Abstract
Objectives
To examine the efficacy of lithium as a mood stabilizer for patients with autism spectrum disorder (ASD).
Experimental Design
A retrospective chart review was performed that examined the use of both extended and immediate release lithium carbonate in patients with ASD that were treated at a single clinical center (CNNH NeuroHealth). Clinical Global Impression (CGI) scales were used to quantify baseline severity of ASD and mood symptoms as well as improvement after treatment with lithium carbonate.
Principle Observations
Our retrospective chart review found that 73.7% (n = 14) of patients with ASD and concomitant maladaptive behaviors experienced “improvement” (CGI-I rating ≤ 3) with the addition of lithium to their treatment regimen. Those with comorbid “ADHD” phenotype were most predictive of an efficacious response (p = 0.038, Odds Ratio 12.2).
Conclusions
Lithium carbonate is a viable, efficacious and well tolerated alternative to various neuroleptics and other psychotropic medications for use as a mood stabilizer for patients with ASD.
Keywords: autism, autism spectrum disorder, lithium, irritability, adhd, self-injury, aggression, obsessive compulsive disorder, bipolar, mood stabilization
Introduction
Even though it did not gain FDA approval until 1970, lithium has been used internationally since the 1950s to treat psychiatric illness, particularly adults with mania in bipolar disorder.1 Outside of bipolar diagnoses, lithium has been found to reduce the risk of suicide in patients with major depressive disorder2 and to decrease severe aggression in hospitalized children with conduct disorders.3 Although it has presented as an effective treatment for both relief of mania and prophylaxis of depression, current trends in medication prescribing for these disorders have shifted away from its use.1 Despite this trend and the tendency in popular opinion to denounce it as ineffective or toxic, a review of published reports on the efficacy of lithium over 25 years reveals that it has continued to be a viable mood stabilizer that yields a substantial decrease in mortality risk for depressed and bipolar patients.4
The exact mechanism of lithium efficacy remains unclear. Elucidating the pharmacodynamic mechanisms of lithium could provide insight into the pathology of mood dysregulation. Various hypotheses have been proposed, including enhancement of presynaptic activity in the serotonergic system, while also preventing dopaminergic or cholinergic supersensitivity by blocking postsynaptic receptors.5 Lithium may also modify cellular calcium or glutamate regulation and homeostasis, as chronic lithium treatment has demonstrated neuroprotective effects against glutamate excitotoxicity by inhibiting NMDAR-mediated calcium influx.6
Other studies suggest that lithium may act on second messenger systems in the brain. One theory is the “inositol depletion hypothesis.” It postulates that since lithium has been shown to inhibit IMPase, it leads to inositol depletion, which could then decrease levels of PIP3 and decrease regeneration of second-messenger IP3. This depletion would then interfere with signaling cascades for PKC and IP3 systems, in effect, blocking some ligand-gated signaling.5 Lithium inhibits IMPase uncompetitively, indicating that it would be most active in systems with the highest substrate concentration, which could explain why lithium works to stabilize mood across several systems and treats both poles of bipolar disorder.5 A study using proton magnetic resonance spectroscopy found that bipolar patients have higher levels of myo-inositol during the manic phase and that the levels were decreased in the anterior cingulate after lithium treatment.7
Lithium was also found to be a competitive inhibitor for glycogen synthase kinase 3 (GSK-3β), a downstream regulator of diverse signaling pathways, as well as a factor in regulating GSK-3β phosphorylation.8 Further, transgenic mice that overexpress GSK-3β have been shown to model manic behavior.9 Other research found that lithium treatment of rats reduced arachidonic acid in brain phospholipids, which suggests that it could possibly also target the arachidonic acid cascade.10 A recent hiPSC study of lithium-responsive BPD neurons found that lithium altered the phosphorylation of CRMP2 (which can be influenced by GSK-3β-dependent pathways), which impacted dendritic spine formation and neural network formation.11 Overall, its complex mechanisms of action allow lithium to have potentially widespread and diverse beneficial effects for mood regulation.
Autism Spectrum Disorder (ASD) is a diagnosis that can be assigned irrespective of biological cause. Thus, the biological heterogeneity of individuals with ASD confounds research in determining effective treatments, particularly pharmacological interventions. A systematic review of psychotropic drug use in individuals with ASD found that median prevalence of its use was 45.7%, and that the median for studies focusing on adults was 61.5%.12
To date, there has been no randomized, controlled study of lithium’s use in ASD. A case study examined two patients who had the same mutation that caused a premature stop codon in exon 21 of the SHANK3 gene, exhibited catatonia-like deterioration, and were diagnosed with ASD. Both patients failed to respond to antipsychotics, benzodiazepines, mood stabilizers, antidepressants, and methylphenidate, but showed remarkable reversal of their symptoms upon treatment with lithium.13 A retrospective follow-up study of 60 ASD children found significant improvement of mood disorder symptoms upon treatment with lithium for social withdrawal, lack of speech production, and abnormal relationships with objects.14
A retrospective chart review of 30 ASD individuals in an inpatient psychiatry unit, 53% with concomitant intellectual disability, found that 43% of the ASD patients treated with lithium were rated as “improved” and 71% of those with two or more pretreatment mood symptoms were rated as “improved”.15 Their analysis showed that those who exhibited elevated mood or mania and were non-responders to atypical antipsychotics showed the most improvement.
Individuals with ASD phenotypes will often have an array of problematic maladaptive behaviors, including aggressions, self-injurious behaviors (SIB), irritability, impulsiveness, and obsessive-compulsive behaviors (OCB).16 Additionally, these maladaptive behaviors can come on suddenly without identifiable provocation or antecedent, and can “shift” rapidly (mood dysregulation). Pharmacological interventions for such maladaptive behavior patterns often rely on an array of psychotropic drugs, including those in the classes of neuroleptic, antiepileptic, psychostimulant, and alpha-adrenergic agonist drugs, occasionally augmented by anti-anxiety agents (benzodiazepines, SSRI). Such medications often cause severe and limiting side effects or are ineffective. We identified a need for an alternative way to stabilize maladaptive behaviors, mood and OCB/perseverative behaviors in ASD patients when neuroleptics or anticonvulsants produce adverse side effects or prove ineffective. In such situations, we hypothesized that lithium may warrant clinical consideration as a relatively safe and effective adjunctive pharmacological therapy.
Materials and Methods
We conducted a retrospective chart review of patients (N = 19) with moderate to severe ASD from a single organization (CNNH NeuroHealth, Voorhees, NJ), who received Lithium Carbonate (immediate or extended release) for treatment of maladaptive behaviors. Variables collected were demographics (age, gender), weight, other diagnoses, previous medications, side effects of previous medications, mood symptoms, date started lithium treatment, duration of lithium treatment, last effective dose of lithium, concomitant medications (at start of treatment and at present), mg/kg dose, blood levels of lithium, side effects, and qualitative data about the effects of lithium treatment [Table 1]. The Clinical Global Impression – Severity (CGI-S) scale was utilized prior to lithium treatment, followed by Clinical Global Impression – Improvement (CGI-I) scale to determine efficacy of lithium treatment.17 CGI-S and CGI-I were completed by one of the authors (Mintz) who is research-trained in utilization of CGI scales. All patient information has been de-identified in conformance with HIPAA requirements.
Table 1. Patient Demographics, Including Concomitant Diagnoses, Maladaptive Behaviors, and CGI-S Scores.
| PT # | AGE | SEX | ASD | ID | OCB | ADHD | OTHER DX | MALADAPTIVE BEHAVIORS | CGI-S |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | F | S | Y | Y | N | GAD, E, MD | PB, Nocturnal maladaptive behaviors | 6 |
| 2 | 27 | M | S | Y | Y | N | E, CP, MVD, SD, I | MST, PB, OCB, agitation | 7 |
| 3 | 25 | M | S | Y | Y | N | E, MD, SD | OB, AG | 7 |
| 4 | 18 | M | S | Y | Y | Y | SD, MD, MVD, GAD | MST, OCB, PB, SIB, decreased need for sleep, decreased interest in pleasurable activity |
7 |
| 5 | 15 | M | S | Y | Y | N | MVD, MD, GAD | OCB, AG, PB, pacing | 7 |
| 6 | 20 | M | S | Y | Y | Y | GAD, O, SD | PB | 5 |
| 7 | 32 | M | M | Y | Y | Y | GAD | PB, OCB | 6 |
| 8 | 19 | M | S | N | Y | Y | GAD, MO, MVD | T, OCB, PB | 5 |
| 9 | 16 | F | S | Y | N | N | SD, MD, MVD, GAD | SIB, AG, SST | 7 |
| 10 | 36 | M | S | Y | Y | Y | O, GAD, SD | PB, OCB, T | 6 |
| 11 | 14 | M | S | N | Y | Y | SD, MO, I | elopement, AG, OT | 7 |
| 12 | 27 | M | S | Y | Y | Y | I, GAD, E | OCB, OT, AG | 6 |
| 13 | 29 | M | S | Y | N | N | I, E, GAD | AG, SIB | 7 |
| 14 | 24 | M | M | N | N | Y | MO, GAD, BD, SD, I, O | OT, SIB | 5 |
| 15 | 17 | M | M | Y | Y | Y | SD, MVD, GAD | OCB, PB, SST, SIB | 5 |
| 16 | 15 | M | S | Y | N | N | I, GAD, SD, MD | OCB, OT, extreme autonomic over-reactivity | 7 |
| 17 | 15 | M | M | N | Y | Y | BD, MO, I, SD | OB, excessive talking, IR, low frustration tolerance, OCB | 4 |
| 18 | 27 | M | S | N | Y | Y | GAD, E, O, SD, MVD | AG | 6 |
| 19 | 16 | M | M | N | Y | Y | MO, SD | AG, OT, physical violence and threats | 4 |
| Overall (Mean or Percent) |
22.1 | 89.5% M | 73.7% S, 26.3% M | 63.2% | 78.9% | 63.2% | Mean 6.0 ± 1.1 |
Abbreviations: F, female; M, male; Y, yes; N, no; M, mild/high functioning ASD; S, moderate/severe ASD; GAD, Generalized Anxiety Disorder; E, Epilepsy; MD, Metabolic Disorder; SD, Sleep Disorder; CP, Cerebral Palsy; I, Impulse Control Disorder; MVD, Movement Disorder; O, Obesity; OCB, Obsessive Compulsive Behavior; ID, Intellectual Disability; BD, Bipolar Disorder; MO, Mood Disorder; AG, Aggressions; OT, Outbursts; IR, Irritability; OB, Oppositional Behaviors; SSB, Self-Injurious Behaviors; MST, Motor Stereotypies; PB, Perseverative Behaviors; T, Tics.
Statistical Analysis
A CGI-I rating of ≤ 3 were considered “improved,” and those > 3 were “not improved.” Because of the relatively small sample size, CGI-I was examined within multiple subsets of the sample by performing Fisher Exact Tests that compared improvement in those with intellectual disability, OCD, ADHD, Blood level ≥ 0.6 mmol/L, ASD-Severe/Moderate, and CGI-S > 5 to those who did not have such diagnoses. Additionally, a paired two sample for means t-test examining concomitant medication before and after lithium treatment was performed.
Results
The cohort was largely male: 17 males and 2 females [Table 1]. Mean age was 22 years and 1 month, with a standard deviation for 6 years and 6 months. 73.7% (n = 14) were diagnosed with ASD – Severe/Moderate, while 26.3% (n = 5) were diagnosed with ASD – Mild/High-Functioning. All patients had episodic and diagnosable mood dysregulation. Concomitant diagnoses included intellectual disability (63.2%; n = 12), ADHD (63.2%; n = 12), obsessive-compulsive disorder (78.9%; n = 15), bipolar disorder (26%; n = 4), epilepsy (32%; n = 6), anxiety (74%; n = 14), and sleep disorder (63%; n = 13) [Figure 1].
Figure 1.
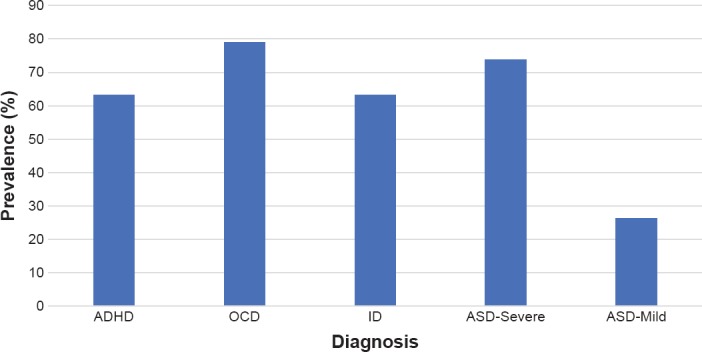
Prevalence of Concomitant Diagnoses within the Sample by Type
The mean duration of lithium treatment was 495 days (SD = 277 days, exclusive of one patient receiving lithium treatment for 3,740 days). A previous review of serum lithium levels for long-term treatment of bipolar disorder found that the optimal response was achieved at 0.6–0.75 mmol/L.18 The mean blood level at most effective lithium dose for our sample was 0.6 ± 0.3 mmol/L. The mean mg/kg dose was 5.3 mg/kg (SD = 1.8). 90.9% of the patients that had a lithium blood level of ≥ 0.6 mmol/L at their optimal dose were in the “improved” category. Although this was not found to be statistically significant (p = 0.11), it is consistent with previous medical literature and lithium dosing recommendations.
Of previous medications taken by patients before lithium treatment, risperidone (42%), fluoxetine (42%), clorazepate (42%), and clonidine (42%) were the most common [Table 2]. 64% of patients had taken a neuroleptic, 58% an anti-anxiety/antidepressant, 58% an antiepileptic drug, and 53% an “ADHD” medication. 32% (n = 6) had adverse side effects to their neuroleptic medication, the most common of which was excessive weight gain (n = 4).
Table 2. Medication History Prior to Lithium by Type.
| MEDICATION | # OF PATIENTS | PERCENT | MEDICATION | # OF PATIENTS | PERCENT |
|---|---|---|---|---|---|
| Neuroleptics | 13 | 65 | Antidepressants | 11 | 55 |
| Risperidone | 8 | 40 | Fluoxetine | 8 | 40 |
| Aripiprazole | 7 | 35 | Sertraline | 3 | 15 |
| Quetiapine | 2 | 10 | Escitalopram | 2 | 10 |
| Anticonvulsants | 12 | 60 | ADHD Medications | 11 | 55 |
| Oxcarbazepine | 4 | 20 | Methylphenidate | 5 | 25 |
| Lamotrigine | 3 | 15 | Guanfacine | 7 | 35 |
| Zonisamide | 3 | 15 | Atomoxetine | 1 | 5 |
| Other | 13 | 65 | |||
| Clorazepate | 8 | 40 | |||
| Clonidine | 8 | 40 | |||
| Hydroxyzine | 3 | 15 |
Lithium was provided as an adjunctive pharmacological agent to an established but suboptimal medication regimen. 42% of patients were simultaneously taking a neuroleptic, 47% an anti-anxiety/antidepressant, 20% an antiepileptic, and 16% an “ADHD” (psychostimulant or alpha-2 adrenergic agonist) medication. A paired two sample for means t-test found no significant difference between the concomitant medications before and after/during optimal lithium treatment (p = 0.125), but the mean decreased from 3.42 medications to 2.89 medications.
42.1% of patients reported side effects to lithium, the most common of which were fatigue (26.3%, n = 5) and tremors (10.5%, n = 2) [Table 3]. However, only three patients discontinued lithium as a result of side effects or loss of efficacy over time (tolerance).
Table 3. Dose Effects of Lithium Treatment.
| PATIENT # | MOST EFFECTIVE DOSE | MG/KG DOSE | BLOOD LEVELS (MMOL/L) | SIDE EFFECTS OF LI | DURATION OF LI TREAMENT (DAYS) | CGI-I | CONCOMITANT MEDICATIONS |
|---|---|---|---|---|---|---|---|
| 1 | 150/300/150 | 3.9 | 0.6 | NR | 201 | 4 | Risperidone, Zonisamide, Buspirone, Diazepam |
| 2 | ER 450 bid | 5.0 | 0.7 | Lethargy | 973 | 1 | Oxcarbazepine, Mirtazapine, Lorazepam, Atenolol, Lisinopril |
| 3 | ER 450 bid | 5.8 | 0.8 | lethargy, tremors at higher doses | 365 | 5 | Betaine, Clorazepate, Leucovorin, Kapvay |
| 4 | 450/300/450 | 7.7 | 1 | NR | 735 | 1 | Fluphenazine, Clonidine, L-Methylfolate, Atarax |
| 5 | 450 bid | 7.7 | 0.8 | fatigue on tid | 763 | 3 | Acetazolamine, Risperidone, Fluoxetine, Clonidine, Lorazepam, Leucovorin |
| 6 | ER 450/300 bid | 3.9 | 0.2 | NR | 488 | 1 | Clonidine |
| 7 | ER 600 bid | 5.6 | 1 | slight right hand tremor | 743 | 2 | Escitalopram, Adderall, Clorazepate, Rantidine |
| 8 | ER 450 bid | 7.8 | 0.8 | NR | 515 | 2 | Creatin |
| 9 | ER 450 bid | 7.3 | 0.6 | NR | 459 | 2 | None |
| 10 | ER 450 bid | 3.2 | 0.6 | lethargy, increased tics | 999 | 3 | Quetiapine, Fluoxetine |
| 11 | 450/300/300 | 5.0 | 0.7 | NR | 880 | 2 | Fluvoxamine |
| 12 | ER 450 bid | 5.0 | 0.6 | NR | 352 | 1 | Quetiapine, Fluoxetine, Clorazepate, Atenolol |
| 13 | ER 450 bid | 6.3 | 0.4 | behaviors worsened | 186 | 5 | Clobazam, Lacosamide, Vagal nerve stimulator |
| 14 | ER 450 bid | 3.5 | 0.7 | dizziness | 3,768 | 3 | Valproic acid, Propranolol, Quetiapine |
| 15 | ER 450 bid | 6.7 | 0.5 | NR | 330 | 1 | Aripriprazole, Fluoxetine, Atomoxetine, Leucovorin |
| 16 | 300 bid | 5.0 | 0.4 | became tolerant | 367 | 4 | Clonidine, Oxcarbazepine |
| 17 | ER 300 bid | 4.2 | 0.1 | NR | 90 | 4 | Thyroid |
| 18 | 150 tid | 1.2 | 0.3 | lethargy | 81 | 3 | Ziprasidone, Topiramate, Clonidine, Fluvoxamine, Trazodone, Lisdexamfetamine |
| 19 | 300/150/300 | 5.6 | 0.2 | NR | 841 | 2 | Guanfacine, Methylphenidate, Aripiprazole |
Note: NR = none reported.
At baseline, the mean CGI-S score was 6.0 (SD = 1). None of the patients in our sample had a CGI-S of less than 4.
CGI-I outcomes found that 73.7% (n = 14) of patients experienced “improvement” (CGI-I ≤ 3) in their maladaptive behaviors and mood symptoms [Table 3]. Additionally, 52.6% (n = 10) of the patients were in the “much improved” and “very much improved” categories (CGI-I ≤ 2). Of those patients with CGI-I ≤ 3, 78.6% (n = 11) had previously failed neuroleptic medication trials or discontinued neuroleptics because of side effects. In patients with CGI-I ≤ 2, 80% (n = 8) failed at least one neuroleptic medication trial prior to lithium treatment or discontinued neuroleptics because of side effects.
91% (n = 11) of patients with concomitant diagnosis of ADHD fell in the improved category. Compared to patients without comorbid ADHD diagnoses, those with comorbid ADHD had statistically significant lithium response rates for the CGI-I (p = 0.038). Although 80% (n = 12) of patients with comorbid OCB improved, this value was not significantly different from those without comorbid OCB (p = 0.27), although there was a high Odds Ratio (3.7). Of note, only 53.8% of those that had a CGI-S > 5 (n = 7) were rated as improved and this was not statistically significant (p = 1), suggesting that the level of severity of illness may not impact the efficacy of lithium [Table 4].
Table 4. Results of a Fisher Exact 2×2 Analysis, with an Alpha Significance of p ≤ 0.05 for the Number of Patients Improved by Concomitant Diagnosis (Compared to Patients Not Improved with that Concomitant Diagnosis). Fisher Exact Test Used Because of the Small Sample Size.
| CRITERIA | NUMBER IMPROVED WITH DIAGNOSIS | % IMPROVED | P-VALUE | ODDS RATIO |
|---|---|---|---|---|
| ID | 9 | 69.2 | 1 | 0.46 |
| OCB | 12 | 80.0 | 0.27 | 3.7 |
| ADHD | 11 | 91.7 | 0.038** | 12.2 |
| Blood Level ≥ 0.6 | 10 | 90.9 | 0.11 | 8.7 |
| ASD Severe/Moderate | 11 | 73.3 | 1 | 0.92 |
| CGI-S > 5 | 7 | 53.8 | 1 | 0.6 |
Case Reports
27-year-old male (Table 1/3, patient #2) with ASD-moderate/severe and moderate intellectual disability experienced extreme motor stereotypies, agitation, and obsessive/compulsive behaviors with severe perseverations. He had previously tried and failed trazodone, quetiapine, aripiprazole, clorazepate, clonidine, fluoxetine, neurontin, methylphenidate, sertraline, chloral hydrate. Quetiapine made his sleep worse, and clorazepate as well as aripiprazole worsened his behaviors. Lithium treatment was started using the extended release formulation, titrating to a dose of 450 mg b.i.d. dose (5.0 mg/kg). His family reported that he seemed to be a “new person.” They observed that motor stereotypies had markedly diminished and various repetitive/“self-stimulatory” behaviors were nearly absent. The patient’s mood improved (“happier”), agitation resolved, and sleep patterns improved. At the steady-state dose, lithium trough blood level was 0.7 mmol/L. CGI-S baseline rating was seven, and after lithium treatment CGI-I rating was one. The patient has tolerated lithium for 973 days (at the time of this manuscript), with only occasional slight lethargy.
18-year-old male (Table 1/3, patient #4) with ASD-moderate/severe and intellectual disability, associated with motor stereotypies, obsessive/compulsive and perseverative behaviors, SIB, mood changes, decreased need for sleep, and anxiety. Previous medication trials included risperidone, sertraline, lorazepam, guanfacine, hydroxyzine, fluoxetine, clorazepate. Risperidone caused a liver enzyme increase and weight gain, while fluoxetine caused a behavioral reaction. Lithium carbonate was started and titrated to 450/300/450 mg t.i.d. The patient experienced a marked decrease in perseverative and stereotyped behaviors. His family reported that he became more aware, social, and engaged. They also reported that he became “happier” and less manic. With lithium, he became more cooperative and compliant, and was able to walk with his mother for three miles a day at times. Lithium dosage was 7.7 mg/kg/day, with a trough blood level of 1.0 mmol/L. Baseline CGI-S was seven, and CGI-I rating with lithium treatment was one. Presently on 735 days of lithium therapy, with no reported side effects.
Discussion
Our retrospective chart review found that 73.7% of patients with ASD and concomitant maladaptive behaviors experienced “improvement” (CGI-I rating of ≤ 3) with the addition of lithium carbonate to their treatment regimen. Although improvements could be seen in those with various forms of mood dysregulation, those with comorbid “ADHD” (p = 0.038, Odds Ratio 12.2) and OCD (Odds Ratio 3.7) phenotypes were most predictive of an efficacious response. Overall, lithium carbonate was found to be relatively well tolerated without major side effects as long as careful monitoring of blood levels and other parameters are done. Additionally, these data demonstrate that lithium carbonate is a viable alternative to various neuroleptics and other psychotropic medications, with an overall favorable side effect profile, particularly for chronic, long-term treatment regimens.
The elevated baseline CGI-S scores demonstrate that this cohort had significantly severe neuropsychiatric illness. Additionally, the cohort had many patients with multiple neurological and neuropsychiatric comorbid diagnoses, as well as many previous and concomitant medication trials, which can confound analyses. The biological heterogeneity of ASD is a further confounder. Nevertheless, in this cohort, ASD-moderate/severe vs. mild/high functioning or CGI-S baseline of > 5 vs. ≤ 5 did not impact lithium efficacy, suggesting that comorbid factors may be more predictive of treatment response than the categorization type of ASD.
This study was limited by its small sample size, retrospective nature, uncontrolled clinical setting, and the biological and clinical heterogeneity of ASD. Additionally, many patients were on polypharmacy, which can create predictable and unpredictable drug-drug interactions.
Conclusions
Lithium carbonate is a viable, efficacious and well tolerated alternative to various neuroleptics and other psychotropic medications for use as a mood stabilizer for patients with ASD. Further investigations, particularly prospective, controlled clinical trials could provide additional information concerning the efficacy and tolerability of lithium carbonate treatment in ASD populations.
Footnotes
Conflict of Interest
The Authors declare that there are no conflicts of interest for the subject matter of this manuscript.
References
- 1.Shortner E. The history of lithium therapy. Bipolar Disord. 2009;11:4–9. doi: 10.1111/j.1399-5618.2009.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzzetta F, Tondo L, Centorrino F, Baldessarini R. Lithium Treatment Reduces Suicide Risk in Recurrent Major Depressive Disorder. J Clin Psychiatry. 2007;68:380–383. doi: 10.4088/jcp.v68n0304. [DOI] [PubMed] [Google Scholar]
- 3.Malone R, Delaney M, Luebbert J, Cater J, Campbell M. A Double-Blind Placebo-Controlled Study of Lithium in Hospitalized Aggressive Children and Adolescents With Conduct Disorder. Arch Gen Psychiatry. 2000;57:649–654. doi: 10.1001/archpsyc.57.7.649. [DOI] [PubMed] [Google Scholar]
- 4.Baldessarini R, Tondo L. Does Lithium Treatment Still Work? Evidence of Stable Responses Over Three Decades. Arch Gen Psychiatry. 2000;57:187–190. doi: 10.1001/archpsyc.57.2.187. [DOI] [PubMed] [Google Scholar]
- 5.Corbella B, Vieta E. Molecular targets of lithium action. Acta Psychiatr Scand. 2003;15:316–340. doi: 10.1046/j.1601-5215.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka S, Hough CJ, Chuang D-M. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-d-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davanzo P, Thomas M, Yue K et al. Decreased Anterior Cingulate Myo-inositol/Creatine Spectroscopy Resonance with Lithium Treatment in Children with Bipolar Disorder. Neuropsychoparhmacol. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 8.Brown K, Tracy D. Lithium: the pharmacodynamic actions of the amazing ion. J Psychopharmacol. 2012;3:163–176. doi: 10.1177/2045125312471963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prickaerts J, Moechars D, Cryns K et al. Transgenic Mice Overexpressing Glycogen Synthase Kinase 3β: A Putative Model of Hyperactivity and Mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport S, Bosetti F. Do Lithium and Anticonvulsants Target the Brain Arachidonic Acid Cascade in Bipolar Disorder? Arch Gen Psychiatry. 2002;59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 11.Tobe B, Crain A, Winquist A et al. Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. PNAS. 2017;114:E4462–E4471. doi: 10.1073/pnas.1700111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobski K, Hofer J, Hoffman F, Bachmann C. Use of psychotropic drugs in patients with autism spectrum disorders: a systematic review. Acta Psychiatr Scand. 2017;135:8–28. doi: 10.1111/acps.12644. [DOI] [PubMed] [Google Scholar]
- 13.Serret S, Thummler S, Dor R, Vesperini S, Santos A, Askenazy F. Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry. 2015;15:1–6. doi: 10.1186/s12888-015-0490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boil J, Donno F, Petza S, Cera F, Balia C, Carucci S, Zuddas A. Medium-term efficacy data of medications in children and adolescents with autism spectrum disorder: an 18 months retrospective follow up study. Eur Neuropsychopharmacol. 2017;27:S1112. [Google Scholar]
- 15.Siegel M, Beresford C, Bunker M et al. Preliminary Investigation of Lithium for Mood Disorder Symptoms in Children and Adolescents with Autism Spectrum Disorder. J Child Adolesc Psychopharmacol. 2014;24:399–402. doi: 10.1089/cap.2014.0019. [DOI] [PubMed] [Google Scholar]
- 16.Robb A. Managing Irritability and Aggression in Autism Spectrum Disorders in Children and Adolescents. Developmental Disabilities. 2010;16:258–264. doi: 10.1002/ddrr.118. [DOI] [PubMed] [Google Scholar]
- 17.Guy W. Clinical Global Impressions Scale. ECDEU Assessment Manual for Psychopharmacology. 1976 [Google Scholar]
- 18.Severus WE, Kleindienst N, Seemuller F, Fangou S, Moller HJ, Griel W. What is the optimal serum lithium level in the long‐term treatment of bipolar disorder – a review? Bipolar Disord. 2008;10:231–237. doi: 10.1111/j.1399-5618.2007.00475.x. [DOI] [PubMed] [Google Scholar]


