Abstract
Neurite plasticity is a critical aspect of brain functional recovery after stroke. Emerging data suggest that Ras-related C3 botulinum toxin substrate 1 (Rac1) plays a central role in axonal regeneration in the injured brain, specifically by stimulating neuronal intrinsic growth and counteracting the growth inhibitory signaling that leads to growth cone collapse. Therefore, we investigated the functional role of Rac1 in axonal regeneration after stroke.
Delayed treatment with a specific Rac1 inhibitor, NSC 23766, worsened functional recovery, which was assessed by the pellet reaching test from day 14 to day 28 after stroke. It additionally reduced axonal density in the peri-infarct zone, assessed 28 days after stroke, with no effect on brain cavity size or on the number of newly formed cells. Accordingly, Rac1 overexpression using lentivirus promoted axonal regeneration and functional recovery after stroke from day 14 to day 28. Rac1 inhibition led to inactivation of pro-regenerative molecules, including mitogen-activated protein kinase kinase (p-MEK)1/2, LIM domain kinase (LIMK)1, and extracellular signal-regulated kinase (p-ERK)1/2 at 14 days after stroke. Inhibition ofRac1 reduced axonal length and number in cultured primary mouse cortical neurons using microfluidic chambers after oxygen-glucose deprivation (OGD) without affecting cell viability. In contrast, inhibition of Rac1 increased levels of glial fibrillary acidic protein, an extrinsic inhibitory signal for axonal growth, after stroke in vivo and in primary astrocytes after OGD.
In conclusion, Rac1 signaling enhances axonal regeneration and improve post-stroke functional recovery in experimental models of stroke.
Keywords: Stroke, Rac1, Axons
Introduction
Stroke is the primary cause of adult long-term disability in both the USA and worldwide [1]. Although the mortality rate of stroke is decreasing, the incidence of stroke is rising, which translates into an increasing prevalence of patients living with persistent disability [2]. Mounting evidence suggests that axonal outgrowth is a critical aspect of functional recovery because it is essential for establishing new neural connections to compensate for the stroke-induced loss of function. Thus, targeting this process has emerged as an alternative and potentially more tractable target to reduce long-term disability after stroke [3]. However, after injury, this axonal regrowth and remodeling in the adult mammalian central nervous system (CNS) is limited, partially because there is a lack of robust activation of intrinsic neuronal properties for outgrowth, but also because the extrinsic glial environment, such as glial scar formation, inhibits axonal regeneration.
Ras-related C3 botulinum toxin substrate 1 (Rac1) is a Rho-related small GTPase that plays a critical role in axonal growth during neuronal development [4]. Rac1 is ubiquitously expressed throughout the brain [5]. However, Rac1 becomes inactivated when bound to guanosine diphosphate (GDP) and activated when bound to guanosine triphosphate (GTP). Interestingly, emerging data suggest a critical role of the Rac1 pathway in axonal regeneration in the injured brain, but no studies have investigated whether Rac1 is a viable target for axonal regeneration after cerebral ischemia. Downstream substrates of Rac1 (ERK1/2, GFAP, i.e.) are implicated in the stimulation of neuronal intrinsic outgrowth properties, as well as counteracting the glial extrinsic environment, which represent the two critical components that govern axonal plasticity in injured brain. In cultured mouse cortical neurons, Rac1 is required for atorvastatin-induced neurite plasticity after glutamate-induced neuronal excitotoxicity [6]. Interestingly, Rac1 levels are increased in peri-infarct regions in rats after stroke, and this elevation persists for 2 weeks after stroke during the critical phase of axonal regeneration and recovery [7]. Therefore, we investigated the functional role of Rac1 in axonal regeneration and its mechanisms in the recovery phase after stroke in mice.
Methods
Animals
The study was approved by the Center for Laboratory Animal Care of the University of Connecticut Health Center and University of Texas Health Science Center, and performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The C57BL/6J WT male mice (7–8 weeks) were from Charles River.
Middle Cerebral Artery Occlusion (MCAO)
MCAO procedure has been described in details previously [8, 9]. Briefly, after a skin incision along the midline of the mouse neck, 90-min MCA occlusion was induced in mice using an intraluminal filament. After occlusion, mice were reperfused by suture withdrawal and then sacrificed 14 or 28 days later. Mice of the sham groups underwent the same procedure as those in the stroke groups, but without occluding the MCA.
Histological Assessment
Brain atrophy measurement was analyzed as previously described [9, 10]. Generally, mice were perfused and fixed with 4% paraformaldehyde 28 days after stroke. The fixed brains were then sliced for cresyl violet staining in accordance with previously published procedures [10,11]. The brain tissue loss was determined following this calculation: % brain tissue lost = 100% × [(contralateral hemisphere area - contralateral ventricular area) - (ipsilateral hemisphere area - ipsilateral ventricular area)]/(contralateral hemisphere area - contralateral ventricular area).
Production of Lentiviral Vector
Lentiviral vectors carrying Rac1 and GFP were generated following the procedures described in our previous publications [12]. The concentrations of the lentivirus were 109 transducing units/mL, which was titered by transducing 293FT cells and counting eGFP-positive colonies [12]. For virus titering, briefly, 293FT cells were cultured on a 24-well plate and lentivirus were added to wells. The eGFP fluorescence was visualized under a fluorescent microscope after incubation. To determine the titer of lentivirus, the overall fraction of cells that were eGFP positive in each well was calculated, multiplied by the corresponding total cell number in each well, then divided by the actual volume of added lentivirus.
Lentivirus Administration In Vivo
Lentiviral vectors encoding Rac1 or GFP were injected into the mouse brains following previously established methods [12]. Briefly, a four-point injection was carried out at the following coordinates: 0.5 mm anterior to the bregma, 2.0 or 3.0 mm lateral (right) to the sagittal suture, and 1.0 or 2.8 mm from the surface of the skull [12]. A total of 1 μL of concentrated lentivirus (109 transducing units/mL) was injected into each position at a rate of 0.5 μL/min with a 30-gauge needle on a 10-μL Hamilton syringe (catalog no. 80010). After the lentivirus was injected, the needle remained in position for 10 min before it was withdrawn. This injection protocol causes minimal damage to the brain tissue according to our previous experience and is accounted for by control groups injected with GFP virus [12].
Drug Administration In Vivo and In Vitro
For in vivo studies, Rac1 inhibitor, NSC23766 (NSC), was administrated daily from days 7 to 13 after stroke (7 days, 4.0 mg/kg, i.p.). Control mice received the equal amount of vehicle. While most of the published work used 2.5 mg/kg/day in mice [13, 14], we chose 4.0 mg/kg/day to ensure an effective inhibition and found no evidence for toxicity at this dose. For in vitro experiments, the concentration of 30 μM NSC was used [15]. BrdU (bromodeoxyuridine) was injected 7 days after stroke (dose, 50 mg/kg, i.p./8 h for 24 h) [16].
Behavior Measurements Using Pellet Reaching Test
Pellet reaching is a well-organized movement involving complex neural control systems. This test has the capability to measure both the motor and sensory functions of the impaired limb and is increasingly used in long-term survival studies for stroke recovery [17–19]. As previously described, the success rate of pellet reaching was calculated according to the following equation (number of successful reaches/total number of reaches × 100). Mice were pre-tested to assess and control for individual differences. All studies were conducted and analyzed by blinded investigators.
Oxygen-Glucose Deprivation (OGD) in Primary Cortical Neurons and Astrocytes
To assess the role of Rac1 in the intrinsic capacity of neurons for axonal outgrowth, we used mouse primary neuronal culture system, where there is no/minimal glial influence. To measure outgrowth, we utilized a microfluidic chamber which separates axons from neuronal soma and permits direct axonal outgrowth monitoring in cortical neurons [20]. Cortical neurons were challenged by OGD for 2 h [21]. Twenty-four hours after OGD, the cells were co-cultured with 30 μM NSC until 96 h after OGD. Axonal elongation and numbers were then analyzed by NFL staining at 96 h after OGD, as others have shown significant axonal regeneration induced by OGD at this time point [20]. Elongating axons were imaged with a fluorescence microscope. The number of axons were counted and the length of each axon was measured by manually tracing each individual axon with ImageJ software. Primary astrocytes were challenged by OGD for 2 h. Twenty-four hours after OGD, the cells were co-cultured with 30 μM NSC until 96 h after OGD.
Western Blot Analysis
Western blot procedures were performed as previously described [1, 23]. For in vivo studies, GFAP, p-LIMK 1, p-ERK1/2, and p-MEK1/2 were analyzed in the brain samples harvested 14 days after stroke. Rac1 in mouse brain was detected 7 days after injection of Rac1 or GFP vectors. For in vitro experiments, glial fibrillary acid protein (GFAP) expression in primary astrocytes after OGD was probed. β-Actin or β-tubulin was employed as a loading control. The integrated optical density (IOD) of each band was measured using ImageJ. The IOD results were normalized by dividing each value by the mean IOD of the control groups.
Immunohistochemistry and Data Quantification
The staining method was described in previous publications [1, 22]. The Neurofilament-L (NFL) and GFAP were stained in mouse brain slices 28 days after stroke. After staining, the images were recorded and analyzed using a Zeiss Axiovert 200M microscope (Carl Zeiss, Oberkochen, Germany) with an X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc., Mississauga, ON, Canada) and Zeiss image acquisition software (Zeiss LSM 510). Identical digital imaging acquisition parameters were used throughout the study. NFL or GFAP were semi-quantified as the integrated optical density (IOD) of green-positive signals in the penumbra of the ischemic hemisphere using ImageJ. The IOD results were normalized by dividing each value by the mean IOD of the control groups.
Statistical Analyses
Data are presented as the mean ± standard error of the mean (SEM). Mean comparisons between the experimental groups were analyzed by one-way or two-way analysis of variance (ANOVA) followed by post hoc tests for multiple comparisons when appropriate. P <0.05 was considered statistically significant by using SPSS 19 software (Chicago, IL, USA). Both behavioral and histological assessments were performed by an investigator blinded to the vectors/drug treatment.
Results
Delayed Inhibition of Rac1 Led to Poorer Functional Recovery and Reduced Axonal Density
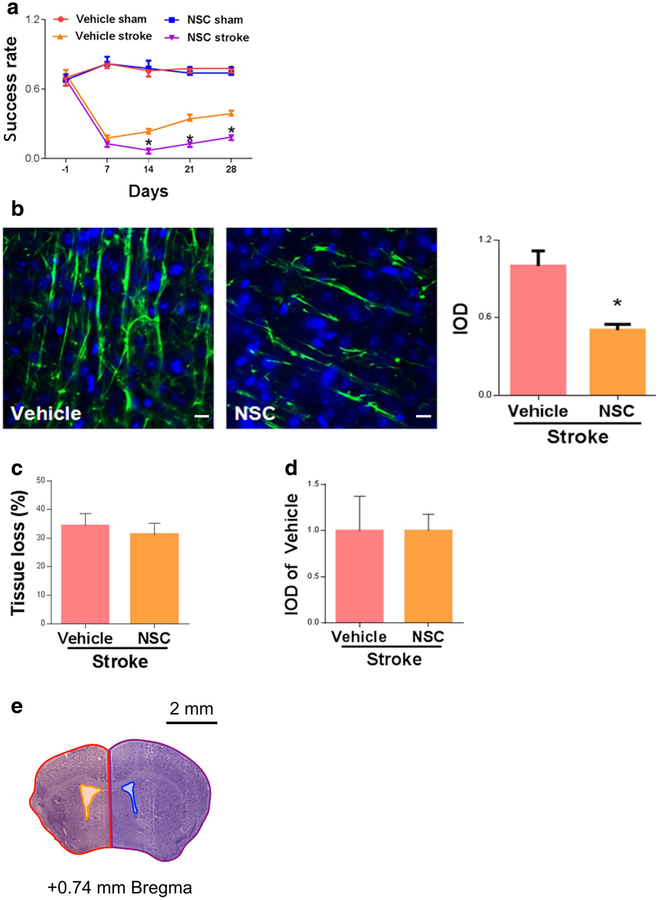
A specific Rac1 inhibitor, NSC23766 (NSC), or vehicle (saline) was administrated to WT mice starting 7 days after MCAO and injected once daily for 7 days (4.0 mg/kg/day). We tested functional motor deficits at 28 days after stroke onset by performing the pellet reaching test. Stroke produced consistent deficits in the pellet reaching test, and gradual recovery was observed in vehicle-treated stroke mice. We found that the delayed inhibition of Rac1 with NSC significantly worsened functional recovery from day 14 to day 28 after stroke (day 28, vehicle 0.39 ± 0.03 vs. NSC 0.18 ± 0.03, n = 7–9, *p < 0.05; Fig. 1a).
Fig. 1.
Delayed inhibition of Rac1 led to poorer recovery and reduced axonal density after stroke. a Functional recovery assessed by pellet reaching test from day 14 to day 28 after stroke. N = 7–9, *p < 0.05. b Axonal density (neurofilament staining)in the peri-infarct zone was assessed on 28 days after stroke. Scale bars: 10 μm. N =4,*p < 0.05. c NSC treatment did not affect tissue loss 28 days after stroke. N =7–9, p > 0.05. d NSC treatment did not reduce BrdU-positive cells at 28 days after stroke. N =4, p > 0.05. e An illustration of how quantification of brain tissue loss was performed after stroke using CV staining. % brain tissue lost = 100% × [(contralateral hemisphere area - contralateral ventricular area) - (ipsilateral hemisphere area - ipsilateral ventricular area)]/(contralateral hemisphere area - contralateral ventricular area). Red area: ipsilateral hemisphere area; yellow area: ipsilateral ventricular area; purple area: contralateral hemisphere area; blue area: contralateral ventricular area. A specific Rac1 inhibitor, NSC23766 (NSC), or vehicle (saline) was administrated to WT mice starting 7 days after MCAO and injected once daily for 7 days (4.0 mg/kg/day). Data were as mean ± SEM
We then studied the effect of delayed Rac1 inhibition in axonal density in the peri-infarct zone. We found that Rac1 inhibitor treatment significantly reduced axonal density (neurofilament staining, NFL) in the peri-infarct zone, assessed at 28 days after stroke (vehicle 1.00 ± 0.12 vs. NSC 0.50 ± 0.04, n = 4, *p < 0.05; Fig. 1b).
Delayed Rac1 Inhibition Did Not Increase Tissue Loss or Reduce the Number of BrdU-Positive Cells
It remains possible that Rac1 inhibition affects brain injury, which would contribute to the changes in functional outcome and axonal density seen in our experiments. The likelihood is slim as NSC was started at 7 days after stroke, when the infarct growth is complete. Regardless, we performed CV staining at 28 days after stroke to examine infarction (cavity size). No differences (vehicle 34.2 ± 4.3% vs. NSC 31.26 ± 3.4%, n = 7–9/group, p > 0.05; Fig. 1c, e) were seen between vehicle and NSC-treated groups. Rac1 has been implicated in neurogenesis, which could potentially contribute to changes in axonal density. To measure neurogenesis, we injected BrdU to label proliferating cells in the brain 7 days after stroke and stained for BrdU-positive cells 28 days after stroke. No difference in BrdU staining in the peri-infarct zone was observed between the vehicle-treated and NSC-treated groups (vehicle 1.00 ± 0.37 vs. NSC 1.00 ± 0.18, n = 4, p > 0.05;Fig. 1d).
Delayed Overexpression of Rac1 Improved Functional Recovery and Increased Axonal Density after Stroke
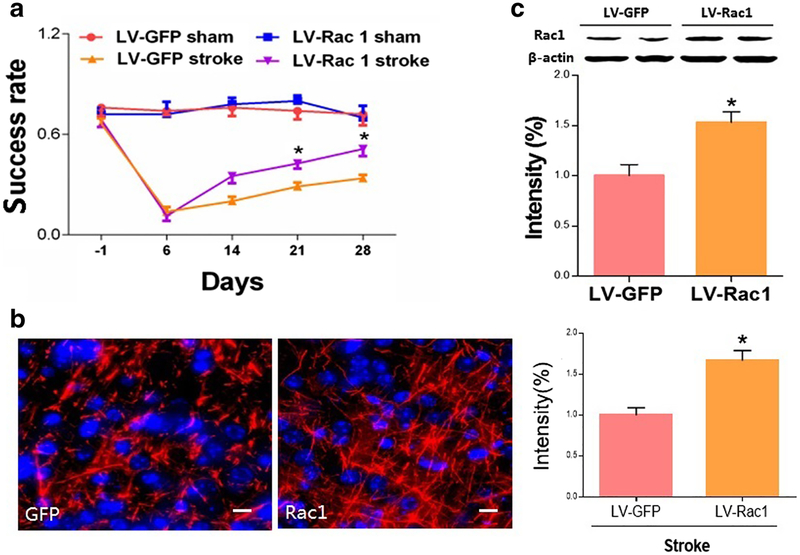
We next studied how Rac1 affects stroke recovery by overexpressing Rac1 using a lentiviral vector carrying the Rac1 gene. We injected highly concentrated lentivirus encoding WT Rac1 at 7 days after stroke into both the cortex and striatum. We found that delayed overexpression of Rac1 improved functional recovery, as assessed by the pellet reaching test from day 14 to day 28 after stroke (day 28, LV-GFP 0.34 ± 0.02 vs. LV-Rac1 0.51 ± 0.04, n = 8, *p < 0.05; Fig. 2a). Axonal density was also significantly increased in the peri-infarct zone in the group with Rac1 overexpression (data LV-GFP 1.00 ± 0.08 vs. LV-Rac1 1.67 ± 0.10, *p<0.05, n = 4, Fig. 2b).
Fig. 2.
Overexpression of Rac1 improved functional recovery and increased axonal density after stroke. a Functional recovery assessed by pellet reaching test from day 14 to day 28 after stroke. N =8, *p < 0.05. b Axonaldensity (neurofilament staining)in the peri-infarct zone was assessed on 28 days after stroke. Scale bars: 10 μm. N =4,*p < 0.05. c Rac1 lentivector significantly increased Rac1 protein levels 7 days after injection in vivo. N =4, *p <0.05. We injected highly concentrated lentivirus encoding WT Rac1 1 week after stroke into both the cortex and striatum. Data were as mean ± SEM
The in vivo effect of the lentivirus on protein expression was confirmed by western blot. To assess this, vectors were injected into both the cortex and striatum in mice at 7 days after stroke. One week after the injection, western blot analysis demonstrated a consistent increase in levels of Rac1 protein in the stroke hemisphere (LV-GFP 1.00 ± 0.11 vs. LV-Rac1 1.53 ± 0.11, n = 4, *p < 0.05; Fig. 2c).
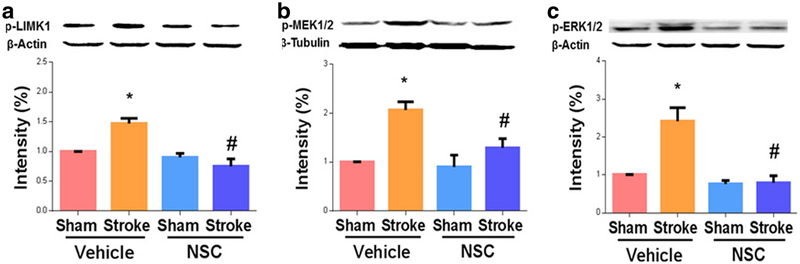
Rac1 Inhibition Reduced P-LIMK 1, P-MEK1/2, and P-ERK1/2 In Vivo
We then moved on to study the mechanisms of Rac1 in stimulating axonal regeneration after stroke. MEK1/2 and ERK1/2 are well-known pro-regenerative molecules, as they stimulate the expression of pro-regenerative genes. Importantly, Rac1 is known to activate MEK (via PAK1), which then phosphorylates ERK. Additionally, Rac1 also targets LIMK1, another potent enhancer of axonal plasticity [22–24]. As expected, we found that delayed inhibition of Rac1 reduced the levels of p-LIMK 1 (day 14, vehicle 1.47 ± 0.09 vs. NSC 0.75 ± 0.12, n = 4, #p < 0.05; Fig. 3a), p-MEK1/2 (day 14, vehicle 2.07 ± 0.17 vs. NSC 1.29 ± 0.19, n = 4, #p < 0.05; Fig. 3b), and p-ERK1/2 (day 14, vehicle 2.42 ± 0.35 vs. NSC 0.79 ± 0.19, n = 4, #p < 0.05; Fig. 3c).
Fig. 3.
Rac1 inhibition reduced p-LIMK 1, P-MEK1/2, and p-ERK1/2 in vivo. A specific Rac1 inhibitor, NSC, or vehicle (saline) was administrated to WT mice starting 7 days after MCAO and injected once daily for 7 days (4.0 mg/kg/day). Samples collected 14 days after stroke. a p-LIMK 1; b p-MEK1/2; c p-ERK1/2. N =4, *p < 0.05 for comparison between sham and stroke group injected with vehicle; #p < 0.05 for comparison between vehicle and NSC group under stroke condition. Data were as mean ± SEM
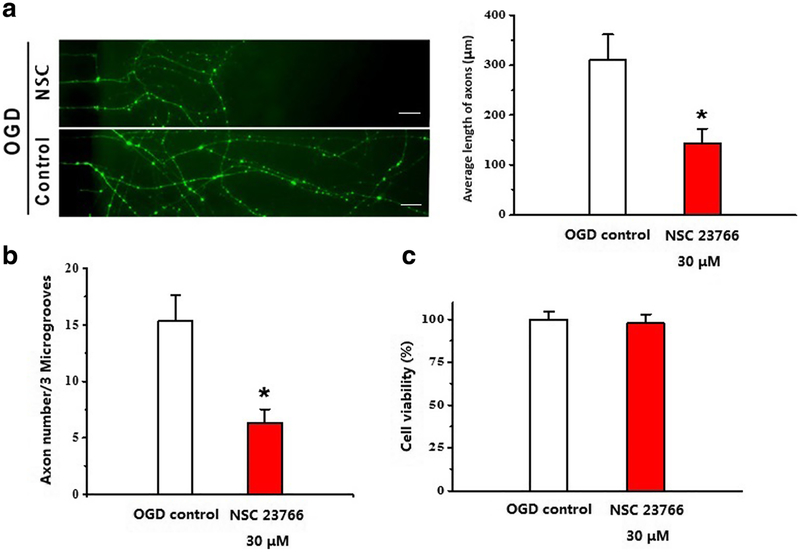
Inhibition of Rac1 Reduced Axonal Length and Number after OGD
We then examined the effect of Rac1 inhibitor (NSC) on axonal outgrowth by using a microfluidic chamber to separate axons from neuronal soma. At 6 days in vitro, cortical neurons were challenged by OGD for 2 h and then co-cultured with 30 μM NSC for 96 h, and axonal elongation was analyzed by immunocytochemical staining of NFL. Rac1 inhibitor significantly suppressed axonal outgrowth both in length (OGD control 309.76 ± 51.79 μm vs. OGD plus NSC 143.95 ± 28.78 μm, *p < 0.05, n = 4; Fig. 4a) and in axon numbers after OGD (OGD control 15.33 ± 2.33 vs. OGD plus NSC 30 μM 6.33 ± 1.20, *p < 0.05; Fig. 4b).
Fig. 4.
Inhibition of Rac1 reduced axonal length and numbers after OGD. Microfluidic chamber was used to separate axons from neuronal soma. At 6 days in vitro, cortical neurons co-cultured with 30 μM NSC23766 for 96 h after challenged by OGD for 2 h, axonal length (a) and number (b) were analyzed by immunocytochemistry NFL staining. N = 4, *p < 0.05. Scale bars: 50 μm. c NSC incubation had no effect on cortical neuron’s cell viability. N =4, p > 0.05. Data were presented as mean ± SEM
At this dosing regimen, NSC incubation had no effect on cortical neuron’s cell viability in vitro (OGD + vehicle 60.3 ± 2.8% vs. OGD + NSC 58.92 ± 3.2%, p > 0.05; Fig. 4c).
Inhibition of Rac1 Increased GFAP after Stroke In Vivo and in Primary Astrocytes after OGD
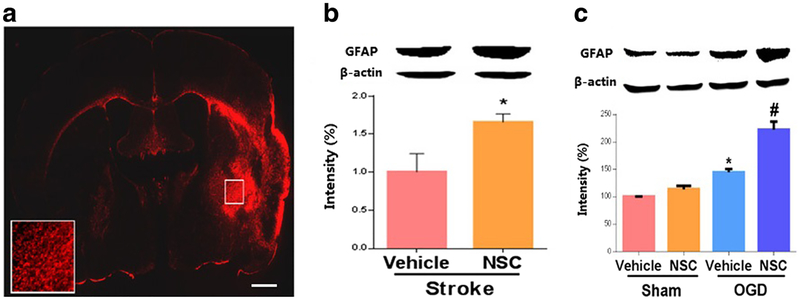
We hypothesized that Rac1 also enhances axonal regeneration by counteracting the growth inhibitory signaling (e.g., glial scar) that leads to growth cone collapse, providing an additional mechanism for improved functional recovery after stroke. Our data showed that GFAP, a marker for glial scar formation and a signal of axonal growth inhibition, had elevated expression in the ischemic hemisphere at 28 days after stroke (Fig. 5a). Furthermore, we observed that the Rac1 inhibitor treated cohort had upregulated levels of GFAP at 14 days after stroke (day 14, vehicle 1.00 ± 0.24 vs. NSC 1.66 ± 0.11, n = 4, *p < 0.05; Fig. 5b).
Fig. 5.
Inhibition of Rac 1 increased levels of GFAP in vivo and in cultured astrocytes after stroke. a GFAP is highly expressed in brains 28 days after stroke. Scale bars: 1 mm. b Rac1 inhibitor upregulated GFAP expression in mice 14 days after stroke. A specific Rac1 inhibitor, NSC, or vehicle (saline) was administrated to WT mice starting 7 days after MCAO and injected once daily for 7 days (4.0 mg/kg/day). N =4, *p <0.05. c Rac1 inhibitor increased levels of GFAP in primary astrocytes after OGD. Cells were challenged by OGD for 2 h. 24 h after OGD, the cells were co-cultured with 30 μM NSC for a total of 96 h. N =4, *p < 0.05 for comparison between sham and OGD group treated with vehicle, #p < 0.05 for comparison between vehicle and NSC group under OGD condition. Data were presented as mean ± SEM
Finally, we then confirmed the effects of Rac1 on GFAP in primary astrocytes after OGD. In line with the in vivo data, we demonstrated that inhibition of Rac1 increased the level of GFAP in astrocytes at 96 h after OGD (vehicle 1.45 ± 0.06 vs. NSC 2.22 ± 0.15, n = 4, #p < 0.05; Fig. 5c).
Discussion
In the present study, we demonstrated that Rac1 plays vital roles in brain plasticity and functional recovery after experimental stroke. Specifically, we have made the following significant findings: (1) Rac1 inhibition led to poorer functional recovery after stroke in long-term survival and reduced axonal density in the penumbra. This treatment did not cause changes in either the brain cavity size or the number of newly divided cells, suggesting that outgrowth of existing neurons is the major contributor to changes in axonal density following treatment. (2) Overexpression of Rac1 promoted improvement of motor and sensory functions, increased long-term survival, and also increased axonal density. (3) Rac1 inhibition reduced the activation of pro-regenerative molecules such as ERK and MEK. (4) Treatment with Rac1 inhibitor exacerbated GFAP expression in vivo and in vitro after stroke, potentially also contributing to reduced axonal outgrowth.
The weak intrinsic growth capacity in neurons and the inhibitory factors from extrinsic glial environments are among the major causes of regenerative failure in the CNS after injury [25, 26]. Rac1 promotes axonal regeneration by counteracting the glial inhibitory signal to axon growth. Rac1 signaling can reduce glial scar formation, a major physical and chemical barrier for axonal growth following stroke [27]. Mounting evidence shows that glial inhibitory signals are the major cause of growth cone collapse and limited CNS axonal regeneration. Reactive astrocytes are the primary contributors of the formation of glial scar, which is a major physical and chemical barrier for axonal outgrowth following injury. Chondroitin sulfate proteoglycans (CSPGs), secreted by the astrocytic scar, are highly inhibitory to regeneration after stroke, and decreased CSPG contributes to improved plasticity in stroke. Secreted CSPG inhibits axonal growth by binding specific receptors expressed on the growth cone membrane, causing the cone to collapse and ultimately leading to axonal retraction. In spinal cord injury models, animals treated with constitutively active Rac1 showed significantly lower expression of GFAP and CSPGs than untreated controls [28]. In line with this report, our study demonstrated that Rac1 inhibition exacerbated GFAP signaling in the brain after stroke using both in vivo and in vitro models.
Our work additionally showed that Rac1 stimulates the intrinsic capacity of neurons for axonal outgrowth. Rac1 upregulates the extracellular signal-regulated kinase (ERK) pathway, which is well known to induce axonal regeneration in the injured central nervous system [29, 30]. This pathway consists of three kinases: rapidly accelerated fibrosarcoma (Raf), mitogen-activated protein/extracellular signal-regulated kinase (MEK), and ERK. Pak1, the downstream target of Rac1, phosphorylates Raf, which then phosphorylates MEK, which in turn phosphorylates ERK [29]. Importantly, ERK1/2 mediates the transcriptional activation of regeneration-associated genes (RAGS). This can occur either directly, when phosphorylated ERK1/2 (p-ERK1/2) translocates from the cytoplasm to the nucleus, or indirectly, when p-ERK1/2 phosphorylates downstream kinases in the cytoplasm which subsequently enter the nucleus [31, 32]. For example, ERK phosphorylates cyclic adenosine monophosphate response element binding protein (CREB). When phosphorylated, CREB induces the transcription of genes important for axonal plasticity processes after CNS injury, such as growth-associated protein 43 (GAP43) [26]. Our work suggests that Rac1 and MEK/ERK interact with each other, although a causative relationship needs to be investigated in the future.
ERK1/2 activation in neurons stimulates pro-regenerative gene expression. However, activation of ERK1/2 in astrocytes may also stimulate gliosis, which would paradoxically hinder axonal regeneration. This effect could be minimal in stroke, as our IHC showed p-ERK1/2 was mostly confined to neuronal cells at 28 days after stroke (data not shown), indicating that ERK1/2 signaling is selectively activated in neurons during the chronic phase of stroke.
Interestingly, the literature shows that Rac1 is pro-regenerative after brain injury; however, other studies have shown that Rac1 inhibition may also provide neuroprotection and reduce injury in the acute phase of injury [33]. To minimize potential effects on acute injury size, we deliberately started Rac1 intervention (both Rac1 inhibitor and lentiviral overexpression) at 7 days after the onset of stroke. This allowed us to hone in on studying the role of Rac1 in brain plasticity. In general, 7 days post-stroke is beyond the window for neuronal protection in this model, as stroke infarction completes within a week after MCA occlusion [34].
One caveat of this study is that axonal regeneration is influenced by factors that can be produced by the peripheral system, such as IL-1 and TGF-beta, as these cytokines affect glial scar formation [35]. Rac1 is also an important mediator of immunity, and inhibition of Rac1 in peripheral blood mononuclear cells from human blood was shown to reduce IL-1 beta release [36]. Although manipulation of Rac1 provides considerable evidence for a combined effect of both central and peripheral responses, brain injection of lentivirus may not allow us to observe peripheral effects. We will closely monitor changes in cytokines using peripheral blood samples. This approach will also help to evaluate the possible role of Rac1 in post-stroke inflammation, as Rac1 is known to participate in the immune response [37, 38].
The translational significance of our findings is high, as we identified a molecule that plays critical roles in enhancing brain plasticity and functional recovery after stroke. Our experiments significantly improved our understanding of the complex axonal growth program that is activated after stroke. Given that the economic burden of post-stroke disability will continue to increase as the population ages and acute stroke care improves, developing novel drugs to improve functional recovery is a growing and urgent public health need. Currently, the only pharmacological treatment for stroke is tissue plasminogen activator (tPA) [2], which has a short therapeutic window, leaving no effective treatment available for the majority of stroke patients. A weak intrinsic regenerative potential in neurons and inhibition from glial cells are major causes of limited axonal regeneration after stroke. Here, we identified a target molecule that stimulates intrinsic components of neuronal growth capacity and simultaneously reduces glial inhibition. Therefore, targeting this molecule may offer robust improvements in functional recovery after stroke. It is especially important in the elderly, the population at greatest risk of stroke, as we have shown an increased astrogliosis occurs in aged individuals after stroke, which is associated with worse recovery in animal models [11].
In summary, we newly demonstrated a critical role of Rac1 in promoting axonal regeneration and brain remodeling after experimental stroke. Our work suggests that Rac1 targets the two key components in axonal rewiring in the post-ischemic brain, both by enhancing the intrinsic properties of neurons to extend axons, as well as by inhibiting glial scar formation in the chronic recovery phase after experimental stroke. Our study suggests that Rac1 signaling is a potential therapeutic target for promoting brain remapping and functional recovery after stroke.
Acknowledgements
This project was generously supported by National Institutes of Health grants R01 NS099628 (J.Li) and R01 NS078446 (J.Li). This project was generously supported by National Institutes of Health grants R01 NS099628 (J.Li) andR01 NS078446 (J.Li).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures were approved by the Center for Laboratory Animal Care of the University of Connecticut Health Center and University of Texas Health Science Center and performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
References
- 1.Liu L, McCullough L, Li J. Genetic deletion of calcium/calmodulin-dependent protein kinase kinase beta (CaMKK beta) or CaMK IV exacerbates stroke outcomes in ovariectomized (OVXed) female mice. BMC Neurosci. 2014;15:118 10.1186/s12868-014-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37(2):259–66. 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Valdes HE, Clarkson AN, Ao Y, Charles AC, Carmichael ST, Sofroniew MV, et al. Memantine enhances recovery from stroke. Stroke. 2014;45(7):2093–100. 10.1161/STROKEAHA.113.004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzetto E, Ettorre M, Pontelli V, Bolomini-Vittori M, Bolognin S, Zorzan S, et al. Rac1 selective activation improves retina ganglion cell survival and regeneration. PLoS One. 2013;8(5):e64350 10.1371/journal.pone.0064350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posada-Duque RA, Velasquez-Carvajal D, Eckert GP, Cardona-Gomez GP. Atorvastatin requires geranylgeranyl transferase-I and Rac1 activation to exert neuronal protection and induce plasticity. Neurochem Int. 2013;62(4):433–45. 10.1016/j.neuint.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Choi DH, Kim JH, Lee KH, Kim HY, Kim YS, Choi WS, et al. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS One. 2015;10(1):e0116814 10.1371/journal.pone.0116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Pu H, Hu X, Wei Z, Hong D, Zhang W, et al. A post-stroke therapeutic regimen with Omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl Stroke Res. 2016;7(6):548–61. 10.1007/s12975-016-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pu H, Jiang X, Hu X, Xia J, Hong D, Zhang W, et al. Delayed docosahexaenoic acid treatment combined with dietary supplementation of Omega-3 fatty acids promotes long-term neurovascular restoration after ischemic stroke. Transl Stroke Res. 2016;7(6): 521–34. 10.1007/s12975-016-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B, Parsha K, Schaar K, Xi X, Aronowski J, Savitz SI. Various cell populations within the mononuclear fraction of bone marrow contribute to the beneficial effects of autologous bone marrow cell therapy in a rodent stroke model. Transl Stroke Res. 2016;7(4):322–30. 10.1007/s12975-016-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–700. 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan H, Denton K, Liu L, Li XJ, Benashski S, McCullough L, et al. Nuclear translocation of histone deacetylase 4 induces neuronal death in stroke. Neurobiol Dis. 2016;91:182–93. 10.1016/j.nbd.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Hou H, Chavez AE, Wang CC, Yang H, Gu H, Siddoway BA, et al. The Rac1 inhibitor NSC23766 suppresses CREB signaling by targeting NMDA receptor function. J Neurosci. 2014;34(42): 14006–12. 10.1523/JNEUROSCI.1659-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veluthakal R, Sidarala V, Kowluru A. NSC23766, aknown inhibitor of Tiam1-Rac1 signaling module, prevents the onset of type 1 diabetes in the NOD mouse model. Cell Physiol Biochem. 2016;39(2): 760–7. 10.1159/000445666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554–65. 10.1016/S0076-6879(06)06043-5. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31(2):413–25. 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linden J, Fassotte L, Tirelli E, Plumier JC, Ferrara A. Assessment of behavioral flexibility after middle cerebral artery occlusion in mice. Behav Brain Res. 2014;258:127–37. 10.1016/j.bbr.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–33. 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alaverdashvili M, Whishaw IQ. A behavioral method for identifying recovery and compensation: hand use in a preclinical stroke model using the single pellet reaching task. Neurosci Biobehav Rev. 2013;37(5):950–67. 10.1016/j.neubiorev.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43(8):2221–8. 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu IC, Kuo PC, Yen JH, Paraiso HC, Curfman ET, Hong-Goka BC, et al. A combination of three repurposed drugs administered at reperfusion as a promising therapy for postischemic brain injury. Transl Stroke Res. 2017;8(6):560–77. 10.1007/s12975-017-0543-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Wu J, Huang J, Zhuo P, Lin Y, Wang L, et al. Electroacupuncture regulates hippocampal synaptic plasticity via miR-134-mediated LIMK1 function in rats with ischemic stroke. Neural Plasticity. 2017;2017:9545646 10.1155/2017/9545646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996(1):55–66. 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 24.Liu HQ, Li WB, Li QJ, Zhang M, Sun XC, Feng RF, et al. Nitric oxide participates in the induction of brain ischemic tolerance via activating ERK1/2 signaling pathways. Neurochem Res. 2006;31 (7):967–74. 10.1007/s11064-006-9102-2. [DOI] [PubMed] [Google Scholar]
- 25.Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Giovanni S Molecular targets for axon regeneration: focus on the intrinsic pathways. Expert Opin Ther Targets. 2009;13(12):1387–98. 10.1517/14728220903307517. [DOI] [PubMed] [Google Scholar]
- 27.Hill JJ, Jin K, Mao XO, Xie L, Greenberg DA. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc Natl Acad Sci U S A. 2012;109(23):9155–60. 10.1073/pnas.1205697109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain A, McKeon RJ, Brady-Kalnay SM, Bellamkonda RV. Sustained delivery of activated rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PLoS One. 2011;6(1):e16135 10.1371/journal.pone.0016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koth AP, Oliveira BR, Parfitt GM, Buonocore Jde Q, Barros DM. Participation of group I p21-activated kinases in neuroplasticity. J Physiol Paris. 2014;108(4–6):270–7. 10.1016/j.jphysparis.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Stern S, Knoll B. CNS axon regeneration inhibitors stimulate an immediate early gene response via MAP kinase-SRF signaling. Mol Brain. 2014;7(1):86 10.1186/s13041-014-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccarelli A, Giustetto M. Role of ERK signaling in activity-dependent modifications of histone proteins. Neuropharmacology. 2014;80:34–44. 10.1016/j.neuropharm.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49(5):296–305. 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Liao J, Ye Z, Huang G, Xu C, Guo Q, Wang E. Delayed treatment with NSC23766 in streptozotocin-induced diabetic rats ameliorates post-ischemic neuronal apoptosis through suppression of mitochondrial p53 translocation. Neuropharmacology. 2014;85:508–16. 10.1016/j.neuropharm.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Schafer DP, McCullough LD. TTC, fluoro-jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179(1):1–8. 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 36.Kuijk LM, Beekman JM, Koster J, Waterham HR, Frenkel J, Coffer PJ. HMG-CoA reductase inhibition induces IL-1beta release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood. 2008;112(9):3563–73. 10.1182/blood-2008-03-144667. [DOI] [PubMed] [Google Scholar]
- 37.Hwaiz R, Hasan Z, Rahman M, Zhang S, Palani K, Syk I, et al. Rac1 signaling regulates sepsis-induced pathologic inflammation in the lung via attenuation of mac-1 expression and CXC chemokine formation. J SurgRes. 2013;183(2):798–807. 10.1016/j.jss.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Taglieri DM, Ushio-Fukai M, Monasky MM. P21-activated kinase in inflammatory and cardiovascular disease. Cell Signal. 2014;26(9):2060–9. 10.1016/j.cellsig.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]