Abstract
Purpose of review
The Roux-en-Y gastric bypass surgery (RYGB) improves glucose control in majority of patients with type 2 diabetes. However, a minority group of individuals develop a life-threatening complication of hyperinsulinemic hypoglycemia. The goal of this review is to identify underlying mechanisms by which RYGB cause hypoglycemia and describe pathogenesis-driven strategies to diagnose and treat this condition.
Recent findings
Gastric bypass leads to higher and earlier peak levels of glucose and lower nadir glucose after eating along with larger insulin and glucagon-like peptide 1 (GLP-1) secretion, resetting the balance between glucose appearance and clearance after this procedure. These weight-loss independent glycemic effects of RYGB have been attributed to changes in ingested glucose appearance as a result of rapid nutrient emptying from stomach pouch to the intestine and increased glucose clearance as a result of prandial hyperinsulinemia. The exaggerated effect of RYGB on postmeal glucose metabolism is a syndrome of postprandial hyperinsulinemic hypoglycemia manifesting in a group of individuals several years after this surgery. Affected patients have larger systemic appearance of ingested glucose and greater postmeal secretion of insulin and GLP-1 compared to those with history of RYGB without symptomatic hypoglycemia. Current evidence supporting a multifactorial model of glucose dysregulation among patients with hypoglycemia will be highlighted in this review.
Summary
Hypoglycemia after RYGB is a life-threatening condition and likely represents the extreme glycemic phenotype of this procedure. Diagnosis is challenging and treatment options are limited.
Keywords: counterregulatory response, gastric bypass surgery, glucose kinetics, hypoglycemia, islet-cell function
INTRODUCTION
Over the last two decades bariatric surgeries have gained popularity given their effectiveness in treatment of obesity and mitigation of its comorbidities [1]. It is now well documented that Roux-en-Y gastric bypass surgery (RYGB) leads to diabetes remission [1]. The dramatic effects of RYGB on the regulation of blood glucose are partly independent of weight loss [2]. The immediate glycemic effects of this procedures are generally explained by faster passage of ingested nutrients into the gut, leading to rapid rise of glucose levels after eating, achieving earlier and higher glycemic peaks followed by lower glucose nadirs [2]. Along with changes in postprandial glucose pattern postmeal secretion of insulin and GLP-1 is also increased after RYGB [3]. The meal-induced hyperinsulinemia typical of RYGB is caused by both enhanced stimulation of β-cells by glucose and GLP-1 [4] and diminished insulin clearance [5]. The glycemic effects of RYGB are exaggerated in a minority group of individuals who suffer from a debilitating condition of postprandial hyperinsulinemic hypoglycemia [3,6].
Despite sporadic case reports of postprandial hypoglycemia after sleeve gastrectomy [7] hypoglycemia after this procedure is much less characterized, and in our experience is likely to be of lesser severity than after gastric bypass. Therefore, this review will focus only on post-RYGB hypoglycemia.
Recent studies have identified multiple pathophysiological factors contributing to hypoglycemia following RYGB, providing important insights into the development of novel ways to treat this condition. Here, we address the known pathogenic mechanisms of hypoglycemia in the context of altered balanced between glucose output and glucose utilization caused by RYGB. We also review the current recommendations regarding diagnosis and treatment of hypoglycemia in this setting.
DIAGNOSIS AND PREVALENCE
Diagnosis of hypoglycemia relies on detecting low glucose values associated with symptoms or signs that are relieved immediately by raising glucose levels (Whipple’s triad [2]). Symptoms of hypoglycemia are nonspecific and traditionally divided into autonomic or neuroglycopenic symptoms, which is attributed to brain glucose deprivation. Hypoglycemia awareness is mainly the result of autonomic nervous system activation, either cholinergic (hunger, sweating, and paresthesia) or adrenergic (palpitation, shakiness, and anxiety) [8]. Neuroglycopenic manifestations of hypoglycemia, however, range from weakness and behavioral changes to confusion, seizure, and loss of consciousness, which are often observable and described by a witness.
It has been well documented that acute reduction of plasma glucose to 55mg/dl (blood glucose <50mg/dL) in persons without history of gastrointestinal surgery during fasting condition triggers symptoms and physiologic responses designed to prevent further decline in glucose [2]. Although, in prandial condition, the glucose threshold for hypoglycemia that provokes physiologic response and symptoms is much less characterized, challenging making the diagnosis of postprandial hypoglycemia. In a large cohort of healthy individuals (N = 650), oral administration of 100 g glucose led to postprandial glucose levels less than 60 mg/dl in 25% and less than 50mg/dl in 10% of studied subjects without any associated symptoms [2]. Furthermore, only 10% of patients with normal gastrointestinal tract suffering from postprandial symptoms consistent with hypoglycemia (N = 118) were found to have low glucose levels when subjected to oral glucose ingestion [2].
Discerning the postmeal hypoglycemia after gastric bypass from those of postprandial asymptomatic low glucose levels or symptoms associated with normal glycemia is even more challenging given a higher frequency of both conditions after this procedure. Evaluation of glucose profile without any information regarding associated symptoms using continuous glucose monitoring system (CGMS) for 5 days demonstrated that 70% of patients with RYGB (N = 40) had at least one episode of interstitial glucose level less than 55mg/dl several years after surgery [2]. Although, examining glucose profile along with associated symptoms showed that 80% of low glucose events (defined as glucose <60mg/dl) were not associated with any symptoms [2]. Also a large number of individuals after RYGB suffer from dumping symptoms that overlap with those of hypoglycemia, particularly autonomic symptoms [9].
Therefore, it is crucial to confirm Whipple’s triad, the presence of symptoms when glucose levels are low (blood glucose <50mg/dl and plasma glucose <55 mg/dl) and absence of symptoms when glucose levels are normal as the very first step after a detailed medical history and physical exam is obtained. This can be done by careful examination of records of documented capillary blood or venous glucose levels during spells and characteristics and severity of associated symptoms and signs, and relationship of spells to meal ingestion, specific trigger foods, or activity. The use of CGMS should be limited for the evaluation of glycemic pattern at the baseline or monitoring the response to treatment because the accuracy of this method in detecting low glucose levels are not optimal [2]. Given the difficulties in distinguishing the symptoms of dumping from those of autonomic symptoms of hypoglycemia, we recommend that diagnosis is made based on neuroglycopenic symptoms. When Whipple’s triad during hypoglycemic event cannot be documented during free-living condition, a mixed-meal test should be utilized to recreate a setting that hypoglycemia occurs. Oral glucose tolerance test is not recommended for the evaluation of postprandial hypoglycemia [2].
Additional diagnostic tests should be considered to exclude malnutrition with excessive weight loss, adrenal insufficiency, or other critical illnesses for ill-appearing patients with hypoglycemia after RYGB. Also, other causes of hypoglycemia such as insulinoma should be excluded in individuals with fasting hypoglycemia, manifesting within the first 6–12 months from surgery or beyond 5 h from prior meal ingestion.
True prevalence of hypoglycemia following RYGB is unknown; however, it is likely underestimated because of complexity of diagnosis and lack of public awareness. In a retrospective survey of electronic medical records of 1206 nondiabetic individuals with prior RYGB, one in 10 individuals were found to have either documented low glucose level (glucose <60mg/dl) or treated with medications for this condition [10]. In the same study, the prevalence of severe hypoglycemia requiring emergency room visit or hospitalization was reported to be less than 1%. These figures are similar to a prospective study in 957 post-RYGB individuals, where the incidence of developing blood glucose less than 50 mg/dl after glucose challenge was approximately 10% during the 5-year follow-up [11]. In contrast, studies using self-report questionnaires report much higher prevalence rates for hypoglycemia, likely due to the overlap of symptoms of dumping and hypoglycemia in this population [12].
PATHOPHYSIOLOGY OF POST-GASTRIC BYPASS HYPOGLYCEMIA
In general, hypoglycemia develops when the total glucose delivery to circulation (ingested carbohydrate and hepatic glucose production) falls behind the total glucose utilization from circulation (by brain, red blood cells, and renal medulla, and insulin sensitive tissues such as muscles). Multiple counterregulatory mechanisms are in place to prevent further disturbance of continuous supply of glucose to the brain from circulation. The two main counter-regulatory responses in person with normal gastrointestinal tract to decreasing glucose concentrations within physiologic range are suppressed insulin secretion and enhanced glucagon release. The goal for these physiologic responses is to minimize glucose utilization and enhance hepatic glucose output preventing further reduction in glycemia [2].
Bypassing the foregut resets the balance between the glucose delivery and utilization, improving postmeal glycemia in most patients while predisposing susceptible individuals to develop hypoglycemia.
Altered glucose delivery after gastric bypass
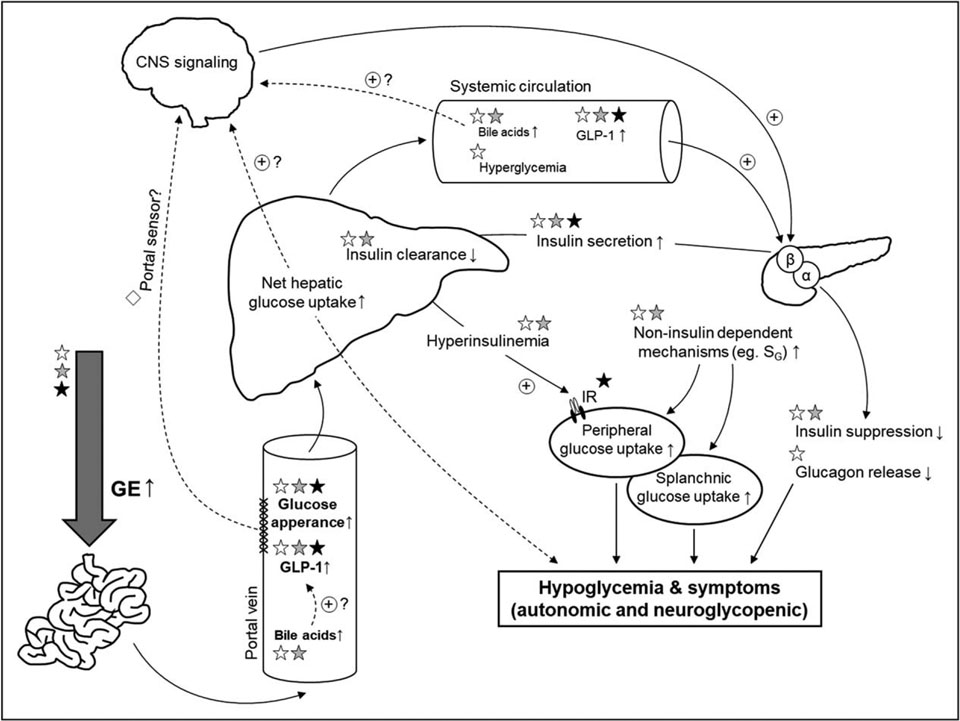
In healthy, nonoperated individuals, the postprandial glucose excursion is determined by the pace of food passage from the stomach into the gut (i.e., 1–4kcal/min) in addition to hepatic and extrahepatic splanchnic glucose uptake [2]. In patients after RYGB, nutrient emptying from the gastric pouch to the gut is accelerated by 100-fold, leading to earlier and higher peak of glucose and lower nadir glucose levels compared to nonoperated individuals [2]. The typical enhanced glucose excursion observed after RYGB is even larger in those with RYGB-related hypoglycemia compared to matched asymptomatic patients with prior history of RYGB (Fig. 1) [13].
FIGURE 1.
Changes in glucose delivery and glucose utilization typical of gastric bypass surgery and the steps that are implicated in the pathogenesis of hypoglycemia after this procedure. The glycemic gradient across the liver contributing to enhanced net hepatic glucose uptake is shown by different font sizes in this figure. ☆, Gastric bypass effect; ☆, Pathophysiologic defect specific to hypoglycemia; ★, Treatment options utilized based on pathogenic factors. CNS, central nervous system; GE, gastric emptying; GLP-1, glucagon-like peptide-1; IR, insulin receptor; SG, glucose effectiveness.
In addition to altered ingested glucose appearance as a result of rerouted gastrointestinal tract, the hepatic net uptake and net output of glucose seems to be also affected by RYGB. Meal ingestion in nonoperated individuals shifts the hepatic net glucose output during fasting state to the net glucose uptake during prandial condition [14]. In patients with T2D 10% weight loss induced by RYGB has shown to lower fasting endogenous glucose output compared to those who achieved similar weight loss by calorie restriction only [15], suggestive of a weight-loss independent effect of RYGB on hepatic glucose metabolism. Also the meal inhibitory effect on endogenous glucose output is significantly larger after RYGB compared with corresponding parameters measured before surgery [16]. The hepatic glucose uptake effect of eating has been attributed to various factors including portal sensory signals that are important in regulation of glucose metabolism; one of the established afferent signals is the negative arterial-portal vein (A–V) glucose gradient [14]. The glycemic gradient across the liver can potentially be augmented as a result of rapid passage of ingested glucose into the intestine after RYGB. Using tracer technique during meal studies we have shown that among patients after RYGB those with hypoglycemia have significantly larger ingested glucose appearance (pre-hepatic) compared to those without hypoglycemia despite similar systemic peak glucose levels [13], suggesting a larger negative A–V glucose gradient in the affected individuals. Therefore, it is plausible that exaggerated glucose concentration gradient across the liver after RYGB contributes to the shift of glucose uptake towards the liver and away from extrahepatic tissues after this procedure in general but to larger extent in those with RYGB-related hypoglycemia.
Altered glucose utilization
Postprandial hyperinsulinemia
Enhanced meal-induced β-cell secretion
In parallel with larger postprandial glucose excursion after RYGB insulin response to meal ingestion shifts to the left and upward, leading to larger and earlier prandial insulin secretion in individuals after this procedure compared to the nonoperated controls [3]. Within patients with prior history of RYGB postmeal insulin secretion is significantly greater in those with hypoglycemia compared to those without [3,6]. Enhanced postprandial insulin secretion in patients with post-RYGB hypoglycemia has been attributed to both larger β-cell sensitivity to increasing glycemia and impaired insulin suppression when glucose concentrations fall below fasting levels [13,17,18]. The blunted β-cell secretory response to the decline of glucose levels have been also reported during short-term studies of induced hypoglycemia in patients after RYGB compared to the nonsurgical control [19,20]. These findings altogether are indicative of dysregulation of β-cell response to hypoglycemia after RYGB interfering with physiologic responses required to minimize glucose utilization during hypoglycemia.
The question remains to be addressed is to what extent the enhanced β-cell secretion after RYGB in general, and particularly in those with hypoglycemia, is the result of larger glycemic stimulus or whether other factors are at play. Postprandial insulin secretion in the nonoperated individuals is tightly regulated by enteroinsular axis activity, which is mainly mediated by insulinotropic gut hormones (GLP-1 and GIP) and neural factors. Growing evidence support that enhanced meal-induced insulin secretion following RYGB is mediated by both hormonal, mainly GLP-1 [4,13,15], and nonhormonal gut factors [20,21].
The well-documented association of postmeal hyperinsulinemia with massive GLP-1 secretion after RYGB [4] raised the possibility that this peptide contributes to enhanced prandial β-cell function after this surgery. Along the same line patients who suffer from hypoglycemia after RYGB were reported to have much larger GLP-1 response to meal ingestion compared to asymptomatic RYGB individuals [3,6,13,17]. Using exendin-(9–39), a potent GLP-1 receptor antagonist, during meal studies has provided conclusive evidence that GLP-1 contribution to postprandial insulin secretion is, in fact, increased after RYGB [4,13]. Furthermore, blocking GLP-1 signaling has shown to correct hypoglycemia in the affected RYGB patients during meal studies [13,22], supporting the pathogenic effect of GLP-1 in this condition.
Bile acids are recently recognized as metabolic regulators, and postmeal bile acid metabolism seems to be changed after RYGB [23]. The role of altered bile acid metabolism or composition after RYGB on glucose metabolism or islet function is still unclear. However, we have recently reported that in two cases with RYGB-related hypoglycemia postprandial unconjugated bile acids are elevated compared to the asymptomatic post-RYGB patients during meal studies that preceded with sham feeding [24]. In this study nadir glucose levels during meal studies were inversely associated with postmeal unconjugated bile acid. Further studies are needed to explore the role of bile acid in glucose metabolism after RYGB.
Altered β-cell morphology
Insulinotropic gut peptides, including GLP-1, have been implicated in regulation of β-cell mass, mainly based on in-vivo and in-vitro studies in animals [2]. Therefore, one of the early hypotheses proposed to explain postprandial hyperinsulinemic hypoglycemia in the setting of RYGB was that increased β-cell mass as a result of increased GLP-1 secretion is the culprit [2,25]. However, the original report of isletcell hypertrophy based on histologic examination of surgical specimens from the hypoglycemia patients treated with partial pancreatectomy has been disputed by subsequent analysis of the same histologic samples when compared with islets from the BMI-matched nonoperated individuals [2]. The role of enhanced β-cell mass in developing hypoglycemia has been also challenged by the failure of partial pancreatectomy to resolve hypoglycemia in the majority of individuals. Thus, current data indicate that the hyperinsulinemia in post-RYGB hypoglycemia patients is mostly caused by larger insulin secretory response to rapid nutrient flux rather than increased β-cell mass.
Decreased postmeal insulin clearance
Peripheral insulin concentrations are determined by the balance between insulin secretion and (mainly hepatic) insulin clearance. Growing evidence indicates that insulin clearance in fasting condition, measured as a ratio between insulin secretion (or C-peptide) to insulin, is significantly increased after RYGB [5,15,26]. However, insulin clearance after meal ingestion, measured as area-under-the-curve (AUC) of insulin secretion to insulin, appears to be reduced after RYGB compared to corresponding values from before surgery [5]. Furthermore, patients with hypoglycemia after RYGB have diminished postmeal insulin clearance by 30% compared to asymptomatic individual after this surgery [3], augmenting postprandial hyperinsulinemia in this condition even further. At this point whether the cause of reduced insulin clearance in these patients is due to inherent variations in insulin internalization or acquired condition due to gastric bypass is largely unknown. However, in one case with prior history of RYGB postmeal insulin clearance measured during oral meal ingestion, condition bypassing the foregut, was lower than that during feeding through a gastrostomy tube, setting that includes the foregut, despite similar GLP-1 and GIP secretion [27]. This observation suggests that bypassing the foregut per se has an effect on insulin clearance that is independent of insulinotropic gut hormones.
Insulin-independent mechanisms
Altered glucose effectiveness
In addition to insulin signaling, glucose tolerance is determined by glucose effectiveness (SG, mass-action effect of glucose), that is the ability of glucose per se to stimulate its own uptake and decrease endogenous glucose production. Patients with prior RYGB have reduced SG during intravenous infusion of step-wise increasing doses of glucose compared with BMI-matched nonsurgical controls [28]. However, affected patients seem to have higher SG, measured using intravenous bolus glucose infusion, compared to asymptomatic patients after RYGB [2]. These observations raise the possibility that the physiologic adaptation of reduced SG following RYGB is lacking in individuals with hypoglycemia; a hypothesis that merits further consideration.
Impaired glucagon response to hypoglycemia
Attenuation of counterregulatory responses could perpetuate hypoglycemia and, however, frequent hypoglycemic episodes lead to blunted counterregulatory responses and unawareness [2]. Glucagon release in response to the decline of glycemia within normal physiologic range stimulates hepatic glucose output in a dose-dependent fashion to avoid hypoglycemia [2]. Although postprandial glucagon response is increased after RYGB [3,13], the postmeal glucagon responses among patients with and without hypoglycemia are almost identical [3,6], indicative of dysregulated a-cell response to hypoglycemia. The blunted glucagon response to hypoglycemia induced by insulin infusion during fasting condition have been well documented by two independent groups of investigators in RYGB-treated patients compared to the same individual before surgery [19] or nonoperated matched controls [20].
TREATMENT
Well designed clinical trials are missing for currently recommended dietary and medical interventions. As reviewed, major pathogenic factors contributing to hypoglycemia after RYGB are increased appearance of ingested glucose to circulation as a result of faster delivery of nutrient to the intestine and enhanced glucose clearance. Therefore, the most effective treatment options are strategies that selectively target the pace of nutrient flux or postmeal insulin secretory response.
Dietary modifications
Given the limitation in the number of available therapeutic options, dietary modifications remain the cornerstone of treatment. Based on basic understanding of carbohydrate absorption from gut, recommendations such as lowering the amount of carbohydrate for every meal (<30 g) or snack (<15 g), avoiding simple carbohydrates with high-glycemic index, and adding protein and fat to every meal and snacks are expected to reduce the glucose excursion and hypoglycemia [29,30]. While avoiding carbohydrate completely is not recommended, it has been documented that lowering the amount of carbohydrate can narrow glucose excursion and increase nadir glucose levels in patients with gastric bypass [31]. In addition to lowering the amount of carbohydrate intake changes in carbohydrate composition, from glucose to fructose, has also been beneficial in increasing nadir glucose levels in patients with post-RYGB hypoglycemia by reducing postmeal glucose spikes [2].
Pharmacotherapy
The use of currently available drugs is mainly based on their effectiveness in treatment of other hypoglycemic conditions such as insulinoma.
Acarbose, an antidiabetic medication, has been used to treat hypoglycemia after RYGB as the first agent started with dietary modification. This intestinal a-glucosidase blocker narrows postmeal glucose excursion and lowers peak glucose by changing digestion and absorption of the luminal carbohydrates. Therefore, the effect of acarbose is relatively selective in reducing postmeal hyperglycemia [2], however utilization of this drug is limited by its gastrointestinal side effects such as flatulence and bloating, especially if the dose is not titrated up gradually.
When dietary modification and acarbose fail to improve frequency or severity of hypoglycemia, medical interventions to reduce insulin secretion are considered as the second line of treatment. Among these agents, diazoxide, a potent vasodilator, inhibits insulin secretion by stimulation of KATP channels in β-cells. Administration of diazoxide in a single case has shown to have some beneficial effects in improving hypoglycemia for several months [2]. Side effects such as hyperglycemia because of systemic suppression of β-cell secretion, peripheral edema, hypotension, and headache limit its use.
Somatostatin analogues, which has been used in insulinoma, bind to somatostatin receptors subtypes 2 and 5 (SST2, SST5) and alter intestinal motility, islet-cell hormonal release and gut hormone secretion simultaneously. Therefore, administration of this compound has double-edge effect as its inhibitory effects on insulin and GLP-1 secretion are favorable in increasing glucose levels whereas suppression of glucagon might not be. In a case of hypoglycemia after RYGB administration of single-dose octreotide (100 μg) before oral glucose challenge increased glucose levels significantly for 5 h as a result of complete inhibition of insulin and GLP-1 responses [2]. This patient was thereafter treated with a long-acting analogue lanreotide and remained symptom-free for a few years. Also, administration of pasireotide versus placebo in a 2-week crossover trial in nine patients with post-RYGB with prior history of symptomatic low glucose levels reduced glucose excursion after oral glucose ingestion [2]. The use of these drugs is limited by their cost as well as gastrointestinal side effects such as diarrhea.
There are also a few investigational drugs in various phases of development. One of these compounds completed phase II clinical trial studies is exendin-(9–39) (Eiger Biopharmaceutical, Palo Alto, CA, USA). The development of this drug was based on earlier observation that hypoglycemia after RYGB was corrected by short-term intravenous infusion of exendin-(9–39) as a result of suppression of postmeal insulin secretion [13,32]. Another investigational treatment for this condition is subcutaneous infusion of glucagon utilizing an event-based open loop system that deliver glucagon in anticipation of hypoglycemia [33]. Finally, blocking the insulin receptor with a human monoclonal antibody XOmA 358 (XOMA Corporation, Berkeley, CA, USA) has been investigated as another option for treatment of hypoglycemia caused by RYGB or other etiologies [34].
Surgical procedures
Surgical interventions are considered for severe life-threatening hypoglycemia who remain symptomatic despite combination of adequate dietary modifications and medical managements. Both gastric bypass reversal and feeding through gastrostomy tube (G-tube) in remnant stomach have been utilized as treatment in refractory hypoglycemia with some benefits by reducing postmeal glucose variability. A summary of published cases treated with reversal surgery reported some improvement in frequency of hypoglycemic events in 12 of 17 affected patients [2]. Glycemic improvement after reversal surgery is often associated with weight regain and delayed gastric emptying, therefore the use of this option is only considered for treatment of refractory cases [35]. Long-term feeding through G-tube is also utilized in some institutions for patients who are not interested in reversal procedure. In a recent study [36], feeding into the remnant stomach by G-tube resulted in markedly reduced glucose excursion and improvement in nadir glucose when compared to oral feeding in six patients with symptomatic post-RYGB hypoglycemia. In the same study, patients underwent a surgical reversal of the RYGB and reported resolution of symptoms during the 20-month follow-up.
Partial pancreatectomy is no longer recommended as a therapeutic option because patients treated with this option experience relapse of postprandial hypoglycemia in several months in spite of developing fasting hyperglycemia as a result of reduced pancreatic mass [2]. Other methods in slowing the gastric emptying after RYGB include gastric pouch outlet restriction and endoscopic plication, although data to evaluate their efficacy in hypoglycemia treatment is lacking.
CONCLUSION
Experimental studies support a multifactorial model of glucose dysregulation among patients with post-RYGB hypoglycemia. Therapeutic options delaying intestinal glucose delivery or suppressing β-cell secretory response have been tried with some success. Further investigation into the long-term effects of RYGB on islet-cells function and glucose metabolism is important for development of strategies to prevent and treat this debilitating complication.
KEY POINTS.
Postprandial hyperinsulinemic hypoglycemia is a challenging late-complication of weight-loss surgery, affecting nearly 10% of patients.
Diagnosis of this condition is complex and requires confirmation of Whipple’s triad.
Patients with RYGB-related hypoglycemia have enhanced systemic appearance of ingested glucose and increased glucose clearance as a result of greater GLP-1 contribution to insulin secretion and lower insulin clearance compared to asymptomatic individuals after this surgery.
Long-term effects of RYGB on islet-cells function may contribute to hypoglycemia in susceptible individuals after this procedure.
Dietary modification and medical and surgical interventions that aim to reduce the postmeal glycemic spikes and insulin secretion are most effective therapeutic strategies.
Acknowledgements
Financial support and sponsorship
H.H. was supported by the grant form Finnish Cultural Foundation (no. 00180071). M.S. was supported by the grant from The National Institute of Diabetes and Digestive and Kidney Diseases (DK105379).
Footnotes
Conflicts of interest
There are no conflicts of interest associated with this manuscript.
REFERENCES
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 7:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab 2018; 8:2815–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab 2014; 6:2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013; 9:3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah A, Holter MM, Rimawi F, et al. Insulin clearance after oral and intravenous glucose following gastric bypass and gastric banding weight loss. Diabetes Care 2019; 2:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 2007; 12:4678–4685. [DOI] [PubMed] [Google Scholar]
- 7.Capristo E, Panunzi S, De Gaetano A, et al. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metab 2018; 6:2136–2146. [DOI] [PubMed] [Google Scholar]
- 8.Yaqub A, Smith EP, Salehi M. Hyperinsulinemic hypoglycemia after gastric bypass surgery: what’s up and what’s down? Int J Obes (Lond) 2017; 42:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emous M, Wolffenbuttel BHR, Totte E, van Beek AP. The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg Obes Relat Dis 2017; 9:1489–1500. [DOI] [PubMed] [Google Scholar]
- 10.Lee CJ, Wood GC, Lazo M, et al. Risk of postgastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity (Silver Spring) 2016; 6:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raverdy V, Baud G, Pigeyre M, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after roux-en-y gastric bypass: a five year longitudinal study. Ann Surg 2016; 5:878–885. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen JB, Pedersen AM, Gribsholt SB, et al. Prevalence, severity, and predictors ofsymptoms ofdumping and hypoglycemia afterRoux-en-Y gastric bypass. Surg Obes Relat Dis 2016; 8:1562–1568. [DOI] [PubMed] [Google Scholar]
- 13.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014; 3:669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 2017; 10:572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetter ML, Wadden TA, Teff KL, et al. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes 2015; 2:434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camastra S, Astiarraga BD, Barbieri C, et al. Beneficial effects of RYGB on β-cell function and hepatic and peripheral insulin sensitivity are maintained seven years after surgery in both diabetic and nondiabetic subjects. Diabetes 2018; Suppl 1:2089-P. [Google Scholar]
- 17.Tharakan G, Behary P, Wewer Albrechtsen NJ, et al. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. Eur J Endocrinol 2017; 6:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poitou C, Bouaziz-Amar E, Genser L, et al. Fasting levels of glicentin are higher in Roux-en-Y gastric bypass patients exhibiting postprandial hypoglycemia during a meal test. Surg Obes Relat Dis 2018; 7:929–935. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson N, Borjesson JL, Sundbom M, et al. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes 2016; 9:2667–2675. [DOI] [PubMed] [Google Scholar]
- 20.Salehi M, Woods SC, D’Alessio DA. Gastric bypass alters both glucose-dependent and glucose-independent regulation of islet hormone secretion. Obesity (Silver Spring) 2015; 10:2046–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi M, Gastaldelli A, D’Alessio DA. Role ofvagal activation in postprandial glucose metabolism after gastric bypass in individuals with and without hypoglycaemia. Diabetes Obes Metab 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig CM, Liu LF, Nguyen T, et al. Efficacy and pharmacokinetics of subcutaneous exendin (9–39) in patients with postbariatric hypoglycaemia. Diabetes Obes Metab 2018; 2:352–361. [DOI] [PubMed] [Google Scholar]
- 23.Khan FH, Kohli R. Bariatric surgery: the rise and fall of bile acids. Surg Obes Relat Dis 2016; 4:770–771. [DOI] [PubMed] [Google Scholar]
- 24.Honka H, D’Alessio DA, DeFronzo RA, Salehi M. Altered bile acid metabolism aftergastric bypass in subjects with and without hypoglycemia. Obesity Week 2018; T-P-3266. [Google Scholar]
- 25.Patti ME, Goldfine AB, Hu J, et al. Heterogeneity of proliferative markers in pancreatic beta-cells of patientswith severe hypoglycemia following Roux-en-Y gastric bypass. Acta Diabetol 2017; 8:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bojsen-Moller KN, Dirksen C, Jorgensen NB, et al. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab 2013; 6:E1066–E1071. [DOI] [PubMed] [Google Scholar]
- 27.Salehi M, Gastaldelli A, D’Alessio DA. Evidence from a single individual that increased plasma GLP-1 and GLP-1-stimulated insulin secretion after gastric bypass are independent of foregut exclusion. Diabetologia 2014; 7:1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab 2018; 4:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suhl E, Anderson-Haynes SE, Mulla C, Patti ME. Medical nutrition therapy for postbariatric hypoglycemia: practical insights. Surg Obes Relat Dis 2017; 5:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Meijeren J, Timmer I, Brandts H, et al. Evaluation of carbohydrate restriction as primary treatment for postgastric bypass hypoglycemia. Surg Obes Relat Dis 2017; 3:404–410. [DOI] [PubMed] [Google Scholar]
- 31.Kandel D, Bojsen-Moller KN, Svane MS, et al. Mechanisms of action of a carbohydrate-reduced, high-protein diet in reducing the risk of postprandial hypoglycemia after Roux-en-Y gastric bypass surgery. Am J Clin Nutr 2019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Craig CM, Liu LF, Deacon CF, et al. Critical role for GLP-1 in symptomatic postbariatric hypoglycaemia. Diabetologia 2017; 3:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laguna Sanz AJ, Mulla CM Fowler KM, et al. Design and clinical evaluation of a novel low-glucose prediction algorithm with mini-dose stable glucagon delivery in post-bariatric hypoglycemia. Diabetes Technol Ther 2018; 2:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson K Single Administration of XOMA 358, an insulin receptor attenuator, improves postmeal and nighttime hypoglycemia profiles in post gastric bypass hypoglycemia (PGBH) Endocrine Society 99th Annual Meeting 2017; Orlando, FL. [Google Scholar]
- 35.Arora I, Patti ME. Can reversal of RYGB also reverse hypoglycemia? Mol Metab 2018; 9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis DB, Khoraki J, Ziemelis M, et al. Roux en Ygastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a nonpancreatic etiology. Mol Metab 2018; 9:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]