Abstract
This study focused on formulating conjugate vaccines targeting oxycodone and heroin for technology transfer, good manufacturing practice (GMP), and clinical evaluation. Lead vaccines used the highly immunogenic carrier protein keyhole limpet hemocyanin (KLH), which poses formulation problems because of its size. To address this barrier to translation, an oxycodone-based hapten conjugated to GMP-grade subunit KLH (OXY-sKLH) and adsorbed on alum adjuvant was studied with regard to carbodiimide coupling reaction time, buffer composition, purification methods for conjugates, conjugate size, state of aggregation, and protein/alum ratio. Vaccine formulations were screened for post-immunization antibody levels and efficacy in reducing oxycodone distribution to the brain in rats. While larger conjugates were more immunogenic, their size prevented characterization of the haptenation ratio by standard analytical methods and sterilization by filtration. To address this issue, conjugation chemistry and vaccine formulation were optimized for maximal efficacy, and conjugate size was measured by dynamic light scattering prior to adsorption to alum. An analogous heroin vaccine (M-sKLH) was also optimized for conjugation chemistry, formulated in alum, and characterized for potency against heroin in rats. Finally, this study found that the efficacy of OXY-sKLH was preserved when co-administered with M-sKLH, supporting the proof of concept for a bivalent vaccine formulation targeting both heroin and oxycodone. This study suggests methods for addressing the unique formulation and characterization challenges posed by conjugating small molecules to sKLH while preserving vaccine efficacy.
Keywords: opioid use disorder, oxycodone, heroin, vaccine, antibody, conjugate, GMP, GLP, FDA
Graphical Abstract
1. INTRODUCTION
The United States is facing public health crisis resulting from widespread opioid use disorders (OUD) and increased incidence of opioid-related overdoses. The incidence of opioid-related fatal overdoses has quadrupled since 19991 and over 42 249 people died from opioid overdose in 2016 alone.2–4 Only a limited subset of the population diagnosed with OUD is receiving medication-assisted treatment.1–4 As a complementary alternative to approved small molecule-based pharmacotherapies, vaccines may offer a safe and cost-effective treatment for OUD.5,6 Vaccines stimulate the patient’s own immune system to produce opioid-specific polyclonal antibodies that selectively bind the target opioid and prevent its distribution across the blood brain barrier, subsequently reducing opioid-induced behavior and other opioid-induced pharmacological undesired effects, such as respiratory depression.5 Although several studies have shown preclinical efficacy of opioid vaccines in mice,7,8 rats,9,10 and human primates,11,12 only one clinical trial has been conducted to date.13 Evaluation of a morphine-based conjugate vaccine showed safety, but only limited information is available,13 suggesting the need for further clinical evaluation of this approach. Ultimately, human studies will answer the question of whether opioid vaccines have the potential to become a viable treatment option for OUD.
Some of the challenges for transitioning vaccine candidates into late-stage preclinical development are as follows: (1) generating a well-characterized hapten–protein conjugate (drug substance) and hapten–protein conjugate adsorbed on adjuvant (drug product) that can be manufactured at the scale required for clinical testing, (2) access to vaccine components produced under good manufacturing practices (GMP), and (3) establishing and meeting release criteria for vaccine products compliant to the guidelines of the Food and Drug Administration (FDA), European Medical Agency (EMA), or other regulatory agencies. Although several candidate vaccines for OUD showed excellent preclinical efficacy, very few were made using GMP components, and their manufacturing processes were optimized and fully characterized.11,14,15
A first-generation vaccine consisting of an oxycodone-based hapten attached to the native keyhole limpet hemocyanin (KLH, decamer or didecamer) carrier protein adsorbed to alum adjuvant (OXY-KLH) has shown extensive preclinical safety and efficacy against oxycodone in mice and rats,16–19 butnative KLH is not available as GMP-grade material and therefore cannot be used in human trials. Because of its large molecular weight, native KLH is also difficult to characterize using standard analytical methods complicating translation of KLH-based vaccines. A second-generation oxycodone vaccine containing a GMP-grade subunit dimer KLH (OXY-dKLH) has shown equivalent efficacy compared to OXY-KLH and OXY conjugated to tetanus toxoid (OXY-TT)9 but has not been fully optimized for scale-up and technology transfer. Finally, the lead OXY hapten has proven effective when conjugated to other carrier proteins including a GMP-grade subunit monomer KLH (sKLH), CRM197, and TT as well as Escherichia coli-expressed CRM- (EcoCRM) and TT-based carriers.20 Because the sKLH was the most accessible GMP-grade carrier to date, our group has been seeking FDA approval for clinical evaluation of the OXY-sKLH, and the primary focus of this study was to facilitate manufacturing of OXY-sKLH adsorbed on alum adjuvant. Because we have previously shown efficacy of the M-KLH, M-sKLH, and M-EcoCRM vaccines targeting heroin and its metabolites in mice and rats,20–22 the secondary goal of this study was to optimize formulation of MsKLH for further development. Finally, the tertiary goal of this study was to test whether both OXY-sKLH and M-sKLH could be co-administered in a bivalent vaccine formulation targeting both heroin and oxycodone. First, OXY-sKLH was conjugated under a range of conditions to optimize the haptenation ratio, conjugate appearance and stability, and subsequently tested for immunogenicity and potency in rats. To allow for sterile filtration, chelants and reducing agents were evaluated for controlling the conjugate size. Reducing the conjugate size effectively decreased the degree of precipitation and loss on filtration but also negatively impacted the immunogenicity of the vaccine. Larger OXY-sKLH conjugates were characterized by dynamic light scattering (DLS) for size and an enzyme-linked immunosorbent assay (ELISA) was developed to measure relative haptenation of conjugates. To optimize M-sKLH, carbodiimide and maleimide coupling chemistries were compared for generation of effective vaccine formulations. In this study, addition of 10% dimethyl sulfoxide (DMSO) during carbodiimide conjugation yielded the most effective M-sKLH formulation. A previous study supported the rationale for developing a bivalent formulation that combines OXY-sKLH and M-sKLH for use as a single vaccine in humans, yet the study focused on a first-generation bivalent formulation containing native KLH and injected intraperitoneally in Freund’s complete and incomplete adjuvants.23 To further advance this concept, in the current study, OXY-sKLH adsorbed on alum was administered IM in combination with M-sKLH to determine whether a bivalent immunization regimen would interfere with its efficacy against oxycodone. Co-administration of OXY-sKLH and M-sKLH was as effective as doubling the dose of OXY-sKLH and more effective than the single dose of immunogen against oxycodone. These studies will advance the OXY-sKLH toward clinical evaluation.
2. MATERIAL AND METHODS
2.1. Synthesis of Oxycodone (OXY) Hapten and Conjugation to sKLH and Bovine Serum Albumin.
The oxycodone-based hapten containing a tetraglycine linker at the C6 position was synthesized as previously described to generate or Li++ salt to scale for manufacturing.20 As part of a technology transfer effort to support synthesis of GMP-grade haptens, the lyophilized form of the OXY(gly)4 hapten was synthesized at 30 g scale at Cambrex, NC. Because the native KLH used in previous studies is not available as the GMP source, the OXY(gly)4 hapten was conjugated to either the GMP-grade monomer KLH (Biosyn, Carlsbad, CA) or the GMP-grade dimer KLH (Stellar, Port Hueneme, CA). Because these KLH subunit formulations were equally immunogenic (Figure S1), and for simplicity, both were labeled as sKLH throughout. In addition, no differences in immunogenicity and efficacy were found when OXY-sKLH containing lyophilized OXY(gly)4 hapten synthesized at 30 g scale was compared with OXY-sKLH containing powder OXY(gly)4 hapten synthesized at 1.54 g scale (Figure S1). For use as the coating antigen in ELISA assays, haptens were conjugated to bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO). All conjugation conditions for the OXY hapten to sKLH are detailed in Table 1. The OXY hapten (range 5.2–52 mM) and ethyl-N′-(3 dimethylaminopropyl)carbodiimide hydrochloride (EDAC, 5.2–208 mM) (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.1 M MES buffer (range pH 4.5–6.0) and reacted for 10 min at room temperature (RT). sKLH or BSA were added and reactions stirred for 3 h at RT followed by ultrafiltration using Amicon filters with 50 or 100 kDa molecular weight cutoff (Merk Millipore, Burlington MA). MES buffer was exchanged with phosphate-buffered saline (PBS) 0.1 M pH 7.2 and the conjugate was stored at +4 °C.
Table 1.
Summary of Conjugation Conditions
| OXY-sKLH batch # | hapten (mM) | EDAC (mM) | EDTA (mM) | TCEP (mM) | NaCl (mM) | sucrose (mM) | quenching condition | storage buffer |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.2 | 208 | PBS pH 7.2 | |||||
| 2 | 5.2 | 208 | H2O 1 mM EDTA | |||||
| 3 | 5.2 | 52 | PBS pH 7.2 | |||||
| 4 | 5.2 | 52 | H2O 1 mM EDTA | |||||
| 5 | 52 | 52 | PBS pH 7.2 | |||||
| 6 | 52 | 52 | H2O 1 mM EDTA | |||||
| 7 | 26 | 26 | H2O 1 mM EDTA | |||||
| 8 | 10.4 | 10.4 | H2O 1 mM EDTA | |||||
| 9 | 5.2 | 5.2 | H2O 1 mM EDTA | |||||
| 10 | 26 | 104 | H2O 1 mM EDTA | |||||
| 11 | 52 | 52 | 1 | 15 | H2O 1 mM EDTA | |||
| 12 | 52 | 52 | 1 | 15 | PBS pH 7.2 | |||
| 13 | 52 | 52 | 15 | H2O 1 mM EDTA | ||||
| 14 | 52 | 52 | 15 | PBS pH 7.2 | ||||
| 15 | 52 | 52 | 1 | H2O 1 mM EDTA | ||||
| 16 | 5.2 | 208 | 1 | H2O 1 mM EDTA | ||||
| 17 | 5.2 | 208 | 15 | H2O 1 mM EDTA | ||||
| 18 | 5.2 | 208 | 1 | 15 | H2O 1 mM EDTA | |||
| 19 | 5.2 | 208 | 15 | PBS pH 7.2 | ||||
| 20 | 5.2 | 208 | 30 | H2O 1 mM EDTA | ||||
| 21 | 5.2 | 208 | 8 | H2O 1 mM EDTA | ||||
| 22 | 5.2 | 208 | 30 | PBS pH 7.2 | ||||
| 23 | 5.2 | 52 | 0.5 | PBS pH 7.2 | ||||
| 24 | 5.2 | 208 | 40 | PBS pH 7.2 | ||||
| 25 | 5.2 | 208 | pH 2.8 | PBS pH 7.2 | ||||
| 26 | 5.2 | 208 | 1% AcOH | PBS pH 7.2 |
2.2. Synthesis of Morphine (M) Hapten and Conjugation to Subunit KLH and BSA.
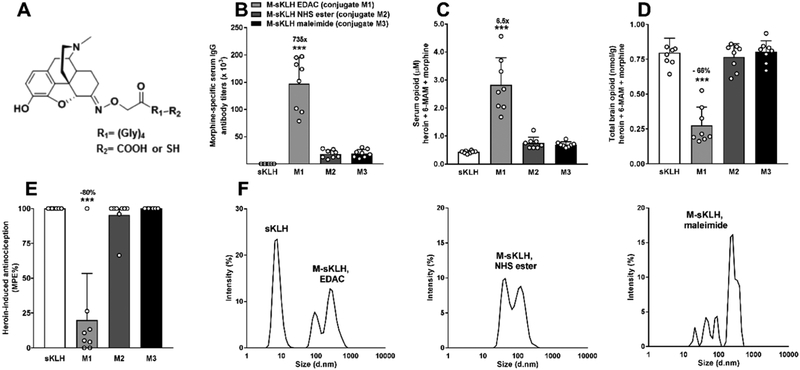
The intermediate morphine hapten structure without the tetraglycine linker was synthesized as previously described.23 A modified version of the M(gly)4OH hapten was generated by adding a terminal thiol group (M(gly)4SH) as previously described for an analogous OXY(gly)4SH.20 M(gly)4OH and M(gly)4SH Li++ haptens were conjugated to either sKLH or BSA as previously described,20,23 using either carbodiimide or maleimide chemistry with minor modifications to avoid precipitation as detailed below. One condition involved conjugation of the M(gly)4OH hapten to sKLH by dissolving 5.2 mM of hapten and 208 mM of EDAC in MES pH 4.5 in the presence of 10% of DMSO (conjugate M1, Figure 5). A second condition involved activation of the M(gly)4OH hapten with 52 mM EDAC in the presence of 128 mM sulfo-NHS (N-hydroxysuccinimide, Thermo Fisher Scientific, Waltham, MA) and 10% DMSO in MES buffer pH 5.0. Upon conversion to the NHS ester, the pH was increased to 7.0 using sodium hydroxide. Conjugations proceeded for 2 h at RT. After 2 h, the conjugate was purified as described in the section above (conjugate M2, Figure 5). A third condition consisted of conjugating M(gly)4SH to maleimide-activated sKLH, which was activated with sulfosuccinimidyl 4-[N-maleimidomethyl] cyclohexane-1-carboxylate (80:1 ratio of maleimide/sKLH moles) (sulfo-SMCC, Thermo Scientific, Waltham, MA) for 2 h at RT as described.20 The maleimide-activated protein was desalted by ultrafiltration. The hapten was dissolved in 715 μL of PBS pH 7.2 containing 1 mM ethylenediaminetetraacetic acid (EDTA) and 50 mM tris(2-carboxyethyl)phosphine (TCEP) (Sigma-Aldrich, St. Louis, MO), added to the sKLH solution and the conjugation proceeded for 4 h at RT. After 4 h, the conjugate was purified by ultrafiltration and stored in PBS pH 7.2 at 4 °C (conjugate M3, Figure 5).
Figure 5.
Characterization, immunogenicity, and efficacy of the M-sKLH vaccine in rats. (A) Morphine-based haptens. (B) Male Holtzman rats (n = 8/group) were immunized by IM injection with 60 μg of M-sKLH and 90 μg of alum on day 0, 21, 42, and 63. Serum was collected on day 70 and morphine-specific antibody titers were measured. On day 77, animals received a SC injection of 1 mg/kg of heroin and were tested on a hotplate set to 54 °C for nociception 30 min later. (C) Serum and (D) brain were collected following (E) hotplate testing. (F) Percent intensity of light scattering of different batches of M-sKLH. Drug distribution and behavioral data are the mean ± SD.
***p < 0.001 compared to control.
2.3. Characterization of Conjugates.
2.3.1. Dynamic Light Scattering.
Analysis was performed using a Zetasizer S90 (Malvern Instruments Inc., Westborough, MA) equipped with a 633 nm laser and an output power in the range of 10–50 mW. The size and diameter of the conjugates were measured under a 173° backscatter. To analyze the aggregation of the conjugate over time, an autopiloted measurement was applied, after manually mixing the sample. Measurements were performed with 75 μL of OXY-sKLH solution in 0.01 M PBS buffer pH 7.2 at a constant temperature of 25 °C using a 40 μL cuvette (Malvern Instruments Inc., Westborough, MA). Data were analyzed using Zetasizer software 7.12 and the raw data were exported to Excel and analyzed with Prism v.7 (Graph pad Software, La Jolla, CA). Analysis of three different OXY-sKLH batches by DLS validated this method (Figure S2). The size distribution of OXY-sKLH was consistent across the three different batches, confirming the reproducibility of the OXY-sKLH conjugation reaction and the DLS as a method to measure the size distribution of these conjugates.
2.3.2. Size Exclusion Chromatography–High-Performance Liquid Chromatography.
Samples were analyzed on a Shimadzu Prominence LC-20AD dual pump system with a SPD-20A UV/vis Spectrophotometer detector (Shimadzu Scientific Instruments Inc., Columbia, MD) using a GE Healthcare Superose 6 Increase column (GE Healthcare Bio-Sciences, Marlborough, MA). The analysis was performed at 280 and 230 nm wavelength using 0.5 mL/min flow rate. A PBS solution pH 7.0 mobile phase was selected to allow good separation, resolution, and for compatibility with solutions contained in samples of interest without hindering resolution and elution of the size standards. Several injections of the mobile phase alone were run throughout the batch to ensure there was no peak carry-over and/or peaks from the mobile phase contributing to the baseline. Run time was determined based on the length of time it took for the size standards to elute (40 min). In instances where an aggregate peak eluted into the next sample injection, run time was extended to include all sample peaks and size standards were run again to verify column efficiency.
2.3.3. Measurement of Hapten Density by ELISA.
Because sKLH is too large to measure the haptenation ratio (number of haptens per protein) using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF), semi-quantitative hapten density was measured using a modified ELISA. Mouse sera was obtained from mice immunized with OXY-CRM197, purified with Protein G (Thermo Fisher Scientific, Waltham, MA), and oxycodone-specific antibodies were coated on a 96-well plate (costar 9018 EIA/RIA 96, Jackson Research Laboratories, Inc., West Grove, PA) at 5 ng/well concentration using 0.5 M carbonate buffer at pH 9.6 and stored overnight at 4 °C. On the following day, the solution was discarded and the plate washed five times with 0.05 M phosphate buffered saline Tween-20 pH 7.2–7.4 (PBST). The plate was blocked with 1% gelatin blocking buffer in PBST. After 1 h, the gelatin was discarded and the plate was washed and stored overnight at 4 °C. On day 3, a standard curve was made using OXY-BSA conjugates with a range of haptenation ratios measured by MALDI-TOF and diluted to 5 μg/mL in PBST (Figure S3). BSA and sKLH controls, as well as OXY-sKLH conjugates with unknown haptenation ratios, were also diluted to 5 μg/mL. All samples were added to the plate in quadruplicate at 100 μL and incubated for 2 h at RT, slowly mixing at 60 rpm. After 2 h, the samples were discarded and the plate was washed. Rat sera which contained oxycodone-specific polyclonal antibodies derived from rats immunized with OXY-TT was diluted 5400× and 100 μL was added to three of the four wells per sample and 100 μL of 1:200 anti-sKLH rat serum was added to the remaining sample well to test for the presence of sKLH. The plate was gently mixed at 60 rpm for 1.5 h RT and incubated without mixing for another 0.5 h before discarding contents and washing with PBST. Secondary antibody Fc-specific goat anti-rat coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was diluted 1:50 000 and 100 μL was added to the plate and stored at 4 °C overnight. The following day, the contents were discarded and the plate was washed with PBST. Enzyme substrate o-phenylenediamine was added to all sample wells (SIGMA-FAST tablet set, Sigma Life Sciences, St Louis, MO). After 30 min of incubation, 2% oxalic acid was added to stop the enzymatic reaction. The plate was read at 492 nm on a BioTek PowerWave XS (BioTek Instruments Inc., Winooski, VT). The OXY-sKLH haptenation ratio was measured based on the OXY-BSA standard curve (function plotted as absorbance at 492 nm vs MALDI-TOF measured haptenation ratio), and the presence of sKLH was observed based on the comparison of the OD values from the OXY-BSA versus OXY-sKLH conjugates. To further qualify this hapten density ELISA assay, result reproducibility was determined within plates on the same day and across different days using representative OXY-sKLH conjugates ranging in the haptenation ratio. The results are reported in Table S1 (Supporting Information).
2.3.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis.
Samples were diluted to 1 μg/mL need concentration here in sterile water (dH2O) and combined with 4× laemmli sample buffer containing 2-mercaptoethanol according to manufacturer’s instructions (BioRad, Hercules, CA). Samples incubated for 10 min at 25 °C and 5 min at 95 °C before loading into a 3–8% tris-acetate protein gel submerged in XT-tricine running buffer (BioRad, Hercules, CA). A large molecular weight (MW) protein ladder (30–60 kDa, Thermo Fisher Scientific, Waltham, MA) was used to guide MW analysis. The gel was run at a constant voltage of 50 V for 30 min followed by 200 V for 2 h. After washing with dH2O several times, the gel was stained in biosafe Coomassie G250 stain (BioRad, Hercules, CA) for 1 h. The gel was then washed in dH2O for 30 min before imaging.
2.4. Experimental Design and Immunization.
2.4.1. Ethics Statement.
These studies were performed following the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal protocols were approved by the Hennepin Healthcare Research Institute Animal Care and Use Committee. Animals were euthanized by CO2 inhalation using AAALAC-approved chambers, and all efforts were made to minimize suffering.
2.4.2. Animals.
Male Holtzman and Sprague–Dawley rats (Envigo, Madison, WI) weighing 200–225 g at the day of arrival were double-housed with 12/12 h standard light/dark cycle and free-fed. Testing occurred during the light phase.
2.4.3. Vaccine Immunogenicity: Antibody Analysis.
Oxycodone or heroin/morphine-specific serum IgG antibody titers were measured using ELISA as previously described.9,23 Briefly, ELISA plates (Costar 9018 EIA/RIA, Jackson Immunoresearch Laboratories Inc., West Grove, PA) were coated with 5 ng/well of BSA conjugates or unconjugated protein control in carbonate buffer at pH 9.6 and blocked with 1% gelatin. Primary antibodies were incubated with goat anti-rat IgG antibodies conjugated to horseradish peroxidase or rabbit antimouse IgG antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to measure immunized rat and mouse sera.
2.4.4. Vaccine Potency: Hotplate Nociception Test and Analysis of Oxycodone and Heroin Distribution to Serum and to the Brain.
The effect of immunization in reducing opioid nociception and opioid distribution to the brain was used to measure vaccine potency in rats challenged with single doses of either oxycodone or heroin (NIDA Drug Supply Program). Rats were habituated to the testing environment for 1 h and then tested on a hotplate (Columbus Instruments, Columbus, OH) set to 54 °C to obtain baseline latencies, a nociceptive response of hindpaw lick or jumping. A maximum cutoff of 60 s was used to avoid injury. Thirty minutes later, rats were injected subcutaneously (SC) with either oxycodone or heroin, and their post-drug latency was obtained 30 min after challenge. Serum and brain samples were collected immediately afterward and oxycodone concentrations were measured by gas chromatography–mass spectrophotometry.17 Heroin, 6-AM, and morphine in serum and brain samples were determined by liquid chromatography–mass spectrophotometry (LC–MS).22 Vaccine efficacy in blocking opioid-induced antinociception was calculated as the percent maximum possible effect (% MPE), calculated as (post-drug latency − pre-drug latency/maximum latency − pre-drug latency) × 100.21
2.4.5. Effect of pH and EDAC Concentration on Efficacy of OXY-sKLH Conjugates.
Rats were vaccinated on days 0, 21, 42, and 63 IM with 60 μg of OXY-sKLH conjugated under a range of pH and EDAC concentrations as defined above (see section 2.1, and summary Table 1) adsorbed to 90 μg of alum adjuvant (Alhydrogel, Brenntag Biosector, Denmark) and 0.01% polysorbate 80 (PS80, Avantor, Center Valley, PA) in a final volume of 0.15 mL. Control rats (n = 10/group) were vaccinated IM with 60 μg of unconjugated sKLH in 90 μg of alum adjuvant in a final volume of 0.15 mL in PBS pH 7.2. On day 70, blood was collected via tail vein for antibody characterization.
2.4.6. Effect of OXY-sKLH Immunogen and Alum Dose on Efficacy.
Rats were vaccinated on days 0, 21, 42, and 63 IM with 0.4 mg/mL OXY-sKLH in 0.6 mg/mL alum adjuvant and 0.01% polysorbate 80 in a final volume of 0.15 mL (30, 60 μg OXY-sKLH and 45, 90 μg alum adjuvant) or 0.3 mL (120 μg OXY-sKLH and 180 μg alum adjuvant). A group of 12 rats was also vaccinated IM with 0.4 mg/mL unconjugated sKLH in 0.6 mg/mL alum adjuvant in a final volume of 0.3 mL (120 μg of sKLH and 180 μg of alum adjuvant). Blood was collected via tail vein on day 70 for serum antibody characterization. On day 77, all rats received 2.25 mg/kg oxycodone SC and were tested for hot plate nociception 30 min later. Immediately following hotplate testing, blood and brain were collected to measure oxycodone concentrations.
2.4.7. Effect of Sterile Filtration on Immunogenicity of OXY-sKLH.
Rats were vaccinated on day 0 IM with 60 μg of OXY-sKLH in 90 μg of alum adjuvant and 0.01% polysorbate 80 in a final volume of 0.15 mL. Control rats were vaccinated IM with 60 μg of unconjugated sKLH in 90 μg of alum adjuvant in a final volume of 0.15 mL in PBS pH 7.2. On day 7, blood was collected via tail vein for antibody characterization.
2.4.8. Effect of TCEP and EDAC on Size and Subsequent Immunogenicity of OXY-sKLH.
Rats were vaccinated on day 0 IM with 60 μg of OXY-sKLH in 90 μg of alum adjuvant and 0.01% polysorbate 80 in a final volume of 0.15 mL. Control rats were vaccinated IM with 60 μg of unconjugated sKLH in 90 μg of alum adjuvant in a final volume of 0.15 mL in PBS pH 7.2. On day 7, blood was collected via tail vein for antibody characterization.
2.4.9. Effect of Conjugation Chemistry on M-sKLH Immunogenicity and Size.
Rats were vaccinated on days 0, 21, 42, and 63 IM with 60 μg M-sKLH in 90 μg of alum adjuvant and 0.01% polysorbate 80 in a final volume of 0.15 mL. This experiment compared M-sKLH conjugates M1, M2, and M3, generated by carbodiimide, carbodiimide in presence of NHS ester, or maleimide. Control rats were vaccinated IM with 60 μg of unconjugated sKLH in 90 μg of alum adjuvant in a final volume of 0.15 mL in PBS pH 7.2. On day 70, blood was collected via tail vein for antibody characterization. On day 77, all groups of rats received 1 mg/kg heroin SC and were tested for hot plate nociception 30 min later. Immediately following testing, blood and brain were collected to measure the concentration of heroin and its metabolites by LC–MS.
2.4.10. Effect of Co-Administration of M-sKLH and OXY-sKLH on Oxycodone Distribution.
Rats were vaccinated on days 0, 21, 42, and 63 IM with 0.4 mg/mL OXY-sKLH, M-sKLH, or OXY-sKLH plus M-sKLH in 0.6 mg/mL alum adjuvant and 0.01% polysorbate 80 in a final volume of 0.15 or 0.3 mL (60 μg of OXY-sKLH, 60 μg of M-sKLH, 60 μg of OXY-sKLH plus 60 μg M-sKLH, and 120 μg of OXY-sKLH in 90 and 180 μg alum adjuvant, respectively). Control rats were vaccinated IM with 0.4 mg/mL unconjugated sKLH in 0.6 mg/mL alum adjuvant in a final volume of 0.15 mL (60 μg of sKLH and 90 μg of alum adjuvant). Blood was collected via tail vein on day 70 for serum antibody characterization. On day 77, all groups of rats received 2.25 mg/kg oxycodone SC and were tested for hot plate nociception 30 min later. Immediately following testing, blood and brain were collected to measure oxycodone concentrations.
2.5. Statistical Analysis.
Data were analyzed using Prism version 7.0 (GraphPad Software, San Diego, CA). The mean antibody titer, serum and brain concentrations, and percentage (%) MPE across groups were analyzed by the one-way ANOVA test paired with Tukey’s multiple comparisons post-hoc test. The group that did not pass the D’Agostino & Pearson normality test was analyzed using the Kruskal–Wallis test.
3. RESULTS
3.1. Experiment 1. Characterizing the Haptenation Ratio of OXY-BSA Conjugates.
Conjugation of oxycodone haptens, OXY(gly)4 Li++ salt and OXY(gly)4 TFA base, to BSA yielded haptenation ratios of 22 and 20, respectively. OXY(gly)4 Li++ salt was subsequently synthesized as a powder or lyophilized, conjugated to BSA, and yielded haptenation ratios of 27 and 28, respectively. Because the lyophilized hapten is easier to reconstitute compared to powder and because it showed the highest haptenation ratio, it was used in subsequent experiments.
3.2. Experiment 2. Effect of pH and EDAC Concentration on the Haptenation Ratio of OXY-BSA and Immunogenicity of OXY-sKLH.
The OXY(gly)4 Li++ hapten conjugated to BSA using MES buffer (4.5–7 pH) yielded higher haptenation ratios at lower pHs (Figure 1A). The OXY(gly)4 Li++ lyophilized hapten was subsequently conjugated to sKLH using MES pH 4.5 and 52 mM EDAC and its immunogenicity was tested in rats. While this vaccine formulation elicited oxycodone-specific serum IgG antibody titers of 68 ± 24 × 103 (mean ± SD, Figure 1B), the conjugate itself precipitated in solution after 2 h from the beginning of the conjugation. To prevent precipitation, conjugation conditions were optimized using OXY-BSA as a model immunogen over a range of EDAC concentrations (52–208 mM) at pH 6 in MES buffer. Increasing the concentration of EDAC during the conjugation of OXY-BSA led to higher haptenation ratios (Figure 1A). OXY-sKLH was then conjugated using 208 mM EDAC in the pH 4.5–6.0 range and showed relative hapten densities of 33–38 as calculated by hapten density ELISA. OXY-sKLH conjugated at lower pH conditions elicited higher oxycodone-specific IgG titers (Figure 1B). The OXY-sKLH conjugated at pH 4.5 showed slight precipitation in solution, whereas the OXY-sKLH conjugated using pH 5.0 and 208 mM EDAC showed higher hapten density and elicited high oxycodone-specific IgG titers. Hence, OXY-sKLH conjugated using pH 5.0 and 208 mM EDAC was chosen as the lead vaccine for all subsequent studies.
Figure 1.
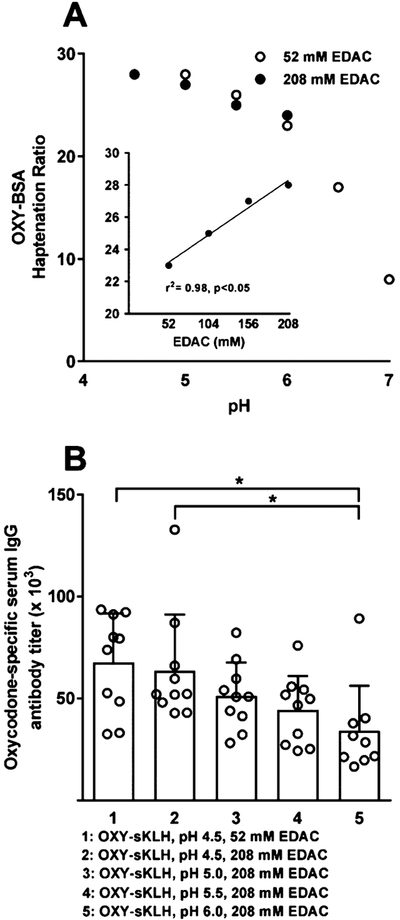
Effect of pH and EDAC concentration on haptenation ratio and immunogenicity. (A) MALDI-TOF was used to calculate haptenation ratios of OXY-BSA conjugated at pH 4.5–7 using 5.2 mM of hapten, 52 or 208 mM EDAC concentrations and (A, inset) at pH 6.0 from 52–208 mM EDAC added to BSA or sKLH for a final concentration of 2.3 or 2.8 mg/mL, respectively. (B) Male Holtzman rats (n = 10/group) were immunized by IM injection with 60 μg of OXY-sKLH and 90 μg of alum on day 0, 21, 42, and 63. Serum was collected on day 70 and oxycodone-specific serum IgG antibody titers were determined by ELISA.
*p < 0.05 brackets indicate group differences.
3.3. Experiment 3. Effect of Dose on Immunogenicity of OXY-sKLH.
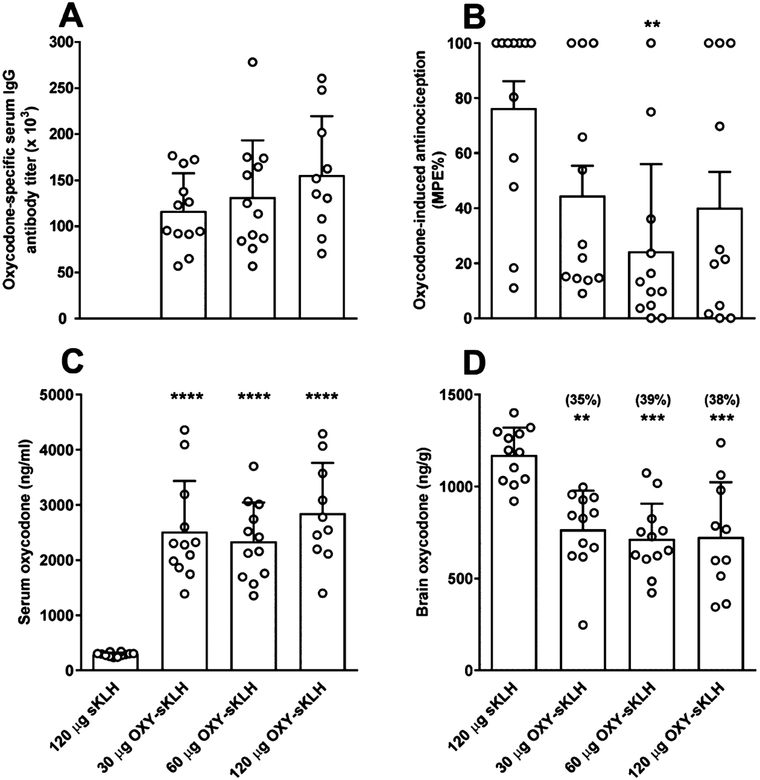
Rats were first vaccinated with 1, 3, 10, and 30 μg of OXY-sKLH to determine the lowest effective dose of vaccine. Only rats that received 10 and 30 μg of OXY-sKLH developed high titers, which were significantly different from titers elicited by 1 and 3 μg of OXY-sKLH, respectively (Figure S4 Panel A). In addition, brain oxycodone concentration was reduced by 38 and 35% in rats that were immunized with 10 and 30 μg of OXY-sKLH, respectively (Figure S4 Panel B). In a subsequent experiment, vaccination with 30, 60, or 120 μg OXY-sKLH elicited oxycodone-specific IgG antibody titers in all vaccinated rats (Figure 2A). Rats that received 60 μg, but not 30 or 120 μg, of OXY-sKLH showed reduced % MPE compared to controls (p < 0.01, Figure 2B). Serum oxycodone concentrations were significantly increased in all groups compared to controls (p < 0.0001, Figure 2C). Brain oxycodone concentrations were significantly reduced in all groups compared to controls (p < 0.01, 0.001, Figure 2D). OXY-sKLH (60 μg) formulated with 90 μg of aluminum was chosen as the lead formulation for subsequent experiments because it was the lowest dose of vaccine that showed the greatest efficacy in the thermal nociception test.
Figure 2.
Oxycodone-specific antibody titers, oxycodone distribution, and antinociception in rats. Male Holtzman rats (n = 12/group) were immunized by IM injection with 30, 60, and 120 μg of OXY-sKLH and 45, 90, and 180 μg of alum, respectively, on day 0, 21, 42, and 63. (A) Serum was collected on day 70 and oxycodone-specific antibody titers were measured. (B) On day 77, animals received a SC injection of 2.25 mg/kg of oxycodone and were tested on a hotplate set to 54 °C for nociception 30 min later. (C) Serum and (D) brain samples were collected immediately following hotplate testing. Numbers above bars represent the percent difference from controls. Distribution and behavioral data are the mean ± SD.
**p < 0.01, ***p < 0.001, ****p < 0.0001 compared to control.
3.4. Experiment 4. Effect of Sterile Filtration on Immunogenicity of OXY-sKLH.
Rats were immunized with OXY-sKLH conjugates that were filtered with either regenerated cellulose (RC) 0.2 μm or RC 0.45 μm filters (Sartorius AG, Germany) and compared to nonfiltered conjugates. Vaccine batches sterile-filtered with 0.2 μm, but not 0.45 μm, filters elicited significantly lower oxycodone-specific antibody titers compared to nonsterile filtered OXY-sKLH (p < 0.05, Figure 3A), whereas 0.45 μm filtration did not affect the size of the drug substance; the conjugate filtered using 0.2 μm showed a reduction in size when analyzed by DLS (Figure 3B), suggesting that OXY-sKLH is too large to be sterile-filtered and 0.2 μm filtration might impact the identity of the drug substance. Sterile filtration through 0.45 μm size filters resulted in a 94% recovery, whereas 0.2 μm size filters resulted in 40% recovery, suggesting that these conjugates may be too large for standard sterile filtration methods. A subsequent experiment compared the effect of purifying OXY-sKLH by either PES (polyethersulfone) or RC membranes on the vaccine’s immunogenicity. Although both PES-and RC-purified conjugates elicited effective antibodies, use of PES membranes resulted in precipitation of the OXY-sKLH, suggesting that RC membranes are more suitable for further development (Figure S5).
Figure 3.
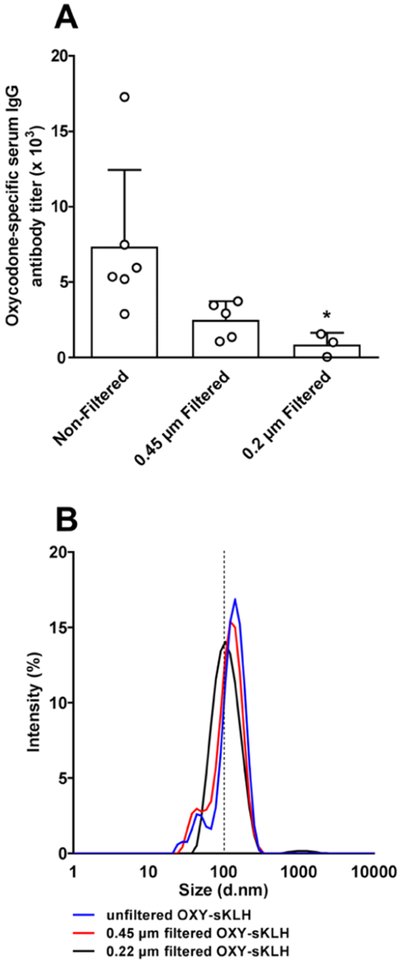
Effect of the filter size on OXY-sKLH immunogenicity and size. (A) Male Holtzman rats (n = 3–6/group) were immunized by IM injection with 60 μg of filtered or unfiltered OXY-sKLH with 90 μg of alum on day 0. Serum was collected on day 7 and oxycodone-specific antibody titers were measured. (B) Percent intensity of light scatter of filtered and nonfiltered OXY-sKLH.
*p < 0.05 compared to the nonfiltered OXY-sKLH.
3.5. Experiment 5. Effect of TCEP and EDAC on Size and Immunogenicity of OXY-sKLH.
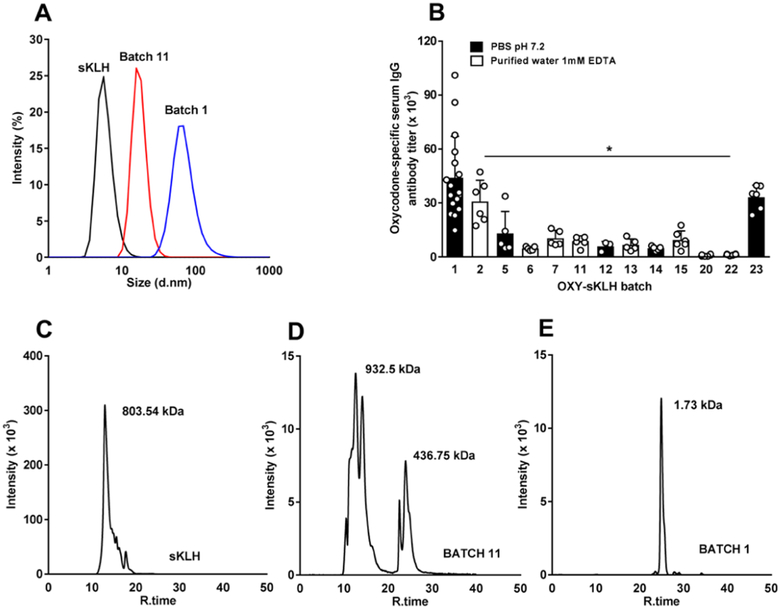
Varying concentrations of TCEP and EDTA were added to the conjugation reactions containing various hapten/EDAC molar ratios (Table 1) to reduce the size of OXY-sKLH and allow subsequent sterile filtration. To further improve conjugate formulation, the vaccine was stored either in PBS 0.1 M pH 7.2 or 1 mM EDTA in water pH 7.2 (Table 1). Batch 11 (Table 1) filtered through a 0.22 μm size filter showed a leftward shift in the DLS curve, indicating a reduction in size compared to batch 1 (Figure 4A). However, batch 11 elicited titers of 8.7 ± 2.7 × 103 (mean ± SD), which were significantly lower than those elicited by batch 1 (p < 0.05), our lead candidate vaccine (Figure 4B). Reduction in size of batch 11 was also confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, with a band around 460 kDa (lane 8, Figure S6) compared to batch 1 which did not migrate into the gel (lane 7, Figure S6). Batches 5, 17, and 19, which were conjugated as described in Table 1, had a band visible near the gel loading well, but the band further down was indistinct. Batch 12 and 18 showed a band around 460 kDa, which was comparable to batch 11. Size exclusion chromatography (SEC) was used to analyze sKLH, the lead conjugate (batch 1), and batch 11. Conjugate 11 had two major peaks. The first peak was similar in size to sKLH (Figure 4C) while the other was approximately 400–450 kDa (Figure 4D). Batch 1 had a single peak close to the MW limits for the column, suggesting that it was too large to be analyzed by SEC (Figure 4E). To circumvent the limitation of sterile-filtering larger conjugates, individual reagents could be sterile-filtered prior to conjugation and manufacturing can be conducted under aspetic conditions.
Figure 4.
Oxycodone-specific antibody titers and DLS. (A) DLS of sKLH and OXY-sKLH batch 1 and 11. (B) Male Holtzman rats (n = 3–17/group) were immunized by IM injection with 60 μg of OXY-sKLH with 90 μg of alum on day 0. Serum was collected on day 7 and oxycodone-specific antibody titers were measured. Data are mean ± SD (C–E) SEC analysis performed on sKLH and OXY-sKLH (batch 1 and 11).
*p < 0.05 compared to batch 1.
3.6. Experiment 6. Effect of Conjugation Chemistry on Heroin/Morphine Vaccine Efficacy.
Conditions identified during optimization of the oxycodone vaccine were applied to the optimization of the M-sKLH vaccine (Figure 5A). Conjugation of M-sKLH was performed using EDAC, EDAC in presence of NHS ester, and maleimide and the relative M-sKLH conjugates were characterized by DLS and then subsequently tested for efficacy in vivo. Conjugation of the lead M(gly)4OH hapten to BSA using EDAC resulted in a haptenation ratio of 29, and the resulting M-sKLH was effective (conjugate M1, Figure 5B). The EDAC coupling reaction was not improved by the presence of sulfo-NHS ester (conjugate M2, Figure 5B, haptenation ratio of 8). Similarly, the use of maleimide chemistry to conjugate the novel M(gly)4SH hapten to BSA (conjugate M3, Figure 5B) showed a haptenation ratio of 7. The DLS panel for M1, 2, and 3 is shown in Figure 5F. Conjugate M2 and M3 are slightly smaller than conjugate M1, maybe due to lower haptenation or reduced cross-linking in solution during the conjugation reaction. Conjugate M1 elicited titers of 147 ± 48 × 103 (mean ± SD), which were significantly higher than those elicited by M2 and M3 (p < 0.001), which elicited titers of 18 196 ± 7525 and 19 025 ± 7356, respectively (Figure 5B). In contrast to M2 and M3, M1 showed a significant increase in serum heroin, 6-AM, and morphine compared to controls (p < 0.001, Figure 5C) following SC administration of 1 mg/kg of heroin. In fact, M1 reduced heroin-induced antinociception by 80% and reduced drug distribution to the brain by 66% compared to M2, M3, and control (p < 0.001 for all comparisons, Figure 5C–E). These data suggest that EDAC-conjugated M-sKLH (M1) is the most effective conjugate vaccine for further development.
3.7. Experiment 7. Effect of Co-administration of Morphine and Oxycodone Vaccine on Immunogenicity and Efficacy.
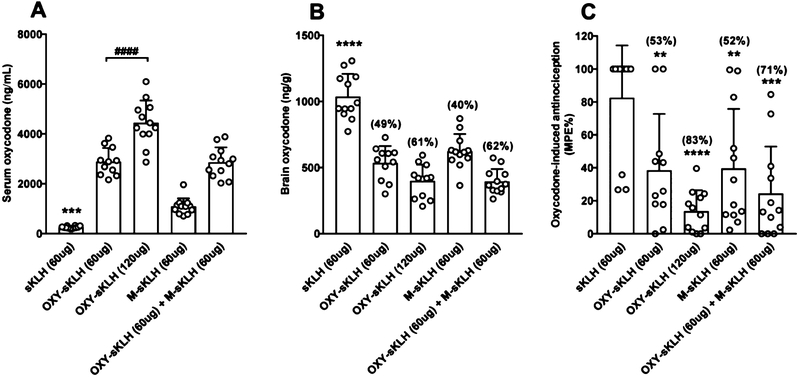
OXY-sKLH and M-sKLH were co-administered to test whether a bivalent vaccine formulation would potentially affect the immunogenicity and efficacy of OXY-sKLH. Vaccination with the monovalent OXY-sKLH at either 60 or 120 μg doses and the bivalent vaccine formulation (60 μg OXY-sKLH and 60 μg M-sKLH) elicited high oxycodone-specific antibody titers. The presence of M-sKLH in the bivalent formulation did not interfere with the development of oxycodone-specific antibody titers (Table 2). Oxycodone-specific antibody titers elicited by the monovalent OXY-sKLH vaccine (60 and 120 μg) cross-reacted with the morphine hapten by 15 and 17%, respectively. Both doses of monovalent OXY-sKLH (60 and 120 μg) and the bivalent vaccine significantly increased the retention of oxycodone in serum compared to the sKLH control group (p < 0.001, Figure 6A). The increase in serum oxycodone elicited by 120 μg of OXY-sKLH was significantly greater than the 60 μg dose of OXY-sKLH, suggesting an effect of immunogen dose (p < 0.0001, Figure 6A). Distribution of oxycodone to the brain was significantly reduced by all monovalent and bivalent vaccine formulations compared to the sKLH control group (p < 0.0001, Figure 6B). The monovalent OXY-sKLH (120 μg) vaccine and bivalent vaccine reduced oxycodone to the brain respectively by 61 and 62% compared to the sKLH group. OXY-sKLH (120 μg) and the bivalent vaccine reduced oxycodone antinociception by 83 and 71%, respectively (p < 0.0001, 0.001, Figure 6C). Vaccination with M-sKLH also produced a significant decrease in the distribution of oxycodone to the brain (Figure 6B), consistent with ELISA titers (Table 2), showing 33% cross-reactivity of anti-morphine antibodies with the oxycodone hapten. M-sKLH also reduced oxycodone antinociception by 52% compared to the KLH control group (p < 0.01, Figure 6C).
Table 2.
Serum, Antibody Titers (×103) and Cross-Reactivity (%)a
| coating immunogen | OXY-sKLH 60 μg | OXY-sKLH 120 μg | M-sKLH 60 μg | M-sKLH + OXY-sKLH 60 μg + 60 μg |
|---|---|---|---|---|
| M-BSA | 31 ± 12 (15%) | 56 ± 18 (17%) | 243 ± 75 | 164 ± 63 |
| OXY-BSA | 213 ± 30b | 334 ± 92b | 80 ± 26 (33%) | 226 ± 71b |
Cross-reactivity of serum antibodies for the nontargeted hapten was calculated by dividing the antibody titers measured using the nontargeted coating antigen by the antibody titers measured using the targeted coating antigen and multiplying by 100 to obtain a percentage.
Compared to the sKLH control group.
Figure 6.
Immunogenicity and efficacy of OXY-sKLH co-administered in a bivalent formulation in rats. Male Holtzman rats (n = 12/group) were immunized on day 0, 21, 42, and 63 by IM injection with 60 μg of OXY-sKLH, 60 μg of M-sKLH, 60 μg of OXY-sKLH plus 60 μg of M-sKLH, and 120 μg of OXY-sKLH adsorbed on either 90 or 180 μg of alum adjuvant, respectively. On day 77, animals received a SC injection of 2.25 mg/kg of oxycodone and were tested on a hotplate set to 54 °C for nociception 30 min later. (A) Serum and (B) brain samples were collected immediately following (C) hotplate testing. Numbers above bars represent the percentage of difference from controls. Drug distribution and behavioral data are the mean ± SD.
**p < 0.01. ***p < 0.001, ****p < 0.0001 compared to control. ####p < 0.0001 brackets indicate group differences. (A) **** OXY-sKLH (120 μg) vs M-sKLH. (B) **** sKLH (60 μg) vs M-sKLH (60 μg), *** OXY-sKLH (120 μg) vs M-sKLH (60 μg), *** M-sKLH (60 μg) vs OXY-sKLH (60 μg) + M-sKLH (60 μg).
4. DISCUSSION
Vaccines offer a promising approach to treat OUD and potentially reduce incidence of fatal overdoses. To date, several vaccine candidates have shown preclinical proof of selectivity and efficacy in reducing the behavioral effects of heroin, oxycodone, hydrocodone, fentanyl, and fentanyl-like compounds9,19,22,24,25 as well as opioid-induced respiratory depression and lethality.8,9,24 One of the biggest challenges to preclinical vaccine development is to generate drug products that can be characterized and manufactured at scale under GMP. Hence, this study focused on further advancing the lead OXY-sKLH and M-sKLH vaccines toward clinical evaluation.
The major findings from this study were as follows: (1) synthesis of OXY(gly)4 hapten as a lithium salt led to a higher haptenation ratio than as TFA salt. A lyophilized version of the OXY(gly)4 hapten synthesized at our CMO site was easier to conjugate to sKLH than the same hapten formulated as powder, (2) increasing the EDAC concentration in the conjugation reaction increased the haptenation ratio and decreased precipitation, (3) when comparing vaccine doses, 60 μg of immunogen was the most effective dose, (4) larger OXY-sKLH conjugates were more immunogenic than smaller conjugates, but filtration through a 0.22 μm filter resulted in significant loss of vaccine and efficacy. As a proposed solution, individual vaccine components could be sterile-filtered prior to conjugation and the conjugation could be conducted under sterile conditions, (5) optimal vaccine efficacy against heroin was achieved by a morphine-based hapten conjugated to the carrier protein using EDAC coupling chemistry in presence of DMSO, and (6) co-administration of 60 μg of OXY-sKLH and 60 μg of M-sKLH yielded a bivalent vaccine that was as effective as doubling the dose of OXY-sKLH (120 μg) and more effective than a single dose of OXY-sKLH (60 μg).
Hapten and linker chemistry can greatly affect vaccine efficacy.11,19,26 Previous studies have shown that opioid vaccines containing haptens conjugated to carrier proteins via tetraglycine linkers using carbodiimide coupling were more effective than structurally similar haptens containing terminal thiol groups for maleimide chemistry.11,17,19 This study showed that OXY(gly)4 synthesized as lithium salt yielded a higher haptenation ratio after conjugation compared to a TFA salt, perhaps because of an interference of TFA during the coupling reaction. It is known that TFA provides carboxyl groups that may interfere with EDAC chemistry. In addition, a lyophilized version of the OXY(gly)4 hapten synthesized at the CMO site yielded a higher haptenation ratio compared to the previously established powder form. In our experience, lyophilized haptens offer the advantage of simplifying manufacturing, greater stability, and greater solubility compared to the same haptens in powder form.
The OXY-sKLH vaccine precipitated when conjugated in MES buffer at pH 4.5 using 52 mM of EDAC, despite a trend for higher haptenation ratios at lower conjugation pH. Adding higher concentrations of EDAC (208 mM) decreased precipitation and increased the haptenation ratio, perhaps creating cross-linking, carrier protein-carrier protein interactions, and stabilizing the conjugate in solution, causing random polymerization of polyproteins.27
In exploring vaccine doses, OXY-sKLH showed to be effective at doses as low as 10 μg, which is within the range of doses previously used in clinical trials.28–30 The 60 μg dose of immunogen was chosen because it was the most effective dose in reducing oxycodone antinociception, even though no differences were found in titers, oxycodone serum, or brain levels when compared to doses of 30 and 120 μg. These results suggest that a possible plateau effect in rats was reached when 120 μg of conjugate and 180 μg of alum adjuvant were used. Considering that opioid users self-reported orally abused oxycodone doses of 2 mg/kg,31,32 the OXY-sKLH vaccine showed preclinical efficacy in reducing antinociception and brain oxycodone levels when immunized rats or mice were challenged with SC doses of oxycodone higher than commonly abused oral doses in humans.
In this study, OXY-sKLH could not be sterilized by filtration without affecting its potency. Sterility is one of the requirements to obtain regulatory approval for human testing. Therefore, sterile filtration of the drug substance was evaluated using 0.2 and 0.45 μm size filters as advised by the FDA.33 Filtration is the only sterilization procedure that could be performed with these heroin and oxycodone vaccines because sterilization by heat would induce aggregation and degradation of the carrier protein. In order to reduce the size and aggregation of the OXY-sKLH conjugate, a chelant and a reducing agent were tested during conjugation as well as quenching strategies. TCEP is typically used as a reducing agent to selectively break disulfide bonds inside or between proteins. In molecular models of KLH, the functional units of the KLH subunits are stabilized by disulfide bonds which seem to be responsible to maintain the integrity of the tertiary structure of KLH.34,35 Use of TCEP during conjugation reduced the OXY-sKLH conjugate size, but also lowered its immunogenicity and efficacy against oxycodone. There is a correlation between size and immunogenicity, but the immunological and biochemical mechanisms are not clear.36 Protein aggregation is known to increase the immune response to antigens.37 Immunization with several batches of nicotine vaccines exhibiting different degrees of aggregation showed the extent of aggregation correlated with individual vaccine efficacy against nicotine in both mice and non-human primates.38–40 Another hypothesis for increased immunogenicity because of the presence of aggregates is that T cell-dependent B cell activation is promoted by repetitive epitopes in carrier proteins or aggregates, which may enhance B cell activation.41,42 In fact, the OXY(gly)4 hapten is effective when conjugated to a variety of carrier proteins, but it is not effective when conjugated to peptides14 or polymers such as ficoll and dextran,20 and its efficacy depends upon CD4+ T cell activation.7,19 Because the size of OXY-sKLH is an essential requirement for retaining vaccine efficacy, filtration of individual components prior to conjugation under sterile conditions could be a viable strategy to achieve sterility without compromising efficacy.
Here, morphine-based haptens were conjugated to sKLH (M-sKLH) using a similar strategy as the lead OXY-sKLH vaccine. We have previously shown that OXY haptens equipped with a tetraglycine linker with a C-term carboxyl group were more effective against oxycodone than OXY haptens containing the same linker with a C-term thiol (−SH) group or a polyethylene glycol (PEG) linker.20 Another group has shown the feasibility of developing an effective heroin vaccine using a PEGylated hapten equipped with a thiol group for maleimide chemistry,15,43 suggesting that linker chemistry should be optimized for each individual hapten or drug target. In this study, M-sKLH conjugated using EDAC had the highest haptenation ratio, which was consistent with another heroin vaccine,11 and effectively reduced the behavioral effects of 1 mg/kg of heroin in rodents. Instead, M-sKLH conjugated using either NHS ester or maleimide chemistry was not effective and therefore discarded from further development. As the M(gly)4 hapten is less soluble in MES buffer than the analogous oxycodone hapten, optimal conjugation required 10% DMSO. DMSO is a nontoxic polar aprotic solvent that dissolves both polar and nonpolar compounds, and it was added to the conjugation reaction to increase the solubility of the morphine hapten and consequently stabilize the O-acylisourea intermediate that is formed when EDAC reacts with carboxylic acid groups present on the morphine hapten.27
Co-administration of OXY-sKLH and M-sKLH (60 μg of each) in a bivalent vaccine formulation was as effective as doubling the OXY-sKLH dose (120 μg) and better than the single OXY-sKLH dose (60 μg). Because of the cross-reactivity shown by the morphine-specific antibodies with the oxycodone hapten (Table 2), it is possible that a B cell population subset may recognize both the OXY and M haptens resulting in an augmented activation of each respective hapten-specific B cell population. These data are consistent with a previous study focusing on combination of OXY-KLH and M-KLH, which showed that M-KLH partially reduced distribution of oxycodone to the brain in rats.23 The current experiment further demonstrated that the addition of M-sKLH did not interfere with the efficacy of OXY-sKLH. The bivalent vaccine increased serum antibody titers compared to the monovalent vaccine, which is consistent with what has been shown previously.23 Combining OXY-sKLH and M-sKLH in a single formulation would appear to be a better option for increasing the efficacy of an oxycodone vaccine than simply increasing the dose because of limitations in the amount of alum adjuvant and immunogen that can be administered clinically. This is an important implication considering that previous addiction vaccines failed in clinical trials because of relatively low and variable antibody titers.28–30 In addition, a bivalent or multivalent vaccine formulation could have an advantage to treat subjects who abuse a range of opioids.
In conclusion, this study identified optimal conditions for further advancement of the OXY-sKLH and M-sKLH candidate vaccines toward manufacturing and IND-enabling studies.
Supplementary Material
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharma-ceut.8b01296.
Oxycodone-specific antibody titers and oxycodone distribution in rats immunized with either OXY-dKLH or OXY-sKLH; OXY-sKLH size distribution as measured by dynamic light scattering; hapten density ELISA standard curve and characterization of BSA carrier protein and OXY-BSA by MALDI-TOF; oxycodonespecific antibody titers and oxycodone brain distribution in rats immunized with increasing OXY-sKLH doses; oxycodone-specific antibody titers and oxycodone distribution in rats immunized with OXY-sKLH purified by filtering membranes of different chemical compositions; and effect of conjugation conditions on size of OXY-sKLH (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Seth P; Scholl L; Rudd RA; Bacon S Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep 2018, 67, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States. https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a1.htm?s_cid=mm6712a1_w, 2015–2016. (accessed 2018). [DOI] [PMC free article] [PubMed]
- (3).Rudd RA; Seth P; David F; Scholl L Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep 2016, 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- (4).Dowell D; Arias E; Kochanek K; Anderson R; Guy GP Jr.; Losby JL; Baldwin G Contribution of Opioid-Involved Poisoning to the Change in Life Expectancy in the United States, 2000–2015. JAMA 2017, 318, 1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pravetoni M Biologics to treat substance use disorders: Current status and new directions. Hum. Vaccines Immunother 2016, 12, 3005–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pentel PR; LeSage MG New directions in nicotine vaccine design and use. Adv. Pharmacol 2014, 69, 553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Laudenbach M; Baruffaldi F; Vervacke JS; Distefano MD; Titcombe PJ; Mueller DL; Tubo NJ; Griffith TS; Pravetoni M The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J. Immunol 2015, 194, 5926–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kimishima A; Wenthur CJ; Zhou B; Janda KD An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem. Biol 2017, 12, 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Raleigh MD; Peterson SJ; Laudenbach M; Baruffaldi F; Carroll FI; Comer SD; Navarro HA; Langston TL; Runyon SP; Winston S; Pravetoni M; Pentel PR Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One 2017, 12, No. e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Li Q-Q; Luo Y-X; Sun C-Y; Xue Y-X; Zhu W-L; Shi H-S; Zhai H-F; Shi J; Lu L A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J. Neurochem 2011, 119, 1271–1281. [DOI] [PubMed] [Google Scholar]
- (11).Bremer PT; Schlosburg JE; Banks ML; Steele FF; Zhou B; Poklis JL; Janda KD Development of a Clinically Viable Heroin Vaccine. J. Am. Chem. Soc 2017, 139, 8601–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bonese KF; Wainer BH; Fitch FW; Rothberg RM; Schuster CR Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974, 252, 708–710. [DOI] [PubMed] [Google Scholar]
- (13).Akbarzadeh A; Norouzian D; Farhangi A; Mehrabi M; Chiani M; Zare D; Saffari Z; Mortazavi M; Nikdel A Immunotherapy of 347 Volunteer Outpatient Morphine Addicts by Human Therapeutic Morphine Vaccine in Kermanshah Province of Iran. J. of Pharmacology and Toxicology 2009, 4, 30–35. [Google Scholar]
- (14).Pravetoni M; Vervacke JS; Distefano MD; Tucker AM; Laudenbach M; Pentel PR Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 2014, 9, No. e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Torres OB; Jalah R; Rice KC; Li F; Antoline JFG; Iyer MR; Jacobson AE; Boutaghou MN; Alving CR; Matyas GR Characterization and optimization of heroin hapten-BSA conjugates: method development for the synthesis of reproducible hapten-based vaccines. Anal. Bioanal. Chem 2014, 406, 5927–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pravetoni M; Pentel PR; Potter DN; Chartoff EH; Tally L; LeSage MG Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 2014, 9, No. e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Pravetoni M; Le Naour M; Harmon TM; Tucker AM; Portoghese PS; Pentel PR An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J. Pharmacol. Exp. Ther 2012, 341, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Laudenbach M; Baruffaldi F; Robinson C; Carter P; Seelig D; Baehr C; Pravetoni M Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci. Rep 2018, 8, 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pravetoni M; Le Naour M; Tucker AM; Harmon TM; Hawley TM; Portoghese PS; Pentel PR Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem 2013, 56, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Baruffaldi F; Huseby Kelcher A; Laudenbach M; Gradinati V; Limkar A; Roslawski M; Birnbaum A; Lees A; Hassler C; Runyon SP; Pravetoni M Pre-clinical efficacy and characterization of candidate vaccines for treatment of opioid use disorders using clinically viable carrier proteins. Mol. Pharmaceutics 2018, 15, 4947–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Raleigh MD; Laudenbach M; Baruffaldi F; Peterson SJ; Roslawski MJ; Birnbaum AK; Carroll FI; Runyon SP; Winston S; Pentel PR; Pravetoni M Opioid Dose- and Route-Dependent Efficacy of Oxycodone and Heroin Vaccines in Rats. J. Pharmacol. Exp. Ther 2018, 365, 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Raleigh MD; Pravetoni M; Harris AC; Birnbaum AK; Pentel PR Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J. Pharmacol. Exp. Ther 2013, 344, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pravetoni M; Raleigh MD; Le Naour M; Tucker AM; Harmon TM; Jones JM; Birnbaum AK; Portoghese PS; Pentel PR Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccines 2012, 30, 4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bremer PT; Kimishima A; Schlosburg JE; Zhou B; Collins KC; Janda KD Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem., Int. Ed 2016, 55, 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sulima A; Jalah R; Antoline JFG; Torres OB; Imler GH; Deschamps JR; Beck Z; Alving CR; Jacobson AE; Rice KC; Matyas GR A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J. Med. Chem 2018, 61, 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Stowe GN; Schlosburg JE; Vendruscolo LF; Edwards S; Misra KK; Schulteis G; Zakhari JS; Koob GF; Janda KD Developing a vaccine against multiple psychoactive targets: a case study of heroin. CNS Neurol. Disord.: Drug Targets 2011, 10, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nakajima N; Ikada Y Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate Chem. 1995, 6, 123–130. [DOI] [PubMed] [Google Scholar]
- (28).Hatsukami DK; Jorenby DE; Gonzales D; Rigotti NA; Glover ED; Oncken CA; Tashkin DP; Reus VI; Akhavain RC; Fahim REF; Kessler PD; Niknian M; Kalnik MW; Rennard SI Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharm. Ther 2011, 89, 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Martell BA; Orson FM; Poling J; Mitchell E; Rossen RD; Gardner T; Kosten TR Cocaine Vaccine for the Treatment of Cocaine Dependence in Methadone-Maintained Patients. Arch. Gen. Psychiatry 2009, 66, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Cornuz J; Zwahlen S; Jungi WF; Osterwalder J; Klingler K; van Melle G; Bangala Y; Guessous I; Müller P; Willers J; Maurer P; Bachmann MF; Cerny T A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 2008, 3, No. e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hays LR A profile of OxyContin addiction. J. Addict. Dis 2004, 23, 1–9. [DOI] [PubMed] [Google Scholar]
- (32).Katz DA; Hays LR; Jellinek MS Adolescent OxyContin Abuse. J. Am. Acad. Child Adolesc. Psychiatr 2004, 43, 231–234. [DOI] [PubMed] [Google Scholar]
- (33).FDA Guidance for Industry. Sterile Drug Products Produced By Aseptic Processing - Current Good Manufacturing Practice. https://www.gmp-compliance.org/guidelines/gmp-guideline/fda-guidance-for-industry-sterile-drug-products-produced-by-aseptic-processing-current-good-manufacturing-practice-september-200, September 2004. (accessed 2018).
- (34).Gatsogiannis C; Markl J Keyhole Limpet Hemocyanin: 9-Å CryoEM Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units. J. Mol. Biol 2009, 385, 963–983. [DOI] [PubMed] [Google Scholar]
- (35).Jaenicke E; Büchler K; Decker H; Markl J; Schröder GF The refined structure of functional unit h of keyhole limpet hemocyanin (KLH1-h) reveals disulfide bridges. IUBMB Life 2011, 63, 183–187. [DOI] [PubMed] [Google Scholar]
- (36).Ratanji KD; Derrick JP; Dearman RJ; Kimber I Immunogenicity of therapeutic proteins: influence of aggregation. J. Immunotoxicol 2014, 11, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hermeling S; Crommelin DJA; Schellekens H; Jiskoot W Structure-immunogenicity relationships of therapeutic proteins. Pharmaceut. Res 2004, 21, 897–903. [DOI] [PubMed] [Google Scholar]
- (38).Thorn JM; Bhattacharya K; Crutcher R; Sperry J; Isele C; Kelly B; Yates L; Zobel J; Zhang N; Davis H; McCluskie M The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice. Vaccines 2017, 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).McCluskie MJ; Thorn J; Gervais DP; Stead DR; Zhang N; Benoit M; Cartier J; Kim I-J; Bhattacharya K; Finneman JI; Merson JR; Davis HL Anti-nicotine vaccines: Comparison of adjuvanted CRM 197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharmacol 2015, 29, 663–671. [DOI] [PubMed] [Google Scholar]
- (40).McCluskie MJ; Thorn J; Mehelic PR; Kolhe P; Bhattacharya K; Finneman JI; Stead DR; Piatchek MB; Zhang N; Chikh G; Cartier J; Evans DM; Merson JR; Davis HL Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int. Immunopharmacol 2015, 25, 518–527. [DOI] [PubMed] [Google Scholar]
- (41).Baker M; Carr F Pre-clinical considerations in the assessment of immunogenicity for protein therapeutics. Curr. Drug Saf 2010, 5, 308–313. [DOI] [PubMed] [Google Scholar]
- (42).Dintzis HM; Dintzis RZ; Vogelstein B Molecular determinants of immunogenicity: the immunon model of immune response. Proc. Natl. Acad. Sci. U.S.A 1976, 73, 3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Jalah R; Torres OB; Mayorov AV; Li F; Antoline JFG; Jacobson AE; Rice KC; Deschamps JR; Beck Z; Alving CR; Matyas GR Efficacy, but Not Antibody Titer or Affinity, of a Heroin Hapten Conjugate Vaccine Correlates with Increasing Hapten Densities on Tetanus Toxoid, but Not on CRM197 Carriers. Bioconjugate Chem. 2015, 26, 1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.