Abstract
Background
Bhasmas are unique Ayurvedic organometallic preparations used for medicinal purpose. Quality of bhasma depends upon quality of starting materials, processing ingredients, meticulous trituration and heating cycle. In Ayurveda, Vanga bhasma is traditional Indian medicine which is an organometallic preparation treated with plant extract. It is especially used in the treatments of diseases related to gastrointestinal tract and genitor urinary system. However detailed characterization studies after synthesis are important which shows authenticity of product.
Objective
The present study deals with the preparation of Vanga bhasma according to the procedure mentioned in the Ayurvedic literature. Synthesized bhasma was characterized by various analytical techniques and also compared with commercial sample.
Material and method
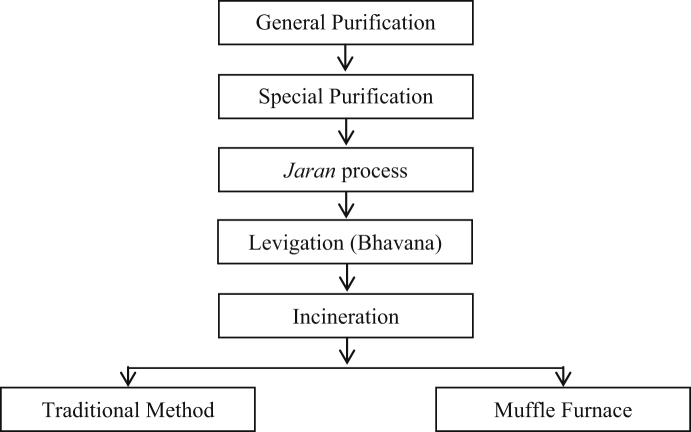
Different steps involved in synthesis of Vanga bhasma include shodhan (purification/detoxification), jaran (heating and stirring), bhavan (levigation) and maran (incineration). Bhasma was incinerated (maran process) by traditional method of heating as well as using muffle furnace. These two products obtained from Maran (incineration) and commercial sample were analyzed for quality control checks, on the parameters described in Ayurvedic texts as well as modern techniques such as TEM, SEM, EDX, XRD, DLS and FTIR were done to find out the nature and form of the drug prepared. The in vitro gastric and gastrointestinal (pancreatic) bioaccessibility of Bhasma were also determined.
Results
The study reveals that the synthesized Bhasma was converted into its nontoxic oxide form and had a highly reduced particle size observed from SEM images. These studies reveal that Vanga Bhasma prepared by traditional method of heating (Sn1) has 50% nanoparticles (150–300 nm range) that prepared by using electric muffle furnace (Sn2) has 100% nanoparticles (50–100 nm range) while commercial samples (Sn3) has 50% nanoparticles (100–300 nm range).
Conclusion
The study confirmed the formation of organometallic compound (SnO2) at the end of the manufacturing process. The percentage bioaccessibility for gastrointestinal digestion is more than the gastric digestion. Hence, it is concluded that Vanga Bhasma can be useful nanomedicine.
Keywords: Vanga Bhasma, Jaran, Levigation, Purification, Characterization, Bio-accessibility
Highlights
-
•
XRD studies reveal crystalline nature of Vanga Bhasma and formation of SnO2.
-
•
SEM studies reveals that Vanga Bhasma prepared by Muffle furnace heating are less than 50 nm.
-
•
TEM analysis of Vanga Bhasma also reveals that bhasma are polycrystalline in nature while commercial sample shows presence of rod like structures.
-
•
The HR-TEM shows that the inter planer distance in particle prepared by traditional method of heating is 0.373 nm while that of bhasma prepared by using the Furnace for heating shows 0.34 nm.
-
•
DLS studies reveal that Vanga Bhasma prepared by traditional method of heating has 50% nanoparticles (150–300 nm range) that prepared by using electric muffle furnace has 100% nanoparticles (50–100 nm range) while commercial sample has 50% nanoparticles (100–300 nm range).
1. Introduction
Ayurvedic formulations are multicomponent mixtures containing plant and animal derived products, minerals and metals [1]. Bhasmas are unique Ayurvedic metallic preparations which are used for medicinal purposes since ancient times [2]. The immunomodulation and ability to target drugs to the site are characteristics of properly prepared organometallic preparations [3], [4], [5]. Properly prepared bhasma is nontoxic, easily absorbable, adaptable and digestible in the body [3], [4], [5]. These bhasmas are generally prescribed with the several other medicines of Ayurveda [6], [7]. The ancient application of nanomedicine in the form of ayurvedic bhasma throws a light on the safer usage of present nanomedicine for living being and environment [6], [8], [9], [10], [11], [12], [13]. Metallic herbal preparations (bhasmas) have some advantages over the plant based drugs such as stability over the longer period, lower dosage, easy storability and easy availability [14]. When the herbometallic mixture is incinerated in the closed vessel, nano-oxide particles are converted into a more favorable oxidation form for human consumption [3], [15], [16].
Vanga bhasma is used in the treatment of genitourinary disorder, diabetes, anemia, asthma gastric ulcers and urinary diseases [17]. The present study deals with the preparation of Vanga Bhasma according to the procedure mentioned in the Ayurvedic literature. The Vanga Bhasma is (Tin based) prepared and analyzed for quality control, on the parameters described in Ayurvedic texts as well as modern technology. The incineration step in synthesis of bhasma was carried out by traditional method of heating as well as using electric muffle furnace. The finally synthesized bhasma was then compared with the marketed sample. The Bhasma was analyzed for quality control checks, on the parameters described in Ayurvedic texts as well as by using modern technology such TEM, SEM, EDX, XRD, FTIR, DLS to find out the nature and form of the bhasma prepared.
2. Materials and methods
The different Materials used for the preparation of Vanga Bhasma are raw vanga (Tin) (Fischer Scientific). Sesame oil (Tila Taila), buttermilk (Takra), cow's urine (Gomutra), powder of Curcuma longa Linn (Haridra Churna) bark, powder of Tamarindus indica Linn root were procured from local retailers. The Kanji, kulattha kwatha (decoction of Dolichos biflorus Linn.), juice of Vitex nigundo leaves (Nirgundi patra swarasa), juice of Aloe barbadensis Miller leaves (Aloe vera kumari swarasa) were prepared in laboratory. All medicinal plants mentioned above in the preparation of Vanga Bhasma were authentified at Agarkar Research Institute, Pune and deposited there.
The preparation of Vanga Bhasma consists steps such as Shodhan, Jaran, Bhavan and Maran [18], [19].
The stepwise procedure is shown in the following block diagram (Fig. 1).
Fig. 1.
Schematic diagram of various steps involved in preparation of Vanga Bhasma.
2.1. Shodhana of Vanga
Traditionally, the ayurvedic drugs are purified through shodhana which is important to reduce the drug toxicities through different physical and chemical processes. Vanga was subjected to shodhana (samanya shodhan and vishesh shodhan).
In samanya shodhan method the raw vanga was heated till it melts and then quenched sequentially in sesame oil, buttermilk, cow's urine, kanji, kullatha kwatha 7 times in each. Then, vishesh shodhan was performed. In vishesh shodhan, Vanga processed in previous step was quenched in juice of Vitex nigundo leaves which was mixed with powder of Curcuma longa Linn (Haridra) [100 g of Haridra powder in 2 L of Vitex Nigundo] for 3 times. At each time fresh liquid was used. These methods of purification are useful to detoxify the raw material. It also modifies the properties of the therapeutic material to enhance their potential [20], [21].
2.2. Jarana of Shuddha Vanga
This process is mainly for the metals of low melting point. This process is responsible for the solidification of Metal and facilitates the process of incineration. The shodhit Vanga was put in an iron vessel & heated over flame till it melted. Then one forth part T. indica Linn. bark powder (50 g) was added to the molten Vanga with stirring and rubbing by iron ladle. The process is repeated till the whole of the Vanga was converted into powder form. The powder is then covered with an earthen saucer and heated strongly for ½ h. After that it was allowed to cool and collected.
2.3. Bhavana of Vanga
Powdered Vanga from Jaran steps was triturated with Aloe vera Tourn ex-Linn (Kumari Swarasa) juice in mortar with the help of pestle till the thick paste was formed which was suitable for making pellets (Chakrikas). Pellets made and kept for shade drying. These pellets were separated into two lots for maran with traditional method of heating and maran with electric muffle furnace heating.
2.4. Maran of Vanga
After proper drying of pellets, these pellets were kept in the big earthen evaporating dishes (sharav) and sealed (sandhi bandhan). Then maran was carried out by traditional method (using cow dung) and by using electric muffle furnace at 600 °C for 3 h.
The incineration with traditional method of heating was carried out using kukkutput, the [pit of 45 cm (l × b × h)] and 60 cow dungs. This process was repeated four times.
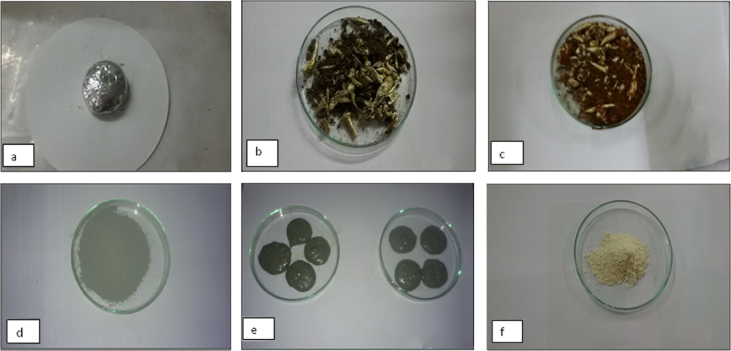
After complete incineration the whitish colored Vanga Bhasma was obtained. This method is useful for conversion of metallic drug from a heavy, hard and rough structure to light, soft, and smooth powder. Also by this method the macro sized particles are reduced to their nano form. This was subjected for physicochemical analysis mentioned in the literature [22], [23] and also analyzed by using modern analytical Techniques. The bhasma was compared with the commercial sample of Vanga Bhasma which is reported on to bottle prepared by same method [18], [19]. Following Photographs show various stages of synthesis of Vanga Bhasma (Fig. 2).
Fig. 2.
Vanga Bhasma images (a) after Melting (b) after Samanya Shodhan (c) after vishesh shodhan (d) after Jaran process (e) after bhavan (f) final product.
2.5. Physical characterization
The prepared bhasma was characterized by traditional method as well as modern analytical method.
Different physical tests mentioned in the literature such as nishchandratvam (lusterless), rekhapurnatvam (particle size enters in the furrows of finger), varitara (floating of product on water) were examined.
The prepared Bhasma was analyzed using various instruments: Fourier transform infrared spectrophotometer (FTIR) (SHIMADZU 8400), Scanning electron microscope (FEI – NovananoSEM-450) with energy dispersive analysis of X-rays (EDAX) (Bruker XSHLASH-6 I30.), X-ray diffractometer (XRD) (SHIMADZU AA-7000), equipped with photo scintillation detector using angular range 2θ = 10–800 at rate of scanning 50/min, ICP-AES (SPECTRO analytical instruments GmbH, Germany, model: 2ARCOS simultaneous ICP spectrometer) Transmission electron microscopy (TecnaiG2U-twin200Kv Lab6FEI Netherlands) and Dynamic light scattering (DLS) (Bruckhaven DLS) to determine organic moieties, to determine particle size, to analyze the surface area, to determine the elemental composition, to study morphology, to determine the crystalline phase.
2.6. Bioaccessibility
Bioaccessibility is the proportion of a nutrient in food that can be utilized for normal body function. We have determined bioaccessibility by in vitro gastric digestion and gastrointestinal digestion method [24].
The chemicals used for determination of Bioaccessibility were Pepsin (Sisco Research Laboratory), Pancreatin 3X extrapure (Sisco Research Laboratory) and Bile salt (Otto Chemicals).
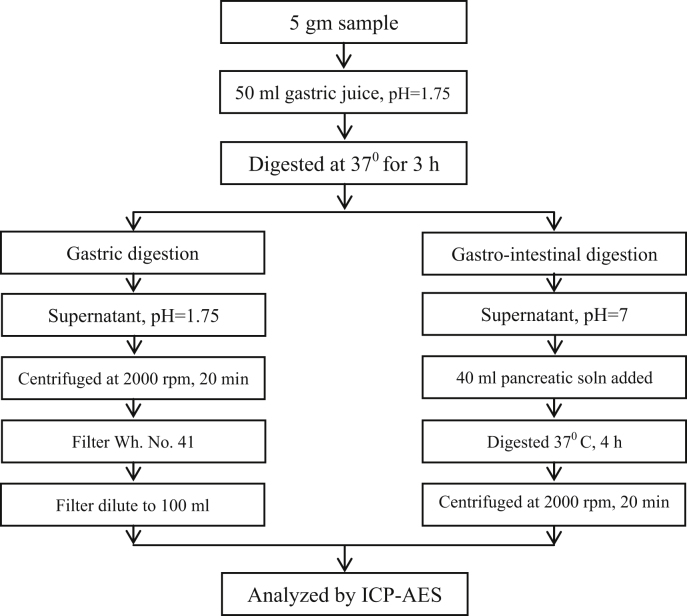
2.6.1. In vitro gastric digestion
For this purpose, accurately weighed 5 g of sample was transferred to a beaker containing 50 mL of gastric juice solution (6% w/v pepsin 100 mL HCl of pH 1.75) and the mixture was shaken vigorously for 2 min. The flask was placed in a water bath at 37 °C on a magnetic stirrer. The reaction mixture was stirred continuously for 3 h. It was then centrifuged for 20 min at 2500 rpm. After that sample was filtered through Whattman No. 41 filter paper. The filtrate was subjected to analysis by using ICP-AES technique.
2.6.2. In vitro gastro-intestinal digestion
For gastro-intestinal digestion the pH of the solution obtained from gastric digestion was adjusted to neutral pH. This is done by drop wise addition of saturated solution of NH4HCO3. To this mixture 45 mL of pancreatic digestion solution (mixture of 2% w/v pancreatin and 0.2% w/v bile salts) was added. The mixture was again digested, as described above, at 37 °C for 4 h. This solution was then centrifuged for 20 min at 2500 rpm. The supernatant was then filtered through Whatman No. 41 filter paper and stored in an airtight container in refrigerator for further analysis.
Stepwise procedure for in-vitro determination of bioaccessibility is shown in following block diagram (Fig. 3).
Fig. 3.
Schematic diagram of in vitro bioaccessibility determination of Vanga Bhasma.
From concentration of elements in gastric and gastro-intestinal digests, the percentage of bioaccessibility (% B) of elements from each sample was calculated by using following formula:
| (1) |
where [GD] = Concentration of element in gastric digest; [GID] = Concentration of element in pancreatic digest and [T] = Total elemental concentration in the sample.
3. Results and discussion
The organoleptic analysis as mentioned in the texts of Ayurveda revealed that it was very soft. The color of the Vanga Bhasma prepared by both methods of heating is milky white. The Bhasma did not produce any taste when kept on tongue, nor did it emit any odor when it was smelt. It indicates that both the bhasmas have complied with all physical tests mentioned in the literature.
Tests such as nishchandratvam (lusterless), rekhapurnatvam (particle size enters in the furrows of finger), varitara (floating of product on water) were positive for the prepared as well as commercial bhasma.
Average weight loss was observed 1.5% in samanya shodhan. Analytically, the Vanga Bhasma is tin dioxide having some other elements such as Ca, Mg, Zn, P, Cu in trace quantity which are incorporated by shodhan of Vanga and process used for heating (Jaran//Maran) [24], [25], [26] wherein T. indica Linn. bark powder and aloe vera kumara swarasa are used.
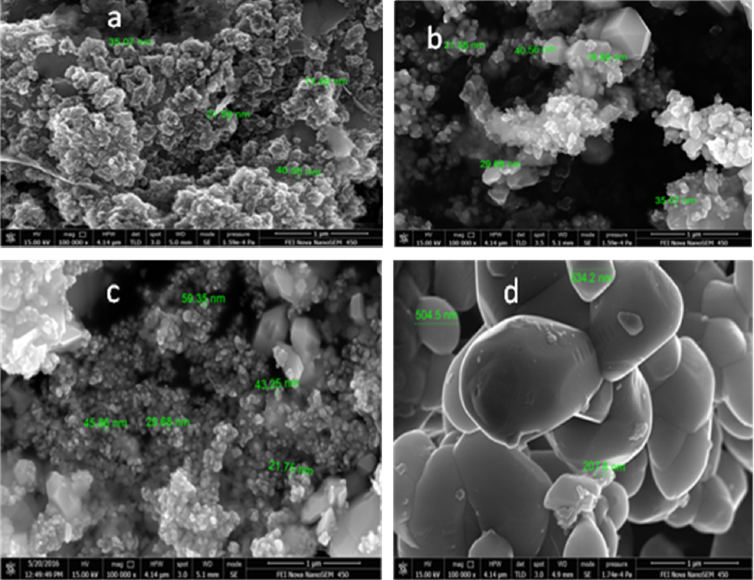
3.1. SEM analysis: surface morphology of bhasmas
As can be seen from Fig. 4 SEM images of Vanga Bhasma particles show granular appearance and porous morphology. These nanoparticles have spherical morphology with the size smaller than 100 nm. The Initial sample shows the rough surface morphology. It is clear from Fig. 4a, that the jaran method helps to convert sample into nanometer size. Bhasma prepared by traditional method of heating (Fig. 4b) and by using muffle furnace heating (Fig. 4c) shows almost same results for SEM. Fig. 4b and c indicates formation of nanoparticles of vanga. While the commercial sample (Fig. 4d) shows larger size than the synthesized bhasma. The commercial sample shows size near to the 500 nm. It was observed that particles are with smooth surface and aggregated. Thus maran (incineration) with electric muffle furnace heating is more calibrated and easy system than traditional method of heating.
Fig. 4.
SEM microgram of Vanga Bhasma (a) after Jaran method (b) prepared by maran with traditional method of heating (c) prepared by maran with electric muffle furnace heating (d) commercial sample.
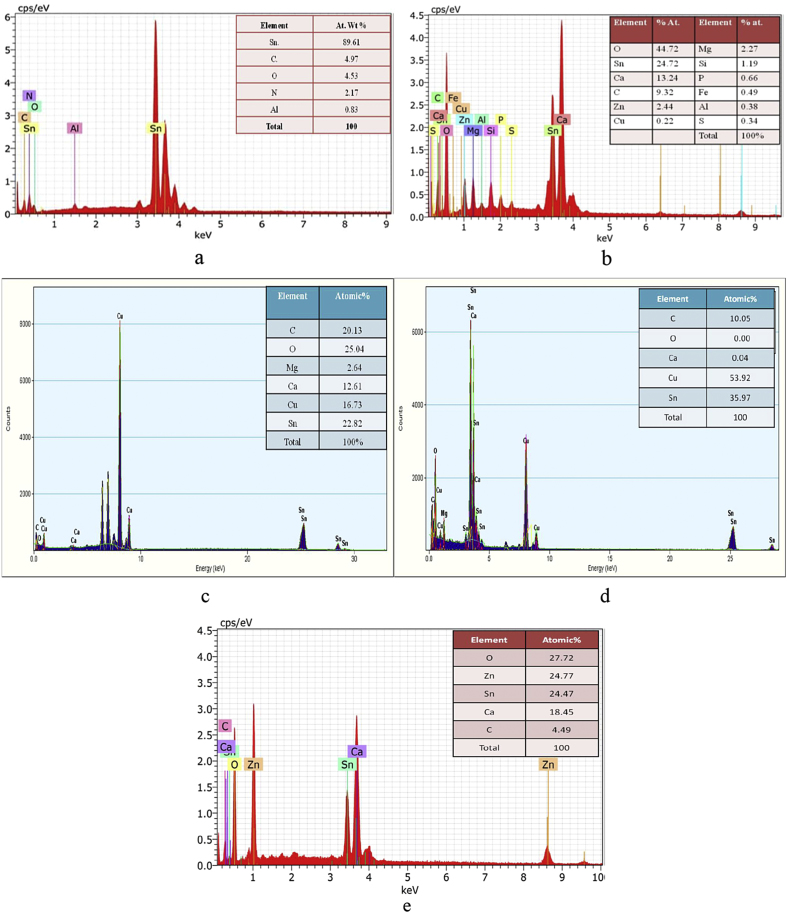
3.2. EDAX analysis
The Major elements present in finally prepared bhasma by both the methods are C, O, Ca, Mg, Cu and Sn. While it is observed that same elements were present in the Bhasma prepared by using electric muffle furnace heating except magnesium. Also the amounts of copper increases in the bhasma prepared using electric muffle furnace heating. Since the concentration of Mg was very less, there may be a possibility of masking it by some other peak. Moreover, we have used copper grids during EDX analysis of prepared Vanga Bhasma therefore copper concentration is higher than expected in bhasma.
EDAX of bhasma after jaran method shows that many elements such as O,Sn,C, Ca, Zn, Mg, Si, P, Fe, Al, S, Cu etc are incorporated in the bhasma. All these elements originate from medicinal plants used during preparation of bhasma [25], [26], [27]. Quantitative inorganic assay shows that tin present in raw material was 89.60% and interestingly, it was decreased in final product to 24.23%, which may be attributed to conversion of some part of the Vanga into Tin oxide form. The substantial reduction is due to addition of other metal oxides, organic materials from herbal source in preparation of the bhasma and continuous oxidation during incineration process of bhasma.
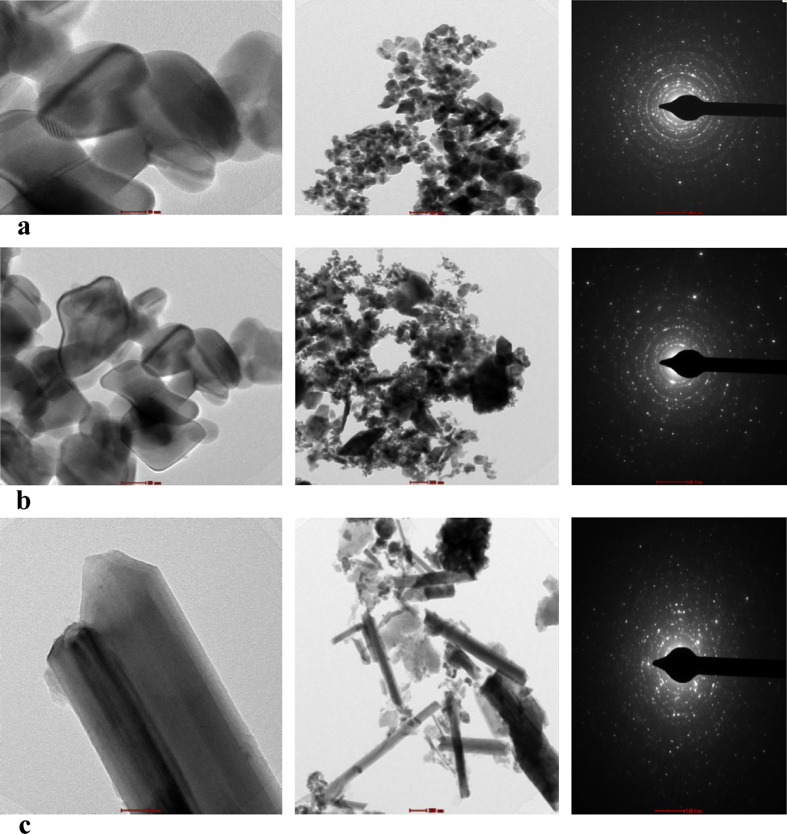
3.3. TEM analysis
TEM study reveals that particle sizes of bhasma prepared by using traditional method of heating and using electric muffle furnace heating were 20 nm (Fig. 6a and b). It is observed that particles of bhasma are uniformly distributed, more clustering. The study also reveals that the particles are uniformly scattered. TEM images clearly show the morphological variation and the nanocrystallite nature of sample. The SAED pattern of diffraction shows that products formed in both the methods are polycrystalline in nature. Similarly commercial sample also polycrystalline particles are aggregated and having smooth surface. Some rod shaped structures also present in the commercial sample, this is may be due to incomplete burning of ingredients used for its preparation.
Fig. 5.
EDAX spectra of Vanga (a) Original Sample (b) after Jaran method (c) prepared by maran with traditional method of heating (d) prepared by maran with electric muffle furnace heating (e) commercial sample.
Fig. 6.
(a) TEM, SAED pattern of Vanga Bhasma after maran with traditional method of heating. (b) TEM, SAED pattern of Vanga Bhasma after maran with electric muffle furnace heating. (c) TEM, SAED pattern of commercial sample of Vanga Bhasma.
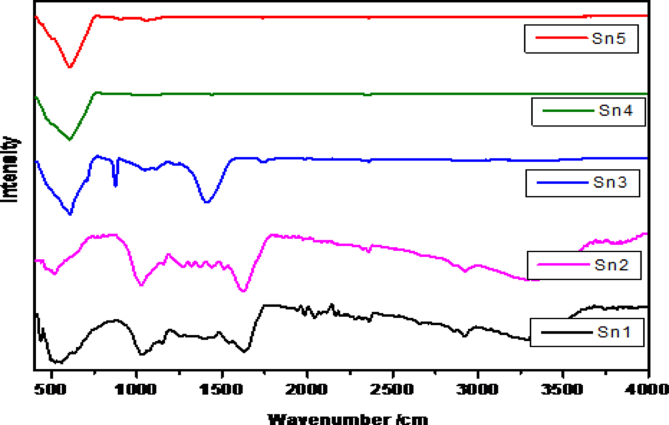
3.4. FTIR analysis of Vanga Bhasma
The FTIR spectra for all the samples are shown in Fig. 7.
Fig. 7.
FTIR spectra for Vanga Bhasma. Sn1 – after shodhan, Sn2 – after jaran, Sn3 – Vanga Bhasma after maran with traditional method of heating, Sn4 – Vanga Bhasma after maran with electric muffle furnace heating, Sn5 – commercial sample.
From Fig. 7 it is observed that, all three spectra (Sn3, Sn4, Sn5) shows 2360.81, 1734.58, 1369.59, 1408.83, 1442.27, 1059.58, 908, 605.90, 873.45, 1793.73, 1111.60 cm−1 which indicate the presence of CH3, OCH3, C O, C C,CH vibration fingerprint region, CO vibration fingerprint region, SnO bonding and SnC bonding. Sn1 and Sn2 are the spectra of the Vanga after detoxification and after jaran method. Which shows the major peaks at 1408.83, 873.45, 607.20, 3331.65, 3632.65, 162.82, 1026.42, 464.16 cm−1 these frequencies are attributed to the bending vibration bond, stretching frequency of OH bond, stretching vibration of SnOH bond, stretching vibration of SnOSn bond. Stretching Vibration of CH, CH3, OCH3, C O, C C bonds etc. Strong peaks at 1408.83, 873.45, 607.20 cm−1 are observed in traditional method of heating sample are due to CH stretching, C C and C O, CCl. These peaks are might be due to high carbon content in the sample.
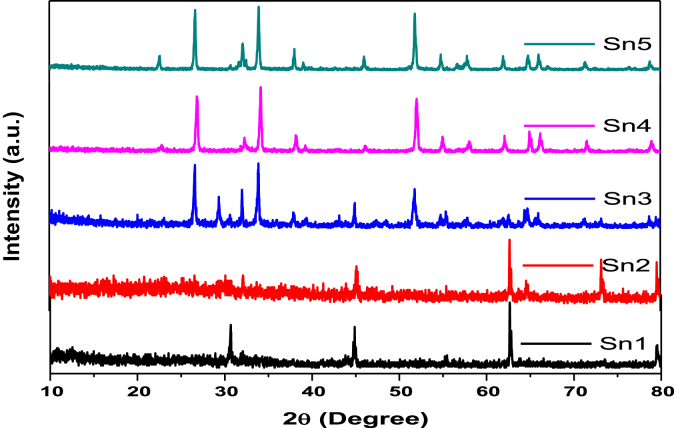
3.5. XRD analysis
It can be seen from Fig. 8 that the peaks match with the diffraction pattern of the tetragonal Tin Oxide (JCPDS 29–1484, 46–1088). The position of main peak in final products Sn4, Sn5 and commercial sample Sn3 is 2θ = 26.67, which is absent in Sn1 and Sn2. Other major peaks are observed at 2θ = 22.56, 34.200, 44.98, 52.04, 65.02, 51.88. Which indicates that, the major planes 110, 101, 200, 211, 220, 002, 310, 112, 301 of SnO2. XRD shows that Vanga Bhasma is crystalline in nature. The major component is Tin Oxide. The predominant peak in sample comprises of SnO2. The crystallite size of bhasma is calculated using Debye–Scherer equation (2)
| (2) |
where, t = Crystallite size; λ = Wavelength; β = Full width of Half Maxima.
Fig. 8.
XRD spectra for Vanga Bhasma. Sn1 – Initial Sample, Sn2 – sample after jaran method, Sn3 – Vanga Bhasma after maran with traditional method of heating, Sn4 – Vanga Bhasma after maran with electric muffle furnace heating, Sn5 – commercial sample.
From Table 1 it is observed that the crystallite size of prepared bhasma is in nanometers.
Table 1.
Crystallite size for major peaks in XRD.
| Vanga Bhasma | 2θ (degree) | Crystallite size (nm) | |
|---|---|---|---|
| Commercial sample | SN3 | 26.64, 33.980, 44.98, 51.860 | 122.58 |
| Prepared by traditional method of heating | SN4 | 26.74, 34.20, 52.04, 65.02 | 52.64 |
| Prepared by electric muffle furnace heating | SN5 | 22.60, 26.60, 32.14, 34.00, 51.88 | 61.27 |
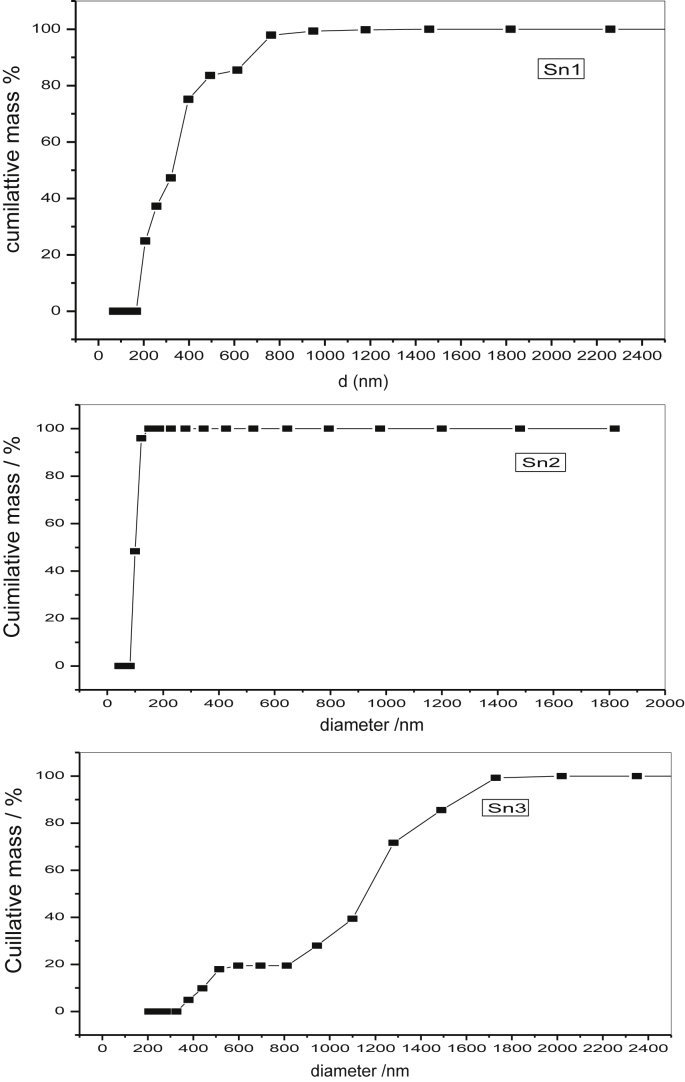
3.6. DLS analysis of Vanga Bhasma
The spectra for DLS analysis of synthesized Vanga Bhasma along with the commercial sample are shown in Fig. 9.
Fig. 9.
DLS spectra for Vanga Bhasma. Sn1 – prepared by traditional method of heating, Sn2 – Vanga Bhasma after maran with electric muffle furnace heating, Sn3 – commercial sample.
These studies reveal that Vanga Bhasma prepared by traditional method of heating (Sn1) has 50% nanoparticles (150–300 nm range) that prepared by using electric muffle furnace (Sn2) has 100% nanoparticles (50–100 nm range) while commercial samples (Sn3) has 50% nanoparticles (100–300 nm range). Further examination of Fig. 9 shows that bhasma prepared by incineration with electric muffle furnace has uniform particle size distribution, while bhasma prepared by incineration with kukkutputa (traditional method) shows trimodal particle distribution. In case of commercial bhasma there is no uniform particle distribution but it is multimodal in nature.
3.7. In vitro bioaccessibility determination
The gastric and gastrointestinal percent bioaccessibility of Vanga Bhasma is shown in following Table 2.
Table 2.
Gastric and gastrointestinal percent bioaccessibility of Vanga Bhasma.
| Vanga Bhasma | Traditionally heated | Heated By muffle furnace | Commercial Bhasma |
|---|---|---|---|
| %Bioaccessibility | |||
| Gastric digestion | 2.03 | 11.74 | 5.01 |
| Gastrointestinal digestion | 6.04 | 14.08 | 9.17 |
From Table 2 It is observed that gastrointestinal percent bioaccessibility of Vanga Bhasma is more as compared to the bioaccessibility of gastric digestion. The bhasma prepared by traditional method of heating shows less bioaccessibility than commercial sample and bhasma prepared by heating with muffle furnace. This is due to remains of some nondigestible metal oxide in the sample because of uneven heating with traditional method.
4. Conclusions
It is observed from SEM Figures that particles of Vanga Bhasma show granular appearance and porous morphology. The study concludes that Vanga Bhasma can be useful nanomedicine if it is prepared by standard method of preparation and analysis. Traditional medicinal system such as Ayurveda serves as excellent template for development of nanomedicine.
Sources of funding
Board of College and University Development, Savitribai Phule Pune University.
Conflict of interest
None.
Acknowledgment
Authors are thankful to Dr. Parag Adhyapak C-MET, Pune for his cooperation. Babita Kale is thankful to Savitribai Phule Pune University for Research stipend.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Mukharjee P.K., Wahile A. Integrated approaches towards drug development from ayurveda and other Indian system of medicine. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Umrani R.D., Paknikar K.M. Ayurvedic medicine Zinc bhasma: physicochemical evaluation, anti-diabetic activity and safety assessment. J Biomed Nanotechnol. 2011;7(1):148–149. doi: 10.1166/jbn.2011.1243. (2) [DOI] [PubMed] [Google Scholar]
- 3.Singh S.K., Gautam D.N., Kumar M., Rai S.B. Synthesis, characterization and histopathological study of lead based Indian traditional drug: Naga bhasma. Indian J Pharm Sci. 2010;72(1):24–30. doi: 10.4103/0250-474X.62232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umrani R.D., Agrawal D.S., Paknikar K.M. Anti-diabetic activity and safety assessment of ayurvedic medicine, Jasad bhasma (zinc ash) in rat. Indian J Exp Biol. 2013;51:811–822. [PubMed] [Google Scholar]
- 5.Verma P., Prasad C.M. Standardization and bioavailability of ayurvedic drug Lauha bhasma part II comparative bioavailability studies. Anc Sci Life. 1995;15:140–145. [PMC free article] [PubMed] [Google Scholar]
- 6.Pal D., Sahu C.K., Haldar A. Bhasma: the ancient Indian nanomedicine. J Adv Pharm Technol Res. 2014;5(1):4–12. doi: 10.4103/2231-4040.126980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari Y.C., Nariya M.B., Galib R., Prajapati P.K. Acute and subchronic toxicity study of Tamra Bhasma (incinerated copper) prepared with and without amritikarana. J Ayurveda Integr Med. 2016;7:23–29. doi: 10.1016/j.jaim.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary A. Ayurvedic bhasma: nanomedicine of ancient India – its global contemporary prospective. J Biomed Nanotechnol. 2011;7(2):68–69. doi: 10.1166/jbn.2011.1205. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar P.K., Chaudhary A.K. Ayurvedic bhasma: the most ancient application of nanomedicine. J Sci Ind Res. 2010;69:901–905. [Google Scholar]
- 10.Kulkarni S.S. Bhasma and nanomedicine. Int Res J Pharm. 2013;4(4):10–16. [Google Scholar]
- 11.Tambekar D.H., Dahikar S.B. Screening antibacterial activity of some bhasma (metal based herbal medicines) against enteric pathogens. Recent Res Sci Technol. 2010;2(10):59–62. [Google Scholar]
- 12.Paul W., Sharma C.H. Blood compatibility study of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2(1):14–22. doi: 10.4103/0974-7788.83183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagtap C.Y., Ashok B.K., Patgiri B.J., Prajapati P.K., Ravishankar B. Acute and subchronic toxicity study of Tamra Bhasma (incinerated copper) prepared from Ashodhita (unpurified) and Shodhita (purified) Tamra in rats. Indian J Pharm. 2013;75(3):346–352. doi: 10.4103/0250-474X.117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A.A., Nair G.C., Reddy A.V.R., Garg A.N. Availability of essential elements in bhasmas: analysis of ayurvedic metallic preparations by INAA. J Radioanal Nucl Chem. 2006;270:173–180. [Google Scholar]
- 15.Wijenyake A., Pitawala A., Bandara R., Abayasekara C. The role of herbometallic preparation in traditional medicine – a review on mica drug processing and pharmaceutical applications. J Ethnopharmacol. 2014;155:1001–1010. doi: 10.1016/j.jep.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Pyrgiotakis G., Bhowmick T.K., Finton K., Suresh A.K., Kane S.G., Bellare R., et al. Cell (A549)-particle (Jasad bhasma) interaction using Raman spectroscopy. J Biopolymers. 2015;89(6):555–563. doi: 10.1002/bip.20947. [DOI] [PubMed] [Google Scholar]
- 17.Baruah H., Praveen R., Chaudhary A.K. Therapeutic uses of vanga bhasma: a critical review. Int J Res Ayurveda Pharma. 2014;5(4):566–570. [Google Scholar]
- 18.Rasendra Sara Sangrah of Sri Gopal Krishnan, Satpute Ashok D, editor. Varanasi Chowkhaba Krishnadas Academy, p. 211.
- 19.Shastri Kasinath., editor. Sadanand Sharma, Rasatarangini. 11th edition. Motilal Banarasidas Publication Delhi; 2009. p. 22. Reprint, Taranga 5/34-35. [Google Scholar]
- 20.Balaji K., Rajendran N., Pemiah B., Krishnaswamy S., Maheswari M.K., Swaminathan S., et al. Scientific validation of different purification steps involved in the preparation of Indian Ayurvedic Medicine, Lauha bhasma. J Ethnopharmacol. 2012;142:98–104. doi: 10.1016/j.jep.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Rajurkar N., Kale B. Synthesis and characterization of Mandur bhasma. Int J Pharm Biol Arch. 2015;6(3):21–28. [Google Scholar]
- 22.Sudhaldev M., Jha C.B. Evolution of the effect of conventionally prepared swarna makshika bhasma on different bio-chemical parameters in experimental animals. J Ayurveda Integr Med. 2015;2(4):187–191. doi: 10.4103/0975-9476.90773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhojashettar S., Poornima B.T., Jadar P.G. Evolution of market sample of ‘Yashada bhasma’ using ‘Namburi phased spot test’. J Ayurveda Integr Med. 2011;2(2):69–71. doi: 10.4103/0975-9476.82524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni S.D., Acharya R., Rajurkar N.S., Reddy A.V.R. Evaluation of bioaccessibility of some essential elements from wheatgrass ( Triticum aestivum L.) by invitro digestion method. Food Chem. 2007;103:681–688. [Google Scholar]
- 25.SK, Shaikh W., Sofia S., Kazi T.G., Usmanghani K., Kabir A., et al. Chemical constituent of Tamarindus indica L. medicinal plant in Sindh. Khanzada Pak J Bot. 2008;40(6):2553–2559. [Google Scholar]
- 26.Sharma D.K., Rai S., Arora S.S., Gupta P.M., Sharma R., Chopra K. Study of trace elements in Aloe vera L. (Aloe barbandiensis Miller) viz. Liliaceae and its biological and environmental importance. J Chem Pharm Res. 2011;3(3):64–68. [Google Scholar]
- 27.Sharma A., Shailajan S. Detection of heavy metals from leaf powders of Annona squamosa Linn., Daturametel Linn. and Vitex negundo Linn. using ICP-OES technique. Env Sci Indian J. 2009;4(4):135–137. [Google Scholar]