Abstract
Objective
During cardiac arrest (CA) resuscitation, an ‘ischaemia-reperfusion’ syndrome occurs leading to multiorgan failure reflected by an increase in blood lactate. Blood lactate is a diagnosis and prognosis biomarker in extracorporeal life support (ECLS), but its kinetic appears more informative to assess a patient’s outcome. The aim of the present study was to describe the prognostic value of blood lactate and lactate clearance (LC) 3 (H3) and 6 h (H6) after the initiation of ECLS in the treatment of refractory CA.
Methods
Patients admitted to the intensive care unit for refractory CA were included. Lactate measurements were performed at the initiation of ECLS (H0) and at H3 and H6 upon the initiation of ECLS. LC was measured from 0 to 3 h (LC03), 0 to 6 h (LC06) and 3 to 6 h (LC36). The primary endpoint was in-hospital mortality within 28 days.
Results
Sixty-six patients were enrolled in the study. Lactate levels were higher in deceased patients. Increased mortality was observed with increasing levels of lactate at H3 and H6 and with decreasing LC03. Using logistic regression, an association was observed between mortality and lactate at H3 with an odds ratio (OR) of 1.21 (95% confidence interval (CI) 1.05–1.42); LC03, OR of 0.93 (95% CI 0.87–0.99) and LC06, OR of 0.96 (95% CI 0.92–0.99).
Conclusion
Blood lactate and LC within the first 3 h of ECLS in refractory CA are associated with mortality. LC is a more relevant parameter than blood lactate, taking into account both the production and elimination of lactate. We suggest to preferentially use LC to assess the patient’s outcome.
Keywords: Cardiac arrest, ECLS, intensive care, lactate, resuscitation
Introduction
Cardiac arrest (CA) induces 300,000 deaths per year in the USA (1). Despite recent progress in basic and advanced life support (BLS and ALS), the survival rate remains low, reaching 8% to 10% at hospital discharge (2). Refractory CA is defined as the failure of ALS (3), considered in some countries as the failure of return of spontaneous circulation after 30 min of cardiopulmonary resuscitation (3, 4). In the last decade, significant progress in therapeutics has been observed in refractory CA (5–7). One of the most notable progress is the use of extracorporeal life support (ECLS), which allowed significant improvement in the survival rate of patients with refractory CA (5–7).
During BLS and ALS, an ‘ischaemia-reperfusion’ syndrome occurs leading to a flow supply-demand mismatch. Consequently, vasoconstriction of peripheral organs results in multiorgan failure (8, 9). An increase in blood lactate, responsible for lactic acidosis (10), reflects reduced perfusion, hypoxia and thus anaerobic metabolism, reflecting demand mismatch and vasoconstriction.
Blood lactate measurement was suggested to reflect the severity of multiorgan failure, but can be influenced by drugs or modification in the hepatic metabolism (11). The benefit of blood lactate was also reported as a diagnosis and prognosis biomarker in sepsis, trauma and in the context of ECLS (12–16). Even though a single measurement of lactate is often used, the kinetic of blood lactate appears more informative to assess a patient’s outcome, reflecting both the production and elimination of lactate. In the perspective to measure decreasing blood lactate concentrations, the elimination of produced blood lactate, lactate clearance (LC), gained interest, reflecting more the adequacy between increased production and/or decreased washout.
Low LC was shown to be associated with increased mortality in various situations, i.e. septic shock, trauma and after cardiac surgery (13, 17–19). Conversely, a high LC, or in other words, the trend towards the re-establishment of the normal blood lactate, decreased mortality (13). Whether blood lactate or LC is a useful tool to evaluate the benefit of ECLS in refractory CA has not yet been clearly assessed.
The aim of the present study was to describe the prognostic value of blood lactate and LC measured at the initiation of ECLS (H0) and at 3 (H3) and 6 h (H6) from the initiation of ECLS in patients with refractory CA treated by ECLS and admitted to the ICU.
Methods
Study population
This observational cohort study was conducted from January 2011 to January 2016 in the intensive care unit (ICU) of Necker Hospital, a French academic hospital. All consecutive patients admitted to the ICU with refractory CA treated by ECLS were included in the study. All causes of out-of-hospital refractory CA were also included. ECLS was initiated in either the pre-hospital setting or directly at ICU admission upon 30 min of ALS.
Patient’s characteristics were collected at ICU admission and included age, weight and size. No-flow, low-flow and length of stay in the ICU were retrieved for all patients. No-flow duration was defined as the time-lapse from collapse to the initiation of CPR, and low-flow duration as the time duration from the initiation of CPR to the cessation of resuscitation measures.
In keeping with the French legislation, our local ethics committee (Comité pour la Protection des Personnes Est 3, Nancy, France) considered that consent of patients was waived for participation in this observational study (no. 17.12.05).
Blood lactate measurements and LC computation
Blood samples were extracted from the artery directly at the initiation of ECLS (H0) and then at 3 (H3) and 6 h (H6) after the initiation of ECLS. Blood samples were immediately analysed by an arterial blood gas analyser (ABL800 FLEX Radiometer©, Denmark) according to the manufacturer’s instructions, including daily calibration and quality control checks.
Blood lactate measurements were performed at the initiation of ECLS (H0) and at 3 (H3) and 6 h (H6) upon the initiation of ECLS. Normal blood lactate was defined as ≤2.2 mmol L−1. Stratification of lactate was performed as follows: ≤2.2, 2.3 to 5.0, 5.1 to 9.9 and ≥10 mmol L−1. These strata were associated with different mortality levels in patients treated by ECLS for refractory CA (20).
Lactate clearance was calculated for the following time periods: 0 to 3 h (LC03), 0 to 6 h (LC06) and 3 to 6 h (LC36) from the initiation of ECLS. It was computed by the following equation: Lactate clearance=([Lactate initial−Lactate delayed]/Lactate initial)*100*Delay−1 (expressed as %/h). The stratification of LC was defined as follows: −20 or lower, −19 to −11, −10 to 0, 1–9, 10–19 and 20% or higher per hour.
Therapeutic management of patients
As previously described, all patients received medical care by the same team of critical care physicians (16). Protocols for the management of critically ill patients did not change over the period study, ensuring no major discrepancies between patients in terms of organ supports and therapies.
Haemodynamic support was achieved by ECLS (Cardiohelp System®, Maquet, Germany), set up via venous-arterial femoral cannulation by two experienced ICU physicians. ECLS was inserted either in the pre-hospital setting or at ICU admission. ECLS flow was adjusted to obtain a mean blood pressure of 50–60 mmHg.
Sedation was started as soon as possible. All patients were sedated using midazolam 0.1 mg kg−1 h−1 and sufentanil 0.2 μg−1 kg−1 h−1 and paralyzed by atracurium 0.1 mg kg−1 h−1 (dose adjusted to obtain a neuromuscular response ≤2 at the ‘trend of four’ monitoring). Sedation status was monitored using a bispectral index (BIS monitor®, Covidien, UK). Ventilation was adjusted to obtain a PaCO2 of 40 mmHg and a PaO2 between 100 and 200 mmHg. Minimum lung ventilation was maintained to avoid pulmonary collapse with a tidal volume of 5 mL kg−1, a respiratory rate of 8 min−1 and positive end-expiratory pressure of 5 cm H2O. ScVO2 was continuously monitored to achieve an ScVO2 >70%. If the return of spontaneous circulation occurred, the haemodynamic status was monitored by echocardiography and continuous cardiac output monitoring devices (Vigileo®, Edwards Lifesciences, USA).
Fluid expansion (fluid administration 30 mL kg−1 day−1 of isotonic saline) and catecholamine (dobutamine 5 μg kg−1 min−1 and norepinephrine) were adjusted to obtain a mean blood pressure between 50 and 60 mm Hg and to prevent pulmonary oedema. Blood transfusion was performed to obtain a target of 10 g dL−1 haemoglobin, 100,000 mm−3 platelets, fibrinogen >1.5 g L−1 and a prothrombin rate >50%.
To prevent coagulation of the ECLS membrane oxygenator, unfractionated heparin was intravenously administered at a low dose during ECLS, with repeated controls to maintain the activated clotting time ratio >2.0. Continuous veno-venous hemodiafiltration was initiated during the first 3 h upon ICU admission.
Mild therapeutic hypothermia was performed during the first 12–24 h following ICU admission. Core body temperature was maintained between 32°C and 34°C using external cooling (ice packs placed on the femoral and humeral vessels) and a heat exchanger device of ECLS.
Statistical analysis
The primary endpoint was in-hospital mortality, defined as death occurring within 28 days after ICU admission.
The analysis followed the guidelines for reporting risk marker (21–23). Comparison of two means was performed using the unpaired Student’s t-test. Comparison of two medians was performed using the Mann-Whitney U test, and comparison of proportions was done using the Fisher’s exact method. Correlation between two variables was assessed using linear regression analysis. Multiple logistic regressions were made to assess the impact of blood lactate and LC on mortality at day 28. Initial blood lactate, LC, age, no-flow duration (duration between CA occurrence and the initiation of BLS or ALS) and low-flow duration (duration between CA occurrence and the initiation of ECLS) were included in the model.
Evaluation of the predictive accuracy of lactate and LC on mortality at day 28 was performed by receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) was determined by a 95% confidence interval (CI), and the optimal cut-off point was assessed using Youden’s approach for lactate and LC.
Data are expressed as mean±SD or median (interquartile range, 25–75) for non-Gaussian variables (D’Agostino-Pearson omnibus test).
All analyses were performed using R 3.4.2 (http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria).
Results
Sixty-six patients were enrolled in the study between January 2011 and January 2017. Table 1 shows the main characteristics of the studied population.
Table 1.
Characteristic of the overall population 3 h upon the initiation of ECLS
| Age (years) | 51±14 |
| Weight (kg) | 89±23 |
| Size (cm) | 175±14 |
| No-flow (min) | 1 (1–11) |
| Low-flow (min) | 29 (93–200) |
| Length of stay in the ICU (days) | 6 (1–68) |
Data are expressed as mean±SD or median (interquartile range, 25–75) for non-Gaussian variables.
SD: standard deviation; ICU: intensive care unit
The mean age of the patients was 51±14 (18–79) years. The median no-flow was 1 (1–11) min, whereas the median low-flow reached 93 (29–200) min. The main suspected aetiology of CA was acute coronary syndrome (67%) (Table 2). Owing to haemodynamic instability despite ECLS, cardiac catheterisation was not performed to all patients, and the decision was left to the physician’s discretion. No patient had previous comorbidities that could affect lactate metabolism. The median length of stay in the ICU was 6 (1–68) days (Table 1).
Table 2.
Aetiologies of cardiac arrest
| Aetiology | n (%) |
| Acute coronary syndrome | 44 (67) |
| Drugs intoxication | 5 (8) |
| Hypertrophic cardiomyopathy | 4 (7) |
| Hypothermia | 4 (7) |
| Pulmonary embolism | 1 (2) |
| Undefined | 6 (9) |
Arterial blood lactate was measured for all patients at H0 (n=66), H3 (n=63) and H6 (n=53) from the initiation of ECLS. No patients were lost to follow-up, and all missing data resulted from early death due to multiple organ failure. Among the 66 patients admitted to the ICU for refractory CA, 40 (61%) died within 28 days.
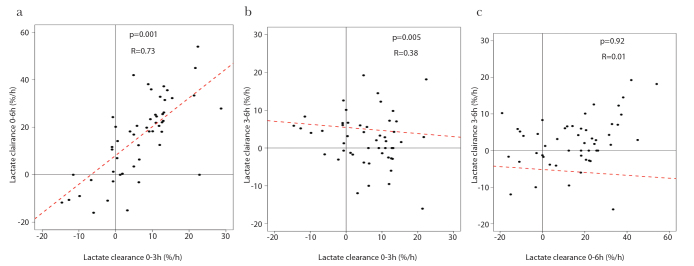
A significant association between LC03 and LC06 was observed, whereas no association was observed between LC03 and LC36 and between LC06 and LC36 (Figure 1).
Figure 1. a–c.
Correlation between sequential measures of LC
LC was calculated between 0 to 3 h (0–3), 0 to 6 h (0–6) and 3 to 6 h (3–6) after the initiation of ECLS. Correlations were evaluated for LC between (a) 0–3, (b) 0–6 and (c) 3–6 and between 0–6 and 3–6 h. LC is expressed as percentage per hour (%/h). LC: lactate clearance.
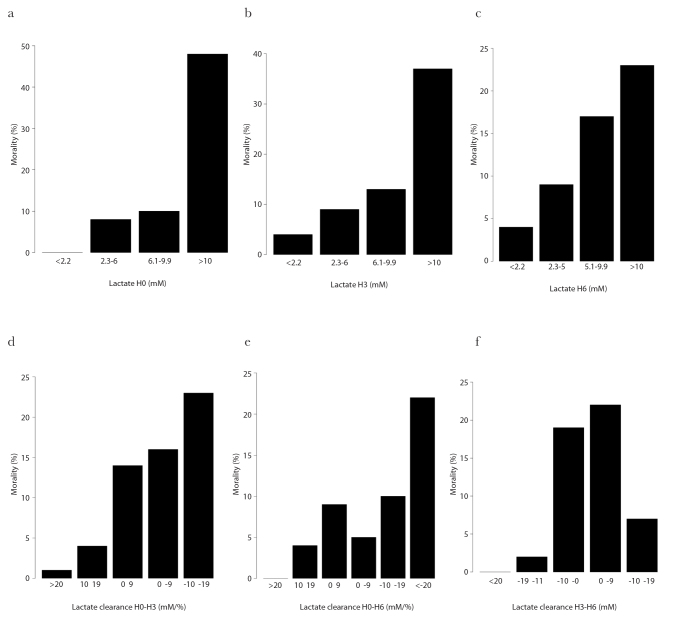
Increased mortality was observed with increasing levels of blood lactate at H0, H3 and H6 from the initiation of ECLS (Figure 2a–c). Mortality increased with decreasing LC within the first 3 h upon the initiation of ECLS (LC03) (Figure 2d). For the other time intervals (3 to 6 and 0 to 6), mortality was fluctuant according to LC with an increase with decreasing LC (Figure 2e and f).
Figure 2. a–f.
Mortality according to blood lactate or LC
Blood lactate was measured at (a) 0 h, (b) 3 h and (c) 6 h after the initiation of ECLS. LC was given for the time intervals between (d) 0 to 3 h (03), (e) 0 to 6 h (06) and (f) 3 to 6 h (36) upon the initiation of ECLS (b). Blood lactate is expressed in micromole (μM). LC is expressed in percentage per hour (%/h) and mortality in percentage (%). LC: lactate clearance.
Blood lactate levels were higher in patients deceased within 28 days at all the measured time points (H0, H3 and H6) in the univariate analysis (Table 3).
Table 3.
Univariate analysis of deceased and alive patients for mortality
| Alive Mean±SD |
Dead Mean±SD |
p | |
|---|---|---|---|
|
| |||
| Age (years) | 49±15 | 52±14 | 0.522 |
| Weight (kg) | 87±26 | 89±22 | 0.734 |
| Size (cm) | 173±20 | 176±9 | 0.364 |
| No-flow (min) | 1 (1–11) | 1 (1–10) | 0.373 |
| Low-flow (min) | 87 (56–139) | 99 (29–200) | 0.21 |
| Length of stay in the ICU (days) | 17 (8–68) | 1 (1–28) | <0.001* |
| Lactate H0 (mmol L−1) | 12.4±6.1 | 14.9±6.3 | <0.001* |
| Lactate H3 (mmol L−1) | 8.3±4.8 | 12.1±5.2 | <0.001* |
| Lactate H6 (mmol L−1) | 7.9±4.3 | 10.4±4.8 | <0.001* |
| LC03 (%/h) | −8.4±10.9 | −3.2±9.5 | <0.001* |
| LC36 (%/h) | −10.9±7.3 | −1.5±1.9 | 0.348 |
| LC06 (%/h) | −20.5±17.5 | −11.1±15.9 | <0.001* |
SD: standard deviation; ICU: intensive care unit
In the univariate analysis, LC was significantly different between alive and deceased patients at 28 days when measured within the first 3 h (LC03) and within the first 6 h (LC06), whereas no difference was found from 3 to 6 h (LC36) upon the initiation of ECLS (Table 3).
No difference was observed in the no-flow and low-flow duration between alive and deceased patients (Table 3).
Using logistic regression models to predict mortality at day 28, blood lactate at H3 (OR [95% CI]=1.21 [1.05–1.42], p=0.01), LC03 (OR [95% CI]=0.93 [0.87–0.99], p=0.03) and LC06 (OR [95% CI]=0.96 [0.92–0.99], p=0.04) remained significant. No significant results were found for blood lactate at H0, H6 and LC36 for mortality at day 28.
ROC curve analysis indicated a cut-off point of 12.8 mmol L−1 for lactate at H3 (AUC [95% CI]=0.72 [0.59–0.85]). A cut-off point of −16%/h was noted for LC03 (AUC [95% CI]=0.69 [0.55–0.83]) and −25%/h for LC06 (AUC [95% CI]=0.66 [0.51–0.82]).
Discussion
In the present study, we observed that blood lactate and LC were associated with mortality at day 28 in patients with refractory CA treated by ECLS. Blood lactate measured at H3 was predictive of mortality at day 28 using logistic regression models, whereas its measurement at the other time points was less informative. In addition, LC within the first 3 h of the initiation of ECLS was found to predict mortality at day 28. LC06 was also found to be significant and most of all correlated with LC03.
These results highlight the potential of lactate measurement to help predict patient’s outcome in refractory CA treated by ECLS. In refractory CA, early biomarkers are needed for the early identification of patients at risk of poor outcome, e.g. mortality. In our study, we suggest that lactate measured at H0 and H3 and LC calculated within the first 3 h could help assess mortality at day 28 in refractory CA treated by ECLS.
As a matter of fact, blood lactate was reported to be a prognosis biomarker in refractory CA treated by ECLS (16). Moreover, its ability to predict mortality was higher than base deficit (16). In actuality, an elevated blood lactate results from either increased production (lactic acidosis) and/or decreased elimination of lactate (LC) (24), found in different types of shock (10). Interestingly, LC is even a more relevant parameter than an absolute value of blood lactate measured at a define time point. It describes both the elimination of produced blood lactate and the ability of the organism to clear lactate by liver metabolism. Blood lactate is a reflection of demand mismatch and vasoconstriction that can lead to lactic acidosis (10) due to inadequate production and/or washout. Lactate reflects the efficiency of blood pressure restoration independently of the method used for ALS. LC was shown to improve the predictive value of lactate for mortality in the context of shock and extracorporeal membrane oxygenation (17, 25–28). Re-establishment of blood lactate to normal or LC was reported to correlate with patient’s outcome (29). However, LC was shown to vary according to the aetiology of CA, administered therapeutics and rate of progression of the disease (25). In this view, the expression ‘lactate shift’ appeared to be more accurate, taking into account both synthesis and clearance of blood lactate and thus avoiding the use of physiological terminology (30). However, lactate shift remains less often used than LC. In any case, both expressions include a dynamic component in the measurement of the biological parameter. Even though blood lactate and LC can predict mortality at definite time points, LC is far more relevant than blood lactate.
In refractory CA treated by ECLS, the need for accurate and early prognosis tools persists, as failure usually occurs early, within the first hours of ALS. Herein, we propose to use LC, measured within the first 3 h of the initiation of ECLS as a hallmark to early assess the prognosis of patients with refractory CA.
The present study has several limitations. First, this is a monocentric study, with a small sample size. Second, this study is observational. Despite the observed relationship between blood lactate and mortality and between LC and mortality at day 28, we cannot conclude in their causality. Third, our study was performed in a country with an efficient pre-hospital system, where physicians are dispatched to the scene and directly initiate treatments, as ECLS. Pre-hospital initiation of ECLS is not performed in all countries, and therefore, our data may not be extrapolated to other settings. Pre-hospital management of patients could potentially interfere on the timing and thus on blood lactate concentrations. Fourth, 20% of the patients died within the first 6 h of ECLS, which may have interfered with our results as analysis was performed on a reduced sample.
Conclusion
The present study reports an association between blood lactate and LC measured within the first 3 h of the initiation of ECLS in refractory CA with mortality at day 28. Early prognosis markers are crucial to improve the management of refractory CA. LC is a dynamic biomarker, gaining wide interest. We suggest to use LC03 to help predict the outcome of patients treated by ECLS. Further studies are required to confirm these results and to discuss the incorporation of LC in the decision-making to pursue or not ECLS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Comité pour la Protection des Personnes Est 3, Nancy, France (no. 17.12.05).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.J.; Design - R.J.; Data Collection and/or Processing - R.J., P.P.; Analysis and/or Interpretation - R.J., A.S.; Writing Manuscript - R.J., A.S.; Critical Review - A.S., P.P., P.C., B.V.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 3.Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, et al. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S315–67. doi: 10.1161/CIR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 4.Recommandations sur les indications de l’assistance circulatoire dans le traitement des arrêts cardiaques réfractaires. Ann Fr Anesth Réanimation. 2009;28:182–6. doi: 10.1016/j.annfar.2008.12.011. [DOI] [Google Scholar]
- 5.Lehot JJ, Long-Him-Nam N, Bastien O. Extracorporeal life support for treating cardiac arrest. Bull Acad Natl Med. 2011;195:2025–36. [PubMed] [Google Scholar]
- 6.Le Guen M, Nicolas-Robin A, Carreira S, Raux M, Leprince P, Riou B, et al. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care Lond Engl. 2011;15:R29. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg. 2012;94:1–7. doi: 10.1016/j.athoracsur.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–79. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Negovsky VA. The second step in resuscitation--the treatment of the “post-resuscitation disease”. Resuscitation. 1972;1:1–7. doi: 10.1016/0300-9572(72)90058-5. [DOI] [PubMed] [Google Scholar]
- 10.Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS. Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma. 2000;48:8–15. doi: 10.1097/00005373-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care Lond Engl. 2005;9:441–53. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221–6. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 13.Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35:584–9. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Sammour T, Kahokehr A, Caldwell S, Hill AG. Venous glucose and arterial lactate as biochemical predictors of mortality in clinically severely injured trauma patients--a comparison with ISS and TRISS. Injury. 2009;40:104–8. doi: 10.1016/j.injury.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Lavery RF, Livingston DH, Tortella BJ, Sambol JT, Slomovitz BM, Siegel JH. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000;190:656–64. doi: 10.1016/S1072-7515(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 16.Jouffroy R, Lamhaut L, Guyard A, Phillipe P, Deluze T, Jaffry M, et al. Base excess and lactate as prognostic indicators for patients treated by extra corporeal life support after out hospital cardiac arrest due to acute coronary syndrome. Resuscitation. 2014;85:1764–8. doi: 10.1016/j.resuscitation.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Régnier MA, Raux M, Le Manach Y, Asencio Y, Gaillard J, Devilliers C, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012;117:1276–88. doi: 10.1097/ALN.0b013e318273349d. [DOI] [PubMed] [Google Scholar]
- 18.Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock Augusta Ga. 2009;32:35–9. doi: 10.1097/SHK.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 19.Murtuza B, Wall D, Reinhardt Z, Stickley J, Stumper O, Jones TJ, et al. The importance of blood lactate clearance as a predictor of early mortality following the modified Norwood procedure. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2011;40:1207–14. doi: 10.1016/j.ejcts.2011.01.081. [DOI] [PubMed] [Google Scholar]
- 20.Mutschler M, Nienaber U, Brockamp T, Wafaisade A, Fabian T, Paffrath T, et al. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit Care. 2013;17:R42. doi: 10.1186/cc12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 24.Revelly JP, Tappy L, Martinez A, Bollmann M, Cayeux MC, Berger MM, et al. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit Care Med. 2005;33:2235–40. doi: 10.1097/01.CCM.0000181525.99295.8F. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Quintairose Silva A, Couto L, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257. doi: 10.1186/s13054-016-1403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Londoño J, Niño C, Díaz J, Morales C, León J, Bernal E, et al. Association of Clinical Hypoperfusion Variables with Lactate Clearance and Hospital Mortality. Shock. 2018;50:286–92. doi: 10.1097/SHK.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 27.Evans AS, Levin MA, Lin HM, Lee K, Weiner MM, Anyanwu A, et al. Prognostic Value of Hyperlactatemia and Lactate Clearance After Mitral Valve Surgery. J Cardiothorac Vasc Anesth. 2018;32:636–43. doi: 10.1053/j.jvca.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Bottiroli M, Decaria D, Maraffi T, Nonini S, Milazzo F, Paino R. Blood lactate normalization following venoarterial ECMO institution for refractory cardiogenic shock. Crit Care. 2015;19(Suppl 1):P146. doi: 10.1186/cc14226. [DOI] [Google Scholar]
- 29.Ioannou N, Terblanche M. Surrogate end points in critical illness research: some way to go yet. Crit Care Med. 2011;39:2561–2. doi: 10.1097/CCM.0b013e31822a581a. [DOI] [PubMed] [Google Scholar]
- 30.Walker CA, Griffith DM, Gray AJ, Datta D, Hay AW. “Lactate Shift,” Rather Than “Lactate Clearance,” for Serial Blood Lactate Monitoring? Crit Care Med. 2015;43:e596. doi: 10.1097/CCM.0000000000001315. [DOI] [PubMed] [Google Scholar]