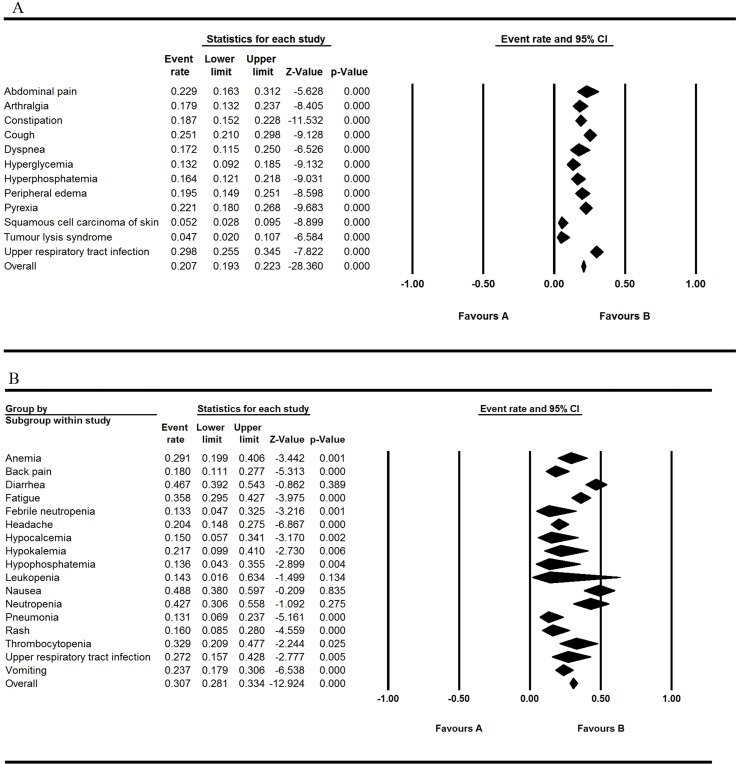
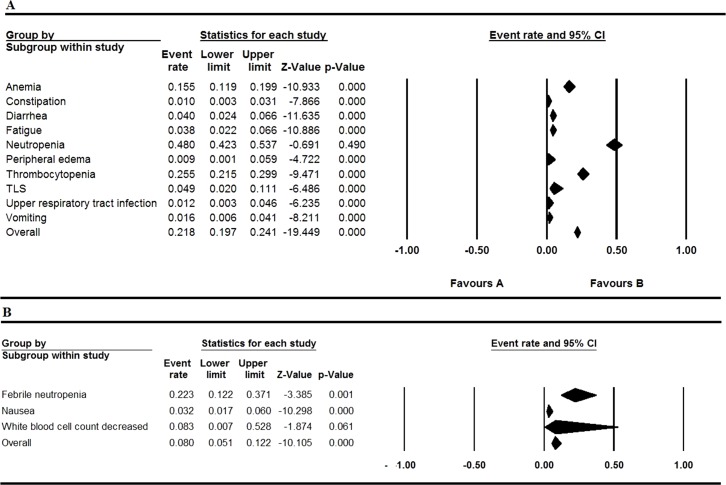
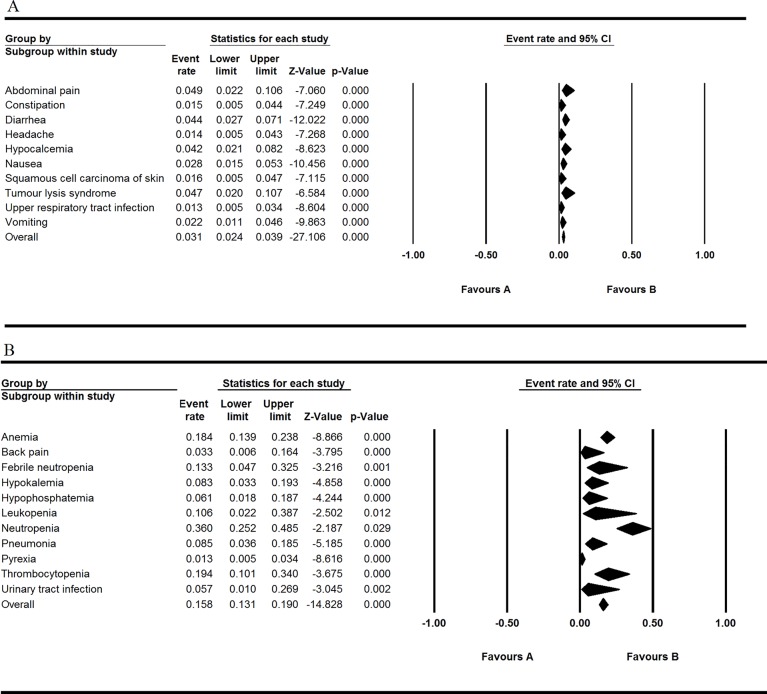
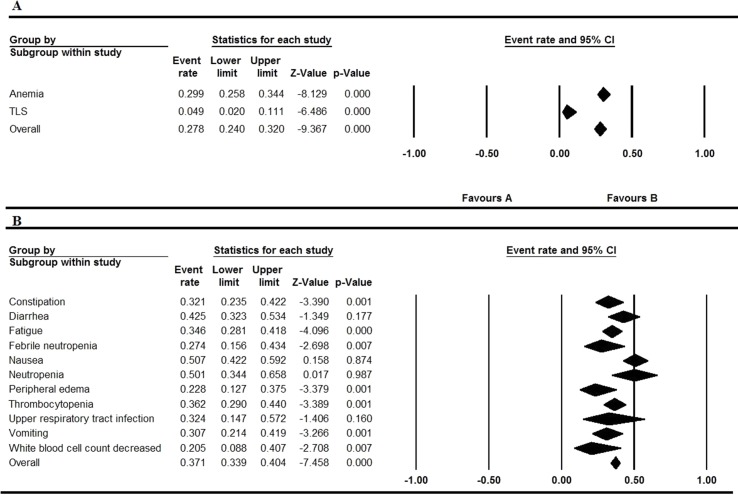
All the involved articles were single arms and reported AEs, so all the articles were used to calculate the AEs rate. The results were presented in Figures 2 to 5 . Figure 2 shows the result of all grade AEs of venetoclax monotherapy (fixed model A, random model B). Figure 3 shows the results of the grade 3 or 4 AEs of venetoclax monotherapy (fixed model A, random model B). Figure 4 shows the results of all grade AEs of venetoclax combination with other therapies (fixed model A, random model B). Figure 5 shows the results of the grade 3 or 4 AEs of venetoclax combination with other therapies (fixed model A, random model B). Table 2 shows the top five most frequently occurred AEs in venetoclax monotherapy.
Figure 2.
Result of all grade adverse events of venetoclax monotherapy. (A) Shows the fixed model and (B) shows the random model.
Figure 5.
Result of the grade 3 or 4 adverse events of venetoclax plus other drugs. (A) Shows the fixed model and (B) shows the random model.
Figure 3.
Result of the grade 3 or 4 adverse events of venetoclax monotherapy. (A) Shows the fixed model and (B) shows the random model.
Figure 4.
Result of all grade adverse eventsof venetoclax plus other drugs. (A) Shows the fixed model and (B) shows the random model.
Table 2.
The top five most common AEs of all the grades and Grade ≥ 3.
| AEs | Any grade | AEs | Grade ≥ 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Event rate (95% CI) | Z value | P value | Statistical method | Event rate (95% CI) | Z value | P value | Statistical method | ||
| Nausea | 0.488 (0.380–0.597) | –0.209 | 0.835 | Random | Neutropenia | 0.360 (0.252–0.485) | –2.187 | 0.029 | Random |
| Diarrhea | 0.467 (0.392–0.543) | –0.862 | 0.389 | Random | Thrombocytopenia | 0.194 (0.101–0.340) | –3.675 | 0.000 | Random |
| Neutropenia | 0.427 (0.306–0.558) | –1.092 | 0.275 | Random | Anemia | 0.184 (0.139–0.238) | –8.866 | 0.000 | Random |
| Fatigue | 0.358 (0.295–0.427) | –3.975 | 0.000 | Random | Febrile neutropenia | 0.133 (0.047–0.325) | –3.216 | 0.001 | Random |
| Thrombocytopenia | 0.329 (0.209–0.477) | –2.244 | 0.025 | Random | Leukopenia | 0.106 (0.022–0.387) | –2.502 | 0.012 | Random |
Venetoclax Monotherapy
After evaluating all the AEs of venetoclax monotherapy patients, nausea, diarrhea, neutropenia, fatigue, and thrombocytopenia were found to be the top five most common AEs of all the grades, and neutropenia, thrombocytopenia, anemia, febrile neutropenia, and leukopenia were the most common AEs of grade ≥ 3 (severe AEs; these results are exhibited in Table 2 ). Of all the reported AEs of monotherapy, the occurrence of thrombocytopenia was relatively high, but it was not the most severe type. Five articles reported the incidence of thrombocytopenia ranging from 0.187 to 0.485, the overall event rate was 0.329 (95% CI 0.209, 0.477), and the grade ≥ 3 (severe AEs) event rate was 0.194 (95% CI 0.101–0.340). Nausea was the most common AE with the highest occurrence rate of 0.488 (95% CI 0.380–0.597), whereas the rate of neutropenia was 0.360 (95% CI 0.252–0.480), which is the most severe AE. Diarrhea and neutropenia were also the frequently reported AEs. With regard to single-arm trial monotherapy, the overall event rate of fatigue was 0.291 (95% CI 0.199–0.406). Despite the rate of neutropenia that reached to 0.360 (95% CI 0.252–0.485), other severe AEs (grade ≥ 3) were relatively rare.
Venetoclax Combination With Other Therapies
Nausea, diarrhea, neutropenia, and thrombocytopenia were still the most common AE occurrence in the patients of all the grades through the analysis. Despite the high rates of neutropenia in 0.480 (95% CI 0.423–0.537), other grade ≥ 3 AEs (severe AEs) were relatively rare. Thrombocytopenia and diarrhea also maintain the overall event rate over 30% of all grades. Upper respiratory tract infection of all grades has the overall event rate of 0.324 (95% CI 0.147–0.572). The overall rate of grade ≥ 3 AEs (severe AEs) is lower than 10% except thrombocytopenia and anemia.
Efficacy
Venetoclax Monotherapy
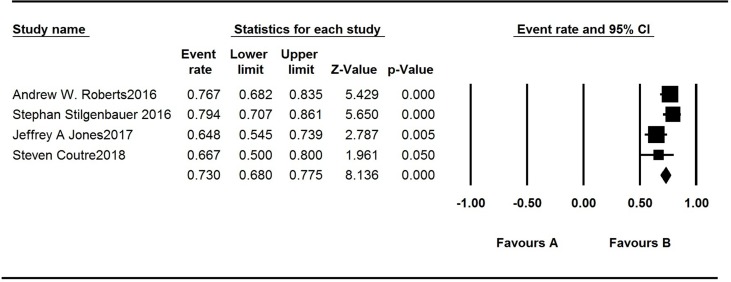
There are seven articles concerning venetoclax monotherapy for treating CLL, MM, acute myelogenous leukemia, and NHL. The patients’ monotherapy survival rates recorded in the articles are listed in Table 3 . PFS is approximately 25 months in studies by Roberts AW and Jones JA. The median first response time of CLL patients in four studies varied greatly. Two studies were 2.5 months, one was 6 weeks, and the other one is 0.8 month. The ORR of the AML patients was 19%, while that among t (11:14) MM patients was 40%. In patients who had been diagnosed as NHL, the ORR was 44%. The highest ORR was reported in patients with mantle cell lymphoma (MCL), which is 75%. The ORR of definite R/R CLL patients ranged from 64.8% to 79.4%, and the overall event rate was 73% ( Figure 6 ), which is quite satisfactory.
Table 3.
The monotherapy survival rates recorded in articles on patients.
| Study | Year | Media first response | Media PFS | Media follow-up | ORR |
|---|---|---|---|---|---|
| Roberts AW | 2016 | 6 weeks (5–24) | 25 months (17–30) | 17 months (1–44) | 79% |
| Coutre S | 2018 | 2.5 months (1.6–8.1) | NM | 14 months (1–29) | 67% |
| Jones JA | 2018 | 2.5 months (1.6–2.6) | 24.7 months (19.2–NT) | 14 months (8–18) | 65% |
| Stilgenbauer S | 2016 | 0.8 months (0.7–1.7) | 6.3 months (4.8–9.8) | 12.1 months (10.1–14.2) | 79.4% |
| Kumar S | 2017 | 9.7 months (7.0–NT) | 2.6 months (1.9–4.7) | 2.5 months (0.2–25) | 21% |
| Konopleva M | 2016 | 144.5 days (83–256) | 2.5 months (1–3) | 63.5 days (14–256) | 19% |
| Davids MS | 2017 | 42 days (30–366) | 6 months (4–10) | 5.3 months (0.2–46.0) | 44% |
NT, not reached; PFS, progression-free survival; NM, not mentioned; ORR, overall response rate.
Figure 6.
Analysis of the overall response rate of the studies.
Venetoclax Combination With Other Therapies
Survival data of the venetoclax plus other drugs are listed in Table 4 (the OS and PFS are listed in Table S1). The response rate of R/R CLL patients treated by venetoclax plus rituximab was 82%. The ORR of the untreated AML patients was 64%, especially in the group of venetoclax plus decitabine and posaconazole in which the ORR was 67%, while that in the group venetoclax plus azacytidine was 59%. The ORR of the R/R MM patients was 67%. When CLL patients treated with venetoclax plus obinutuzumab, the ORR was high to 95%, and even all the untreated patients were in complete remission. According to Arnon P. Kater’s study, both PFS and OS of the venetoclax–rituximab arm were superior to the bendamustine–rituximab arm. Three year PFS of patients treated with venetoclax combined rituximab was 71.4%.
Table 4.
Survival data of the venetoclax plus other drugs.
| Study | Treatment | Disease | ORR |
|---|---|---|---|
| Philippe Moreau | Venetoclax plus bortezomib and dexamethasone | Relapsed or refractory multiple myeloma | 67% |
| Prof John F Seymour | Venetoclax plus rituximab | Relapsed or refractory chronic lymphocytic leukemia | 82%* |
| Courtney D DiNardo A | Venetoclax plus decitabine | Untreated acute myeloid leukemia | 65% |
| Courtney D DiNardo B | Venetoclax plus azacitidine | Untreated acute myeloid leukemia | 59% |
| Courtney D DiNardo C | Venetoclax plus decitabine and posaconazole | Untreated acute myeloid leukemia | 67% |
| Courtney D DiNardo | Untreated acute myeloid leukemia | 64% | |
| Ibrahim Aldoss | Venetoclax plus decitabine or 5-azacitidine | Relapsed or refractory acute myeloid leukemia | 64% |
| Paula Cramer | Venetoclax plus obinutuzumab | Chronic lymphocytic leukemia | 95% |
| Andrew H.Wei | Venetoclax plus cytarabine | Untreated acute myeloid leukemia | 54% (CR) |
| Kerry A. Rogers | Venetoclax plus obinutuzumab and ibrutinib | Relapsed or refractory chronic lymphocytic leukemia | 92% |
| S. de Vos | Venetoclax plus bendamustine and rituximab | Relapsed or refractory non-Hodgkin lymphoma | 65% |
| Andrew D. Zelenetz | Ventoclax plus R/G-CHOP | Non-Hodgkin lymphoma | 87.5% |
| Ian W. Flinn | Venetoclax plus obinutuzumab | Chronic lymphocytic leukemia | 95% (R/R), 100 (1L) |
| Arnon P. Kater | Venetoclax plus rituximab | Relapsed or refractory chronic lymphocytic leukemia | NM |
ORR, overall response rate; NM, not mentioned; CR, complete remission. *This article provided the rate of response.
Assessment of Study Quality and Publication Bias
Review Manager 5.3 was used to assess the methodological quality of all the involved studies. Figures S1 and S2 show the risk of bias graph and risk of bias summary of the researches. The non-randomized studies were assessed by the methodological index for non-randomized studies score; most of the studies get a satisfactory score ( Table 1 ), except a retrospect study that gets 10.
Discussion
Venetoclax is a highly selective oral BCL2 inhibitor with high sensitivity in patients with CLL/SLL, including patients who were treated with first-line and second-line chemotherapy or having 17p deletion (Levy and Claxton, 2017). As the first BCL-2 inhibitor approved by the US Food and Drug Administration, BCL-2 inhibitor was effective in CLL and recommended for R/R CLL after being treated by ibrutinib according to National Comprehensive Cancer Network. It was also regarded as the second-line medicine in MCL patients. Clinical trials of venetoclax in ER- and BCL2-positive metastatic breast cancer were reported (Lok et al., 2019), and BCL-2 is a potential target in treating nasopharyngeal carcinoma (Wang et al., 2018) and small cell lung cancer (Inoue-Yamauchi et al., 2017; Lam et al., 2017; Lochmann et al., 2018). This study investigated the efficacy and safety of venetoclax in hematological malignancies.
With regard to the safety, we analyzed the AEs of patients taking venetoclax orally. The most common AEs were diarrhea, nausea, neutropenia, fatigue, thrombocytopenia, anemia, cough, pyrexia, and abdominal pain. Nausea occurred in almost half of the patients, while these patients rarely experienced severe nausea. Severe AEs are relatively rare except for neutropenia among monotherapy patients. Thrombocytopenia and anemia were the most severe AEs among the venetoclax plus other therapies. Notably, hematologic toxicities were the most frequent severe AE. The ORR of the monotherapy patients was surprisingly encouraging, with three quarters of the patients benefiting.
TLS is an important toxic effect in the first-in-human phase 1 study in patients who had been diagnosed as R/R CLL or SLL (Roberts et al., 2016). The risk of TLS has relevance with the rapid CLL cells reduction, which can be alleviated by gradual dose escalation therapy (Howard et al., 2011; Souers et al., 2013; King et al., 2017). The safety evaluation of some other clinical trials suggested initiating with 20-mg venetoclax for 1 week followed by no additional intensive prophylactic measures (Roberts et al., 2016). Comparatively, TLS rarely happened to CLL patients in this therapeutic scheme even in venetoclax combined with other drugs (Coutre et al., 2018; Jones et al., 2018). Except for CLL patients, TLS events were not observed in MM, NHL, or AML patients (Konopleva et al., 2016; Davids et al., 2017; Kumar et al., 2017; Parikh et al., 2018). Diarrhea and nausea are the most common AEs of chemotherapy (Gralla et al., 1981) and so as venetoclax, although they are generally mild and few that appear as severe AEs. Except for anemia, the occurrence rates of grade 3 or 4 febrile neutropenia and thrombocytopenia are under 0.1. The occurrence rate of anemia in R/R CLL patients was significantly higher than untreated when venetoclax combined with obinutuzumab (Cramer et al., 2018; Flinn et al., 2019), and the reason needs further exploration.
About 40% patients experienced neutropenia after taking venetoclax, and most of them went through grade 3 or 4 AE. Fortunately, none of these patients quit the treatment because of severe neutropenia. Most patients with neutropenia received growth factor or reduced the venetoclax dose (Wierda et al., 2005; Robak et al., 2010). It is also suggested that patients receive intermittent treatment with neutrophil growth factor since this method enabled continuous venetoclax treatment in majority of the patients with grade 4 neutropenia. Neutropenia and TLS were reported in only one study, while TLS is not severe and it could be relieved by growth factor (Seymour et al., 2017). Some researches reported that the occurrence of neutropenia in venetoclax monotherapy was consistent with previous chemotherapy or immunotherapies in patients with R/R CLL (Hallek et al., 2010). The rate of neutropenia in NHL patients was less than that in CLL patients, while severe AEs were more common (Davids et al., 2017). The condition of MM patients was similar to that of NHL patients (Kumar et al., 2017). In addition, neutropenia is rare in R/R AML patients (Konopleva et al., 2016).
In our research, the ORR of venetoclax monotherapy can reach 73%, while the combination of lenalidomide and rituximab used in R/R CLL patients was only 66%, and the combination of bendamustine and rituximab remained less effective. There are two involved articles that mentioned deletion of chromosome 17p (del[17p]) in patients with CLL. The past articles reported that these del[17p] patients had quite a bad prognosis and ORR. When patients received ibrutinib and idelalisib treatment, del[17p] was a high risk factor, leading patients discontinuation of treatment (Maddocks et al., 2015). The first trial of venetoclax showed that the response rate of the R/R CLL patients with deletion 17p was 71%; about 16% achieved a complete response (Roberts et al., 2016). In another study, all the involved patients were R/R CLL patients with deletion [17p]; the ORR reached 79.4% (Stilgenbauer et al., 2016). Venetoclax is worthy to be recommended to the CLL patients with deletion [17p]. Patients with MCL have the highest ORR (75%) of all the NHL patients, which is similar to the Bruton tyrosine kinase inhibitor ibrutinib (Wang et al., 2013). The ORRs of other NHL patients were more inferior compared with the MCL monotherapy patients. When venetoclax was combined with other therapies, the response rate of diffuse large B-cell lymphoma and follicular lymphoma was promising compared with historical treatment (Marcus et al., 2017; Vitolo et al., 2017). While the response rate of different subtypes was quite different, the response rate of venetoclax was also influenced by various combining methods. Survival rates of elderly patients with R/R AML were quite poor (Pinto et al., 2001). The ORRs of the R/R AML in elderly patients differed tremendously among the reported studies. In our studies, the ORR was 19% with oral monotherapy. It was reported that over 20% remission rates were rarely acquired without other intense cytotoxic chemotherapy and inpatient care (Breems et al., 2005; Thol et al., 2015). When venetoclax plus decitabine or 5-azacitidine was used in R/R AML patients, the ORR was over 60% (Aldoss et al., 2018). Venetoclax combined with other drugs was also used in untreated AML. To elderly patients, venetoclax plus low dose cytarabine gets more favorable results than decitabine and azacitidine monotherapy treatment relatively (Kantarjian et al., 2012; Dombret et al., 2015; Wei et al., 2019). The ORR of MM patients treated with venetoclax plus bortezomib and dexamethasone was satisfactory with tolerable AEs (Moreau et al., 2017). When venetoclax combined with obinutuzumab, the patients had a quite satisfactory ORR; nearly all the patients benefit from this method.
Compared with venetoclax monotherapy, venetoclax combined with other therapies did not obviously increase the rate of thrombocytopenia, neutropenia, and anemia, while the rates of peripheral edema and upper respiratory tract infection increased among patients applying two or more therapies. The ratio of grades 3 to 4 thrombocytopenia slightly increased in the patients using venetoclax plus decitabine. Thrombocytopenia had been reported as the most frequent severe AEs of decitabine (He et al., 2017). In conclusion, the combination with other therapies was also well tolerated. There was no significance between the monotherapy and combination groups.
In our study, we admit that several limitations are inevitable. The dose of the drug was different between individual trials, and some AEs were dose dependent. Some of the studies chose dose-escalation method to evaluate the characters of the drug. In addition, all the involved studies were single-armed studies without double-blinded randomized controlled trials. Lastly, studies rarely use the same treatment method; it is hard to say which one is better for the patients. More researches were expected. Although the patients are all in advanced stages, the degree of malignancy was dispersive, which might have aroused bias to the final analysis.
Conclusion
In conclusion, venetoclax is a mild and efficient drug in treating advanced hematological malignancy specially fit for the CLL patients with del[17p]. Neutropenia is relatively severe among the patients, and it can be alleviated by reducing dose or some other ways. Few TLS occurred after taking the drugs. The AEs aroused by venetoclax and combination are well tolerable. The use of venetoclax monotherapy or its combination with other therapies is desirable in hematological malignancy.
Contribution to the Field Statement
Venetoclax has shown effectiveness in the treatment of patients with hematological malignancy. However, toxicities and clinical benefits of venetoclax in cancer patients are not well defined. To systematically review the safety and efficacy of venetoclax in the treatment of patients, we retrieved all the relevant clinical trials on the adverse events (AEs) and survival outcomes of venetoclax through PubMed. Ten eligible studies involving a total of 726 patients met our meta-analysis criteria. After analysis of the data, we found that venetoclax is a mild and efficient drug in treating advanced hematological malignancy that is specially fit for the CLL patients with del[17p], and AEs are well tolerable. Few tumor lysis syndrome (TLS) occurred after taking the drugs. The AEs aroused by venetoclax and combination are well tolerable. Therefore, the use of venetoclax monotherapy or its combination with other therapies is desirable in hematological malignancy.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
QL and XM mainly generated the idea. QL and LC collected the data and wrote the manuscript. HJ and HL helped correct the grammar and carried out some statistical work. KS and QL mainly did some statistical work. YC helped in collecting the data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00697/full#supplementary-material
References
- Aldoss I., Yang D., Aribi A., Ali H., Sandhu K., Al Malki M. M., et al. (2018). Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 103 (9), e404–404e407. 10.3324/haematol.2018.188094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C. (2003). The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol. Immunol. 39 (11), 615–647. 10.1016/S0161-5890(02)00252-3 [DOI] [PubMed] [Google Scholar]
- Breems D. A., Van Putten W. L., Huijgens P. C., Ossenkoppele G.J., Verhoef G. E., Verdonck L. F., et al. (2005). Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 23 (9), 1969–1978. 10.1200/JCO.2005.06.027 [DOI] [PubMed] [Google Scholar]
- Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., et al. (2006). Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 9 (5), 351–365. 10.1016/j.ccr.2006.03.027 [DOI] [PubMed] [Google Scholar]
- Cimmino A., Calin G. A., Fabbri M., Iorio M. V., Ferracin M., Shimizu M., et al. (2005). miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. U. S. A. 102 (39), 13944–13949. 10.1073/pnas.0506654102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutre S., Choi M., Furman R. R., Eradat H., Heffner L., Jones J. A., et al. (2018). Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood 131 (15), 1704–1711. 10.1182/blood-2017-06-788133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P., von Tresckow J., Bahlo J., Robrecht S., Langerbeins P., Al-Sawaf O., et al. (2018). Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 19 (9), 1215–1228. 10.1016/S1470-2045(18)30414-5 [DOI] [PubMed] [Google Scholar]
- Davids M. S., Roberts A. W., Seymour J. F., Pagel J. M., Kahl B. S., Wierda W. G., et al. (2017). Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 35 (8), 826–833. 10.1200/JCO.2016.70.4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos S., Swinnen L. J., Wang D., Reid E., Fowler N., Cordero J., et al. (2018). Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann. Oncol. 29 (9), 1932–1938. 10.1093/annonc/mdy256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo C. D., Pratz K. W., Letai A., Jonas B. A., Wei A. H., Thirman M., et al. (2018). Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 19 (2), 216–228. 10.1016/S1470-2045(18)30010-X [DOI] [PubMed] [Google Scholar]
- Dombret H., Seymour J. F., Butrym A., Wierzbowska A., Selleslag D., Jang J. H., et al. (2015). International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126 (3), 291–299. 10.1182/blood-2015-01-621664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn I. W., Gribben J. G., Dyer M. J. S., Wierda W., Maris M. B., Furman R. R., et al. (2019). Phase 1b study of venetoclax–obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood. 10.1182/blood-2019-01-896290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla R. J., Itri L. M., Pisko S. E., Squillante A. E., Kelsen D. P., Braun D. W., et al. (1981). Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 305 (16), 905–909. 10.1056/NEJM198110153051601 [DOI] [PubMed] [Google Scholar]
- Green D. R., Walczak H. (2013). Apoptosis therapy: driving cancers down the road to ruin. Nat. Med. 19 (2), 131–133. 10.1038/nm.3076 [DOI] [PubMed] [Google Scholar]
- Hallek M., Fischer K., Fingerle-Rowson G., Fink A. M., Busch R., Mayer J., et al. (2010). Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet, 2010 376 (9747), 1164–1174. 10.1016/S0140-6736(10)61381-5 [DOI] [PubMed] [Google Scholar]
- He P. F., Zhou J. D., Yao D. M., Ma J. C., Wen X. M., Zhang Z. H., et al. (2017). Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Oncotarget 8 (25), 41498–41507. 10.18632/oncotarget.17241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. C., Jones D. P., Pui C. H. (2011). The tumor lysis syndrome. N. Engl. J. Med. 364 (19), 1844–1854. 10.1056/NEJMra0904569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Yamauchi A., Jeng P. S., Kim K., Chen H. C., Han S., Ganesan Y. T., et al. (2017). Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat. Commun. 8, 16078. 10.1038/ncomms16078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. A., Mato A. R., Wierda W. G., Davids M. S., Choi M., Cheson B. D., et al. (2018). Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 19 (1), 65–75. 10.1016/S1470-2045(17)30909-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H. M., Thomas X. G., Dmoszynska A., Wierzbowska A., Mazur G., Mayer J., et al. (2012). Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 30 (21), 2670–2677. 10.1200/JCO.2011.38.9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater A. P., Seymour J. F., Hillmen P., Eichhorst B., Langerak A. W., Owen C., et al. (2019). Fixed duration of venetoclax–rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J. Clin. Oncol. 37 (4), 269–277. 10.1200/JCO.18.01580 [DOI] [PubMed] [Google Scholar]
- King A. C., Peterson T. J., Horvat T. Z., Rodriguez M., Tang L. A. (2017). Venetoclax: a first-in-class oral BCL-2 inhibitor for the management of lymphoid malignancies. Ann. Pharmacother. 51 (5), 410–416. 10.1177/1060028016685803 [DOI] [PubMed] [Google Scholar]
- Konopleva M., Pollyea D. A., Potluri J., Chyla B., Hogdal L., Busman T., et al. (2016). Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 6 (10), 1106–1117. 10.1158/2159-8290.CD-16-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kaufman J. L., Gasparetto C., Mikhael J., Vij R., Pegourie B., et al. (2017). Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130 (22), 2401–2409. 10.1182/blood-2017-06-788786 [DOI] [PubMed] [Google Scholar]
- Lam L. T., Lin X., Faivre E. J., Yang Z., Huang X., Wilcox D. M., et al. (2017). Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitors. Mol. Cancer. Ther. 16 (8), 1511–1520. 10.1158/1535-7163.MCT-16-0459 [DOI] [PubMed] [Google Scholar]
- Levy M. A., Claxton D. F. (2017). Therapeutic inhibition of BCL-2 and related family members. Expert. Opin. Investig. Drugs. 26 (3), 293–301. 10.1080/13543784.2017.1290078 [DOI] [PubMed] [Google Scholar]
- Lochmann T. L., Floros K. V., Naseri M., Powell K. M., Cook W., March R. J., et al. (2018). Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin. Cancer Res. 24 (2), 360–369. 10.1158/1078-0432.CCR-17-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S. W., Whittle J. R., Vaillant F., Teh C. E., Lo L. L., Policheni A. N., et al. (2019). A phase Ib dose-escalation and expansion study of the BCL2 Inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 9 (3), 354–369. 10.1158/2159-8290.CD-18-1151 [DOI] [PubMed] [Google Scholar]
- Maddocks K. J., Ruppert A. S., Lozanski G., Heerema N. A., Zhao W., Abruzzo L., et al. (2015). Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 1 (1), 80–7. 10.1001/jamaoncol.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R., Davies A., Ando K., Klapper W., Opat S., Owen C., et al. (2017). Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 377 (14), 1331–1344. 10.1056/NEJMoa1614598 [DOI] [PubMed] [Google Scholar]
- Marschitz I., Tinhofer I., Hittmair A., Egle A., Kos M., Greil R. (2000). Analysis of Bcl-2 protein expression in chronic lymphocytic leukemia. Am. J. Clin. Pathol. 113 (2), 219–229. 10.1309/491W-L1TN-UFQX-T61B [DOI] [PubMed] [Google Scholar]
- Mason K. D., Carpinelli M. R., Fletcher J. I., Collinge J. E., Hilton A. A., Ellis S., et al. (2007). Programmed anuclear cell death delimits platelet life span. Cell 128 (6), 1173–1186. 10.1016/j.cell.2007.01.037 [DOI] [PubMed] [Google Scholar]
- Moreau P., Chanan-Khan A., Roberts A. W., Agarwal A. B., Facon T., Kumar S., et al. (2017). Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 130 (22), 2392–2400. 10.1182/blood-2017-06-788323 [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., et al. (2005). An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435 (7042), 677–681. 10.1038/nature03579 [DOI] [PubMed] [Google Scholar]
- Parikh A., Gopalakrishnan S., Freise K. J., Verdugo M. E., Menon R. M., Mensing S., et al. (2018). Exposure-response evaluations of venetoclax efficacy and safety in patients with non-Hodgkin lymphoma. Leuk. Lymphoma 59 (4), 871–879. 10.1080/10428194.2017.1361024 [DOI] [PubMed] [Google Scholar]
- Pinto A., Zagonel V., Ferrara F. (2001). Acute myeloid leukemia in the elderly: biology and therapeutic strategies. Crit. Rev. Oncol. Hematol. 39 (3), 275–287. 10.1016/S1040-8428(00)00122-0 [DOI] [PubMed] [Google Scholar]
- Robak T., Dmoszynska A., Solal-Céligny P., Warzocha K., Loscertales J., Catalano J., et al. (2010). Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 28 (10), 1756–1765. 10.1200/JCO.2009.26.4556 [DOI] [PubMed] [Google Scholar]
- Roberts A. W., Seymour J. F., Brown J. R., Wierda W. G., Kipps T. J., Khaw S. L., et al. (2012). Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 30 (5), 488–496. 10.1200/JCO.2011.34.7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. W., Davids M. S., Pagel J. M., Kahl B. S., Puvvada S. D., Gerecitano J. F., et al. (2016). Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374 (4), 311–322. 10.1056/NEJMoa1513257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L. E., Plunkett W., McConnell K., Keating M. J., McDonnell T. J. (1996). Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia 10 (3), 456–459. 10.3109/10428199609067578 [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Huang Y., Ruppert A. S., Awan F. T., Heerema N. A., Hoffman C., et al. (2018). Phase 1b study of obinutuzumab, ibrutinib, and venetoclax in relapsed and refractory chronic lymphocytic leukemia. Blood 132 (15), 1568–1572. 10.1182/blood-2018-05-853564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin C. M., Hann C. L., Garon E. B., Ribeiro de Oliveira M., Bonomi P. D., Camidge D. R., et al. (2012). Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 18 (11), 3163–3169. 10.1158/1078-0432.CCR-11-3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour J. F., Ma S., Brander D. M., Choi M. Y., Barrientos J., Davids M. S., et al. (2017). Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 18 (2), 230–240. 10.1016/S1470-2045(17)30012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73 (9), 712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- Souers A. J., Leverson J. D., Boghaert E. R., Ackler S. L., Catron N. D., Chen J., et al. (2013). ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19 (2), 202–208. 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- Stilgenbauer S., Eichhorst B., Schetelig J., Coutre S., Seymour J. F., Munir T., et al. (2016). Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 17 (6), 768–778. 10.1016/S1470-2045(16)30019-5 [DOI] [PubMed] [Google Scholar]
- Thol F., Schlenk R. F., Heuser M., Ganser A. (2015). How I treat refractory and early relapsed acute myeloid leukemia. Blood 126 (3), 319–327. 10.1182/blood-2014-10-551911 [DOI] [PubMed] [Google Scholar]
- Touzeau C., Dousset C., Le Gouill S., Sampath D., Leverson J. D., Souers A. J., et al. (2014). The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 28 (1), 210–212. 10.1038/leu.2013.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft M. F., Wei A. H., Mason K. D., Vandenberg C. J., Chen L., Czabotar P. E., et al. (2006). The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 10 (5), 389–399. 10.1016/j.ccr.2006.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo U., Trněný M., Belada D., Burke J. M., Carella A. M., Chua N., et al. (2017). Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J. Clin. Oncol. 35 (31), 3529–3537. 10.1200/JCO.2017.73.3402 [DOI] [PubMed] [Google Scholar]
- Wang M. L., Rule S., Martin P., Goy A., Auer R., Kahl B. S., et al. (2013). Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369 (6), 507–516. 10.1056/NEJMoa1306220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Fan X., Song J., Wu H., Han J., et al. (2018). ABT-199-mediated inhibition of Bcl-2 as a potential therapeutic strategy for nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 503 (3), 1214–1220. 10.1016/j.bbrc.2018.07.027 [DOI] [PubMed] [Google Scholar]
- Wei A. H., Strickland S. A., Hou J. Z., Fiedler W., Lin T. L., Walter R. B., et al. (2019). Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J. Clin. Oncol. 37 (15), 1277–1284. 10.1200/JCO.18.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda W., O’Brien S., Wen S., Faderl S., Garcia-Manero G., Thomas D., et al. (2005). Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J. Clin. Oncol. 23 (18), 4070–4078. 10.1200/JCO.2005.12.516 [DOI] [PubMed] [Google Scholar]
- Willis S., Day C. L., Hinds M. G., Huang D. C. (2003). The Bcl-2-regulated apoptotic pathway. J. Cell Sci. 116 (Pt 20), 4053–4056. 10.1242/jcs.00754 [DOI] [PubMed] [Google Scholar]
- Zelenetz A. D., Salles G., Mason K. D., Casulo C., Le Gouill S., Sehn L. H., et al. (2019). Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood 133 (18), 1964–1976. 10.1182/blood-2018-11-880526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.