Abstract
Objective
Previous research suggests attention and white matter (WM) abnormalities in individuals with mucopolysaccharidosis type I (MPS I); this cross-sectional comparison is one of the first to examine the relationship of WM structural abnormalities as measured by corpus callosum (CC) volumes with attention scores to evaluate this relationship in a larger sample of patients with MPS I.
Methods
Volumetric MRI data and performance on a computerized measure of sustained attention were compared for 18 participants with the severe form of MPS I (MPS IH), 18 participants with the attenuated form of MPS I (MPS IATT), and 60 typically developing age-matched controls.
Results
The MPS I groups showed below-average mean attention scores (p < 0.001) and smaller CC volumes (p < 0.001) than controls. No significant associations were found between attention performance and CC volume for controls. Attention was associated with posterior CC volumes in the participants with MPS IH (p = 0.053) and total (p = 0.007) and anterior (p < 0.001) CC volumes in participants with MPS IATT.
Conclusions
We found that attention and CC volumes were reduced in participants with MPS I compared to typically developing controls. Smaller CC volumes in participants with MPS I were associated with decreased attention; such an association was not seen in controls. While hematopoietic cell transplantation used to treat MPS IH may compound these effects, attention difficulties were also seen in the MPS IATT group, suggesting that disease effects contribute substantially to the clinical attentional difficulties seen in this population.
We investigated abnormalities in attention and white matter (WM) in mucopolysaccharidosis (MPS) type I, given atypical WM findings in canine models1,2 and our recent studies of MPS I and II.3,4 MPS I is an autosomal recessive metabolic disorder in which lysosomal-enzyme (α-l-iduronidase) deficiency promotes cellular accumulation of glycosaminoglycans, resulting in multisystem dysfunction.4 Now recognized as a continuum of severity,4 3 phenotypes have been described. Early cognitive and physical decline and childhood mortality characterize the severest form, Hurler syndrome (MPS IH). Hematopoietic cell transplantation (HCT) decreases mortality and morbidity.4 Hurler-Scheie and Scheie syndromes have a later onset, slower progression, and enzyme replacement therapy (ERT) treatment.5 Without quantified biological distinctions, we combined them into an attenuated group (MPS IATT).
Caregivers of children with MPS I report concerns such as lack of focus and poor processing, suggesting attention difficulties. In patients with MPS I, inattention was found to be associated with WM abnormality as measured by diffusion tensor imaging (DTI) in the corpus callosum (CC).5 Smaller CC volumes have been found in children with attention-deficit/hyperactivity disorder (ADHD) compared to controls.6,7 Because the HCT preparative regimen has been shown to affect WM integrity and attention in other populations,8,9 transplantation for MPS IH may compound disease-related abnormalities.
We associate an attention measure with CC volumes in patients with MPS IH and MPS IATT. We hypothesized that attention test performance will be impaired in both groups compared to controls, with MPS IH most impaired; that CC volumes will be smaller in both MPS groups than controls but smallest in MPS IH; and that attention will be associated with CC volumes.
Methods
Participants
Eighteen participants with MPS IH and 18 with MPS IATT meeting the inclusion criteria from the pool of participants in the multicenter study Longitudinal Studies of Brain Structure and Function in MPS Disorders (U54NS065768) of the Lysosomal Disease Network (Rare Disease Clinical Research Network) were compared to 60 typically developing age-matched controls enrolled in 4 other Institutional Review Board–approved studies collecting attention data and MRI data on the same scanner during the same period as the current study.10–13
All participants with MPS I who met the inclusion criteria were used. Criteria included the following: (1) participants were between 5 and 22 years of age; (2) all participants with MPS IH had undergone HCT with or without ERT; (3) all participants with MPS IATT were currently treated with ERT, and (4) as indicated by the protocol, all participants had completed attention testing and a structural MRI within 3 months of each other.
Standard protocol approvals, registrations, and patient consents
Institutional ethics standards committees on human experimentation approved all studies from which participants were drawn. Institutional Review Board–approved written informed consent was obtained from all individuals or legal guardians; assent was obtained from children and those ≥18 years of age with legal guardians.
Measures
MRI acquisition and processing
MRIs were acquired with a harmonized brain MRI protocol at each center for 3T Siemens (Trio or Skyra; Siemens, Malvern, PA) or Philips (at 1 center; Philips, Best, the Netherlands) scanners. Each participating center submitted quality-control scans to ensure that scan sequences were acceptable for analyses. Volumetric MRI data were acquired with comparable sequences for the control participants. In the postprocessing period, motion correction techniques were used to correct slight movement artifact.
Magnetization-prepared rapid acquisition with gradient echo sequences were used. All scans were centrally analyzed at the University of Minnesota. Automated segmentation of brain structures was carried out with FreeSurfer Image Analysis Suite, version 5.3.14 All analyzed images were inspected for accuracy because morphologic abnormalities are known to cause gray matter/WM segmentation failure in patients with MPS. Scans in which segmentation was aberrant were manually adjusted and reprocessed. Midanterior, anterior, central, midposterior, and posterior CC sections were automatically delineated by FreeSurfer. Regions were analyzed by summing the midanterior and anterior regions to represent the anterior region and the midposterior and posterior regions to represent the posterior region. The anterior, central, and posterior region values were summed for a total CC volume.
Attention testing
Attention testing for the participants with MPS I was conducted with the Test of Variables of Attention (TOVA), a computerized continuous-performance task15 that has been suggested to be useful for assessing attention symptoms in individuals ≥5.5 years of age.16 The TOVA is a 21.6-minute test consisting of randomly presented geometric targets; the individual presses a microswitch when the target is presented and inhibits pressing the switch for the nontarget. For children 4 to 5.49 years old, the task is shorter, lasting 10.8 minutes. Instructions are simultaneously presented in text and auditory format by the computer before each administration. A brief practice test before the full test ensures task understanding. An examiner remains in the room during the administration. Stimuli are presented for 100 milliseconds with targets presented at varied time intervals.16 We selected all standard, relevant variables of attention yielded by the TOVA corresponding to those used for control participants, including failures to respond to targets (omission errors [OE]), responses to nontargets (commission errors [CE]), reaction time for correct responses (RT), and consistency of the reaction time (VRT). Raw scores and standard scores are yielded for each variable. The visual TOVA is normed on 1,596 healthy individuals 4 to >80 years of age, stratified by age and sex.17 For participants with MPS I from 5 to 7 years of age, the raw scores from a 10-minute version of the TOVA were compared to scores from a same-age healthy control group who had the 10-minute TOVA, an abbreviated measure of IQ, and an MRI.13
For the control participants ≥10 years of age, the Conners’ Continuous Performance Task II (CCPT) was used.18 Like the TOVA, the CCPT is a computerized continuous-performance task that assesses attention in individuals ≥6 years old. The individual presses the space bar to the target letters and inhibits responding to the nontarget letter. Instructions are provided in text on the screen; the examiner can read them if the individual cannot read them on his or her own. A brief practice test administered before the full test ensures understanding. Interstimulus intervals of targets vary among 1, 2, and 4 seconds.18 Normative data are from 1,920 healthy individuals.18 CCPT scores have been found to be correlated with observations of inattentive/hyperactive behavior during the administration of the CCPT.19 Although the targets differ, the same measurement parameters (OE, CE, RT, and VRT) were used from the CCPT and transformed so that, like the TOVA, higher scores indicated better performance. Combination of variables from both the TOVA and the CCPT has precedent in other peer-reviewed studies.3,20
Statistical analyses
No effect size was chosen; all participants were part of a prospective natural history study of MPS. There was not a power analysis. All participants meeting inclusion criteria were included. No data were missing for our outcome variables. Descriptive statistics, including mean and SD for continuous variables and frequency for categorical variables, were tabulated for controls and the MPS groups. Group mean differences were evaluated with a t test with unequal variance and Welch degrees of freedom for confidence intervals (CIs) and p values. First-order linear trends of the association between brain substructure volumes and neuropsychological metrics and between neuropsychological scores and age were based on least-squares simple regression estimates. Adjusted analyses were similarly based on multiple linear regression and the t distribution with corresponding model degrees of freedom for CIs and p values. All analyses were conducted with R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria).21
Data availability
All data were entered into the Data Management and Coordinating Center (DMCC) for the Rare Diseases Clinical Research Network. The DMCC is a secure clinical data management system that collects and stores data on a variety of rare diseases from organizations across the United States. The data are held in the DMCC for 5 years, after which they are released to the database of Genotypes and Phenotypes.
Results
Participants
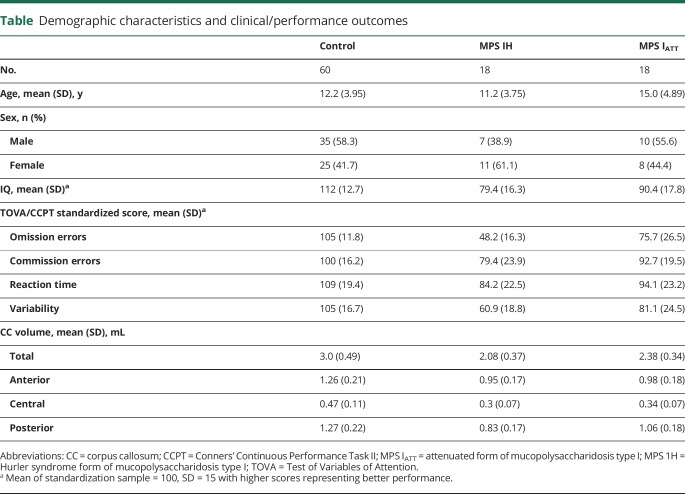
Characteristics were summarized by phenotype (MPS IH or MPS IATT) and the control group (table). Age and IQ differed between groups. The MPS IATT group was slightly older in mean age than the other 2 groups. The MPS IH group had a below-average mean IQ. The mean IQ of the MPS IATT group was within the average range but lower than the expected mean of 100, while the mean IQ of the control group was also within the average range but higher than the expected mean.
Table.
Demographic characteristics and clinical/performance outcomes
Six patients with MPS IATT had 1 L238Q mutation. Previously, this mutation (when paired with a severe nonsense mutation or deletion) had been found to be associated with low IQ and psychiatric disorder.22 This group had greater attention difficulties compared with other participants with MPS IATT (OE: −31.20 mean standard score difference, 95% CI −49.94 to −12.47, p = 0.001; VRT: −20.26 mean standard score difference, 95% CI −36.90 to −3.63, p = 0.017). Because significant differences in attention were seen between the participants with MPS IATT without the mutation and those with MPS IH (OE: −45.87 mean standard score difference, 95% CI −57.19 to −34.55, p < 0.001; VAR: −34.21 mean standard score difference, 95% CI −46.06 to −22.35, p < 0.001), all patients with MPS IATT were included in the following analyses.
Attention performance
Results were similar with or without adjustment for IQ. Without adjustment for IQ, both MPS I groups had a mean TOVA performance below the average range of the age-based standardization sample (defined as within 1 SD of the population mean, 85–115, with higher scores equaling better performance) on OE and VRT (figure 1). Only the MPS IH group performed outside the average range on CE and RT. The control group performed in the average range on all parameters. For participants with MPS IH, OE was significantly lower than for participants with MPS IATT and controls (p < 0.001), and VRT was significantly lower than for participants with MPS IATT (p = 0.004) and controls (p < 0.001). Participants with MPS IATT were significantly lower than controls in OE (p < 0.001), RT (p = 0.014), and VRT (p < 0.001).
Figure 1. Mean attention test scores for each domain and confidence intervals for MPS IH, MPS IATT, and control groups.
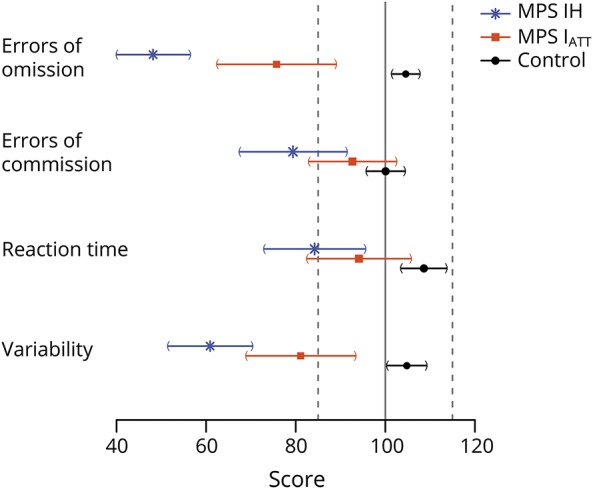
Score values are on the x-axis, with the mean standardized score (100) denoted by solid vertical line and ±1 SD (15) denoted by dashed vertical line. MPS IATT = attenuated form of mucopolysaccharidosis type I; MPS 1H = Hurler syndrome form of mucopolysaccharidosis type I.
CC volumes
CC volume was positively correlated with age in both controls and participants with MPS IATT; however, the patients with MPS IH did not show an association between volume and age (figure 2). Because the MPS I groups differed in age and age is associated with CC volume, all comparisons of CC volumes between groups were adjusted for age and sex (figure 3). Volumes were significantly smaller (p < 0.001) in participants with MPS IH and MPS IATT than in controls for total and all segmented regions of the CC. In a comparison of the 2 MPS groups, significant differences were found in the posterior region only, with the volumes of the participants with MPS IH being significantly smaller than that of participants with MPS IATT (p = 0.009).
Figure 2. Participant's age in years (x-axis) and associated volume (in mL) of their entire corpus callosum (y-axis) along with a regression line for each group as a whole (MPS IH, MPS IATT, and controls).
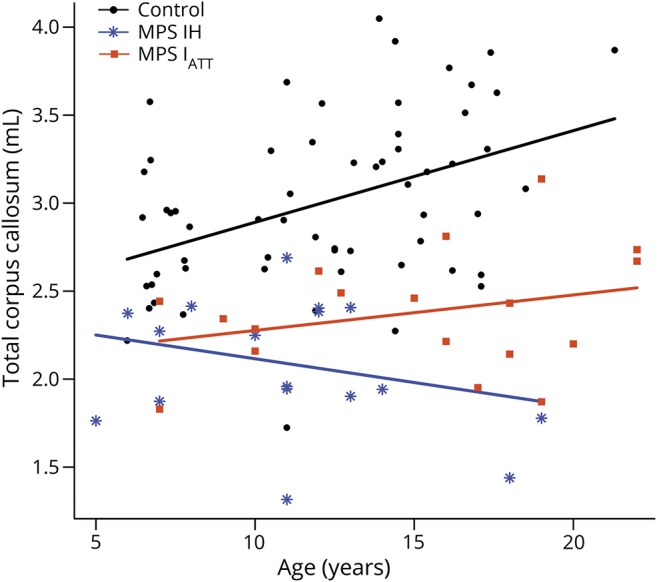
MPS IATT = attenuated form of mucopolysaccharidosis type I; MPS 1H = Hurler syndrome form of mucopolysaccharidosis type I.
Figure 3. Mean total CC volume and volumes of anterior, central, and posterior regions of the CC volume (in mL) for the MPS IH, MPS IATT, and control groups.
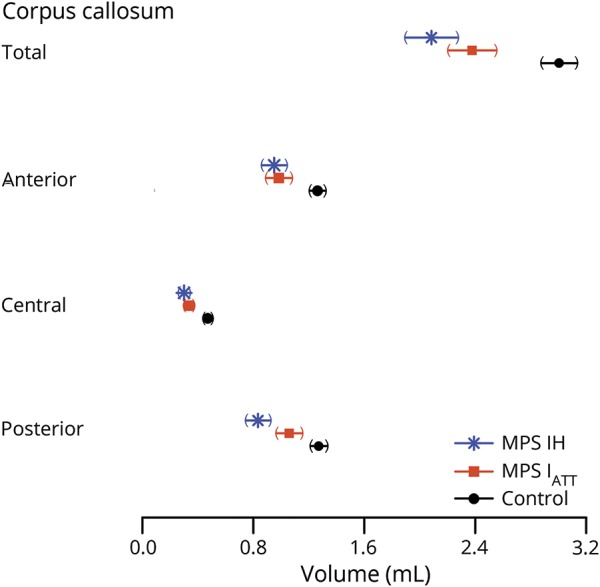
CC = corpus callosum; MPS IATT = attenuated form of mucopolysaccharidosis type I; MPS 1H = Hurler syndrome form of mucopolysaccharidosis type I.
Association between volumes and attention
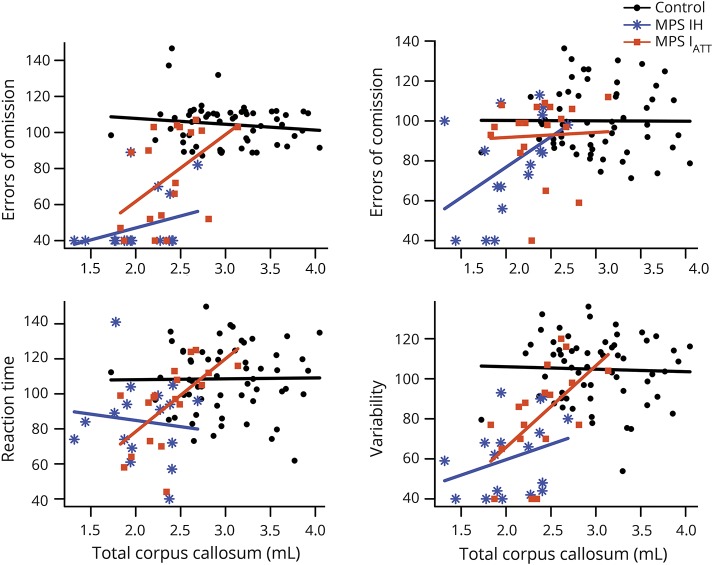
In the MPS IH group, higher scores on OE were associated with larger posterior CC volumes (35.64 per 1 mL, 95% CI −0.53 to 71.80, p = 0.053), higher CE scores were associated with larger central CC volumes (179.92 per 1 mL, 95% CI 68.27–291.58, p = 0.002), and higher scores on VRT were associated with increased central CC volumes (84.06 per 1 mL, 95% CI 0.03–168.09, p = 0.050). Other associations were of similar magnitude although not statistically significant.
For MPS IATT, higher scores on OE were associated with larger total (37.14 per 1 mL, 95% CI 10.24–64.04, p = 0.007) and anterior (75.83 per 1 mL, 95% CI 37.48–114.18, p < 0.001) CC volumes; higher scores on RT were associated with larger total (41.62 per 1 mL, 95% CI 20.44–62.80, p < 0.001), anterior (53.33 per 1 mL, 95% CI 4.37–102.29, p = 0.033), and posterior (83.31 per mL, 95% CI 52.02–114.59, p < 0.001) CC volumes; and higher scores on VRT were associated with larger total (40.77 per 1 mL, 95% CI 19.34–62.19, p < 0.001), anterior (66.34 per 1 mL, 95% CI 18.14–114.55, p = 0.007), and posterior (74.91 per 1 mL, 95% CI 32.82–117.00, <0.001) CC volumes (figure 4). Other associations were of similar magnitude although not statistically significant.
Figure 4. Association between attention scores and CC volumes.
Each graph depicts a separate attention domain. Attention score (y-axis) is a standard score with a population mean of 100 and SD of 15. Associated corpus callosum (CC) volume is in milliliters (x-axis). These are plotted for each participant along with a regression line for each group as a whole (Hurler syndrome form of mucopolysaccharidosis type I [MPS IH], attenuated form of mucopolysaccharidosis type I [MPS IATT], and controls).
No significant associations were found between attention performance and CC volume for controls
Discussion
We found that attention and CC volumes, independently of age, were significantly reduced in participants with MPS I compared to typically developing controls. Greater impairment in attention was seen in the severe compared to the attenuated phenotype. Posterior CC volumes in participants with MPS IH were significantly smaller than in those with MPS IATT. Attention and CC volume associations were found for both patient groups but not the control group.
The CC was chosen as the focus of our analyses because it has been found to be smaller in a canine model of MPS I23 and previously was found to be associated with attention in other patient populations3,24 and in an exploratory study of MPS IH using DTI.5 CC volumes were positively associated with age in controls and patients with MPS IATT but not in patients with MPS IH. For MPS IH, other factors, possibly related to HCT treatment,8,9 especially at a young age,8,25 or pathophysiologic differences,26 may affect WM development.
Our results suggest that smaller CC volumes in MPS I are associated with decreased attention. This association has been found in individuals with other neurodevelopmental disorders such as ADHD6,27,28 and in those with strokes secondary to sickle cell disease.29 In addition, evidence is strong that HCT for malignancies results in WM damage, implicated in poor attention as a late effect of treatment.8,9 Therefore, HCT for MPS IH is likely to have similar effects on the WM integrity and attention. However, HCT is not the treatment for MPS IATT, but WM abnormalities and poor attention are nonetheless evident, indicating that HCT alone is not the explanation. Such attention difficulties are also seen in other MPS disorders such as MPS II and III, which are not treated with HCT. Disease effects appear to contribute substantially to the clinical attentional difficulties seen in MPS I.
While these effect sizes are large in our small sample, we may have missed additional relationships that could have been found with a larger sample size. However, a small sample size is an unavoidable factor in rare-disease research. Other limitations of this study include an inability to match controls on the basis of age, sex, and IQ to our participant groups, which would have reduced our group sizes greatly. IQ differences between the patient group and controls are inevitable because IQ in patients with MPS IH and in those with the attenuated L238Q mutation is lower than in the normative population as a component of the disease. For our study, finding healthy controls with lower IQs was not feasible. In addition, our control participants had a higher than average IQ with a small range and may not be an ideal, generalizable comparison group.
Another limitation is that a computerized continuous-performance task does not perfectly quantify attention in all its aspects. In addition, participants 5 to 7 years of age were given a 10-minute version of the TOVA. Ordinarily, a longer version is given to those ≥5.5 years of age. In our experience, 5- to 7-year-olds with MPS I were often unable to perform on the 22-minute version due to fatigue and poor sustained attention. This determined the decision to use the 10-minute version in the gathering of control data for children 5 to 7 years of age. These control data have not been published.
An additional limitation is that 2 different computerized tasks were used for this study; however, both purport to measure the same variables. Each measure also has a different means of response. For the TOVA, a small microswitch, accurate to ±1 millisecond, is used. For the CCPT, the individual presses the keyboard space bar to respond. While the small response switch may have been difficult to use efficiently given the carpal tunnel and finger contractures common to those with MPS I, because all those with MPS completed the TOVA, there should not be within-group variance secondary to the differences in physical response. It may be that the increased VRT and slower RT represents the effect of the orthopedic issues in MPS IATT. Finally, while this study uses volumetric data to estimate potential functional effects of disease, structural volume alone is a relatively coarse metric. Future work should incorporate DTI or resting-state fMRI as an additional measure of brain effect.
Clinically, attention is a skill important to every aspect of conscious functioning. It is critical for learning and memory, academic success, efficiency, productivity, and even interpersonal facility. While the disease process itself is likely associated with the attention and WM abnormalities, for children with MPS IH, HCT is likely compounding these abnormalities. A less neurotoxic treatment applied early enough to spare WM development is needed, and advances have been made toward this goal.30 For the MPS IATT, treatments that reach the brain are needed because ERT presumably does not cross the blood-brain barrier.31 Until alternative treatments that are applied earlier and ultimately promote improved WM development are developed, addressing the attention problems in individuals with MPS I through behavioral, educational, and medical means may reduce adverse effects of inattention on academic, vocational, and interpersonal success. Although performance on attention measures is not diagnostic of an ADHD, like other individuals with attention difficulties, those with MPS I will benefit from environmental supports, parent training, skills training, and behavior therapy to help make focusing and sustaining attention less effortful. Given that stimulant medication has been found to be efficacious for other individuals with attention problems, it is possible that, pending cardiac status and monitoring, such treatment could also help those with MPS I found to be responsive. Although the degree of responsiveness and the side-effect profile for any medication depend on the individual patient, individuals with attention deficits due to other conditions such as those with autism spectrum disorders32 or fetal alcohol spectrum disorders33 may also have a different responsiveness to ADHD medications. For those with MPS I, anecdotal caregiver report suggests benefit in some, but not all, children.
This is one of the first studies to systematically demonstrate, in a well-characterized sample of young patients with MPS I (both attenuated and severe), an association between attention deficits and decreased CC volumes. This study not only further delineates the clinical phenotype but also provides further evidence of WM neuropathology in MPS I.
Acknowledgment
The authors thank Brianna Yund, MS (University of Wisconsin–Milwaukee), for her role in collecting neuropsychological data.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- CI
confidence interval
- CC
corpus callosum
- CCPT
Conners’ Continuous Performance Task II
- CE
commission errors
- DMCC
Data Management and Coordinating Center
- DTI
diffusion tensor imaging
- ERT
enzyme replacement therapy
- HCT
hematopoietic cell transplantation
- MPS
mucopolysaccharidosis
- MPS IATT
attenuated form of mucopolysaccharidosis type I
- MPS IH
Hurler syndrome form of mucopolysaccharidosis type I
- OE
omission errors
- TOVA
Test of Variables of Attention
- VRT
consistency of the reaction time
- WM
white matter
Author contributions
K.E. King: wrote first draft of paper, revised and edited draft. K.D. Rudser: statistical analysis, including writing/editing of that section, generated graphics, revised and edited draft. I. Nestrasil: leadership role on the scientific aspects of neuroimaging, including writing/editing of that section, revised and edited draft, lead control MRI project for participants <8 years old. V. Kovac: scientific analysis of neuroimaging data (FreeSurfer analysis and development of methods for manual adjustment in MPS I), acquired images and quality assurance of incoming data from other sites, collected MRI controls for <8 years of age, edited draft. K.A. Delaney: leadership role in neuropsychological testing, neuropsychological data analysis, edited draft. J.R. Wozniak, B.A. Mueller, and K.O. Lim: collected and analyzed control MRI data for participants >8 years old. Julie Eisengart: leadership role in neuropsychological testing, neuropsychological data analysis, edited draft. E.G. Mamak and J. Raiman: leadership and data collection at Hospital for Sick Children, edited draft. N. Ali and S. Cagle: leadership and data collection at Emory University, edited draft. P. Harmatz: leadership and data collection at Children's Hospital Oakland, edited draft. Chester Whitley: provided scientific expertise in genetics, edited draft. Elsa Shapiro: directed research, assisted in writing paper with extensive revising and editing.
Study funding
This study was funded by the Genzyme/Sanofi Corp, Rare Diseases Clinical Research Network, Lysosomal Disease Network, NIH U54NS065768, and the resources of the Center for Magnetic Resonance Research and the Center for Neurobehavioral Development. Control data were supported by the NIH (5P41RR008079, 5K12RR023247, P30-NS057091, and MO1-RR00400; K24 MH071434, K24 DA028773, RO1-MH61744, R01-AA12479, and RO1-MH63407 to Master Drug Data Base), Medical Investigation of Neurodevelopmental Disorders Institute, and Gillette Children's Research Fund, Shire (4- to7-year-olds study), and Clinical and Translational Science Institute grant support (UL1TR000114 from the National Center for Advancing Translational Sciences [NCATS] of the NIH). This publication was also supported in part by the NIH NCATS through UCSF-CTSI grant UL1 TR000004 (Dr. Harmatz). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Study data were collected and managed with REDCap electronic data capture tools hosted at the University of Minnesota. The Lysosomal Disease Network (U54NS065768) is a part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, and NCATS. This consortium is funded through a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke, and the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure
K.E. King is a consultant for Shire Plc; has received research support from Shire, Sanofi Genzyme, and Alexion Pharmaceuticals, Inc; and has had previous contract work for Shapiro Neuropsychology Consulting. K.D. Rudser reports no disclosures relevant to the manuscript. I. Nestrasil is a consultant for Armagen, Biomarin, RegenxBio, ICON, Bioclinica, and Quantims and received research support from Biomarin, Sanofi Genzyme, and Shire. V. Kovac reports no disclosures relevant to the manuscript. K. Delaney is an employee of BioMarin Pharmaceuticals. J.R. Wozniak, B.A. Mueller, and K.O. Lim report no disclosures relevant to the manuscript. J.B. Eisengart reports consulting fees for Regenxbio, Armagen, Sanofi Genzyme, and Velocity; contract work for Shapiro Neuropsychology Consulting; travel support from Regenxbio and the UK MPS Society; and research support with Sangamo and the National MPS Society. E.G. Mamak receives or has received an educational grant from Shire, Biomarin, Genzyme, and Actelion; travel grants from Shire; and honoraria from Genzyme and Shire. J. Raiman has received speakers fees and travel support or research support from Shire, Sanofi Genzyme, Biomarin, and Alexion. N. Ali has received research support from Shire, Sanofi Genzyme, BioMarin, Amicus, and Pfizer, as well as lecturers' honoraria from Sanofi Genzyme, BioMarin, and Amicus. S. Cagle has received speaker and consultant honoraria from BioMarin, Sanofi Genzyme, and Shire. P. Harmatz reports consulting fees from BioMarin, Inventiva, Armagen, PTC, and RegenixBio, as well as consulting fees, travel fees, and honoraria from Chiesi, Shire, Sanofi-Genzyme, and Alexion. C.B. Whitley reports NIH funding, including for the Lysosomal Disease Network (Rare Diseases Clinical Research Network) (NIH U54NS065768). E.G. Shapiro reports consulting fees from Shapiro Neuropsychology Consulting, LLC. Go to Neurology.org/N for full disclosures.
References
- 1.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middleton DM, Li JY, Chen SD, et al. Diffusion tensor imaging findings suggestive of white matter alterations in a canine model of mucopolysaccharidosis type I. Neuroradiol J 2018;31:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yund B, Rudser K, Ahmed A, et al. Cognitive, medical, and neuroimaging characteristics of attenuated mucopolysaccharidosis type II. Mol Genet Metab 2015;114:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro EG, Nestrasil I, Rudser K, et al. Neurocognition across the spectrum of mucopolysaccharidosis type I: age, severity, and treatment. Mol Genet Metab 2015;116:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro E, Guler OE, Rudser K, et al. An exploratory study of brain function and structure in mucopolysaccharidosis type I: long term observations following hematopoietic cell transplantation (HCT). Mol Genet Metab 2012;107:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil 1991;24:141–146. [DOI] [PubMed] [Google Scholar]
- 7.Semrud-Clikeman M, Filipek PA, Biederman J, et al. Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry 1994;33:875–881. [DOI] [PubMed] [Google Scholar]
- 8.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer 2009;52:159–164. [DOI] [PubMed] [Google Scholar]
- 9.Anderson FS, Kunin-Batson AS, Perkins JL, Scott Baker K. White versus gray matter function as seen on neuropsychological testing following bone marrow transplant for acute leukemia in childhood. Neuropsychiatr Dis Treat 2008;4:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol 2007;22:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wozniak JR, Muetzel RL, Mueller BA, et al. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res 2009;33:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak JR, Mueller BA, Ward EE, Lim KO, Day JW. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: a diffusion tensor imaging study. Neuromuscul Disord 2011;21:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovac V, Yund B, Ahmed A, Rudser K, Shapiro E, Nestrasil I. The development of brain and neurocognitive function in typically developing children ages 4–7 years. Mol Genet Metab 2014;111:S49. [Google Scholar]
- 14.Fischl B. FreeSurfer. Neuroimage 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Test of Variables of Attention (Version 7.3) [computer program]. Los Alamitos: TOVA Co; 2007. [Google Scholar]
- 16.Forbes GB. Clinical utility of the Test of Variables of Attention (TOVA) in the diagnosis of attention-deficit/hyperactivity disorder. J Clin Psychol 1998;54:461–476. [DOI] [PubMed] [Google Scholar]
- 17.Leark RA, Greenberg LM, Kindschi CL, Dupuy TR, Hughes SJ. Test of Variables of Attention Professional Manual. 844 ed. Los Alamitos, CA: The TOVA Company; 2008. [Google Scholar]
- 18.Conners' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. [computer program]. North Tonwanda: Mutli-Health Systems; 2000. [Google Scholar]
- 19.Weis R, Totten SJ. Ecological validity of the Conners' Continuous Performance Test II in a school-based sample. J Psychoeducational Assess 2004;22:47–61. [Google Scholar]
- 20.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol 2001;23:545–559. [DOI] [PubMed] [Google Scholar]
- 21.R Computing Team. R: A Language and Environment for Statistical Computing. 3.2.4 ed. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 22.Ahmed A, Whitley CB, Cooksley R, et al. Neurocognitive and neuropsychiatric phenotypes associated with the mutation L238Q of the α-L-iduronidase gene in Hurler-Scheie syndrome. Mol Genet Metab 2014;111:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vite CH, Nestrasil I, Mlikotic A, et al. Features of brain MRI in dogs with treated and untreated mucopolysaccharidosis type I. Comp Med 2013;63:163–173. [PMC free article] [PubMed] [Google Scholar]
- 24.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer 2006;106:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects After Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transpl 2012;18:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos D, Monaga M. Mucopolysaccharidosis type I: current knowledge on its pathophysiological mechanisms. Metab Brain Dis 2012;27:121–129. [DOI] [PubMed] [Google Scholar]
- 27.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology 2003;17:496–506. [DOI] [PubMed] [Google Scholar]
- 28.Luders E, Narr KL, Hamilton LS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry 2009;65:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc 2006;12:24–33. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh A, Miller W, Orchard PJ, et al. Enzyme replacement therapy prior to haematopoietic stem cell transplantation in mucopolysaccharidosis type I: 10 year combined experience of 2 centres. Mol Genet Metab 2016;117:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldenhoven M, Boelens JJ, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol Blood Marrow Transpl 2008;14:485–498. [DOI] [PubMed] [Google Scholar]
- 32.Reiersen AM, Todd RD. Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment. Expert Rev Neurother 2008;8:657–669. [DOI] [PubMed] [Google Scholar]
- 33.Petrenko CL, Alto ME. Interventions in fetal alcohol spectrum disorders: an international perspective. Eur J Med Genet 2017;60:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were entered into the Data Management and Coordinating Center (DMCC) for the Rare Diseases Clinical Research Network. The DMCC is a secure clinical data management system that collects and stores data on a variety of rare diseases from organizations across the United States. The data are held in the DMCC for 5 years, after which they are released to the database of Genotypes and Phenotypes.