Abstract
Background
Few stroke patients have reported improvements after Ayurvedic massage. Unfortunately, there is a dearth of indexed literature to support the use of this in rehabilitation.
Objectives
To objectively measure the differences between patients with stroke who received Ayurvedic massage in addition to standard Physiotherapy (PT) versus those who received only standard PT.
Materials and methods
The study was a prospective case control study, retrospectively analysed. The setting was a tertiary level hospital with neuro-rehabilitation unit. Fifty-two patients undergoing acute inpatient rehabilitation were prospectively followed post stroke. They were self-selected one month from the event for Ayurvedic massage with regular PT or PT alone. Twenty five received Ayurvedic massage with PT and twenty seven received only PT. All participants completed treatment. Information related to age, gender, National Institute of Health Stroke Scale result, number of co-morbidities, and whether cases were deemed simple or complex were taken at baseline. All patients received 6 hours of physical therapy averaged over a week. Massage was delivered daily for a total of 10 sessions followed by steam application.
Results
Patients were categorized as simple or complicated stroke based on events prior to rehabilitation. Both simple and complicated patients who received Ayurvedic massage had lower MAS and need for antispastic drugs, achieved standing with minimal assistance sooner, and had better locomotion at discharge. All these differences were significant.
Conclusion
Utilizing Ayurvedic massage in post stroke patients with flaccidity can promote faster standing with minimal assistance and lead to less need for antispastic drugs at discharge.
Keywords: Stroke, Massage, Ayurveda, Spasticity
1. Introduction
After acute stroke, rehabilitation measures form the mainstay of ongoing care. In the early stages of the Upper Motor Neuron (UMN) syndrome flaccidity predominates. As a result, patients are dependant for transfers and locomotion. Families of patients with hemiparesis felt that when their member is medically stable and able to stand with minimal assistance caring in a home setting with a visiting therapist is a feasible option [1]. One of the limitations to rehabilitation services is the delay in improvement when limbs are flaccid. While there are physiotherapy (PT) options to treat UMN related hypotonia, its benefits are appreciated once selective voluntary motor control and spasticity emerge. In our prior observations it was found that some patients who were flaccid and received early Ayurvedic massage regained tone sooner than those who did not. This observational study was done to quantify and compare that change to our standard treatment regimen.
Post stroke 30% of patients develop the UMN syndrome [2]. While spasticity onset can be anytime in the post-stroke period [3] it begins within six weeks of stroke onset in about 25% of patients [4]. Strong predictors for developing spasticity are a high degree of paresis and hypoesthesia at stroke onset [5], [6]. Other risk factors leading to spasticity are; more severe paresis at 16 weeks compared to first week, Modified Ashworth Scale (MAS) 2 in at least one joint within 6 weeks after stroke, more than two joints affected by increased muscle tone, hemispasticity within 6 weeks after stroke and lower Barthel Index [4].
The quantity of regained selective voluntary motor control on the other hand is related to lesion location, severity, and rehabilitation.
The basic pathophysiology is irregular neuronal reorganization after a brain lesion [7], [8]. The result of this may be increased activity in the muscles and exaggerated reflex responses to peripheral stimulation. This occurs due to disinhibition of the normal reflexes, release of primitive reflexes and active tonic stretch reflex [9], [10]. Stimulation of fast twitch motor neurons along with increased transmission between interneurons (due to diminished frontal inhibition) can promote spasticity [11]. Indeed early walking post stroke is facilitated by spasticity. Therefore modalities that enable this can possibly reduce length of stay.
Few articles in Pubmed detail ‘massage stroke’ patients [12], [13], [14]. 59.3% of patients in India having stroke will see an Ayurvedic physician at some point in their care [15]. Publications about Ayurvedic massage (Abhyanga) in hemiplegia (Pakshaghata) were found by searching for ‘Paskshagata' pdf’ in a web search engine. All the articles addressed the Vata imbalance causing the stroke, and ranged from case reports to case series [17], [18], [19], [20], [21]. They were conducted in Ayurvedic centres, and did not include standard allopathic management. Patients enrolled were taken anywhere from 4 days to 2 years from onset. As none of the patients taken in the first 30 days required critical care it can be assumed the strokes were mild to moderate in severity. While all used clinical features, only two utilized radiology to localize the stroke and none used National Institute Stroke Scale (NIHSS). One combined Abhyanga with ingested Ayurvedic medication [18]. Of all these one compared to a placebo [19], and another to the standard of care. None used PT as a comparison or as part of the treatment.
In Pakshaghatha, Abhyanga (oil massage) removes the Srothorodha (clogging of channels) by virtue of its quality of Vatahahara swabhava (pacifying quality), and the Prabhava (imperceptible quality) of the medicaments used for the Abhyanga. Swedana karma (steam bath) corrects the vathavaigunya (vitiated Vata) and brings about srothosudhi (opens up the channels), re-establishing Doshasamyatha (balanced state of Doshas). The Virechana karma (Purgation) and Vasthi karma (medicated enema) which follows the Abhyanga completes the sodhana (flushing out) whence the medications given orally removes all the factors that led to Dhoshavaigunya (Imbalance of Doshas) which originally caused the Srothorodha (blocking or clogging of channels) manifesting the disease [16]. As the oils have multiple components, a separate Pubmed search was carried out to find the publications specifically relating individual reagents in our oils and stroke outcomes (see Table 1).
Table 1.
Oil sources and their possible effects.
| Effect | Agent |
|---|---|
| Lowers blood pressure | Hordeum vulgare[23], Brassica juncea[24] |
| Controls diabetes | Aegle marmelos[25] |
| Anti-oxidant | Withania somnifera, Valeriana wallichi,Pterocarpus santalinus[26] |
| Neuroprotective | Sesame seed [22], Tribulus terrestris[27], Asparagus [28], Moringa oleifera[29] |
| Lipid lowering | Terminalia chebula[30] |
The problem with utilizing Ayurveda as a treatment modality in studies of patient care is that internal variability exists within the field. This arises due to presence of variable components in Ayurvedic preparations using the same source variable preparation techniques leading to properties of these components changing, dosing and application differences. We have used only one Vaidyan and his treatment regimen to have a control on other variabilities.
2. Methods
Patients admitted to the Neuro-rehabilitation service between the years 2014–2017 were followed. All patients were admitted for their first stroke which was diagnosed both clinically and localized with Magnetic Resonance Imaging (MRI) or Computerized Tomography (CT). All had ischemic strokes resulting in hemiplegia. Massage as an adjuvant was offered if the patient was one month from stroke onset, first stroke, Brunnstrom stage 1, had MAS 0 and was interested in receiving it. The Ayurvedic Physician determined the patient's Prakriti based on doshaja lakshana (dosha indicators) from Asthanga Hridaya [16]. Patients who didn't have the following contraindications (active infection, malnutrition, dehydration, constipation/diarrhea) were taken up for massage, and formed the cases group. Patients who did not get massage (controls) were otherwise similar except they expressed lack of interest. No one was excluded for having Abhyanga contraindications. NIHSS was taken at baseline.
All outcome measures were taken at admission to rehabilitation and at discharge, except weeks to standing with minimal assistance. Weeks to standing with minimal assistance was taken from stroke onset. In case this was not achieved the value was set as the combined length of stay (under Neurology and PMR). Patients were further categorized as complicated if they: underwent decompressive hemicraniectomy, had coronary events related to Cerebrovascular Accident (CVA), on tracheostomy, had multidrug resistant (MDR) nosocomial infections, and/or Congestive Heart Failure (CHF) with Ejection Fraction <30%. Primary outcome measures were Brunnstrom leg progression, MAS at knee flexors and ankle plantar flexors, Weeks from stroke onset to standing with minimal assistance, Functional Independence Measure (FIM) for walking, and antispastics at discharge. Baclofen was added when: MAS was 3, patient had painful flexor spasms, or if there was ankle clonus impairing standing balance. The Brunnstrom leg progression was chosen as post-stroke both spasticity and selective voluntary motor control emerge together and this scale helps differentiate the two.
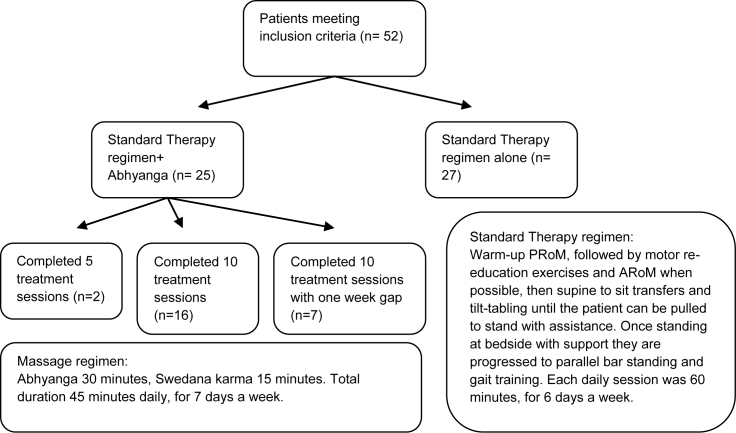
Standard Therapy regimen- All patients received at least 6 h of therapy averaged in a week. This consisted of Passive and Active Range of Movement (RoM), tilt tabling, gait training in parallel bars, and a variety of therapist guided, self/bystander assisted exercises to promote motor recovery (see Fig. 1).
Fig. 1.
Study protocol flowchart. Footnotes: PRoM- Passive Range of Movement, ARoM- Active Range of Movement.
Abhyanga (Ayurvedic massage)- The patient was first assessed by the Ayurvedic Physician to determine their predominant constitution (prakriti). Based on this mild (balya) medicated oil was warmed and rubbed with gentle pressure from the neck down by two massage therapists simultaneously, from the upper limbs to trunk, then lower limbs for 30 min while on a wooden table. Following this the patient was exposed to steam for 15 min either via a chamber or hose. In Vata predominant Prakriti Dhanwantharam oil was used, in Pitha Pindathailam oil, and in Kapha Karpasasthyadi oil. The treatment consisted of 5 or 10 sessions. Treatment number was determined by progression in therapy. Those who achieved standing sooner stopped massage at 5 sessions, whereas those who did not received 10 sessions. (see Fig. 1).
Student's t-test was used to calculate the differences between groups.
3. Results
Twenty five patients received Ayurvedic massage one month from stroke onset. Twenty seven patients chose not to get massage. Three other patients (not included in the analysis) received the massage and PT anywhere from 4 to 48 months post-stroke. Their outcomes are mentioned in the discussion.
Of the cases the mean NIHSS was 13.44. Eighteen were simple strokes and seven were deemed complicated. Radiologically twenty-four had a lesion in the MCA distribution, and one had unilateral pontine involvement. Of the common co-morbidities twenty-two had Hypertension (HTN), and sixteen had Diabetes Mellitus type 2 (DM II). Of the uncommon co-morbidities: one had Rheumatic heart disease, two had Parkinsons's disease, one had Chronic Obstructive Pulmonary Disease, one had Chronic Kidney Disease, one had Normal Pressure Hydrocephalus, and one had Atrial fibrillation. All patients were deemed to have a vata dosha imbalance.
In the control group the mean NIHSS was 13.22. Sixteen were simple strokes and eleven were deemed complicated. Radiologically twenty-three had a lesion in the MCA distribution, and three had unilateral pontine involvement Of the common co-morbidities eighteen had HTN, and fourteen had DM II. Of the uncommon co-morbidities: two had Rheumatic heart disease, one had Chronic Kidney Disease, one had Systemic Lupus Erythematosus, two had Hepatitis C Viral infections in remission, one had Atrial fibrillation, and one had Dilated Cardiomyopathy.
There was a significant difference between the two groups for mean age favouring the control group. Otherwise the groups were similar at baseline (see Table 2 for details).
Table 2.
Baseline characteristics.
| Cases | Controls | p value | |
|---|---|---|---|
| age | 65 ± 13 | 51.2 ± 12.3 | |
| male | 18 | 19 | |
| female | 7 | 8 | |
| left sided | 17 | 12 | |
| right sided | 7 | 12 | |
| brainstem | 1 | 3 | |
| NIHSS | 13.22 | 13.44 | 0.88 |
| simple | 18 | 16 | |
| complicated | 7 | 11 |
In the case group individuals were of the following predominant dosha: Vatha 10, Pitha 8 and Kapha 7. No side effects of massage were noted. Two patients received 5 sessions and the remainder twenty-three received 10. Four patients had a seven day gap in massage due concomitant to nosocomial UTI, but the remainder had no break in care.
With respect to primary outcome measures, in the case group there was net change in Brunnstrom-leg which was not significant (p = 0.47) versus the control. The change in MAS was significant (p value < 0.001) favouring the massage group over control group. The case group also took less time to stand with minimal assistance (p value = 0.001). At discharge FIM score for walking was better in the massage group versus than the control group (p value = 0.007) (see Table 2). No measures of secondary outcome was observed.
Regardless of improvements three patients from the case group and, six patients from the control group only achieved standing with moderate assistance or less at discharge. To correct for their weeks to stand with minimal assistance the value was set as their entire length of stay from admission to discharge home. Three case group patients did not achieve standing with minimal assistance, nor did five from the control group. Two of the patients in the case group were on Baclofen at discharge versus nine in the control group. Two of the case group received 5 sessions whereas the others all got 10 sessions.
4. Discussion
Based on the findings, it can be stated that the followed treatment modality promoted faster gains possibly by either modulating interneuron activity promoting spasticity or improving selective voluntary motor control. As spasticity is caused by unregulated alpha moto-neuron overactivity, its variable presentations are related to interneuron activity. It was believed that the Abhyanga modulated this activity yielding better outcomes. Considering the low numbers of the uncommon co-morbidities and the similar distribution between both groups along with the relatively similar baseline NIHSS scores it is unlikely they had a significant confounding effect. Regardless the same patients in each group with these uncommon co-morbidities were also the same patients that often did not progress to walking with minimal assistance (more so in controls than cases). While their medication side effects could interfere with outcomes, as the number of such patients was similar along with outcomes overall, it is unlikely this was relevant here. Though there was no significant difference in Brunnstrom staging the patients receiving Abhyanga had lower Ashworth scores and less need for antispastic drugs. The irregularity with Brunnstrom may be due to low sample size. They also stood sooner and were walking with less support than the control group at discharge. It can be inferred that there was possibly more recovery of selective voluntary motor control rather than spasticity. It can also be proposed that the spasticity was relatively better controlled (less need for medication) in the case group as there was faster standing and better walking noted. It cannot be definitively stated these were due to design limitations. Despite the gains, a peripheral treatment cannot explain signs of central recovery. A potential reason for this seeming better response may be that early recovery of some crude voluntary motor function may dissuade the emergence of spasticity overall thereby allowing other areas of the brain to facilitate task relearning. From an Ayurvedic standpoint, the oil components were absorbed through the body and effect their therapeutic action. There were no major differences between patients in the massage group outcomes when taking into account their predominant prakriti. It was believed that the oils restored internal homeostasis, hence promoting recovery. As overall outcome difference between the three predominant Prakriti in the case group was minimum, it was taken as negligible. NIHSS did correlate to severity as those with worse scores had these uncommon co-morbidities and often did not achieve walking.
Some interesting contradictions to the mentioned result with respect to infratentorial stroke (brainstem) was observed. In total there were four such patients. One was a case and the others were control. The individual who received Abhyanga had outcome measures similar to that of the control group.
Another unique observation concerned three patients (not included in this study) who were more than 12 months post event and had spasticity. After botulinum toxin injection they self referred themselves for the same treatment within 4–8 weeks and the following was noted. All had a baseline MAS of 1 that became 3 after the massage. This is in supportive of our hypothesis that interneuron overactivity in spastic patients being contributed by Abhyanga.
The following are the limitations to this case series: sampling bias, lack of blinding, inability to generalize outcomes. As the focus was on function and not risk factors, biochemical markers were not used. (all patients had routine lab work, but values at discharge were not repeated). No correlation of structural (CT/MRI) lesions to function was studied. While most treatment regimens addressed on aggravated Vata dosha, as thre present study regimen was a preparatory cleansing, it addressed on Prakriti rather than Samprapti ghataka (components of pathology) which is needed for the complete Ayurvedic treatment. As the protocol used to determine Prakriti, we used Ashtanga Hridaya proforma. The study was not designed to look into the specific differences per Prakriti dosa or doshaja substratification. All the patients were screened, examined, sorted and followed by the same Ayurvedic physician. Outcome measures were done by the same Allopathic Physician (see Table 3).
Table 3.
Statistical outcomes.
| Variable | group | pre | post | difference | p value |
|---|---|---|---|---|---|
| Brunnstrom leg | cases | 0 | 3.67 ± 0.7 | 3.52 ± 1.0 | .47 |
| control | 0 | 3.33 ± 0.68 | 3.33 ± 0.68 | ||
| MAS | cases | 0.13 ± 0.34 | 1.17 ± 0.48 | 1.0 ± 0.65 | 0.001 |
| control | 0.0 ± 0.0 | 2.56 ± 0.64 | 2.56 ± 0.64 | ||
| weeks to stand min A | cases | 6.0 ± 2.16 | 0.001 | ||
| control | 9.42 ± 4.55 | ||||
| FIM walking d/c | cases | 5.1 ± 1.1 | 0.007 | ||
| control | 4.2 ± 1.2 |
Footnotes: MAS- Modified Ashworth Scale, FIM- Functional Independence Measure, weeks to stand with min A- Assistance.
This is the first publication to prospectively follow patients immediately post stroke receiving Ayurvedic massage compared to a standard allopathic control.
5. Conclusion
Ayurvedic massage was well tolerated in self-selected patients who were one month post stroke. These self-selected patients improved faster in standing and had better locomotion at discharge.
Sources of funding
None.
Conflict of interest
None.
Acknowledgements
The authors acknowledges the valuable contributions of Dr. Micheal Andary and Dr. Veena Menon.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Rodgers H., Price C. Stroke unit care, inpatient rehabilitation and early supported discharge. Clin Med (Lond) 2017;17(2):173–177. doi: 10.7861/clinmedicine.17-2-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer N.H., Esquenazi A. Muscle overactivity and movement dysfunction in the upper motoneuron syndrome. Phys Med Rehabilit Clin North Am. 2003;14:855–883. doi: 10.1016/s1047-9651(03)00093-7. [vii–viii] [DOI] [PubMed] [Google Scholar]
- 3.Ward A.B. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol. 2012;19:21–27. doi: 10.1111/j.1468-1331.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- 4.Wissel J., Schelosky L.D., Scott J., Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010;257:1067–1072. doi: 10.1007/s00415-010-5463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban P.P., Wolf T., Uebele M., Marx J.J., Wissel J. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016–2020. doi: 10.1161/STROKEAHA.110.581991. [DOI] [PubMed] [Google Scholar]
- 6.Coupar F., Pollock A., Rowe P., Weir C., Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012;26:291–313. doi: 10.1177/0269215511420305. [DOI] [PubMed] [Google Scholar]
- 7.Sheean G. The pathophysiology of spasticity. Eur J Neurol. 2002;9:3–9. doi: 10.1046/j.1468-1331.2002.0090s1003.x. [DOI] [PubMed] [Google Scholar]
- 8.Binkofski F., Seitz R.J., Arnold S., Classen J., Freund H.J. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–470. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- 9.Ivanhoe C., Reistetter T. Spasticity: the misunderstood part of the upper motor neuron syndrome. Am J Phys Med Rehabil. 2004;83:S3–S9. doi: 10.1097/01.phm.0000141125.28611.3e. [DOI] [PubMed] [Google Scholar]
- 10.Pandyan A., Gregoric M., Barnes M. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27:2–6. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- 11.Marque P., Roques C.F. Facilitation of transmission in heteronymous group II pathways in spastic hemiplegic patients. J Neurol Neurosurg Psychiatr. 2001;70:36–42. doi: 10.1136/jnnp.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y.J., Cheng J. Effectiveness and safety of Chinese massage therapy (Tui Na) on post-stroke spasticity: a prospective multicenter randomized controlled trial. Clin Rehabil. 2016;31(7):904–912. doi: 10.1177/0269215516663009. [DOI] [PubMed] [Google Scholar]
- 13.Thanakiatpinyo T., Kuptniratsaikul V. The efficacy of traditional Thai massage in decreasing spasticity in elderly stroke patients. Clin Interv Aging. 2014;9:1311–1319. doi: 10.2147/CIA.S66416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibbritt D., Srithong K. Rehabilitation of stroke patients using traditional Thai massage, herbal treatments and physical therapies. Zhong Xi Yi Jie He Xue Bao. 2012;10(7):743–750. doi: 10.3736/jcim20120704. [DOI] [PubMed] [Google Scholar]
- 15.Pandian J.D., Liu M., Misbach J., Venketasubramanian N. Int J Stroke. 2011;6(6):541–543. doi: 10.1111/j.1747-4949.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- 16.Moorthy S. Oriental Publishers; 2004. Sareerasthanam, the Ashtanga Hridaya, Varanasi. [Google Scholar]
- 17.Patel J., Patel K.B. A non-randomized observational clinical study on ayurvedic management of pakshaghata. J Biol Sci Opin. 2015;3(5) [Google Scholar]
- 18.Sharma L.K., Maheswar T. A clinical study on pakshaghata with a combination of ekanga veera ras, masha taila and shastikashali pinda sweda. J Ayurvedic Sci. 2004;25(1) [Google Scholar]
- 19.Ediriweera R.H.S.S., Perera M.S.S. Clinical study on the efficacy of chandra kalka with mahadalu anupanaya in the management of pakshaghata. Ayurveda. 2011;32(1) doi: 10.4103/0974-8520.85720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil R.B., Landge S.K., Patil M.V., Gayal R.R. Evaluation of efficacy of mahamasha taila abhyanga, Shashtik shali pinda sweda, and mustadi rajyapana basti in the management of pakshaghata. Int J Ayurvedic Herbal Med. 2017;7:4. [Google Scholar]
- 21.Sushma P., Sadanandam C. Evaluation of efficacy of shirovasthi in the management of pakshaghata: a pilot study. Int J Adv Res. 2016;4(8) [Google Scholar]
- 22.Jamarkattel-Pandit N., Pandit N.R., Kim H., Bu Y. Neuroprotective effect of defatted sesame seeds extract against in vitro and in vivo ischemic neuronal damage. Planta Med. 2010;76(1):20–26. doi: 10.1055/s-0029-1185903. 2009. [DOI] [PubMed] [Google Scholar]
- 23.Ardiansyah, Komai M. Fermented barley extract supplementation ameliorates metabolic state in stroke-prone spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2015;79(11):1876–1883. doi: 10.1080/09168451.2015.1052772. 2015. [DOI] [PubMed] [Google Scholar]
- 24.Tuekpe M.K., Ariizumi M. Potassium excretion in healthy Japanese women was increased by a dietary intervention utilizing home-parcel delivery of Okinawan vegetables. Hypertens Res. 2006;29(6):389–396. doi: 10.1291/hypres.29.389. [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi S., Aggarwal A. Indigenous drugs in ischemic heart disease in patients with diabetes. J Alternative Compl Med. 2009;15(11):1215–1221. doi: 10.1089/acm.2009.0187. [DOI] [PubMed] [Google Scholar]
- 26.Mazzio E.A., Bauer D., Mendonca P., Soliman K.F. Natural product HTP screening for attenuation of cytokine-induced neutrophil chemo attractants (CINCs) and NO2- in LPS/IFNγ activated glioma cells. J Neuroimmunol. 2017 15;302:10–19. doi: 10.1016/j.jneuroim.2016.11.012. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liguori C., Sallustio F. Development of collateral veins as a favorable prognostic factor for complete recovery in cerebral venous thrombosis due to Tribulus terrestris. Int J Stroke. 2015;10(6):E66–E67. doi: 10.1111/ijs.12572. [DOI] [PubMed] [Google Scholar]
- 28.Jalsrai A., Becker A. The neuroprotective effects and possible mechanism of action of a methanol extract from Asparagus cochinchinensis: in vitro and in vivo studies. Neuroscience. 2016 13;322:452–463. doi: 10.1016/j.neuroscience.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 29.Kirisattayakul W., Jittiwat J. Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxid Med Cell Longev. 2013;2013:951415. doi: 10.1155/2013/951415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy M.M., Adeghate E. Anti-hyperlipidemic effect of methanol bark extract of Terminalia chebula in male albino Wistar rats. B Pharm Biol. 2015;53(8):1133–1140. doi: 10.3109/13880209.2014.962058. [DOI] [PubMed] [Google Scholar]