Abstract
Angiogenesis is critical for breast cancer progression. Overexpression of HER-2/neu receptors occur in 25-30% of breast cancers, and treatment with trastuzumab inhibits HER-2-overexpressing tumor growth. Notably, HER-2-mediated signaling enhances vascular endothelial growth factor (VEGF) secretion to increase tumor-associated angiogenesis. Squalamine (aminosterol compound) suppresses VEGF-induced activation of kinases in vascular endothelial cells and inhibits tumor-associated angiogenesis. We assessed antitumor effects of squalamine either alone or with trastuzumab in nude mice bearing breast tumor xenografts without (MCF-7) or with HER2-overexpression (MCF-7/HER-2). Squalamine alone inhibited progression of MCF-7 tumors lacking HER2 overexpression, and squalamine combined with trastuzumab elicited marked inhibition of MCF-7/HER2 growth exceeding that of trastuzumab alone. MCF-7/HER-2 cells secrete higher levels of VEGF than MCF-7 cells, but squalamine elicited no growth inhibition of either MCF-7/HER-2 or MCF-7 cells in vitro. However, squalamine did stop growth of human umbilical vein endothelial cells (HUVECs) and reduced VEGF-induced endothelial tube-like formations in vitro. These effects correlated with blockade of focal adhesion kinase phosphorylation and stress fiber assembly in HUVECs. Thus, squalamine effectively inhibits growth of breast cancers with or without HER-2-overexpression, an effect due in part to blockade of tumor-associated angiogenesis.
Keywords: Tumor-associated angiogenesis, Breast cancer, VEGF, Squalamine, Trastuzumab, MCF-7
1. Introduction
Despite an increase in early detection, improved surgical treatment, radiotherapy and drug therapy, breast cancer remains a major cause of mortality among women worldwide. The overexpression of HER-2/neu proto-oncogene, which encodes a 185 kDa transmembrane receptor tyrosine kinase with homology to epidermal growth factor receptor [1, 2], has been found in 25-30% of human breast cancers and correlates with poor clinical outcome [3–8]. Trastuzumab (HerceptinR), a humanized monoclonal antibody specific for the extracellular domain of HER-2 receptor, has shown effectiveness as a single agent as well as in combination with chemotherapeutic agents [9, 10]. HER-2 receptor-mediated signaling is also known to enhance secretion of vascular endothelial growth factor (VEGF), eliciting increased tumor-associated angiogenesis that is critical for tumor growth and progression [11–19]. Consequently, the use of antiangiogenic therapy such as bevacizumab (Avastin), a humanized monoclonal antibody that inhibits VEGF, alone and in combination with HER2-targeted therapies has been investigated [11–13, 15–17, 19]. To date, randomized clinical trials of dual therapy with bevacizumab and trastuzumab have not demonstrated an additional overall survival benefit of adding bevacizumab to trastuzumab and/or docetaxel chemotherapy despite some improvement in progression-free survival [11, 12, 17, 19, 20]. However, alternative antiangiogenic agents that have a different mechanism of action have shown significant antitumor activity in several malignancies [13, 21–28]. Thus, squalamine, an aminosterol isolated originally from dogfish shark liver, has been shown to exhibit potent antiangiogenic activity due to the selective inhibition of new blood vessel formation [25, 29–31]. As VEGF is integral to the pathogenesis of neovascular age-related macular degeneration, early phase clinical trials of squalamine for this condition are underway [32–34], Further, squalamine has also been reported to be effective in blocking tumor progression in several preclinical xenograft models, including breast [28, 31, 35], ovarian [24, 36], lung [23, 26], brain [27] and prostate [37] cancers. Additive antitumor effects have been demonstrated with squalamine in combination with chemotherapeutic agents such as cisplatin, carboplatin, cyclophosphamide and 5-fluorouracil [24, 26, 28]. In Phase I trials, squalamine administered IV was determined to be well-tolerated by patients and not associated with major toxicities at recommended dose levels [21]. In more advanced clinical trials, squalamine in combination with chemotherapy was also reported to be well-tolerated and demonstrated significant clinical benefit for treatment of patients with either non-small cell lung or ovarian cancers [22, 23, 36].
This study evaluates whether the in vivo use of either squalamine alone or combined with trastuzumab provides additional antitumor efficacy against human breast cancer cell xenografts with or without HER-2/neu-overexpression, respectively. Further, we have investigated potential molecular mechanisms by which squalamine may exert antiangiogenic effects. Our results indicate that squalamine administered alone inhibits the progression of breast tumors lacking HER2-overexpression. Furthermore, squalamine, particulalry in combination with trastuzumab, significantly suppresses the growth of HER2-overexpressing tumors in vivo, exceeding the inhibition expected with trastuzumab treatment alone. This antitumor effect of squalamine appears to be due in part to its blockade of tumor-associated angiogenesis stimulated by vascular endothelial growth factors. This action may be mediated mainly by inhibition of VEGF-induced phosphorylation of p42/p44 MAP kinase [24] and focal adhesion kinase (FAK), which, in turn, disrupts the critical assembly of stress fibers in tumor-associated vascular endothelial cells [38–41].
2. Materials and Methods
2.1. Reagents
Trastuzumab (Herceptin®; lyophilized, sterile powder) was purchased from Genentech, Inc. (South San Francisco, CA). The lyophilized recombinant human VEGF was obtained from PeproTech (Rocky Hill, NJ). Chemically synthesized squalamine was provided by Genaera Pharmaceuticals Inc. (Plymouth Meeting, PA).
2.2. Cell lines
MCF-7 cells (American Type Culture Collection, Rockville, MD) stably transfected with a vector containing the full-length cDNA of human HER-2 gene (MCF7/HER2) were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS) [42,43,45]. Parental control MCF-7 cells were used and maintained as described before [42, 43]. Human umbilical vein endothelial cells (HUVECs) (BioWhittaker, Inc., Walkersville, MD) were routinely grown in Endothelial Cell Basal Medium (EBM®, BioWhittaker, Inc.) supplemented with 2% fetal bovine serum, 10 ng/ml hEGF, 1.0 μg/ml hydrocortisone, 50 μg/ml gentamicin, 50 ng/ml amphotericin B, and 12 mg/ml bovine brain extract (BBE). Cultured HUVECs were serum starved overnight (>12 h) prior to experimental use.
2.3. Human tumor xenografts in nude mice
Animals were housed in a pathogen-free environment with controlled light and humidity and received food and water ad libitum. All animal experiments were approved and followed the guidelines and regulations of our Institutional Animal Care and Use Committee. MCF-7/HER2 cells were inoculated subcutaneously at 2 × 107 cells/animal into the dorsal area of 6-week-old female athymic mice (Harlan Sprague-Dawley, Indianapolis, IN) primed with 1.7 mg s.c. estradiol-17β (E2β) in a biodegradable binder pellet (Innovative Research of America, Sarasota, FL) beginning 3 days prior to cell inoculation [44, 45]. Treatment was initiated when tumors grew to 50-80 mm3. Animals were randomized by weight and tumor size at the start of the experiment, with 5-7 animals included in each treatment group. Trastuzumab (loading dose, 8 mg/kg; then 4 mg/kg weekly thereafter), and squalamine (2 mg/kg daily for 28 days) were administered by intraperitoneal injection. Vehicle injections in the control group (n=5) were given on an identical treatment protocol as squalamine. Tumors were measured using calipers on the days indicated, and tumor volume was calculated as the product of length × width × height as before [43, 46–51]. In an independent parallel experiment, mice were sacrificed at day seven of the treatment, and the tumors resected, fixed in 37% formalin and embedded in paraffin for immunohistochemical staining. Furthermore, experiments were performed using parental control MCF-7 cells without HER2-overexpression. MCF-7 cells were inoculated subcutaneously at 2 × 107 cells/animal into the dorsal area of 6-week-old female athymic mice primed with 1.7 mg s.c. estradiol-17β in a biodegradable binder pellet beginning 3 days prior to cell inoculation [44, 45]. The treatment protocol was the same as that used for MCF-7/HER2 cells.
2.4. Immunohistochemical staining and microvessel counting
Endothelial cells were stained with a rabbit antiserum against Von Willebrand Factor (vWf), (Dako, Carpinteria, CA) [15]. To evaluate microvessel quantitation, slides were scanned at low-power magnification (×40 to ×100) to identify areas with the highest number of vessels. Five areas per tumor considered to have the highest densities were selected and counted at ×200 power magnification, and mean values ± SEM were recorded. Any brown-staining endothelial cells or cluster of endothelial cells with or without a lumen, clearly separated from adjacent microvessels, tumor cells, and other connective tissue elements, was considered to be individual vessels.
2.5. In vitro cell proliferation
HUVEC cells (1 × 104) and MCF-7 cells (1 × 105) were seeded in 6-well cell culture plates in duplicate. The cells were allowed to attach overnight in the appropriate complete media. The medium was then removed and replaced with either fresh complete medium, medium supplemented with VEGF, squalamine or VEGF plus squalamine. Cells were dispersed in trypsin, resuspended in PBS, and counted on the indicated days with a hemocytometer [44, 45].
2.6. Vascular endothelial cell tube-like formation in vitro and Binary Mapping
HUVECs were maintained in 24-well plates previously coated with Geltrex (Thermo Fisher Scientific, Waltham, MA). Cells were incubated in Vasculife complete medium without VEGF (Lifeline Cell Technology, Oceanside, CA) in the presence of 1 μM squalamine, 50 ng/ml VEGF (Lifeline Cell Technology), a combination of squalamine and VEGF or vehicle control. After 12-18 hours, cells were stained with 4 μg/mL Calcein AM dye (Thermo Fisher Scientific) and incubated at 37°C for 30 minutes. Tube formations were observed under a fluorescent microscope. Photographs were obtained at 40X magnification, and tube-like formations were quantified using the ImageJ Angiogenesis Analyzer (http://rsb.info.nih.gov/ij/) [52, 53]. The angiogenesis analyzer allows the network analysis of photos from endothelial tube assays based on fluorescence imaging using calcein dye. Images were converted to binary images and traced. A trace of the overall network of tubes was then generated. Once this map is produced, the angiogenesis analyzer quantifies and measures the number of segments, mesh areas, junctions and nodes. Additionally, the software also measures the length of the aforementioned elements. Quantification of all these elements was obtained from at least three different experiments and quantified for statistical analysis.
2.7. Detection of phospho-focal adhesion kinase (FAK) activity by gel electrophoresis and Western immunoblot
HUVEC cells (Lifeline Cell Technologies, Danvers, MA) were grown in 100 mm culture dishes to 80-90% confluence in complete HUVEC cell medium Vasculife (Lifeline Cell Technologies) without VEGF. Before adding VEGF (50 ng/ml) for 10 minutes at 37°C, cells were incubated in the presence or absence of 1 μM squalamine for 1 hour. Appropriate controls were also prepared. After treatment, cells were rinsed with PBS 3 times and placed on ice before lysing in 100 μl cold RIPA buffer (Thermo Fisher Scientific) in the presence of protease inhibitors (Thermo Fisher Scientific). Total protein concentration was determined by a standard Bradford Assay. Protein samples (50 μg/lane) were loaded on a 4-12% precast Tris-Glycine gel . Proteins were then transferred to a PVDF membrane and immunoblotted with anti-phosphoFAK (Y397) and anti-total FAK monoclonal antibodies (Cell Signaling Technology). Bands were detected using the Pierce ECL Western Blotting Substrate blocked with milk (Thermo Fisher Scientific).
2.8. Measurement of extracellular VEGF protein by Elisa and Western immunoblot
MCF-7 breast cancer cells were cultured under serum-free conditions for 24 h and treated in the presence or absence of squalamine (1.6 μM). Conditioned medium was collected after 24 h. The amount of secreted VEGF was measured in the conditioned medium with a VEGF-specific sandwich-ELISA assay (R & D Systems Inc., Minneapolis, MN) according to the manufacturer’s instructions [44]. VEGF protein levels were normalized for protein content in the conditioned medium. For Western immunoblotting MCF7/PAR and MCF7/HER2 cells were grown in vitro and treated with increasing doses of squalamine. After 48 hours supernatant was collected and concentrated using amicon Ultra-15 centrifugal filter devices (Thermo Fisher Scientific). Western immunoblots were done using anti-VEGF antibody (Thermo Fisher cat #MA5-12184).
2.9. Confocal microscopy for phosphor-FAK detection
HUVECs were grown on glass coverslips. They were fixed with 3.7% formaldehyde and permeabilized with 100% acetone. Phospho-FAK was detected using a rabbit polyclonal antibody anti-FAK [pY397] (Biosource International; Camarillo, CA). Phospho-FAK antigen-antibody complexes were detected with fluorescein anti-rabbit IgG (H+L) (VECTOR Laboratories; Burlingame, CA). F-actin was assessed using rhodamine-conjugated phalloidin (0.165 μM) (Molecular Probe, Inc.; Eugene, OR). After repeated washes with PBS, coverslips were mounted onto glass microscope slides and viewed with a Leica TCS SP MP Inverted Confocal Microscope [40, 41].
2.10. Statistics.
Statistical differences regarding in vitro cell proliferation, VEGF secretion and microvessel density were analyzed using Student’s t-test. ANOVA was used for comparison of tumor xenograft volumes. All results were expressed as mean ± SEM. P < 0.05 was considered as statistically significant.
3. Results
3.1. Squalamine inhibits growth in vivo of MCF-7 breast tumors with or without HER-2 overexpression
The antitumor activity of squalamine was evaluated using MCF-7 tumor xenografts in vivo. MCF-7/HER2 cells were first implanted subcutaneously. When tumors grew to 50-75 mm3, animals were treated with control, trastuzumab alone (8 mg/kg, loading dose, and 4 mg/kg weekly thereafter), squalamine alone (2 mg/kg) on days 1-28, or the combination of trastuzumab plus squalamine. Treatments were terminated after day 28. Squalamine treatment alone significantly retarded the growth of tumors as compared to controls (P<0.001). However, a more profound and sustained regression of tumors was observed with the combination of squalamine and trastuzumab as compared to control tumors that grew rapidly to more than 600-700 mm3 in the course of the experiment (P < 0.001; Fig 1). In parallel experiments using parental MCF-7 tumor xenografts that do not overexpress HER-2 receptors, treatment with squalamine alone, but not trastuzumab alone, also significantly reduced tumor growth in vivo (P<0.001; cf. [24]). Notably, the antitumor effect of squalamine alone appears proportionately greater in HER2-overexpressing tumor xenografts than that observed in tumors without HER2-overexpression.
Figure 1.
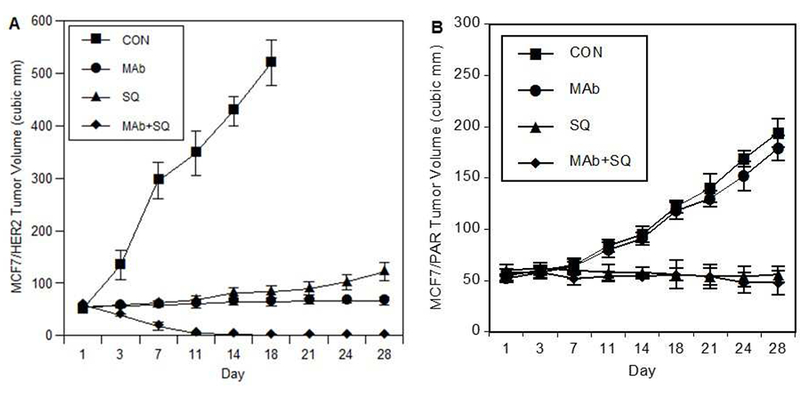
Squalamine inhibits growth of human breast tumor xenografts in vivo. A) Squalamine inhibits growth of MCF-7/HER2 breast tumor xenografts in vivo and enhances antitumor effects of trastuzumab. MCF-7/HER-2-overexpressing breast cancer cells were subcutaneously inoculated in nude mice. After 7 days, animals with tumors of comparable size were randomized to treatment with trastuzumab (Mab; 8 mg/kg loading dose followed by a 4 mg/kg dose administrated weekly thereafter), squalamine (SQ; 2 mg/kg on days 1-28), or trastuzumab administrated in combination with squalamine (Mab+SQ). The control group (CON) received appropriate vehicle injections on days 1-28. Animals with tumors exceeding 500-mm3 were sacrificed as per our approved institutional animal research committee protocol. After day 28, the experiment was terminated. Results are expressed as mean ± SEM. Antitumor effects of MAb and SQ alone groups were significantly different from those of the CON group; and antitumor effects of the MAb+SQ combination treatment group were significantly different from those of CON and from either MAb or SQ treatments as single agents (all at P < 0.01). B) Squalamine inhibits growth of MCF-7 breast tumor xenografts but does not enhance effects of trastuzumab in tumors without HER2 overexpression. MCF-7 cells were inoculated subcutaneously as above, and animals with tumors of comparable size were randomized to treatment with control (CON), trastuzumab (MAb), squalamine (SQ) or a combination of these two agents (MAb+SQ). The control group received appropriate vehicle injections during the course of the study as in A) above. After day 28, the experiment was terminated, with results expressed as mean ± SEM. Antitumor effects of SQ alone or MAb+SQ groups were both significantly different from those of the CON group (P<0.001); but antitumor effects of the MAb treatment alone as expected were not significantly different from those of the CON group.
3.2. Squalamine suppresses MCF-7/HER-2 breast xenograft-associated angiogenesis in vivo.
Immunohistochemical analysis of the vascularization of tumor xenografts performed postmortem by staining with polyclonal antibody against endothelial cell-specific von Willebrand factor demonstrated a decreased microvessel density, as counted from five different high-power fields (×200), in 7-day tumor tissues obtained from mice with squalamine administered either alone or in combination with trastuzumab, as compared with control or trastuzumab alone-treated tumor tissues (see Fig. 2). Microvessel density was quantitated based on data from three mice in each treatment group.
Figure 2.

Immunohistochemical staining for vascular endothelial cells in MCF-7/HER-2 xenograft tissues obtained from nude mice treated for seven days with either squalamine (SQ) or trastuzumab (MAb) administered alone or in combination (MAb/SQ). Microvessels were stained using a polyclonal antibody against vWf and were randomly counted from 5 different high-power fields (×200) per mouse tumor. The values are represented as means ± SEM of microvessel density per high-power field (n = 3). Treatment with squalamine (SQ) alone or a combination of squalamine with trastuzumab (MAb/SQ) elicited a significant reduction in microvessel density as compared to controls (CON) or to trastuzumab (MAb) alone (P < 0.01, by student’s t-test).
3.3. Squalamine does not affect production of VEGF by MCF-7 breast cancer cells with or without HER-2 gene overexpression.
To determine whether HER-2 overexpression may contribute to angiogenesis in breast cancer by promoting VEGF production, we evaluated HER-2 effects on VEGF secretion. Parent and HER-2 overexpressing breast MCF-7 cancer cells were incubated for 48 hours in vitro, and secretion of VEGF into conditioned media was determined by enzyme-linked immunosorbant assay (ELISA). MCF-7 cells overexpressing HER-2 gene had significantly increased levels of VEGF secretion as compared to that found in parental MCF-7 cells, a results consistant with that reported previously in ovarian cancer cells with and without HER2 overexpression [24]. Moreover, treatment with squalamine elicited no significant effect on secretion of VEGF by the breast tumor cells in vitro (Fig. 3A). These results were further confirmed by detection of VEGF in the extracellular media of MCF7/PAR and MCF7/HER2 cells after squalamine treatment by use of gel electrophoresis and Western immunoblot (Fig. 3B).
Figure 3.

VEGF secretion by MCF-7 breast cancer cells with or without HER-2/neu gene overexpression. A) Cells were grown in vitro, either with control medium (Con) or medium containing 1.6 μM squalamine (SQ). After 48 hours, media were collected, and VEGF levels were measured by ELISA assay. Data are represented as mean ± SEM. VEGF levels secreted by MCF-7/HER2 (MCF/HER2) cells were significantly different than those secreted by MCF-7 parental cells without HER2-overexpression (MCF/PAR) when treated with either control or squalamine (P < 0.05). Addition of squalamine did not affect the level of VEGF secretion by either MCF/PAR or MCF/HER2 cells.
B) Western immunoblot showing VEGF expression levels after cells were treated with varying doses of squalamine. MCF7/PAR and MCF7/HER2 cells were grown in vitro and treated with increasing doses of squalamine. After 48 hours, the supernatant was collected and concentrated as described in methods. Western immunoblots were done using anti-VEGF antibody (Thermo Fisher). The results indicate no difference in the expression levels of VEGF secreted into the extracellular medium after squalamine treatment.
3.4. Squalamine blocks VEGF-induced proliferation of HUVECs in vitro, but does not directly affect proliferation of MCF-7/HER-2 cells.
To further assess the biological mechanism for the antiangiogenic and antitumor effect of squalamine seen in MCF-7/HER-2 breast tumor xenografts, HUVECs were grown in vitro in the presence of 50 ng/ml of VEGF, 3.2 μM of squalamine and combinations of VEGF and different concentrations of squalamine. A significant inhibition of VEGF-induced vascular endothelial cell proliferation by squalamine was clearly evident at day 8. This growth-suppressive effect of squalamine is dose-dependent with maximal suppression at 3.2 μM. Doses of 1.6 μM and as low as 160 nM also showed significant suppression of endothelial cell growth (Fig. 4a). In contrast, squalamine had no direct inhibitory effect on the proliferation of human breast MCF-7 cancer cells, either with HER2 overexpression (Fig. 4B) or without HER2 overexpression (data not shown).
Figure 4.

In vitro HUVEC and MCF-7/HER-2 cell proliferation. a) Squalamine inhibits VEGF-induced proliferation of endothelial cells in vitro. HUVECs were grown in the presence of VEGF (50 ng/ml), squalamine (SQ; 3.2 μM), combinations of VEGF with squalamine (VEGF/SQ 3.2 μM ; VEGF/SQ 1.6 μM; VEGF /SQ 0.16 μ M and VEGF/SQ 0.016 μM), or control medium alone (Con). Results show that VEGF alone, but not SQ alone, stimulates HUVEC proliferation by day 8 (P<0.001); while SQ elicits a dose-dependent reduction in VEGF-induced HUVEC proliferation at doses ranging from 0.16 to 3.2 μM by day 8 (P<0.01). b) Squalamine does not directly affect growth of MCF-7/HER-2 breast cancer cells in vitro. Results show that cell numbers of MCF-7/HER2 cells are unchanged by incubation with either VEGF or SQ as compared to controls (CON) (P>0.05). All data are from duplicate determinations of cell numbers and the values are represented as mean ± SEM from 3 independent experiments.
3.5. Squalamine suppresses VEGF-stimulated capillary tube-like formation by HUVECs in vitro.
HUVECs were cultured in serum-free medium on the surface of Geltrex with the indicated treatments. vehicle, squalamine (1 μM), VEGF (50 ng/ml) or a combination of VEGF (50 ng/ml) with squalamine (1 μM). After 18 h incubation, endothelial cell tubular structures were photographed and analyzed by using Angiogenesis Analyzer ImageJ as described in methods. As shown in Figure 5, the results reveal representative capillary tube like formations as well as the skeletons of tubular networks that identify established master segments, meshes, master junctions, branches, nodes and segments. HUVEC cells in those plates in which the networked capillary tubes were more effectively inhibited by squalamine showed a marked alteration in their shape and size, in contrast with the more characteristic spindle-shaped cells that form capillary-like tubes either in the absence of squalamine or in the presence of very low doses of squalamine. It is apparent that VEGF alone promotes enhanced capillary tube-like formation as compared to controls while treatment with squalamine in combination with VEGF disrupts the expected VEGF-induced capillary tube-like formations (Fig. 5).
Figure 5.

Regression of VEGF-induced HUVEC tube-like structures after squalamine treatment. HUVECs were cultured in serum-free medium on the surface of Geltrex with the indicated treatments. A) vehicle (CONTROL); B) squalamine 1 μM (SQUAL); C) VEGF 50 ng/ml; D) VEGF (50 ng/ml) + squalamine (1 μM) (SQ+VEGF). After 18 h incubation, endothelial cell tubular structures were observed under a fluorescent microscope and photographed with a 20× objective (top panels) and analyzed by Angiogenesis Analyzer ImageJ as described in methods. Middle and lower panels show corresponding skeletons of tubular networks identifying master segments (orange), meshes (blue sky), master junctions (red), branches (green), nodes (red surrounded by blue) and segments (magenta). E) and F) ImageJ analyzed parameters of tube formation E) node number, F) number of master segments, meshes and master junctions were calculated. Values are the mean and SEM calculated from triplicates in 3 independent experiments (n=3, *p<0.05; **p<0.01). HUVEC cells in those plates in which the networked capillary tubes were more effectively inhibited by squalamine showed marked alteration in their shape and size, in contrast with the more characteristic spindle-shaped cells that form capillary-like tubes either in the absence of squalamine or in the presence of very low doses of squalamine.
3.6. Squalamine disrupts F-actin stress fibers and blocks VEGF-induced phosphorylation of focal adhesion kinase in HUVECs.
Next, we assessed the effect of squalamine on endothelial cell architecture to further understand the mechanisms by which squalamine inhibits endothelial cell proliferation and capillary tube-like formation. HUVECs were stimulated with 50 ng/mL VEGF in the absence or presence of squalamine. Thereafter, the organization of actin filaments in stress fibers and the activation of FAK were evaluated by using both confocal microscopy and Western blots methods. The results indicate that VEGF induced significant activation of FAK (Fig. 6c1 and 6c3) as compared to cells treated with controls (Fig. 6a1 and 6a3) or with squalamine alone (Fig. 6b1 and 6b3). In addition, actin filaments appear less organized and chaotic in control cells (Fig. 6a2 and 6a3) and in cells treated with squalamine alone (Fig. 6b2 and 6b3). In contrast, treatment with VEGF for 10 min induced a marked reorganization of the actin filaments into more defined trans-cytoplasmic stress fibers (Fig. 6c2 and 6c3). Notably, squalamine treatment essentially inhibited formation of trans-cytoplasmic stress fibers as well as VEGF-induced phosphorylation of FAK when both agents were administered in combination (Fig. 6d2 and 6d3).
Figure 6.

Squalamine blocks FAK phosphorylation and formation of actin stress fibers in HUVECs exposed to VEGF. a) HUVECs were made quiescent and either treated with a) control (CON); or b) treated with squalamine (SQ; 3.2 μ M) alone for 60 min; c) treated with 50 ng/ml VEGF for 10 min; and compared with d) cells pre-treated with squalamine (3.2 μ M) for 60 min and 50 ng/ml VEGF for 10 min. FAK phosphorylation (green signal) was detected using a polyclonal antibody anti-FAK [pY397]; and F-actin (red signal) was detected using rhodamine-conjugated phalloidin. Cells were examined by confocal microscopy and DAPI was used for nuclear staining (blue). Results show that VEGF stimulates FAK phosphorylation (c1) as compared to control (a1) and squalamine (b1) treatment, while this effect is inhibited by combined treatment with squalamine (d1). Further, VEGF also induces a reorganization of actin stress fibers (c2) as compared to control (a2) and squalamine (b2) treatment, while this action is reversed by dual treatment with squalamine (d2). The lower panel (a3-d3) presents an overlay of the green and red signals for FAK and F-actin, respectively for each of the treatment groups. The bulk of HUVECs responded to VEGF-induced FAK phosphorylation as reported by others [78] as well as to squalamine-dependent disruption of VEGF-induced FAK phosphorylation. Representative fields are shown, based on data obtained in 5 different experiments.
Results obtained by confocal microscopy were confirmed by use of gel electrophoresis and Western immunoblot experiments. Incubation of HUVEC cells with VEGF increased FAK (Tyr397) phosphorylation that was inhibited when administered in the presence of squalamine (Fig. 7A). Inhibition of FAK phosphorylation (Tyr397) was dose-dependent in the presence of increasing concentrations of squalamine (Fig. 7B), thus confirming independent results obtained by confocal microscopy.
Figure 7.

Squalamine blocks FAK phosphorylation in HUVECs exposed to VEGF. HUVECs were incubated in the presence of VEGF, squalamine or a combination of both VEGF and squalamine A) HUVEC cells were treated with control vehicle (VH), VEGF (50 ng/ml), squalamine (SQ; 1 μM) or VEGF in combination with squalamine (VEGF+SQ). Lysates were prepared and processed as described in materials and methods. Western blotting was done with monoclonal antibodies against phosphoFAK (upper panel) and total FAK (lower panel). B) HUVEC cells were treated with control vehicle (VH) or increasing concentrations of squalamine from 0.001- 1 μM for 1 hour in vitro. Immunoblotting was done with anti-phospho (Y397) FAK. For loading control, membranes were stripped and reprobed with anti total FAK.
4. Discussion
Tumor-associated angiogenesis is a prognostic factor that helps to identify patients with breast cancer at high risk for disease recurrence and death. Several studies have clearly demonstrated that the intratumoral microvessel density of breast carcinoma is associated with aggressive tumor growth, invasion and further may serve to predict the response to chemotherapeutics [54–57]. Overexpression of the HER-2 growth factor receptor also correlates with poor clinical outcome in breast cancer [3,4, 8–10, 49], and HER-2-overexpression is closely associated with increased tumor-associated angiogenesis and increased expression of VEGF [9, 10, 15, 58, 59]. We have confirmed that human MCF-7 breast cancer cells with HER-2 gene overexpression exhibit enhanced secretion of VEGF as compared with parental cells lacking HER-2 overexpression (Fig. 3) [15,24]. Expression of VEGF is also associated with worse prognosis in breast cancers that lack HER-2 overexpression [60–63]. Hence, angiogenesis is a key component of breast cancer growth, invasion and metastasis, and inhibition of angiogenesis has been an attractive strategy for breast cancer treatment, particularly using bevacizumab [12, 13, 15, 16, 18]. However, despite increased response rates in both the metastatic and neoadjuvant setting, bevacizumab has failed to show any overall survival benefit [64–67]. In addition, randomized clinical trials of dual therapy with bevacizumab and trastuzumab have not demonstrated an additional overall survival benefit of adding bevacizumab to trastuzumab and/or docetaxel chemotherapy despite some improvement in progression-free survival [11, 12, 16, 17, 19, 20, 68]. We postulate that further identification of antiangiogenic agents that may function by different mechanisms may help to address this problem.
Our results provide evidence that the combination of trastuzumab plus squalamine significantly suppresses the growth of MCF-7/HER-2 breast tumor xenografts in vivo. Most of the animals in the dual treatment group showed complete macroscopic remission of tumor xenografts at 28 days after initiation of the combined treatment. In addition, administration of squalamine alone elicits a partial reduction in tumor xenograft size as compared to appropriate controls (Fig. 1A). Such in vivo effects of squalamine directly correlated with a reduction of blood vessel formation (about 70%; P <0.01) in the tumors as demonstrated by immunohistochemical analysis of tumor vascularization by staining with antibody against endothelial cell-specific von Willebrand Factor (Fig. 2). Of special note, treatment with squalamine alone, but not trastuzumab alone, also reduced the growth of MCF-7 tumor xenografts without HER-2 gene overexpression (Fig. 1B). The inhibitory action of squalamine alone in MCF-7 cells is proportionately less than the marked suppression observed in tumors with HER2-overexpression.
The antiangiogenic activity of squalamine appears to be mediated by a direct effect on vascular endothelial cells. We demonstrated that squalamine was able to block VEGF-induced HUVEC proliferation in vitro in a dose-dependent manner (Fig. 4a), but squalamine had no direct effect on either parental or HER-2 overexpressing MCF-7 human breast cancer cell proliferation, nor on the levels of VEGF production by these cancer cells. The current findings confirm earlier reports (cf. [24]) that HER-2-overexpressing tumors produce significantly greater levels of VEGF than corresponding tumors that lack HER2-overexpression, thereby implicating a role for VEGF in the antiangiogenic activity of squalamine. The suppression of VEGF-induced HUVEC proliferation in vitro by squalamine was then investigated with additional experiments that demonstrated that squalamine suppressed VEGF-stimulated capillary tube-like formation by HUVECs , also in a dose-dependent manner.
The current findings are consistent with independent reports showing that squalamine inhibits tumor-associated angiogenesis and malignant growth in multiple animal models [21–36]. This effect is considered to be mediated, at least in part, by blocking mitogen-induced proliferation and migration of endothelial cells, thus preventing neovascularization of tumors. Overall, squalamine has been found to have no observable effects on unstimulated endothelial cells, is not directly cytotoxic to tumor cells and does not alter mitogen production by tumor cells [cf. [24, 27]. To further clarify the mechanism by which squalamine exerts its antiangiogenic effect, we examined the assembly of actin into trans-cytoplasmic stress fibers and the activation of focal adhesion kinase by use of confocal fluorescence microscopy [38–41]. Squalamine inhibited both the VEGF-induced phosphorylation of FAK and the organization of actin filaments into stress fibers. Focal adhesions are points of tight adhesion which provide a structural link between the cytoskeleton in the inner side of the endothelial cell and the extracellular matrix on the outer side. They are assembled following the recruitment of several signaling molecules such as talin, paxillin, vinculin, tensin and α-actinin, orchestrated by activation of the key molecule, FAK [69]. Prior reports have shown that squalamine also blocks VEGF-induced phosphorylation of SAPK2/p38 MAP kinase, which in turn modulates the activation of HSP27 in human endothelial cells [40,41]. HSP27 acts as an F-actin cap-binding protein and shows a phosphorylation-modulated inhibitory function on F-actin polymerization [70–73]. The rapid phosphorylation of HSP27 upon activation of SAPK2/p38 would release its binding to actin allowing polymerization which then contributes, in tight coordination with assembly of focal adhesions, to the formation of stress fibers in cells exposed to various agonists, such as VEGF and TGF-β, among others [40, 41, 70, 71]. The integrity of stress fibers as well as focal adhesion structures is vital for the angiogenesis process, because it permits the migration of endothelial cells and their subsequent attachment to the surrounding extracellular matrix in order to begin the formation of new tubular structures [24, 25, 27, 40, 41]. We propose that squalamine blocks angiogenesis by first inhibiting FAK and potentially p38 MAP kinase phosphorylation [40, 41] and subsequent assembly of focal adhesion, actin filament polymerization and anchorage, affecting in this way the stress fiber formation in endothelial cells. This will affect, in turn, the migration and organization of these cells in newly-growing blood vessels. In support of these results, Williams et al. [74] and Connolly et al. [32] also report that squalamine induces disorganization of F-actin stress fibers and a concomitant reduction of detectable cell adhesion molecule VE-cadherin on the endothelial cell surface.
In a previous report, we also determined that squalamine blocks VEGF-stimulated tyrosine phosphorylation of MAP kinase in HUVEC cells in vitro [24]. This effect was observed in quiescent HUVEC cells upon treatment with VEGF in combination with squalamine for 10-30 min in vitro. Squalamine inhibition of endothelial cell proliferation may also result in part from its interaction with endothelial cell surface proton pumps, thus altering intracellular pH and impeding downstream signaling by VEGF and possibly other vascular growth factors [75–77]. Alternatively, calcium-dependent cell signaling following exposure to growth factors such as VEGF may be dysregulated when squalamine forces intracellular redistribution of calmodulin [32]. Irrespective of which specific mechanism is operating, we find that squalamine blocks VEGF-stimulated proliferation of human umbilical vein endothelial cells in vitro, while it does not directly interfere with growth of tumor cells or production of VEGF by these tumor cells in vitro. VEGF has been shown to initiate biologic responses by binding with receptor tyrosine kinases, including Flt-1, Flk-1/KDR and neuropilin, present at the surface of endothelial cells [78–80]. The proliferative action of VEGF in endothelial cells is associated with the subsequent VEGF-induced tyrosine phosphorylation and stimulation in concert of focal adhesion kinase (FAK) and MAP kinases, including p38 MAP kinase, ERK-1 (p44 MAPK) and ERK-2 (p42 MAPK) [41, 80, 81]. On testing the assumption that blockade of endothelial cell proliferation by squalamine may occur, in part, by suppression of MAP kinase signaling cascades induced by VEGF in endothelial cells, we found that squalamine significantly curbs VEGF-stimulated phosphorylation of MAP kinase isoforms p42 and p44 in HUVEC [24]. Thus, squalamine may prevent endothelial cell growth and associated angiogenesis by interrupting critical signal transduction necessary for vascular endothelial cell activation necessary for tumor-associated angiogenesis [54–57].
In previous studies, we suggested that combinations of treatments incorporating agents that work by different mechanisms might elicit a more complete suppression of tumor growth and tumor-associated angiogenesis [24]. Consistent with this notion, Izumi et al. explored the possibility that trastuzumab treatment elicited a rearrangement of the tumor vascular network to more closely resemble normal networks, thereby facilitating drug delivery to previously inaccessible regions [82] . Therefore, dual therapy with trastuzumab plus squalamine could offer a more optimal combination for breast cancer, as we demonstrate in the current experimental model focused on human breast cancers overexpressing HER-2 receptors. Treatment with squalamine in breast tumors with or without HER-2-overexpression, potentially including triple-negative subtypes [61, 67] may be a reasonable option to consider going forward because squalamine lactate for clinical use is reported to be safe and well-tolerated and shows evidence of antitumor activity in patients afflicted with non-small cell lung cancer and ovarian cancer in Phase I-II trials [21–23].
In conclusion, results of this study show that the antiangiogenic steroidal compound squalamine effectively suppresses the growth of human breast cancer cells with or without HER-2 receptor overexpression when administered alone and especially when administered in combination with trastuzumab in HER-2-overexpressing tumors using preclinical models in vivo. The antitumor effect exerted by squalamine is due, in part, to blockade of VEGF-induced phosphorylation of ERK-1 (p44 MAPK) and ERK-2 (p42 MAPK) as well as focal adhesion kinase, affecting, in turn, assembly of actin filaments into trans-cytoplasmic stress fibers in endothelial cells and the process of tumor-associated angiogenesis [56–58, 82].
Highlights.
Squalamine (SQ) is an aminosterol that blocks VEGF-induced angiogenesis.
HER2-overexpressing tumor cells secrete high levels of VEGF.
Dual therapy with SQ and Herceptin markedly stops HER2+ breast tumor growth in vivo.
SQ alone also reduces progression of HER2-negative breast tumor cells in vivo.
Acknowledgments
We thank Dr. Jon Williams and Dr. Kenneth Holroyd from Genaera Pharmaceuticals for providing pharmaceutical grade squalamine and for helpful advice. Dr. Michael Zasloff also contributed useful discussions. This work was supported by funds from the National Institutes of Health (NIH)/National Cancer Institute Partnership to Eliminate Cancer Health Disparities [U54 CA-14393], California Breast Cancer Research Program IDEA Awards [16IB-0042 and 18IB-0034], US Army Medical Research and Materiel Command [DAMD17-03-1-0381], Stiles Program in Integrative Oncology, Hickey Family Foundation, Robert Wood Johnson Foundation Nurse Faculty Scholar Award [69352] and Tower Cancer Research-Jessica M. Berman Breast Cancer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Richard J. Pietras has consulted with Astra-Zeneca, Pfizer and Genentech. The remaining authors declare no conflicts of interest.
References
- [1].Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al. , Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene, Science 230(4730) (1985) 1132–1139. [DOI] [PubMed] [Google Scholar]
- [2].Semba K, Kamata N, Toyoshima K, Yamamoto T, A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma, Proc Natl Acad Sci U S A 82(19) (1985) 6497–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harris JR, Lippman ME, Veronesi U, Willett W, Breast cancer (3), N Engl J Med 327(7) (1992) 473–480. [DOI] [PubMed] [Google Scholar]
- [4].Lemoine NR, Staddon S, Dickson C, Barnes DM, Gullick WJ, Absence of activating transmembrane mutations in the c-erbB-2 proto-oncogene in human breast cancer, Oncogene 5(2) (1990) 237–239. [PubMed] [Google Scholar]
- [5].Press MF, Slamon DJ, Flom KJ, Park J, Zhou JY, Bernstein L, Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens, J Clin Oncol 20(14) (2002) 3095–3105. [DOI] [PubMed] [Google Scholar]
- [6].Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P, Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group, J Clin Oncol 11(10) (1993) 1936–1942. [DOI] [PubMed] [Google Scholar]
- [7].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL, Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene, Science 235(4785) (1987) 177–182. [DOI] [PubMed] [Google Scholar]
- [8].Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. , Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer, Science 244(4905) (1989) 707–712. [DOI] [PubMed] [Google Scholar]
- [9].Baselga J, Current and planned clinical trials with trastuzumab (Herceptin), Semin Oncol 27(5 Suppl 9) (2000) 27–32. [PubMed] [Google Scholar]
- [10].Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L, Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2, N Engl J Med 344(11) (2001) 783–792. [DOI] [PubMed] [Google Scholar]
- [11].Falchook GS, Moulder S, Naing A, Wheler JJ, Hong DS, Piha-Paul SA, Tsimberidou AM, Fu S, Zinner R, Janku F, Jiang Y, Huang M, Parkhurst KL, Kurzrock R, A phase I trial of combination trastuzumab, lapatinib, and bevacizumab in patients with advanced cancer, Invest New Drugs 33(1) (2015) 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V, AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer, J Clin Oncol 31(14) (2013) 1719–1725. [DOI] [PubMed] [Google Scholar]
- [13].Kerbel RS, Reappraising antiangiogenic therapy for breast cancer, Breast 20 Suppl 3 (2011) S56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS, Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors, Am J Pathol 151(6) (1997) 1523–1530. [PMC free article] [PubMed] [Google Scholar]
- [15].Pietras RJ, Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature, Breast J 9(5) (2003) 361–373. [DOI] [PubMed] [Google Scholar]
- [16].Rugo HS, Chien AJ, Franco SX, Stopeck AT, Glencer A, Lahiri S, Arbushites MC, Scott J, Park JW, Hudis C, Nulsen B, Dickler MN, A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer, Breast Cancer Res Treat 134(1) (2012) 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwartzberg LS, Badarinath S, Keaton MR, Childs BH, Phase II multicenter study of docetaxel and bevacizumab with or without trastuzumab as first-line treatment for patients with metastatic breast cancer, Clin Breast Cancer 14(3) (2014) 161–168. [DOI] [PubMed] [Google Scholar]
- [18].Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, Meloche S, Alaoui-Jamali MA, Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis, Oncogene 19(31) (2000) 3460–3469. [DOI] [PubMed] [Google Scholar]
- [19].Zhao M, Pan X, Layman R, Lustberg MB, Mrozek E, Macrae ER, Wesolowski R, Carothers S, Puhalla S, Shapiro CL, Ramaswamy B, A Phase II study of bevacizumab in combination with trastuzumab and docetaxel in HER2 positive metastatic breast cancer, Invest New Drugs 32(6) (2014) 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumler I, Christiansen OG, Nielsen DL, A systematic review of bevacizumab efficacy in breast cancer, Cancer Treat Rev 40(8) (2014) 960–973. [DOI] [PubMed] [Google Scholar]
- [21].Bhargava P, Marshall JL, Dahut W, Rizvi N, Trocky N, Williams JI, Hait H, Song S, Holroyd KJ, Hawkins MJ, A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers, Clin Cancer Res 7(12) (2001) 3912–3919. [PubMed] [Google Scholar]
- [22].Hao D, Hammond LA, Eckhardt SG, Patnaik A, Takimoto CH, Schwartz GH, Goetz AD, Tolcher W, McCreery HA, Mamun K, Williams JI, Holroyd KJ, Rowinsky EK, A Phase I and pharmacokinetic study of squalamine, an aminosterol angiogenesis inhibitor, Clin Cancer Res 9(7) (2003) 2465–2471. [PubMed] [Google Scholar]
- [23].Herbst RS, Hammond LA, Carbone DP, Tran HT, Holroyd KJ, Desai A, Williams JI, Bekele N, Hait H, Allgood V, Solomon S, Schiller JH, A phase I/IIA trial of continuous five-day infusion of squalamine lactate (MSI-1256F) plus carboplatin and paclitaxel in patients with advanced non-small cell lung cancer, Clin Cancer Res 9(11) (2003) 4108–4115. [PubMed] [Google Scholar]
- [24].Li D, Williams JI, Pietras RJ, Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression, Oncogene 21(18) (2002) 2805–2814. [DOI] [PubMed] [Google Scholar]
- [25].Pietras RJ, Weinberg OK, Antiangiogenic Steroids in Human Cancer Therapy, Evid Based Complement Alternat Med 2(1) (2005) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schiller JH, Bittner G, Potentiation of platinum antitumor effects in human lung tumor xenografts by the angiogenesis inhibitor squalamine: effects on tumor neovascularization, Clin Cancer Res 5(12) (1999) 4287–4294. [PubMed] [Google Scholar]
- [27].Sills AK Jr., Williams JI, Tyler BM, Epstein DS, Sipos EP, Davis JD, McLane MP, Pitchford S, Cheshire K, Gannon FH, Kinney WA, Chao TL, Donowitz M, Laterra J, Zasloff M, Brem H, Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature, Cancer Res 58(13) (1998) 2784–2792. [PubMed] [Google Scholar]
- [28].Teicher BA, Williams JI, Takeuchi H, Ara G, Herbst RS, Buxton D, Potential of the aminosterol, squalamine in combination therapy in the rat 13,762 mammary carcinoma and the murine Lewis lung carcinoma, Anticancer Res 18(4A) (1998) 2567–2573. [PubMed] [Google Scholar]
- [29].Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN Jr., McCrimmon D, Zasloff M, Squalamine: an aminosterol antibiotic from the shark, Proc Natl Acad Sci U S A 90(4) (1993) 1354–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rao MN, Shinnar AE, Noecker LA, Chao TL, Feibush B, Snyder B, Sharkansky I, Sarkahian A, Zhang X, Jones SR, Kinney WA, Zasloff M, Aminosterols from the dogfish shark Squalus acanthias, J Nat Prod 63(5) (2000) 631–635. [DOI] [PubMed] [Google Scholar]
- [31].Williams JI, Weitman S, Gonzalez CM, Jundt CH, Marty J, Stringer SD, Holroyd KJ, McLane MP, Chen Q, Zasloff M, Von Hoff DD, Squalamine treatment of human tumors in nu/nu mice enhances platinum-based chemotherapies, Clin Cancer Res 7(3) (2001) 724–733. [PubMed] [Google Scholar]
- [32].Connolly B, Desai A, Garcia CA, Thomas E, Gast MJ, Squalamine lactate for exudative age-related macular degeneration, Ophthalmol Clin North Am 19(3) (2006) 381–391, vi. [DOI] [PubMed] [Google Scholar]
- [33].Pecen PE, Kaiser PK, Current phase 1/2 research for neovascular age-related macular degeneration, Curr Opin Ophthalmol 26(3) (2015) 188–193. [DOI] [PubMed] [Google Scholar]
- [34].Wroblewski JJ, Hu AY, Topical Squalamine 0.2% and Intravitreal Ranibizumab 0.5 mg as Combination Therapy for Macular Edema Due to Branch and Central Retinal Vein Occlusion: An Open-Label, Randomized Study, Ophthalmic Surg Lasers Imaging Retina 47(10) (2016) 914–923. [DOI] [PubMed] [Google Scholar]
- [35].Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ, Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer, J Natl Cancer Inst 96(10) (2004) 739–749. [DOI] [PubMed] [Google Scholar]
- [36].Davidson S, Chap L, Pietras RJ, Astrow A, Gajewski W, Brader F, Petrone M, Desai A, Solomon S, Holroyd K, Major F, Adler L and Cohn A. A Phase IIA trial of continuous 5-day infusions of MSI-1256F (squalamine lactate) plus carboplatin for therapy of persistent or recurrent advanced ovarian cancer., Proc. Am. Soc. Clin. Oncol 878 (2002). [Google Scholar]
- [37].Sokoloff MH, Rinker-Schaeffer CW, Chung LW, Brendler CB, Adjunctive therapy for men with high risk localized and locally advanced prostate cancer: targeting disseminated tumor cells, J Urol 172(6 Pt 2) (2004) 2539–2544. [DOI] [PubMed] [Google Scholar]
- [38].Huot J, Houle F, Marceau F, Landry J, Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells, Circ Res 80(3) (1997) 383–392. [DOI] [PubMed] [Google Scholar]
- [39].Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J, SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis, J Cell Biol 143(5) (1998) 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J, Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase, J Biol Chem 275(14) (2000) 10661–10672. [DOI] [PubMed] [Google Scholar]
- [41].Rousseau S, Houle F, Landry J, Huot J, p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells, Oncogene 15(18) (1997) 2169–2177. [DOI] [PubMed] [Google Scholar]
- [42].Chazin VR, Kaleko M, Miller AD, Slamon DJ, Transformation mediated by the human HER-2 gene independent of the epidermal growth factor receptor, Oncogene 7(9) (1992) 1859–1866. [PubMed] [Google Scholar]
- [43].Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ, Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells, Oncogene 9(7) (1994) 1829–1838. [PubMed] [Google Scholar]
- [44].Aguilar Z, Akita RW, Finn RS, Ramos BL, Pegram MD, Kabbinavar FF, Pietras RJ, Pisacane P, Sliwkowski MX, Slamon DJ, Biologic effects of heregulin/neu differentiation factor on normal and malignant human breast and ovarian epithelial cells, Oncogene 18(44) (1999) 6050–6062. [DOI] [PubMed] [Google Scholar]
- [45].Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ, HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells, Oncogene 10(12) (1995) 2435–2446. [PubMed] [Google Scholar]
- [46].Berkelhammer J, Luethans TN, Hook RR Jr., Oxenhandler RW, Phenotypic instability of mouse melanomas after propagation in vivo and in vitro, Cancer Res 46(6) (1986) 2923–2928. [PubMed] [Google Scholar]
- [47].Garon EB, Pietras RJ, Finn RS, Kamranpour N, Pitts S, Marquez-Garban DC, Desai AJ, Dering J, Hosmer W, von Euw EM, Dubinett SM, Slamon DJ, Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer, J Thorac Oncol 8(3) (2013) 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hill JH, Plant RL, Harris DM, Grossweiner LI, Rok B, Seter AJ, The nude mouse xenograft system: a model for photodetection and photodynamic therapy in head and neck squamous cell carcinoma, Am J Otolaryngol 7(1) (1986) 17–27. [DOI] [PubMed] [Google Scholar]
- [49].Lockshin A, Kozielski T, Stehlin JS Jr., Prediction of anticancer activity by tumor uptake of radiolabeled monoclonal antibody, Cancer Lett 30(1) (1986) 1–9. [DOI] [PubMed] [Google Scholar]
- [50].Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D, Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers, Oncogene 18(13) (1999) 2241–2251. [DOI] [PubMed] [Google Scholar]
- [51].Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ, Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs, Oncogene 17(17) (1998) 2235–2249. [DOI] [PubMed] [Google Scholar]
- [52].Chevalier F, Lavergne M, Negroni E, Ferratge S, Carpentier G, Gilbert-Sirieix M, Sineriz F, Uzan G, Albanese P, Glycosaminoglycan mimetic improves enrichment and cell functions of human endothelial progenitor cell colonies, Stem Cell Res 12(3) (2014) 703–715. [DOI] [PubMed] [Google Scholar]
- [53].Ferratge S, Ha G, Carpentier G, Arouche N, Bascetin R, Muller L, Germain S, Uzan G, Initial clonogenic potential of human endothelial progenitor cells is predictive of their further properties and establishes a functional hierarchy related to immaturity, Stem Cell Res 21 (2017) 148–159. [DOI] [PubMed] [Google Scholar]
- [54].Bevilacqua P, Barbareschi M, Verderio P, Boracchi P, Caffo O, Dalla Palma P, Meli S, Weidner N, Gasparini G, Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma--results of a multiparametric study, Breast Cancer Res Treat 36(2) (1995) 205–217. [DOI] [PubMed] [Google Scholar]
- [55].Jacquemier JD, Penault-Llorca FM, Bertucci F, Sun ZZ, Houvenaeghel GF, Geneix JA, Puig BD, Bardou VJ, Hassoun JA, Birnbaum D, PJ. Viens, Angiogenesis as a prognostic marker in breast carcinoma with conventional adjuvant chemotherapy: a multiparametric and immunohistochemical analysis, J Pathol 184(2) (1998) 130–135. [DOI] [PubMed] [Google Scholar]
- [56].Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G, Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma, J Natl Cancer Inst 84(24) (1992) 1875–1887. [DOI] [PubMed] [Google Scholar]
- [57].Weidner N, Semple JP, Welch WR, Folkman J, Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma, N Engl J Med 324(1) (1991) 1–8. [DOI] [PubMed] [Google Scholar]
- [58].Alameddine RS, Otrock ZK, Awada A, Shamseddine A, Crosstalk between HER2 signaling and angiogenesis in breast cancer: molecular basis, clinical applications and challenges, Curr Opin Oncol 25(3) (2013) 313–324. [DOI] [PubMed] [Google Scholar]
- [59].Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL, HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression, Mol Cell Biol 21(12) (2001) 3995–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fakhrejahani E, Toi M, Antiangiogenesis therapy for breast cancer: an update and perspectives from clinical trials, Jpn J Clin Oncol 44(3) (2014) 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ribatti D, Nico B, Ruggieri S, Tamma R, Simone G, Mangia A, Angiogenesis and Antiangiogenesis in Triple-Negative Breast cancer, Transl Oncol 9(5) (2016) 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tawara K, Scott H, Emathinger J, Ide A, Fox R, Greiner D, LaJoie D, Hedeen D, Nandakumar M, Oler AJ, Holzer R, Jorcyk C, Co-Expression of VEGF and IL-6 Family Cytokines is Associated with Decreased Survival in HER2 Negative Breast Cancer Patients: Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion, Transl Oncol 12(2) (2018) 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zajkowska M, Gacuta E, Kozlowska S, Lubowicka E, Glazewska EK, Chrostek L, Szmitkowski M, Pawlowski P, Zbucka-Kretowska M, Lawicki S, Diagnostic power of VEGF, MMP-9 and TIMP-1 in patients with breast cancer. A multivariate statistical analysis with ROC curve, Adv Med Sci 64(1) (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [64].Miller KD, O’Neill A, Gradishar W, Hobday TJ, Goldstein LJ, Mayer IA, Bloom S, Brufsky AM, Tevaarwerk AJ, Sparano JA, Le-Lindqwister NA, Hendricks CB, Northfelt DW, Dang CT, Sledge GW Jr., Double-Blind Phase III Trial of Adjuvant Chemotherapy With and Without Bevacizumab in Patients With Lymph Node-Positive and High-Risk Lymph Node-Negative Breast Cancer (E5103), J Clin Oncol 36(25) (2018) 2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Redondo A, Ramos Vazquez M, Manso L, Gil Gil MJ, Garau Llinas I, Garcia-Garre E, Rodriguez CA, Chacon JI, Lopez-Vivanco G, Long-term response to first-line bevacizumab-based therapy in patients with metastatic breast cancer: results of the observational “LORENA” study, Onco Targets Ther 11 (2018) 5845–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sini V, Cassano A, Corsi D, De Laurentiis M, Gamucci T, Mauri M, Naso G, Roselli M, Ruggeri EM, Tonini G, Vici P, Zampa G, Marchetti P, Bevacizumab as first-line treatment in HER2-negative advanced breast cancer: pros and cons, Tumori 102(5) (2016) 472–480. [DOI] [PubMed] [Google Scholar]
- [67].Varella L, Abraham J, Kruse M, Revisiting the Role of Bevacizumab in the Treatment of Breast Cancer, Semin Oncol 44(4) (2017) 273–285. [DOI] [PubMed] [Google Scholar]
- [68].Huemer F, Gampenrieder SP, Schlattau A, Greil R, Overcoming resistance against HER2-targeting agents in fifth-line therapy: is there still a place for bevacizumab in HER2+ breast cancer?, Clin Breast Cancer 14(1) (2014) e17–20. [DOI] [PubMed] [Google Scholar]
- [69].Burridge K, Chrzanowska-Wodnicka M, Focal adhesions, contractility, and signaling, Annu Rev Cell Dev Biol 12 (1996) 463–518. [DOI] [PubMed] [Google Scholar]
- [70].Huot J, Houle F, Spitz DR, Landry J, HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress, Cancer Res 56(2) (1996) 273–279. [PubMed] [Google Scholar]
- [71].Huot J, Lambert H, Lavoie JN, Guimond A, Houle F, Landry J, Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27, Eur J Biochem 227(1–2) (1995) 416–427. [DOI] [PubMed] [Google Scholar]
- [72].Lavoie JN, Hickey E, Weber LA, Landry J, Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27, J Biol Chem 268(32) (1993) 24210–24214. [PubMed] [Google Scholar]
- [73].Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J, Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27, Mol Cell Biol 15(1) (1995) 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Williams JI, Squalamine- a new angiostatic steroid, Humana Press, Plymouth, MA, 1998. [Google Scholar]
- [75].Akhter S, Nath SK, Tse CM, Williams J, Zasloff M, Donowitz M, Squalamine, a novel cationic steroid, specifically inhibits the brush-border Na+/H+ exchanger isoform NHE3, Am J Physiol 276(1 Pt 1) (1999) C136–144. [DOI] [PubMed] [Google Scholar]
- [76].Eckhardt SG, Angiogenesis inhibitors as cancer therapy, Hosp Pract (1995) 34(1) (1999) 63–68, 77,–69, 83–64. [DOI] [PubMed] [Google Scholar]
- [77].Perni M, Galvagnion C, Maltsev A, Meisl G, Muller MB, Challa PK, Kirkegaard JB, Flagmeier P, Cohen SI, Cascella R, Chen SW, Limbocker R, Sormanni P, Heller GT, Aprile FA, Cremades N, Cecchi C, Chiti F, Nollen EA, Knowles TP, Vendruscolo M, Bax A, Zasloff M, Dobson CM, A natural product inhibits the initiation of alpha-synuclein aggregation and suppresses its toxicity, Proc Natl Acad Sci U S A 114(6) (2017) E1009–E1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Abedi H, Zachary I, Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells, J Biol Chem 272(24) (1997) 15442–15451. [DOI] [PubMed] [Google Scholar]
- [79].Mustonen T, Alitalo K, Endothelial receptor tyrosine kinases involved in angiogenesis, J Cell Biol 129(4) (1995) 895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Soker S, Fidder H, Neufeld G, Klagsbrun M, Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain, J Biol Chem 271(10) (1996) 5761–5767. [DOI] [PubMed] [Google Scholar]
- [81].Kroll J, Waltenberger J, The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells, J Biol Chem 272(51) (1997) 32521–32527. [DOI] [PubMed] [Google Scholar]
- [82].Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK, Tumour biology: herceptin acts as an anti-angiogenic cocktail, Nature 416(6878) (2002) 279–280. [DOI] [PubMed] [Google Scholar]


