Abstract
Nearly 130 years after the first insights into the existence of mitochondria, new rolesassociated with these organelles continue to emerge. As essential hubs that dictate cell fate, mitochondria integrate cell physiology, signaling pathways and metabolism. Thus, recent research has focused on understanding how these multifaceted functions can be used to improve inflammatory responses and prevent cellular dysfunction. Here, we describe the role of mitochondria on the development and function of immune cells, highlighting metabolic aspects and pointing out some metabolic- independent features of mitochondria that sustain cell function.
Keywords: Immunometabolism, Mitochondrial function, Cell fate, And immune cells
Abbreviations
- •NO2
Nitrogen Dioxide Radical
- 2DG
2-Deoxy-d-Glucose
- APC
Antigen-Presenting Cell
- ASCs
Antibody-Secreting Cells
- ATP
Adenosine Triphosphate
- Bcl-2
B-cell Lymphoma 2
- BCR
B Cell Receptor
- CARKL
Carbohydrate Kinase-Like Protein
- CoA
Coenzyme A
- CPT
Carnitine Palmitoyltransferase
- CSR
Class-Switch Recombination
- DAMPs
Danger-Associated Molecular Patterns
- DCs
Dendritic Cells
- Drp1
Dynamin-Related Protein 1
- ETC
Electron Transport Chain
- FADH2
Reduced Flavin Adenine Dinucleotide
- FAO
Fatty Acid Oxidation
- FAS
Fatty Acid Synthesis
- FFA
Free Fatty Acids
- fMLP
N-Formylmethionine-Leucyl-Phenylalanine
- FO
Follicular
- GC
Germinal Center
- GSH
Glutathione
- GM-CSF
Granulocyte-Macrophage Colony-Stimulating Factor
- GPx
Glutathione Peroxidase
- GSSG
Oxidized Glutathione
- GTPases
Guanosine Triphosphatases
- GLUT1
Glucose transporter 1
- H2O2
Hydrogen Peroxide
- HBP
Hexosamine Biosynthetic Pathway
- HIF-1α
Hypoxia-Inducible Factor 1α
- HO•
Hydroxyl
- HOCl
Hypochlorous Acid
- IFN-γ
Interferon-γ
- IL
Interleukin
- IMM
Inner Mitochondrial Membrane
- KEAP1
Kelch-Like ECH-Associated Protein 1
- LLPCs
Long-Lived Plasma Cells
- MCU
Mitochondrial Calcium Uniporter
- MiD
Mitochondrial Dynamics Proteins
- MFF
Mitochondrial Fission Factor
- MnSOD
Manganese-Dependent Superoxide Dismutase
- MPO
Myeloperoxidase
- Mfn
Mitofusin
- mtDNA
Mitochondrial DNA
- mtGSH
Mitochondrial Glutathione
- mTOR
Mammalian Target of Rapamycin
- mtROS
Mitochondrial Reactive Oxygen Species
- MZ
Marginal Zone
- NADH
Reduced Nicotinamide Adenine Dinucleotide
- NETs
Neutrophil Extracellular Traps
- NLRP3
Nucleotide-Binding Oligomerization Domain-Like Receptor Pyrin Domain-Containing-3
- NO•
Nitric Oxide
- NOS
Nitric Oxide Synthase
- Nox
NADPH Oxidase
- Nrf2
Nuclear Factor (Erythroid-Derived 2)-Like-2 Factor
- O-GlcNAc
O-linked N-Acetyl Glucosamine
- O2•-
Superoxide Anion
- OMM
Outer Mitochondria Membrane
- ONO-
Nitrite
- ONOO-
Peroxynitrite
- Opa1
Optic Atrophy 1 Protein
- OXPHOS
Oxidative Phosphorylation
- ONOOH
Peroxynitrous Acid
- PAMPs
Pathogen-Associated Molecular Patterns
- PGC-1β
Peroxisome Proliferator-Activated Receptor γ–Coactivator-1β
- PINK1
PTEN-Induced Putative Kinase 1
- PMA
Phorbol 12-myristate 13-Acetate
- PPP
Pentose Phosphate Pathway
- PRRs
Pattern Recognition Receptors
- RNS
Reactive Nitrogen Species
- SDH
Succinate Dehydrogenase
- SK3
Small Conductance Calcium-Activated Potassium Channel 3
- SLPCs
Short-Lived Plasma Cells
- SOD
Superoxide Dismutase
- STAT6
Signal Transducer and Activator of Transcription 6
- T4SS
Type IV Secretion System
- TCA
Tricarboxylic Acid
- TCR
T Cell Receptor
- Th
Helper T Cell
- TLRs
Toll-Like Receptors
- Treg
Regulatory T Cell
- UDP-GlcNAc
Uridine Diphosphate-N-Acetyl Glucosamine
- xCT
Cystine/glutamate transporter
1. Introduction
Cellular metabolism has been an extensively explored field during the past decade. The understanding of how cells use energy to perform their functions has attracted the attention of scientists, especially with regard to metabolic-related diseases such as obesity, diabetes, and cancer [[1], [2], [3], [4]]. Classically, these conditions not only change whole-body metabolism, but also impair the inflammatory responses. Thus, immunometabolism has emerged as a potential new field of inquiry in order to investigate how the metabolic alterations affect immune cells.
The immune system comprises a family of heterogeneous cells with multiple roles during homeostasis and inflammation in a tissue-specific manner. Recent studies have shown that different immune cell subtypes use distinct metabolic programs to perform their functions. For instance, effector T cells prioritize aerobic glycolysis during anabolic metabolism to balance the synthesis of macromolecules and the generation of energy to support it [5]. Conversely, memory T cells, as well as regulatory T cells (Treg), prioritize fatty acid oxidation (FAO also called β-oxidation) to support the energy demand for survival and function [5].
As first hypothesized by Richard Altmann in 1890 [6], the generation of useful metabolic energy is provided mainly by mitochondria in virtually all eukaryotic cells. However, the concept that the mitochondria are only powerhouses of cells has changed due to a myriad of other roles this membrane-bound organelle can perform. In immune cells, for instance, mitochondria can also regulate cell development, activation, proliferation, differentiation, and death [7,8], which directly impact cell fate and fitness. Moreover, mitochondria are dynamic organelles and can change their morphology and position in the cells through coordinated cycles of fission and fusion to regulate their own and functions and cell metabolism [9]. This process is called mitochondrial dynamics. Another important aspect of the mitochondrial physiology is the local production of oxidants or more commonly referred to as “reactive oxygen species” (mtROS). They were first described as byproducts of the electron transport chain (ETC) and implicated in oxidative damage [[10], [11], [12], [13]], but are also important signaling molecules for cell activation when produced in low quantities [10,[14], [15], [16]].
Thus, the importance of mitochondria goes beyond energy production. Mitochondria can orchestrate immunity by modulating both metabolic and physiologic states in different types of immune cells. In this review, we will focus on how mitochondria drive the development and function of immune cells, highlighting their main metabolic features and pointing out other metabolism-independent roles of mitochondria that sustain cell function.
2. Immune cells metabolism: an overview
Immune cells need energy stored at adenosine triphosphate (ATP) to grow and perform their functions. Glucose is the most studied fuel for cells. After uptake, glucose undergoes a series of 10 consecutive reactions [reviewed in Ref. [17]] in the cytoplasm to be converted into pyruvate and in the process producing two molecules of ATPs [18]. Pyruvate can be either transported into the mitochondria by mitochondrial pyruvate carriers or converted into lactate in the cytoplasm by lactate dehydrogenase (LDH). During the latter reaction, the cofactor nicotinamide adenine dinucleotide (NADH) is oxidized, thus restoring cytosolic NAD+ levels to allow continued glycolytic flux. Lactate production, first reported as a pathway that is activated only under anaerobic conditions, can also occur in the presence of oxygen [19]. In 1923, Otto Heinrich Warburg observed that glucose consumption by oxygen-exposed tumor cells was elevated compared to normal cells [20]. This event is called aerobic glycolysis or Warburg's effect. In general, aerobic glycolysis is the preferred metabolic pathway in activated immune cells [5].
Another important glucose-related metabolic pathway is the hexosamine biosynthetic pathway (HBP). This pathway regulates post-translational modifications (PTMs) such as phosphorylation, acetylation, glycosylation, ubiquitination, acetylation, and hydroxylation [[21], [22], [23]]. PTMs modify protein structure and function, modulating cell signaling pathways, for example [[23], [24], [25]]. HBP is linked to the glycolytic pathway by fructose-6-phosphate. In the presence of glutamine, fructose-6-phosphate generates glucosamine-6-phosphate [26]. Then, after subsequent enzymatic steps involving acetyl-CoA produced during fatty acid metabolism and from pyrimidine biosynthesis products, uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) is generated [26]. UDP-GlcNAc is the substrate for O- and N-glycosylations, producing complex glycoconjugates, such as glycoproteins and proteoglycans [27]. These glycosylations are associated with conventional secretory pathways dependent on the Golgi apparatus and endoplasmic reticulum [23,28]. However, in 1984 Torres and Hart described a non-canonical glycosylation mechanism involving the association of O-linked N-acetyl Glucosamine (O-GlcNAc) with cytoplasmic, nuclear and mitochondrial proteins [29]. This special form of glycosylation plays important roles in metabolic homeostasis, stem cell biology, signaling pathways, transcription factors function, and immune cell maintenance [29]. Defects in O-GlcNAc are linked to the development of cancer, diabetes, Alzheimer's disease, and other disorders [[30], [31], [32]].
As stated above, the pyruvate produced from glucose metabolism can be transported into the mitochondria and be oxidized by pyruvate dehydrogenase (PDH), generating acetyl-CoA that enters the tricarboxylic acid (TCA) cycle. In the TCA cycle, acetyl-CoA is further oxidized and generates key intermediates that can also be used as precursors of complex molecules. Some of the TCA reactions produce NADH and flavin adenine dinucleotide (FADH2) to fuel the ETC, which in turn couples the electron transfer to the pumping of protons across the inner mitochondrial membrane (IMM) to create an electrochemical proton gradient. ATP synthase uses the potential energy from the protons when they move from high-to low-potential sides of the mitochondrial membrane to generate ATP [33,34]. This pathway is called oxidative phosphorylation (OXPHOS) and produces 36 ATPs per mole of glucose.
In addition, to provide intermediates for the biosynthesis of macromolecules, some TCA cycle-derived molecules can act as signaling molecules and even play microbicidal roles [[35], [36], [37]]. The TCA cycle in M1 macrophages and dendritic cells (DCs), for example, is not complete. The “broken TCA cycle” diverts some of its intermediates to other pathways. This can lead to the accumulation of citrate and succinate, for instance, in these cells [38]. When citrate accumulates in the mitochondrial matrix, it can be transported from the mitochondria to the cytosol by the mitochondrial citrate carrier [39]. Once in the cytosol, citrate is oxidized to acetyl-CoA, which leads to de novo lipogenesis. This is crucial to the expansion of organelles, such as the endoplasmic reticulum and Golgi, which is required for increased protein production and secretion. This is important during DCs activation [39,40]. To replenish the citrate in mitochondria and fuel the TCA cycle, the cells use other metabolites to produce more citrate through anaplerotic reactions [36]. For instance, glutamine metabolism can generate the intermediate α-ketoglutarate via glutaminolysis, allowing the TCA cycle to proceed [38].
Succinate is formed by the oxidation of succinyl-CoA via succinyl thiokinase (also called succinyl-CoA synthetase) and is oxidized to fumarate in complex II of the ETC by succinate dehydrogenase (SDH) and in the process FAD is reduced to FADH2. FADH2 can be oxidized again to FAD by the iron-sulfur (Fe-S) center of the SDH. This process produces both superoxide anion (O2•-) and hydrogen peroxide (H2O2). A break in the TCA can occur during the conversion of succinate to fumarate by SDH, leading to succinate accumulation in the mitochondria and cytosol. Succinate has a well-established function in macrophage polarization [41]. Pro-inflammatory M1 macrophages are characterized by increased availability of succinate in the cytosol, where it acts to inhibit prolyl hydroxylases. Prolyl hydroxylases are responsible for the degradation of the hypoxia-inducible factor 1α (HIF-1α), leading to its stabilization [41]. Moreover, succinate stimulates DCs via succinate receptor 1 through the induction of intracellular calcium mobilization and enhancing DCs migration and cytokines secretion [35]. In order to restrain the pro-inflammatory role of succinate another TCA cycle-derived molecule, itaconate, is produced from cataplerosis of cis-aconitate [42]. Itaconate has anti-inflammatory properties and regulates both metabolism and effector functions of macrophages through the inhibition of succinate oxidation by SDH [42].
FAO is a catabolic pathway which converts long-chain fatty acids into acyl-CoA [43]. Mitochondria can import fatty acids from the cytosol through carnitine palmitoyltransferase I (CPT1), which is located in the outer mitochondria membrane (OMM), and carnitine palmitoyltransferase II (CPT2), located in the IMM [44]. In the mitochondrial matrix, acyl-CoA is oxidized into acetyl-CoA, fueling the TCA cycle. Moreover, FAO is not only important for energy production because it is also involved in T cell and macrophage fate decision, as will be discussed below [[45], [46], [47], [48]]. To investigate FAO pathway, researchers widely use the CPT1 irreversible inhibitor etomoxir. However, caution is needed in interpreting results that use this drug since high doses of etomoxir also inhibit complex I of the ETC and deplete free CoA [[49], [50], [51]].
Further, amino acid metabolism, especially glutamine metabolism, is critical for immune cell development and response [38]. Leukocytes can use glutamine in the cytoplasm to divert glucose from glycolysis to HBP, as previously mentioned, or through glutaminolysis to fuel the TCA cycle [5]. Moreover, glutamine is used by immune cells to provide considerable amounts of NADPH [52]. Usually, the canonical pathway to generate NADPH is via the pentose phosphate pathway (PPP) [53,54]. This pathway can occur in parallel with aerobic glycolysis depending on glucose-6-phosphate (G6P) availability. When glucose is converted into G6P during glycolysis, carbon flux can be directed via glucose-6-phosphate dehydrogenase (G6PD) to the PPP, reducing NADP+ into NADPH, and producing nucleotides, nucleic acids, and amino acids precursors [[54], [55], [56]].
Our understanding of the involvement and requirement of these different metabolic pathways in immune cells were facilitated by studies involving isotopically labeled metabolites by tracking the carbon flux into different intermediates under both physiological and pathological states.
3. Mitochondria: the central hub in innate and adaptive immune cells
Mitochondrion is a double-membrane-bound organelle. The OMM contains multiple proteins, such as mitochondrial voltage-dependent anion channel (VDAC), also called mitochondrial porin [57], mitochondrial antiviral signaling protein (MAVS) involved in virus recognition [58], regulators of mitochondrial dynamics, such as mitofusin1 and 2 (Mfn-1 and 2) [59,60], and antiapoptotic proteins, such as B-cell lymphoma 2 (Bcl-2) [61,62], among others [63,64]. The main role of VDAC is to facilitate the diffusion of small molecules and ions through the OMM, serving as a connection between the mitochondria and the cytosol. VDAC participates actively in necrotic and apoptotic cell death processes, which involve changes in mitochondria membrane permeability [57]. Thus, OMM can be seen as a signaling platform that directly connects the mitochondria with other molecules present in the cell. The IMM, in turn, is impermeable to the majority of small molecules and ions. It contains the ETC and other membrane transporters [41]. The mitochondrial matrix is enclosed by the IMM, has a viscous consistency and contains mtDNA, ribosomes, nucleotides and soluble enzymes [65].
Mitochondria are also involved in cell death. In addition to apoptosis and necrosis, mitochondria participate in pyroptosis, another inflammatory type of cell death that is dependent on inflammasome activation [66]. The inflammasome is a cytosolic high-molecular-weight protein complex that is able to sense pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). The most well-characterized inflammasome is the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NLRP3) [67]. mtDNA released into the cytosol is a DAMP that induces inflammasome oligomerization and activation in macrophages and T cells [68]. This activation leads to the cleavage of pro-interleukin (IL)-1β and 18 to their active forms, leading to pyroptosis [69]. mtDNA can also be released extracellularly as web-like structures by T and B lymphocytes, and neutrophils [70,71]. These webs boost the antiviral defense or trap and kill larger pathogens. The mechanisms by which these cells release mtDNA will be described below.
4. Mitochondrial dynamics
Mitochondria are dynamic organelles that are constantly undergoing fission or fragmentation, fusion or elongation, and trafficking [72]. These processes are in accordance with cell energy necessity, in other words, low-proliferative cells and decreased the production of ATP by OXPHOS are associated with mitochondrial fission, and the opposite for mitochondrial fusion [73]. In contrast, M2 macrophages have elongated mitochondria [73]. A similar differential pattern is observed for memory T lymphocytes (fused mitochondria) and activated T lymphocytes (fragmented mitochondria) [74]. This indicates that mitochondrial dynamics can be regulated by and also regulates the polarization status of immune cells.
The four major proteins involved in mitochondrial dynamics are members of dynamin-like guanosine triphosphatases (GTPases), which include: Mfn1 and 2, optic atrophy 1 (Opa1) and dynamin-related protein 1 (Drp1) [75]. Mfn1 and Mfn2 are localized to the OMM, and Opa1 is localized to the IMM [75]. Mitochondrial fusion is mediated by the tethering of two mitochondria. Mfn 1 and 2 mediate the fusion of the two OMMs, followed by Opa1-dependent IMM fusion [76]. Mitochondria fusion occurs under starvation conditions or higher OXPHOS activity. This increases cristae formation and provides more surface area for OXPHOS and FAO.
On the other hand, nutrient excess, severe stress, and impaired OXPHOS lead to mitochondrial fission [74]. In such conditions, the cytosolic Drp1 is dynamically recruited to the OMM, where it binds to anchor proteins in the mitochondrial surface including mitochondrial fission factor (MFF) and the mitochondrial dynamics proteins 49 and 51 (MiD49 and MiD51) [72]. The binding of Drp1 with these proteins cause Drp1 oligomerization and the subsequent formation of a ring-like structure around the mitochondria, which leads to the narrowing of the membrane [76]. The mechanism by which each immune cell type trigger changes in mitochondria dynamics will be discussed later in this review.
The cell function is also influenced by the mitochondrial distribution within cells and this organization depends on their interaction with the cytoskeleton and molecular motors [75]. The movement in the cell depends on miro-anchoring proteins in the OMM that interact with molecular motors (kinesin or dynein) in the microtubules [72]. The trafficking of mitochondria increases the chances for random mitochondrial interactions [72]. Furthermore, we believe that the size of mitochondria may impact mitochondrial transport across the cell. In this sense, small mitochondria can be transported easier than larger ones.
5. Oxidants production: a double-edged sword
Oxidants in immune cells can be produced by NADPH oxidase (Nox), mitochondria and peroxisomes and are essential for antibacterial host defense and signaling pathways [77,78]. Nox is a multimeric protein complex present in phagocytes (neutrophils, macrophages, and DCs). After cell activation, cytosolic NADPH is recruited to the plasma or phagosome membranes, thus allowing Nox assembly and activation. An oxidative burst is produced when molecular oxygen is oxidized by activated Nox into the radical anion superoxide O2•-. This is a crucial event for the elimination of engulfed pathogens by phagocytes [79]. There are several members of the Nox family [80]. Nox1 is most common in colon epithelial cells.
Nox2 is the most abundant and main isoform in immune cells, Nox4 is found mainly in fibroblasts and vascular cells, and Nox5 is regulated by Ca2+ and is expressed in spleen, kidney, vascular cells and lymphoid tissue [79].
Peroxisomes are ubiquitous organelles primarily involved with lipid metabolism, FAO and fatty acid synthesis (FAS) [81,82]. Peroxisomes are one of the main sources of oxidants, mainly H2O2, and are intimately related to mitochondrial physiology and structure through peroxisome-mitochondrial communication via vesicular trafficking, intraorganellar diffusion or physical contact [83,84]. At the same time, peroxisomes have the complete machinery to maintain cellular redox balance. Besides peroxisomes, mitochondria are also crucial sources of oxidants [85,86]. mtROS are produced from the leakage of electrons in the ETC, mainly at complexes I and III [10], although complex II can also produce O2•- [10]. Complex I and II produce mtROS only in the mitochondria matrix, but complex III produces mtROS in both the matrix and intermembrane spaces [10,87,88]. O2•- is mostly converted into H2O2, which can be further converted into hydroxyl radicals (HO•) [89]. In this reaction, transition metal ions, such as iron (Fe) or copper (Cu), can catalyze the formation of HO•. This reaction was proposed by the physical chemist, Fritz Haber and his student, Joseph Weiss, in 1934. The Haber–Weiss reaction (catalyzed by iron) describes how highly reactive HO• is generated in live organisms [90]:
| Fe3+ + O2•- → Fe2+ + O2 |
| Fe2+ + H2O2 → Fe3+ + HO• + OH- (Fenton reaction) |
| O2•- + H2O2 → O2 + HO• + OH- |
Hydroxyl radicals are very unstable and highly reactive [91] and can damage lipids, proteins, and DNA in the cells [92]. O2•- and H2O2 are more stable and less reactive compared to hydroxyl radicals but can also damage cellular components [92]. These oxidants directly kill pathogens by causing oxidative damage to biomolecules or by stimulating pathogen elimination via non-oxidative mechanisms, including the activation of pattern recognition receptors, autophagy, web-like structures formation in neutrophils (neutrophil extracellular traps – NETs), and T-lymphocyte responses [93].
Another important oxidant with a potential role as a microbicidal agent is hypochlorous acid (HOCl). Myeloperoxidase (MPO) promotes the formation of HOCl in phagosomes from H2O2 in the presence of chloride anion (Cl-). MPO is a peroxidase enzyme present at high levels in primary granules of neutrophils [[94], [95], [96]]. HOCl is a potent oxidant and, is highly cytotoxic [[94], [95], [96]].
Since these reactive oxygen species are toxic to cells, antioxidant pathways have evolved to neutralize the molecules. The most common antioxidant machinery in immune cells is comprised of glutathione peroxidases (GPx), superoxide dismutases (SOD), and catalase [97]. First, O2•- is converted to H2O2 through copper zinc-dependent SOD (CuZnSOD) activity or manganese-dependent SOD (MnSOD) [97]. Likewise, the mitochondrial-produced ONOO- can be neutralized by MnSOD. Subsequently, H2O2 is converted into H2O by catalase or in a GPx-dependent manner [97]. Due to the lack of catalase in the mitochondria, the conversion of H2O2 into H2O is dependent on GPx and mitochondrial glutathione (mtGSH). Once GSH is the substrate to GPx, mtGSH is oxidized by GPx to generate oxidized glutathione (GSSG) [97,98]. At the same time, GPx neutralizes H2O2 generating H2O. mtGSH also neutralizes ONOO-/ONOOH and •NO2. Later, GSSG is converted into its reduced form, GSH, by the NADPH-dependent GSSG reductase. GSH is a tripeptide synthesized in the cytosol, and can also be found in the ER, nucleus, and mitochondria [97,99]. Its concentration in the mitochondria is similar to that in the cytosol (10–14 mM) [97,99,100], allowing the detoxification of the oxidants produced during mitochondrial respiration and avoiding mitochondrial damage. In addition to its function as a major antioxidant, GSH can also play a role in immune cell reprogramming, proliferation, and activation [97,[101], [102], [103]]. A recent study showed that GSH can regulate glycolysis and support glutamine metabolism, essential to T cell proliferation [101].
Another class of oxidants involved in both cell damage and immune cell fate decision are the reactive nitrogen species (RNS). Important RNS are nitric oxide (NO•), peroxynitrite (ONOO-) and nitrogen dioxide radical (•NO2). NO• is enzymatically synthesized during the conversion of l-arginine into l-citrulline by NO synthase (NOS) [104,105]. There are three distinct cellular isoforms of NOS: endothelial (eNOS), neuronal (nNOS), and inducible NOS (iNOS) ([106]. NO• can react with O2•- and form ONOO-, a strong oxidant and nitrating agent that can diffuse from the cytosol to the mitochondria [107,108]. ONOO- can react with important mitochondrial components causing oxidation, nitration or nitrosation of proteins. This leads to caspase activation and, consequently, apoptosis [109]. In addition, ONOO- reacts with carbon dioxide, generating •NO2 or spontaneously decomposes to •NO2 and HO•. ONOO- also reacts with a hydrogen ion (H+), generating peroxynitrous acid (ONOOH). Similar to reactive oxygen species, the RNS are toxic to the cells if produced in excess and must be neutralized by complex antioxidant machinery. One component of this machinery is an important antioxidant enzyme called peroxiredoxin-5 (Prx-5). This thiol-dependent monomeric peroxidase has an important role in inflammation [84,110]. Prx-5 has cytoprotective function against endogenous and exogenous peroxides, especially alkyl hydroperoxides and ONOO-, and also H2O2 [110]. The expression of Prx-5 in macrophages and microglia increases after pro-inflammatory stimulation [111,112]. In microglia, oxidants are important agents for their activation and therefore have an important role in neurodegenerative diseases [111]. Park et al. showed that Prx-5 is a negative regulator of excessive mitochondrial fission because Prx-5 inhibits the dephosphorylation of Drp-1 that is induced by the Ca2+/calcineurin pathway [111].
In this sense, the synthesis of oxidants in immune cells is crucial not only to host defense but also for cell signaling, function, and fate decision. Likewise, the neutralization of oxidants is vital to avoid mitochondria and cell damage.
6. Mitochondrial dysfunction and mitophagy
Mitochondrial dysfunction is commonly coupled with the loss of ETC efficiency, resulting in decreased ATP production [113]. However, abnormalities in any mitochondrial process, such as generation and detoxification of mtROS, apoptosis, and decrease in mitochondrial calcium uptake from the cytosol can cause mitochondrial dysfunction [113]. Mitochondrial dysfunction can activate the NLRP3 inflammasome in immune cells [114]. This activation is induced by mtROS or mtDNA, which promotes the secretion of IL-1β and cell death by pyroptosis [115]. Thus, impaired mitochondria function is closely linked to inflammation, neurodegenerative disorders and cancer development [116,117]. One mechanism by which cells prevent mitochondria dysfunction is through mitophagy [118]. This process is a specific form of macroautophagy and involves the removal of damaged mitochondria to maintain the overall mitochondria functionality [118,119]. Mitophagy is crucial because mitochondria are one of the main sources of oxidants and, consequently are overexposed to oxidant-mediated damage [120]. This specific type of autophagy is regulated by two main proteins, PTEN-induced putative kinase 1 (PINK1), localized on the OMM, and Parkin, an E3-ubiquitin ligase localized in the cytosol [121]. After activation, Parkin mediates the polyubiquitination of proteins associated with the OMM, such as Mfn-1 and 2. This results in mitochondria sequestration by the autophagosome [[122], [123], [124], [125]]. Autophagosome engulfs the mitochondria and fuse with lysosomes to degrade damaged mitochondria [118]. This process prevents exacerbated activation of inflammatory signaling pathways [118,126]. The importance of mitochondria integrity and functionality is notable and can be seen by the variety of diseases related to mitochondria dysfunction, such as neurodegenerative (Parkinson, Alzheimer and Huntington's disease), cardiovascular (cardiomyopathy, cardiac hypertrophy) liver diseases (diabetes, fatty liver disease) and cancer [118,126]. Thus, mitophagy is a cell survival mechanism crucial to prevent non-functional mitochondria causing cellular disorders by eliminating dysfunctional mitochondria.
7. Neutrophils
Neutrophils are quick-responder cells during the inflammatory response and belong to the so-called innate immune system. They sense pathogens and quickly respond to stress situations [127]. Chemoattractants induce the migration of neutrophils into inflammatory sites by a series of molecular mechanisms that coordinate cell chemotaxis, involving cytoskeleton and cell membrane rearrangement [128]. Once in the site of inflammation, they recognize, phagocytose and kill microorganisms through different mechanisms, such as degranulation, oxidant production or releasing NETs [129].
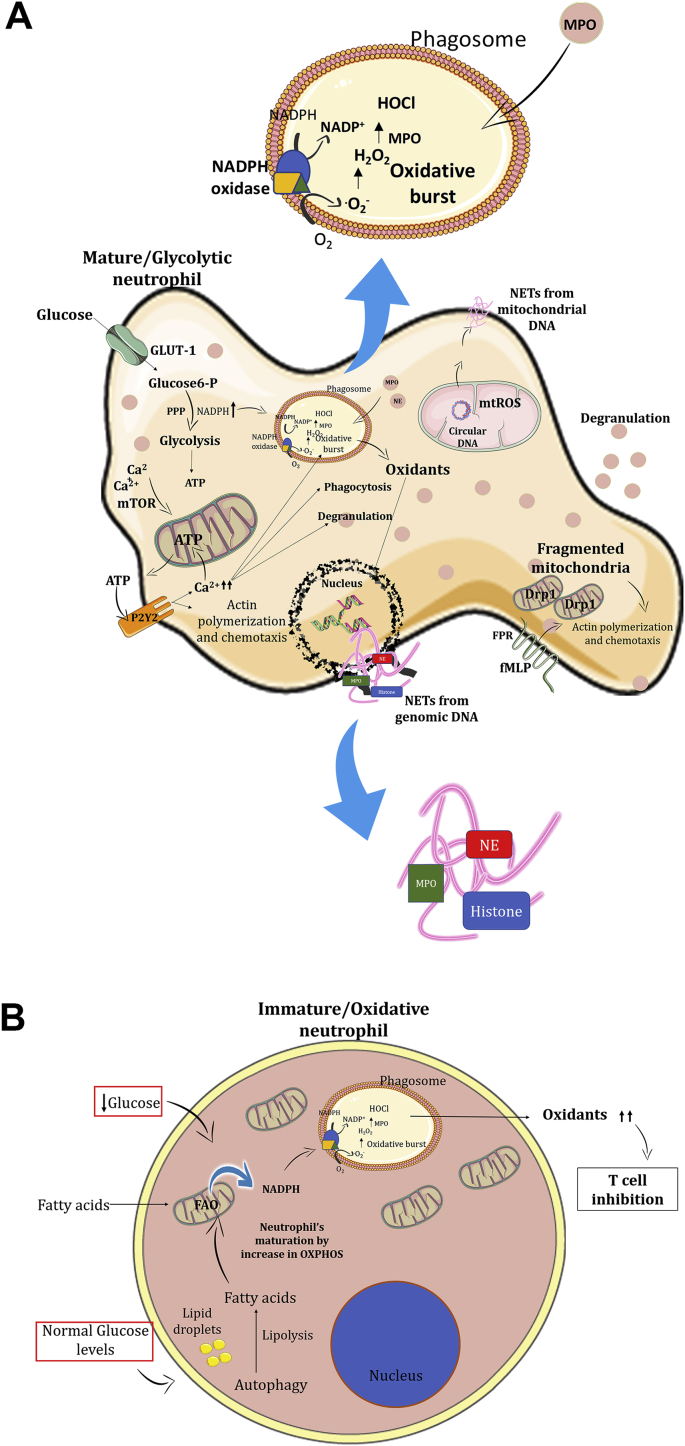
Recently, the importance of neutrophils beyond the acute phase of inflammation has become evident. Neutrophils can modulate the function of cells of both innate and adaptive immunity by secreting soluble mediators or via cell-cell contact [128]. As a result, interest in neutrophil metabolic requirements has grown. However, so far, little is known regarding the metabolic profile of neutrophils as compared to other immune cells. A growing body of evidence suggests that neutrophils contain few mitochondria and are purely glycolytic [[130], [131], [132], [133]]. In contrast, Riffelmacher and colleagues observed that autophagy is crucial for neutrophil maturation since it limits glycolysis and increases OXPHOS [134]. Autophagy is intimately related to neutrophil metabolism and differentiation [[134], [135], [136]]. By using genetically modified mice lacking Atg7 (an essential autophagy protein) in hematopoietic precursors, neutrophils showed an immature phenotype. During granulopoiesis, neutrophils undergo a metabolic shift from glycolysis to free fatty acids (FFAs)-dependent OXPHOS and this metabolic reprogramming was not observed in Atg7-deficient neutrophils [134]. Thus, since the autophagy can promote lipid breakdown through lipolysis to provide FFAs to feed OXPHOS, Atg7-deficient neutrophils accumulate lipid droplets and have reduced FFA levels, remaining immature (Fig. 1A) [134]. Although a previous study suggested that autophagy is not essential for neutrophil maturation [135], Riffelmacher and colleagues comment that difference in the results from the two studies may be related to the use of different mouse strains [134].
Fig. 1.
Immature and mature neutrophils have different metabolic requirements. A. In the presence of normal glucose levels, immature neutrophils undergo autophagy to a proper maturation through FAO associated with OXPHOS. In the same way, during a low glucose levels context (tumoral environment), immature neutrophils prioritize FAO associated with OXPHOS to increase NADPH levels and consequently oxidants production. Excess of oxidants leads to T cell inhibition, contributing to tumor growth. B. Mature neutrophils prioritize aerobic glycolysis and generate NADPH from PPP. NADPH is the Nox2 substrate to produce oxidants inside the phagosome and crucial to eliminate phagocyted pathogens. Oxidants from Nox2 are also important to NETs formation from genomic DNA while mtROS are important to mitochondrial NETs production. Intracellular Ca2+ levels stimulate mitochondrial ATP production by the mTOR pathway. The ATP acts in an autocrine way by binding the P2Y2 receptors in the neutrophils stimulating intracellular Ca2+ mobilization and chemotaxis. Mitochondrial fission also contributes to neutrophil chemotaxis.
Another recent study reinforces that aerobic glycolysis is the preferable metabolic pathway for mature neutrophils [137]. Rice and colleagues showed that immature subsets of neutrophils (Ly6Gint, c-Kit+/CXCR2-) have a higher number of mitochondria and a higher OXPHOS rate after stimulation with phorbol 12-myristate 13-acetate (PMA) or after treatment with a competitive inhibitor of glucose, 2-deoxy-d-glucose (2DG). 2DG competes with glucose to enter into the cells and inhibits the hexokinase, the first enzyme in the glycolysis pathway [137]. Interestingly, cancer patients often have immature subsets of immune cells in the peripheral blood and their neutrophils have increased mitochondrial content and OXPHOS activity [137]. In this report, the authors also showed that oxidative neutrophils use mitochondria to maintain intracellular NADPH levels via FAO and, consequently, the increased production of oxidants by Nox2 (Fig. 1A) [137]. In this context, these oxidative neutrophils could promote tumor progression by inhibiting T cells due to excessive oxidant production, especially in a microenvironment where glucose content is limited [137].
Usually, glycolytic neutrophils produce O2•- by Nox2 via PPP [53,54]. In neutrophils, other oxidants generated in the phagosome, mainly H2O2 and HOCl, are crucial to kill phagocytosed microorganisms [138]. HOCl, as previously mentioned, is a highly reactive product generated by MPO during the oxidative burst. However, oxidants and MPO are not only related to the oxidative burst. They are also essential to NET formation [139]. This most recent neutrophil effector function was uncovered in 2004 by Brinkmann and coworkers when they observed that neutrophils died in a particular way after bacterial stimulation [139]. Classically, NETs are composed of chromatin fibers with intracellular enzymes/proteins - MPO, elastase, and histone (Fig. 1B) [139]. This effector function can immobilize and kill extracellular pathogens or prevent the spreading of microorganisms [139]. The release of NETs occurs by decondensation of nuclear chromatin and the disintegration of nuclear envelope culminating with cellular membrane rupture and extravasation of intracellular content of the neutrophil. This specific type of cell death in neutrophils is called NETosis [140]. It differs from apoptosis and necrosis by its caspase independence, morphological characteristics (NETosis involves plasma membrane rupture, while apoptosis forms membrane blebs) and dependence on oxidants [139,141]. So far, two distinct types of NETosis have described: Nox-dependent and Nox-independent pathways [142]. Nox-dependent NETosis is the classical type of NETs release and is well described after PMA stimulation in vitro [143]. The process starts 1 h after PMA stimulation and requires oxidants production by Nox2. Nox-independent NETosis pathway requires mtROS generation [139,144,145] and an increase in intracellular calcium concentration [142,146,147]. Douda and colleagues observed that calcium ionophore-induced NETosis is rapid (occurs in less than 1 h), is NADPH-oxidase independent, is mediated by small conductance of calcium-activated potassium channel 3 (SK3) and relies on mtROS production [142]. Due to the exacerbated increase in intracellular Ca2+ concentrations (induced by calcium ionophores, for instance), mitochondria produce elevated mtROS levels, which trigger NET formation in the absence of Nox2-derived oxidants [148]. Importantly, in both types of NETosis described above, cellular membrane rupture and neutrophil death occur [139,141,142]. However, a different type of NETs release was suggested by Youssef and colleagues [71]. Using confocal microscopy, they showed that neutrophils stimulated with granulocyte-macrophage-colony-stimulating factor (GM-CSF) and complement component 5a (C5a) remain alive after NETs release [71]. They claim that it is because the chromatin source is not nuclear but mitochondrial [71]. They also demonstrate the dependence of oxidant production for generating mitochondrial NETs as well as in classical NETosis (Fig. 1B) [71]. Recently, the same authors showed that Opa1 is required for ATP production through aerobic glycolysis in neutrophils [149]. Mitochondria-derived ATP is important for microtubule network formation, which is crucial to NETs formation [149]. This suggests that Opa1 is required to release NETs [149].
Regarding the metabolic requirements for NETs release, several studies have shown that NET formation and release is an aerobic glycolysis-dependent process [150,151] and any manipulation that disrupts glycolysis inhibits NETs release. In 2014, Rodríguez-Espinosa et al. suggested a metabolic diversity to NET formation: the early phase, that comprises chromatin decondensation, is not strictly dependent on exogenous glucose. However, exogenous glucose and the aerobic glycolysis are necessary for the late phase that comprises the release of web-like structures [151].
Although mitochondria and cell metabolism play a role in NETs release, they are also important in well-described neutrophils functions, such as phagocytosis, degranulation, and chemotaxis. Recently, Bao and colleagues demonstrated that mitochondria-derived ATP is transported extracellularly and activates purinergic receptors, such as P2Y2, in an autocrine manner, resulting in neutrophil activation [152,153]. This activation is mediated by an increase in intracellular Ca2+ levels leading to an amplification of mitochondrial ATP production [152,153]. Increased ATP production provides positive feedback of ATP binding to P2Y2 and sustains the neutrophil oxidative burst, degranulation, and phagocytosis (Fig. 1B) [152,153]. Mitochondrial ATP burst can be regulated by the mammalian target of rapamycin (mTOR) signaling pathway, which controls mitochondrial Ca2+ uptake [153]. The inhibition of mTOR complex 1 (mTORC1) or both mTORC1 and mTORC2 limits mitochondria-derived ATP production and consequently neutrophil chemotaxis [153]. Recently, a study using a zebrafish model indicated that a mitochondrial network plays an indispensable role in the regulation of neutrophil motility in vivo [154]. Using a transgenic zebrafish lineage, they disrupted the mtDNA polymerase specifically in neutrophils and observed a reduced velocity in neutrophil interstitial migration [154]. One of the consequences of mtDNA polymerase dysfunction is the loss of the ETC proteins that are encoded by mtDNA. To demonstrate that this is due to the loss of ETC function they used specific inhibitors to disrupt the mtROS production, such as rotenone (complex I) and antimycin (complex III) and observed inhibition of neutrophil motility [154]. Consistent with the participation of mitochondria in neutrophil chemotaxis, another research group suggested that mitochondrial dynamics are involved in neutrophil's actin polarization and chemotaxis [155]. They showed that the activation of the mitochondrial calcium uniporter (MCU), a well-known Ca2+ transporter located in the IMM, enhances the polarization and chemotaxis of neutrophils stimulated by N-formylmethionine-leucyl-phenylalanine (fMLP) or IL-8. In the same way, Ru360, an MCU inhibitor, decreases and even abrogates neutrophil polarization and chemotaxis [155]. Analysis of mitochondrial morphology using a MitoTracker® Red probe coupled confocal analysis showed that in fMLP-stimulated neutrophils the mitochondrial anatomy changed from elongated/fused (unstimulated) to fragmented [155]. The treatment of neutrophils with Ru360 prevented mitochondria fragmentation and, consequently, neutrophil polarization and chemotaxis [155]. Treatment with the Drp1 inhibitor MDIVI-1 abrogated neutrophil polarization and chemotaxis, confirming the role of mitochondrial dynamic in these mechanisms (Fig. 1B) [155].
Neutrophils modulate their own metabolism in accordance with environmental cues. Recent studies have highlighted the importance of mitochondria in several neutrophils functions, including chemotaxis. Certainly, there is much more to be discovered but these recent studies using novel technologies have enhanced our understanding of the immunometabolism of these cells.
8. Macrophages
Macrophages are immune cells derived from the yolk sac and fetal liver during the early stages of development and from bone marrow-derived monocytes after birth [156]. The majority of the adult tissue macrophages originate from embryonic precursors [156]. However, during immune responses bone marrow-derived monocytes can migrate from the blood into the tissues, where they acquire specific macrophage profiles and intermingle with resident macrophages to remove the foreign material, depending on the cytokine milieu found in the inflammatory microenvironment [157]. The majority of the studies focusing on macrophage immunometabolism have been done in vitro using macrophage colony-stimulating factor (M-CSF or CSF-1) to differentiate bone-marrow progenitor cells into macrophages (also known as M0). Differentiated macrophages can be divided into two important classes. (i) M1 or classically activated macrophages are associated with tissue proinflammatory responses and microbial killing. They are induced in vitro by treating M0 macrophages with LPS and interferon-γ (IFN-γ). (ii) M2 or alternatively activated macrophages are associated with resolution of inflammation, wound healing and resistance to helminth infections [[158], [159], [160]]. They are induced in vitro by IL-4.
8.1. M1 macrophages
During tissue injury, foreign molecules or pathogens (bacteria, virus or toxins) are recognized by resident innate cells (mainly dendritic cells and macrophages). This leads to the secretion of cytokines and chemokines to recruit additional inflammatory monocytes from the blood. These monocytes recognize infectious microorganisms-related molecules (e.g., LPS) and inflammation-related cytokines (TNF-α, IL-1β and/or IFN-γ) in the milieu, and then differentiate into M1 macrophages. These cells produce inflammatory cytokines (IL-1β, TNF and IL-6) and oxidants (NO and ROS). One important marker of M1 activation in vitro is an increase in iNOS and consequently production of NO (see Oxidants production: a double-edged sword). Another important source of oxidants is the mitochondria recruitment to phagosomes to promote the delivery of oxidants to augment the bactericidal activity of macrophages [161].
Although the proinflammatory profile of M1 macrophages was characterized decades ago, only recently have the metabolic pathways involved in this activation been examined. One of the firsts established findings was that M1 macrophages have increased glucose uptake, mostly by increasing the transcription and translocation of glucose transporter 1 (GLUT1) to the plasma membrane [162,163]. The uptake of glucose is associated with increased glycolytic flux. However, interestingly, M1 macrophages use glycolysis to produce lactate, a process named aerobic glycolysis [164]. The importance of glycolysis for M1 macrophages has been presented in several reviews [see Ref. [165]], therefore we will focus on the mitochondrial aspects involved in classical activation.
Although most of the pyruvate is converted to lactate, a portion goes to the mitochondria to feed the TCA cycle. However, in M1 macrophages the metabolic intermediates citrate and succinate accumulate due to two interruptions in enzymatic reactions resulting in breaks in the TCA cycle. The breaks are a consequence of downregulation of both isocitrate dehydrogenase (IDH) and SDH. The accumulation of citrate was identified as a consequence of downregulation of IDH, the enzyme that catalyzes the conversion of isocitrate to α-ketoglutarate [166]. This metabolite can be transported to the cytosol, where will be used for the production of oxidants, prostaglandins and fatty acids [42,167]. Alternatively, it can be used to synthesize itaconate [167], a metabolite with antibacterial properties described in the 1970s [168]. However, recently it has been shown that following LPS stimulation itaconate can leave the mitochondria and modify the Kelch-like ECH-associated protein 1 (KEAP1) via alkylation. This enables the nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2) to initiate an anti-inflammatory program and induce the expression of anti-oxidant genes [169]. It is interesting to observe that itaconate acts as a negative-feedback loop in the inflammation induced by LPS, acting mainly in late time points. Furthermore, itaconate can also inhibit SDH, leading to decreased fumarate levels and succinate accumulation (the second break) [161]. It is important to point out that during M1 polarization, the main source of succinate is glutamine-dependent anaplerosis [41]. As previously discussed, when succinate is in the cytosol, it attenuates the degradation of HIF-1α by prolyl hydroxylases. Macrophages treated with succinate have increased HIF-1α levels, enhancing its migration into the nucleus resulting in increased transcription of IL-1β and glycolytic genes, such as the Ldh subunit a, pyruvate kinase isozyme M2 (Pkm2), hexokinases I and II, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 enzyme (Pfkfb3) [38,41,42] (Fig. 2A).
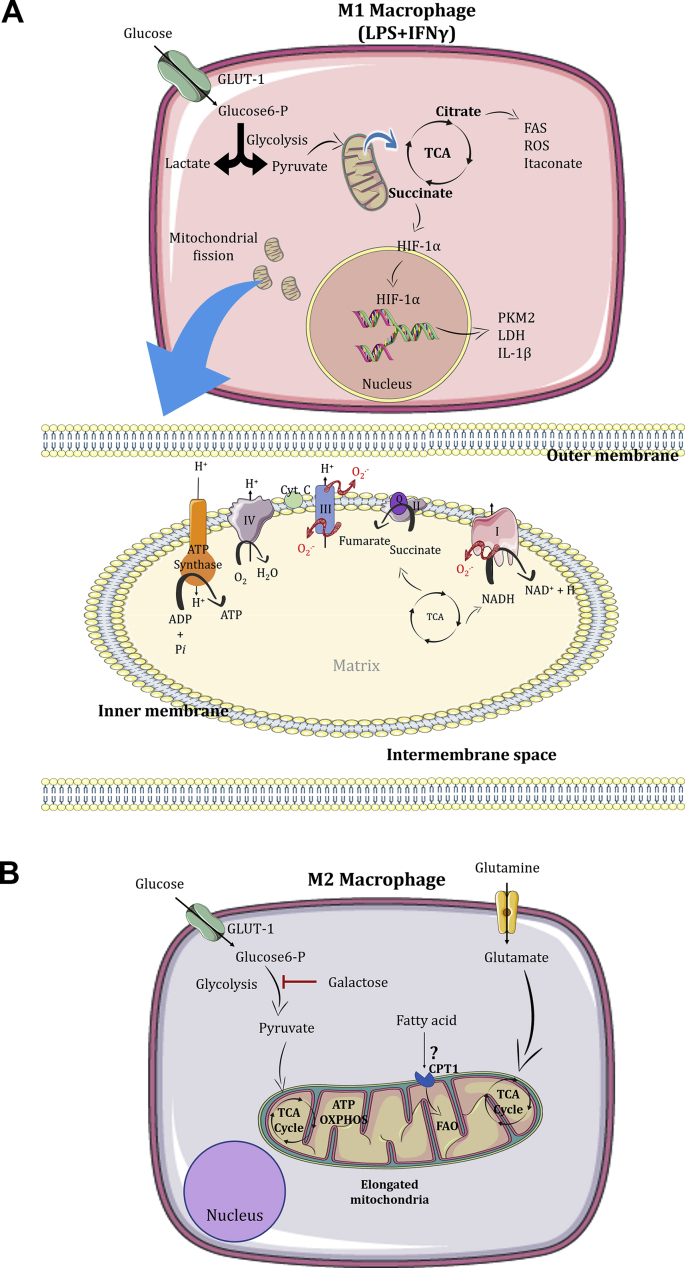
Fig. 2.
Metabolism of macrophages. A. Under LPS and IFN-γ stimuli, macrophages preferentially use glucose to produce lactate. In the mitochondria, citrate can be exported and used to produce fatty acids and itaconate. The mitochondria increase the production of oxidants and succinate, which are capable to stabilize the transcription factor HIF-1α in the cytosol, triggering the transcription of HIF-1α-target genes. The M1 macrophage is characterized by mitochondria fission. B. Metabolism of M2 macrophages. Alternatively activated macrophages (M2) use glucose and glutamine to feed the TCA cycle and produce ATP by oxidative phosphorylation. These cells are known to have elongated mitochondria, and consequently more efficient energy production. The use of fatty acid oxidation and glycolysis by M2 macrophages are under review in the literature.
Upon M1 stimuli, the protons that are pumped out from complexes I, III and IV of the ETC are not used to produce ATP by ATP synthase [170]. Interestingly, the activity of complex I, II and IV are decreased during M1 polarization, leading to an increase in the ΔΨm [170,171]. The consequence of complex II (SDH) suppression is the accumulation of succinate along with high proton motive force that leads to a reversal on the electron flow at complex I, in a process referred to as reverse electron transport (RET) [37] (Fig. 2A). Succinate accumulation and RET leads to an increased production of oxidants, which is known to stabilize HIF-1α [170]. Similar to the effects observed with itaconate treatment, pharmacological inhibition of SDH using dimethyl malonate (DMM) blocks RET mtROS production, HIF-1α stabilization and induction of IL-1β, while increasing anti-inflammatory gene expression [170]. Furthermore, a study using M1 macrophages with a specific knock-out of immune responsive gene 1 (Irg1), a mitochondrial-associated enzyme that converts cis-aconitate to itaconate, observed more HIF-1α stabilization, and production IL-1β than in the M1 from WT mice [42]. These results raise the possibility that HIF-1α - IL-1β axis is a consequence of the efficiency and directionality of the ETC rather than signaling through succinate accumulation.
Although the majority of the literature has used macrophage cell lines or bone marrow-derived macrophages to describe the interplay between metabolism and macrophage function, the extent to which these findings translate into the diverse spectrum of macrophage phenotypes in vivo remains to be defined. Artyomov et al. presented an extensive comparison between M1 bone marrow-derived macrophages and peritoneal macrophages [69]. Both macrophages are highly glycolytic upon LPS treatment [69]. However, peritoneal macrophages also display increased OXPHOS activity, using the high levels of acetyl-CoA available to supply the TCA cycle. This is not observed in bone marrow-derived macrophages [69].
8.2. M2 macrophages
The induction of M2 macrophages is observed under several conditions, such as helminthic infection and allergy. In addition, when a tissue injury occurs, these cells are responsible for the resolution phase. They mediate wound healing by the secretion of anti-inflammatory cytokines and by promoting the migration and proliferation of matrix cells, in a mechanism not totally understood [172]. In each response mentioned above, groups of immune cells (e.g. eosinophils, mastocytes, and T helper 2 cells) secrete type 2 cytokines, such as IL-4, and IL-13. It was recently established that IL-4 and M-CSF drive mTORC2 activation and phosphorylation/activation of the signal transducer and activator of transcription 6 (STAT6), which in turn induces interferon regulatory factor 4 (IRF4) and STAT6 M2 markers [173].
The initial metabolism studies described that M2 macrophages prefer FAO to fuel mitochondrial activity [174,175]. However, this concept was recently challenged by investigations describing that FAO is dispensable for M2 macrophage polarization [49,176]. Importantly, researchers frequently use 200 μM of etomoxir to inhibit FAO [49]. At this high concentration, etomoxir was shown to also affect adenine nucleotide translocase (an enzyme that catalyzes ADP/ATP exchange across the IMM) and complex I of the ETC. In addition, excess etomoxir leads to the formation of etomoxiryl-CoA, which depletes intracellular free CoA levels, thus blunting cell metabolism [49]. At low concentrations (<3 μM), etomoxir still inhibits CPT-1 but does not affect these other metabolic processes. Divakaruni et al. also showed that in macrophages lacking both CPT1 and CPT2 proteins, etomoxir did not affect M2 polarization, indicating that FAO is dispensable for M2 polarization [49] (Fig. 2B). We speculate that M2 macrophages use FAO to maintain their metabolism after polarization. This recently published data started a new chapter in M2 macrophages polarization and metabolism and other groups will need to investigate the real necessity of FAO for M2 polarization and review the concentrations of classical modulators and their off-targets effects.
The second wave of studies showed that glucose can also fuel OXPHOS in M2 macrophages. Interestingly, after 16–24 h of stimulation with IL-4, the glucose consumption is increased in these macrophages. In 2016, two important studies showed that different subunits of mTOR are important for the upregulation of glucose. Covarrubias et al. showed that IL-4 induces an increase in glucose uptake in a time-dependent manner, in which AKT and mTORC1 act in parallel to support this change and are essential regulators of M2 markers. Huang et al. showed that mTORC2 and IRF4, but not mTORC1, are responsible to enhances glucose utilization in M2 macrophages [173,177]. To test this hypothesis, these two groups and the majority of the studies in the literature have used 2DG as a readout for glycolysis inhibition [173,177]. After these studies, Wang et al. showed that the doses of 2DG used also have important off-target effects, altering not just glycolysis, but also OXPHOS and ATP levels [178]. They showed that pre-treatment of bone marrow-derived macrophages with 2DG blocks the STAT6 signaling pathway induced by IL-4, which is required for M2 macrophage polarization [178]. To better understand the effects of glycolysis inhibition, the authors used macrophages cultured with IL-4 in either glucose-free medium or containing galactose instead of glucose as the main carbon source (which render glycolysis functionally ineffective, with no ATP produced). They observed that the absence of glycolysis does not affect the expression of M2 markers, OXPHOS, and intracellular ATP levels [178]. It is important to point out that glutamine can fuel the TCA cycle, and it has been shown that under glucose depletion or the presence of galactose, M2 macrophages utilize this metabolite [178]. These results indicate that both aerobic glycolysis and glucose-derived acetyl-CoA are not required for M2 polarization (Fig. 2B). Further, they highlight the importance of cautious data interpretation with the use of 2DG in the literature regarding M2 polarized macrophages.
One of the most important functions of macrophages is the clearance of apoptotic cells. While the mechanisms related to phagocytosis of apoptotic cells are well known, Wang et al. demonstrated that mitochondrial fission occurs during this process. Drp1 is essential for degradation of the apoptotic cells in phagolysosomes [179]. Indeed, Escoll et al. used Legionella pneumophila, a Gram-negative intracellular bacterium, to infect human macrophages and showed that the presence of type IV secretion system triggers mitochondrial fission in a mechanism that is dependent on Drp1 [180]. Although the range of stimuli in macrophages is extensive, the literature has shown that M1 phenotype accumulates fragmented mitochondria and M2 macrophages elongated mitochondria [73] (Fig. 2).
8.3. Resident macrophages
The milieu in which macrophages are found can directly impact their metabolism. In the context of a tumor microenvironment, cancer cells produce large amounts of lactate, as the final product of aerobic glycolysis. Lactate induces HIF-1α expression and M2 polarization of macrophages thus promoting tumor growth [181]. Moreover, during obesity, adipocytes release pro-inflammatory factors like C-C motif chemokine ligand 2 (CCL2), TNF and FFAs that induce the recruitment and activation of adipose tissue macrophages [182]. Adipose tissue macrophages acquire a “metabolically activated” phenotype after high-fat diet-induced obesity [183]. This is accompanied by increased glycolysis and oxygen consumption in comparison to lean adipose tissue macrophages [184]. The production of proinflammatory factors by macrophages amplifies an inflammatory pathway that blocks insulin action in adipocytes, and consequently contributing to insulin resistance [185,186].
Although the metabolism of these distinct phenotypes of macrophages has been extensively studied, the metabolism adaptation of tissue-resident macrophages is still poorly understood. However, mitochondria occupy a central place during macrophage polarization, controlling metabolism and signaling pathways in this cell. Future studies are necessary to delineate how mitochondria contribute to the metabolism of these cells during activation.
9. Dendritic cells
DCs are specialized cells that recognize pathogens via pattern recognition receptors (PRRs), phagocytose microorganisms, process antigens and present antigens via major histocompatibility complex to activate T lymphocytes. The efficiency of these processes depends on the ability of DCs to migrate from the tissue to the tissue-draining lymph nodes through the lymphatic vessels [39,187]. DCs can be generated in vitro by treating human peripheral blood monocytes with GM-CSF and IL-4 or treating mouse bone-marrow-derived cells with only GM-CSF(39). Although there is a variety of DCs in tissues, the majority of the studies on DCs differentiation have been performed by using these in vitro approaches [39].
The activation of DCs with TLR agonists leads to increased glucose consumption via aerobic glycolysis [164,188]. It is important to point that, as in macrophages, the majority of the literature uses high doses of 2DG to inhibit glycolysis and therefore this glycolytic dependency will likely be reassessed in the next few years. However, it is known that the acetyl-CoA derived from glycolysis induces the production of citrate in the mitochondria. In DCs citrate is exported to the cytosol via mitochondrial citrate carrier Slc25a1 [189]. Once in the cytosol, citrate accumulation together with higher levels of PPP-derived NADPH increases FAS [39] (Fig. 3). FAS is required to fulfill the requirements of the endoplasmic reticulum and Golgi to support the high demand for protein synthesis and secretion [40].
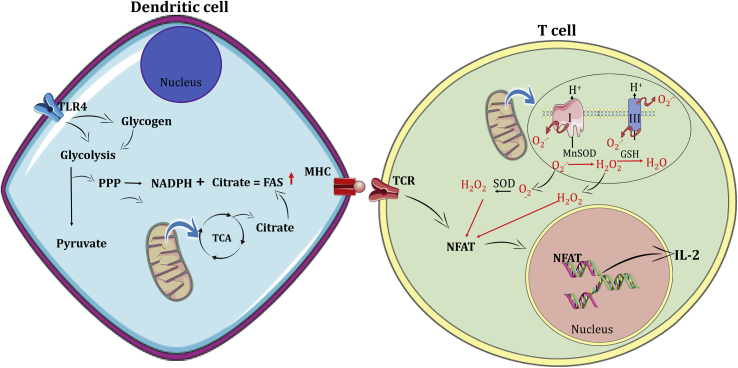
Fig. 3.
Metabolism of dendritic cells and T lymphocytes. Inflammatory signals drive glycolysis, increasing glucose uptake and glycogenosis in DCs. The conversion from glucose to pyruvate increases citrate levels in the mitochondria, which can be exported to the cytosol. The PPP generates NADPH, used in the FAS. FAS are used to generate membranes to the endoplasmic reticulum and Golgi complex, in an NADPH-dependent manner, both necessary to increase protein synthesis. Dendritic cells process antigens and present them using the MHC at the cell surface where they can be recognized by the TCR. This event engages ROS production, which facilitates the activation of nuclear factor of activated T cell (NFAT) activation. This transcription factor induces IL-2, a T cell growth cytokine.
Although some of the glucose is used to produce FA, DCs also use OXPHOS in the early stages of activation [190]. It is known that TLR activation of DCs have increased expression of iNOS and consequently production of NO [191]. However, NO is a potent inhibitor of the complex IV of the ETC [191], consequently decreasing OXPHOS-generated ATP. Interestingly, 24 h after stimulation, DCs have increased NO production, reduced TCA cycle activity and lower levels of oxygen consumption, but increased glycolytic capacity (Warburg metabolism) [191]. A recent finding showed that DCs have intracellular glycogen stores that support the early steps of TLR (one PRR subtype) activation [192]. In the first 3 h of activation, the breakdown of glycogen stores increases the oxygen consumption rate (OCR) and citrate accumulation in the cytosol to fuel FAS. After this early stage of activation, DCs decrease OXPHOS activity [191,192] (Fig. 3). Thus, the changes in DCs metabolism are supported by intracellular stores of glycogen associated with glucose import. The increase in NO production is also an essential step for the degradation of pathogens by DCs.
Despite the fact that OXPHOS is important for the early stages of activation, mitochondria have been showed to be essential for DCs differentiation. The inhibition of mitochondrial respiration in monocytes by the ETC inhibitor rotenone blunts DCs differentiation induced by GM-CSF [193,194]. One important aspect of DCs development is that during the initial stages (3 days after GM-CSF stimuli), DCs present lower levels of oxidants and are highly responsive to TLR stimulation as assessed by the upregulation of activation markers (such as CD80, CD88) and induction T cell proliferation upon antigen presentation [195]. In contrast, 6 days after GM-CSF treatment, DCs have higher levels of oxidants, increased release of H2O2 and decreased capacity to stimulate T cell [195]. This indicates that mitochondria have a central role during DCs differentiation [193,194].
In general, there are three major DCs subtypes: classical DCs, monocyte-derived DCs and plasmacytoid or tolerogenic DCs. These subtypes may have different metabolic demands [39,187]. Tolerogenic DCs, which are commonly found in tumors where they function to inhibit T cell function to favor tumor progression, use lipid metabolism to fuel OXPHOS and produce the necessary ATP [196,197]. In the same way, antigen presentation by plasmacytoid DCs is regulated by mitochondria through a ROS-dependent mechanism. The reduced mtROS levels in these cells decrease their cross-presentation capacity to generate CD8+ T-cell responses in vivo [198].
Little is known about mitochondrial dynamics in DCs. Ryu et al. showed that GM-CSF-induced immature DCs have increased levels of Mfn2 and Opa1 [199], suggesting that before differentiation DCs have fused mitochondria. On the other hand, Del Prete et al. described that after LPS-induced differentiation and activation, DCs have an increased number of condensed mitochondria [194]. However, the mechanisms by which mitochondria dynamics influence antigen presentation is still not elucidated.
For us, it is clear that little is known about DCs metabolism, because the literature has focused on the effects of TLR activation. However, DCs present a great diversity and a range of different PAMPs are recognized by them. Even though mitochondrial metabolism is remarkably important for them, new studies are necessary to better understand how mitochondria morphology and metabolism influence the function of DCs during immune responses, particularly in the in vivo context.
10. T lymphocytes
T lymphocytes originate from bone marrow-derived hematopoietic stem cells and migrate to the thymus, where beta chain rearrangement and selection happens. After this stage, it is known that double-positive thymocytes have increased expression of Glut1, indicative of increased glycolysis [200]. During the early stages of development, T lymphocyte metabolism has been studied only after positive and negative selection checkpoints. After these maturation steps, T lymphocytes migrate to the periphery. These naïve T lymphocytes can be CD4+CD8- (also called as helper T cells) or CD4-CD8+ (or cytotoxic T cells). Naïve T lymphocytes express low levels of the activation marker CD44 and high levels of the lymph node homing receptors CD62L and CCR7 [201].
APCs expressing processed peptides migrate to the lymph nodes, where they encounter naïve T lymphocytes. The interaction between the cells promotes T cell activation and a rapid metabolic shift from OXPHOS towards aerobic glycolysis in a mechanism dependent on increased activity of pyruvate dehydrogenase kinase 1 that inhibits pyruvate transport to the mitochondria [202]. TCR activation along with CD28-mediated co-stimulation leads to a rapid increase in Glut1 expression and consequent glucose uptake [47,203,204]. Indeed, it was shown that T cell activation using both anti-CD3 and anti-CD28 antibodies switches T lymphocytes metabolism from FAO and pyruvate oxidation via the TCA cycle to aerobic glycolysis, PPP and glutamine oxidation [205].
Activated CD4+ T lymphocytes can be differentiated into distinct helper T cells. There are four major subsets of CD4+ cells: Th1, Th2, and Th17 are effector T cells, while the Treg prevents autoimmunity and exacerbated immune responses. It is well accepted that Th1, Th2, Th17, and cytotoxic CD8+ T lymphocytes use aerobic glycolysis to promote their effector function and differentiation [38]. Increased aerobic glycolysis generates metabolites that are substrates for nucleotide, amino acid, and lipid biosynthetic pathways to produce molecules required for cell division [206,207].
As presented above, glycolysis is essential for effector T cells action. However, it was shown that T lymphocytes can use glycolysis or OXPHOS to promote cell proliferation and survival of naïve T cell [209,210]. CD3/CD28 stimulation in glucose-free medium and using pyruvate or α-ketoglutarate to feed the TCA cycle was sufficient to induce T cell activation [211]. While Th2 and Th17 cells depend on glutamine metabolism to enrich TCA cycle intermediates and to promote differentiation, in Th1 cells it does not occur [212,213]. On the other hand, in Th1 cells, glutaminolysis is essential to promote IFN-γ production, which suggests the importance of the TCA cycle not in differentiation, but in effector function [210]. Furthermore, the blockade of ATP synthase is sufficient to abrogate the proliferation of T lymphocytes [210]. Therefore, the ATP produced by mitochondria is sufficient to support T cell metabolism [211]. It is also known that Treg depends on higher levels of OXPHOS and FAO, with decreased glycolytic flux, compared to Th17 cells [47]. t is interesting to observe that inhibition of FAO by etomoxir did not affect effector T lymphocyte proliferation but impaired Treg differentiation [48,208]. However, it is important to point out that, as in macrophages, the use of etomoxir in T lymphocytes is under review in literature. Raud et al. used genetic animal models with CPT1a specifically deleted in T lymphocytes to show that FAO was not necessary for effector and memory T lymphocytes or Treg development [50].
The formation of membranes are essential for T cell proliferation and immunological synapses between Th cells and APCs. In this context, FAS is also important for T cell development. At the immunological synapse, a cytoskeletal-dependent mitochondrial redistribution occurs to allow for immunological synapse formation. Calcium influx increases in these mitochondria close to immunological synapse, allowing calcium-dependent activation and clonal expansion in Th lymphocytes [211,212]. The deletion or inhibition of acetyl-CoA carboxylase 1, the enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA impairs Th17 and CD8+ T cell induction and proliferation [[213], [214], [215], [216]]. However, to our knowledge, the role of FAS in other T subsets is not clear. Interestingly, FAO is preferentially used by non-inflammatory and tolerogenic immune cells, whereas FAS is predominantly observed in inflammatory adaptive cells.
Although adaptive immune cells do not use ROS for their effector functions, these molecules can act as signaling messengers in several pathways. During TCR cross-linking mtROS are generated within 15 min [210,217]. During T cell activation, low, physiologically relevant levels of ROS are generated, i.e., an H2O2-mediated oxidative signal, which facilitates activation of the ROS-dependent transcription factors NF-kB and NFAT, both essential for T cell activation [12,218,219]. This oxidative signal is indispensable for T cell activation [12,217,220]. Different enzymatic sources, such as the respiratory chain [[218], [219], [220], [221]], lipoxygenases [222], Nox2 and dual oxidase 2 [223,224], have been described as participating in T cell activation-triggered ROS production. In a series of important studies, Kaminski et al. showed that TCR-induced PKC activation drives ETC complex-I-mediated mtROS production. The O2•- released into the mitochondrial matrix is converted into H2O2 by MnSOD. This H2O2 diffuses into the cytoplasm to act as an oxidative signal mediator that is essential for T cell activation-induced gene expression (e. g., IL-2, IL-4, CD95 ligand) [[218], [219], [220],225] (Fig. 3). The production of mtROS from complex III of the ETC is also important for T cell signaling in CD8+ and CD4+ T lymphocytes in vivo [210]. Even though the role of mtROS has been explored, future studies will be needed to clarify the targets of mtROS during T cell activation.
Following activation of an immune response, a large number of lymphocytes can be found secreting high quantities of cytokines and chemokines. This can be dangerous to the host tissues if these cells are not finely regulated. The reduction in the antigen load leads to apoptosis of effector T cell by two mechanisms: (a) the “extrinsic pathway” that is dependent on death receptors (e.g. TNF receptor family) and leads to cleavage of caspases 8 and 10. (b) or the “intrinsic pathway” that is triggered through damage to mitochondrial membranes, endoplasmic reticulum stress and initiator caspase 9 [226]. The majority of the effector T lymphocytes undergo apoptosis, while memory T lymphocytes survive and restore their lipid oxidation metabolism [201]. Memory CD8+ and CD4+ T lymphocytes display increased mitochondrial number and spare respiratory capacity, which is the maximal mitochondrial respiratory capacity available to a cell to produce energy under conditions of increased work or stress [48,227,228]. Interestingly, CD8+ memory T lymphocytes do not take up external FA. Rather, these cells synthesize FA by using glucose-derived carbon, which is incorporated into triacylglycerol TAG in the ER and stored as neutral lipids in lysosomes. The lysosomal lipolysis can be used to generate FA for FAO [229]. This work was the first to describe the futile metabolic cycling of FAS and FAO in immune cells [229].
One important aspect of T lymphocyte differentiation is mitochondrial dynamics. T cell activation increases the mitochondrial mass and ΔΨm [230]. Buck et al. showed that the mitochondria of memory CD8+ T lymphocytes were elongated [74]. The same article showed that the deficiency of OPA1 causes defective development of memory T cell in vitro and in vivo and that this is accompanied by decreased OXPHOS [74]. One important aspect of mitochondria fusion is that even in effector T lymphocytes culture conditions, the induction of fusion confers a memory T cell phenotype. In contrast, effector T lymphocytes show fission of mitochondria [74]. Based on these observations, we speculate that mitochondrial dynamics can define T lymphocyte fate. Regarding the mitochondrial dynamics during CD4+ T cell development, more studies are necessary to determine the underlying mechanisms. For example, little is known about T cell mitochondrial dynamics in vivo and how the immune response modulates the metabolism of these cells.
The metabolism of the most studied CD4+ and CD8+ cells have many questions to be solved. Another important aspect is that T lymphocytes are plastic, therefore understanding the metabolism of these various T lymphocyte subsets is essential to the next steps in the treatment of several diseases.
11. B cells
B cells are components of the adaptive immune response. In addition to being derived from lymphoid-committed precursors in the bone marrow, B cells share other features with T cells such as antigen recognition through individual antigen receptors (B cell receptor or BCR), coordinated developmental progression, costimulatory requirements for activation and the generation of memory cells [231]. However, the stages of B cell development, mechanisms of activation, effector functions and transcriptional profile are quite different from those found in T cells. B cells can act as APCs and respond to infectious pathogens earlier than T lymphocytes since they have functional PRRs (e.g, TLRs and Nod-like receptors), although the subtypes of these PRRs can differ between mice and humans [232,233]. More recently, human B cells have been described as capable of releasing mtDNA into the extracellular environment after treatment with the TLR9 agonist class C CpG in a dose-dependent manner (Fig. 4A) [70]. This B cell-derived webs have different morphology, composition, and function from those produced by neutrophils and are essentially responsible for activating anti-viral responses via induction of type I IFNs. Interestingly, the web releasing process is independent of the BCR, TLR9, cGAS/STING, AIM-2 and oxidant signaling pathways [70].
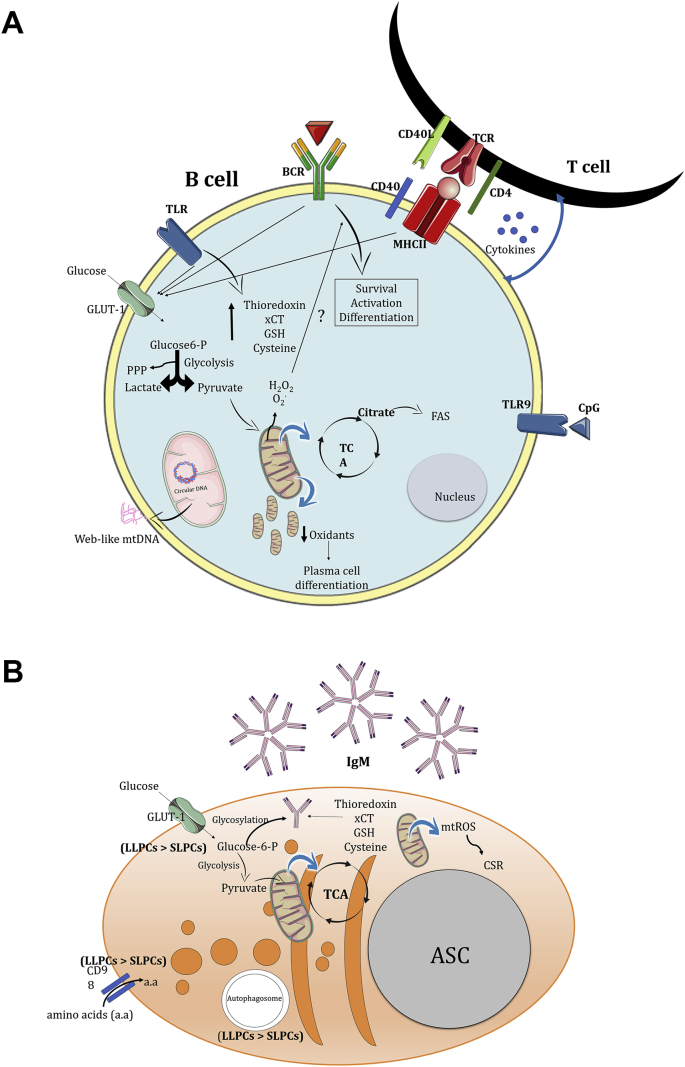
Fig. 4.
Essential metabolism and mitochondrial regulation on B cells (A) and Antibody-Secreting Cells (B). A. B cells are activated through T cell-independent or -dependent mechanisms. B cell activation increases both aerobic glycolysis and OXPHOS rates, enhancing the expression of the glucose transporter Glut1 and mitochondrial mass. Part of the TCA cycle fueling is used to de novo lipid biosynthesis via citrate transport from the mitochondria into the cytoplasm. B cell activation promotes both oxidants and antioxidants production, which the balance regulates B cell survival, effector functions and cell fate. Higher mtROS levels are important for sustaining both mitochondrial activity and mass, activating the CSR. On the other hand, lower mtROS levels were directly associated with plasma cell differentiation. Moreover, B cell activation also increases the mitochondria number and change their anatomy from an elongated to a rounded shape. Recently, CpG-stimulated TLR9 in B cells causes mtDNA releasing into the extracellular environment and its associated in stimulating anti-viral responses via type I IFN production. B. ASCs have high metabolic demand in order to produce antibodies. Part of the glucose is used to glycosylate antibodies and intracellular membrane expansion. Higher antioxidant activity is associated with increased IgM secretion by ASCs. Different subtypes of plasma cells show different metabolic profiles. LLPCs show increased glucose uptake, CD98 amino acid transporter expression and autophagosome mass compared to SLPCs. a.a: amino acids.
ASC: Antibody-Secreting Cell, BCR, B Cell Receptor, CSR, Class-Switch Recombination, FAS, Fatty Acid Synthesis, GSH, Glutathione, LLPCs, Long-Lived Plasma Cells, OXPHOS, Oxidative Phosphorylation, PPP, Pentose Phosphate Pathway, SLPCs, Short-Lived Plasma Cells, TCR, T Cell Receptor, TLR, Toll-Like Receptor, TCA, Tricarboxylic Acid Cycle, xCT, Cystine/glutamate transporter.
Although B cells perform a variety of functions as stated above, they are well known for their ability to produce and secrete polyreactive and antigen-specific antibodies after differentiating into plasmablasts and plasma cells. They are collectively referred to as antibody-secreting cells (ASCs) [234]. There are five major antibody isotypes (IgM, IgG, IgA, IgD, and IgE), whose structures and functions have been reviewed elsewhere [235].
B cells are activated through T cell-independent or -dependent mechanisms. The former includes the participation of pleiotropic cytokines (IL-6, TNF, IL-1) and the recognition of PAMPs via PRRs (e.g., TLR4, TLR7 or TLR9). T-dependent B cell responses occur when B cells obtain help from CD4+ T cells, generating more specific antibody responses. T cells are primed by recognizing antigens presented by B cells, which in turn, further activate the cognate B cell [234]. Activation of the PI3K-Akt-mTORC1 axis is important to generate ASCs in a process that is also dependent on mtDNA replication, mitochondrial remodeling, and metabolic switching. The β isoform of protein kinase C (PKCβ) and the R-Ras2 (a Ras superfamily GTPase) are essential to activate mTORC1 after BCR triggering [236,237].
B cell subsets include B1 and B2 cells. The B1 cells (also subclassified into B1a and B1b B cells) populate the pleural and peritoneal cavities, and the mucosal system. They secrete polyreactive antibodies and are primarily derived from fetal liver precursors, although the bone marrow can produce some B1 cells with less efficacy throughout life [238,239]. The limited de novo production of B1 cells may be a putative consequence of their evolutionary advantage of self-renewal in the periphery by mechanisms partially dependent on autophagy [240].
B2 cells are subdivided into follicular (FO) and marginal zone (MZ) B cells and are continuously produced in the bone marrow [239,241]. Committed B cell lineage precursors in the bone marrow (e.g. early pro-B cells, late pro-B cells, large pre-B cells, and small pre-B cells) have high metabolic rates but show some differences at each developmental stage. This includes distinct glucose uptake rates, oxidant production and mitochondrial mass [242]. These metabolic dynamics are tightly regulated by pre-BCR signaling (an important checkpoint at the pro-B/pre-B transitional stage), which its activation causes the highest glucose uptake and oxidants production, but no change in mitochondrial mass [242]. Furthermore, it has also been shown that any metabolic disturbance during the earliest stages impairs B cell development [242]. After leaving the bone marrow, immature B cells reach the secondary lymphoid tissues to complete their maturation process.
FO B cells are recirculating cells expressing monoreactive BCRs and home to the follicles in sites close to T cell zones in both the spleen and lymph nodes [241]. This location facilitates the bi-directional communication between B and T cells and, consequently, specific and sustained immune responses. The MZ B cells, however, inhabit the splenic marginal sinus, have polyreactive BCR and play mixed functions by performing T cell-independent and -dependent responses through rapid antibody production after recognizing PAMPs or via antigen presentation to CD4+ T cells localized in the splenic follicles, respectively [243].
Following cross-talk between B and T cells, B cells may become blasts and undergo class-switch recombination (CSR) to differentiate into ASCs (short-lived plasma cells – SLPCs) without forming a germinal center (GC) reactions in a process called “extrafollicular response” [244]. On the other hand, antigen-activated B cells may enter the follicles and initiate GC responses to yield high proliferative rate, high-affinity antibody production, inducing CSR and generating both memory B cells or long-lived plasma cells (LLPCs) [231]. GC reactions have a high energy demand and mitochondrial biogenesis is needed to support these activation steps. GC B cells have high HIF-1α-dependent glycolytic activity [245] that is sustained by the hypoxic microenvironment in the light zone of GC [246]. Moreover, the metabolic sensor glycogen synthase kinase 3 (GSK3) was described as an important regulator of oxidant production during the GC reactions to prevent the overproduction and consequent activation of apoptosis signaling pathways [245]. In addition, the CSR increases both mitochondrial mass and activity, and ROS production [247].
The investigations into the metabolic profiles of B cells subtypes have only recently started. In general, the metabolism of total splenic B cells is distinct from T cells in both the quiescence and activation states. Caro-Maldonado et al. showed that B cells have higher glycolytic activity than T cells in the resting state [248]. After activation, both glycolysis and OXPHOS rates increase proportionately in B cells, without the metabolic shift observed in T lymphocytes. B cell activation also increases the expression of the glucose transporter Glut1 and mitochondrial mass (Fig. 4A) [248]. Uptake of extracellular glucose is essential for B cell proliferation, intracellular membrane network expansion (required for antibody production) and for ASC marker expression (CD138 and Blimp-1) [249]. In fact, mice with B cells deficiency of Glut1 failed to produce total and specific IgM or IgG 7 days after immunization with NP-ovalbumin [248]. However, a study using RNAseq and isotopic labeling approaches concluded that glycolysis is dispensable for B cell activation, whilst biosynthesis of ribonucleotides, OXPHOS and mitochondrial dynamic remodeling are required [250]. Naïve B cells have a low number of mitochondria and they have an elongated shape. On the other hand, activated B cells have rounded shape mitochondria (Fig. 4A). This indicates that activated B cells may prioritize mitochondria number instead of the most efficient metabolism-related anatomy in order to be transmitted to daughter cells during clonal expansion. Moreover, the same study showed that mtDNA remains unchanged even with the increased number of mitochondria in activated B cells [250].
A more recent study has shown that aerobic glycolysis, TCA, PPP, and FAS are more active in B1 cells than B2 cells [240]. This high metabolic activity may be associated with the rapid response of B1 cells. Corroborating this hypothesis, IgA-producing plasma cells also have high metabolic activity (predominantly glycolytic) in the gut, since they are continuously producing antibodies as frontline surveillance at mucosal surfaces [251]. Additionally, B1a cells with impaired autophagy function due to deficiency of Atg7 show decreased metabolic activity and mitochondrial dysfunction [240]. Although there are some indications that MZ B cells have higher glucose uptake rates than FO B cells [245], the understanding of which metabolic pathways support the activation of B cells and their subtypes still require further investigation. In addition, no study has described the contribution of HBP to support the development, differentiation and activation of B cells.
B cells produce regulated amounts of H2O2 to amplify and maintain the BCR signaling pathway (Fig. 4A) [252]. This is particularly important for GC reactions. In fact, one study demonstrated that GC B cells are more sensitive to H2O2 compared to other B cell subtypes (mature B cells, memory B cells and plasma blasts) as determined by higher levels of phosphorylated proteins associated with BCR activation [253]. Furthermore, the source of oxidant production following BCR activation changes over time. In the first 2 h of stimulation, the production is mediated by Nox2, whereas after this period the main source of oxidants is mitochondrial respiration [254]. Oxidant production during these both stages is important to regulate the BCR-activated B cell proliferation since the treatment with N-acetylcysteine impairs their proliferation rate. However, the use of gp91-deficient B cells, which lack of functional Nox activity, showed that O2•- and other ROS-derived oxidants constrain the proliferation of BCR-stimulated B cells and limits T cell-independent B cell responses [255]. One recent study also described that Duox1 (an H2O2 producer)-deficient B cells had unchanged antibody production but increased proliferative rate [256]. Thus, additional studies are necessary to confirm the impact of oxidant production on B cell proliferation.
T cell-independent B cell activation also increases oxidant production as observed in LPS-stimulated B cells [257]. At the same time, B cells upregulate the antioxidant response, including the production of thioredoxin, the cystine transporter xCT and nonprotein thiols, mainly GSH and cysteine [257]. This redox balance seems to be essential to regulate B cell development and function. Oxidant production is required for their differentiation (Fig. 4A), whereas the higher antioxidant activity increases IgM secretion in ASCs (Fig. 4B) [257]. However, one study showed that OXPHOS, but not ROS production, is essential for antibody synthesis [258].
Increasing efforts have been made to understand the metabolic profile of plasma cells (mainly LLPCs) as well as the factors that impact GC responses and memory generation [246]. These cells are potential therapeutic targets since they have increased high-affinity antibody production capacity and long-lasting responses. Lam et al. showed that LLPCs take up more glucose than SLPCs [259]. It was further confirmed that this glucose is fundamental for pyruvate generation and its consequent use by the mitochondria for maintenance of LLPCs, since the absence of a functional mitochondrial pyruvate carrier decreased the frequency of LLPCs in the bone marrow. Moreover, a recent study emphasized the importance of immunometabolism in describing ASC subpopulations and other immune cells. It has been shown that LLPCs and SLPCs have very similar transcriptional programs, but different metabolic profiles [260]. The former showed higher glucose uptake, greater expression of the CD98 amino acid transporter, and higher autophagosome mass compared to the latter (Fig. 4B). Thus, it becomes necessary to investigate which non-transcriptional factors regulate the longevity of plasma cells. The understanding of mitochondrial dynamics of memory B cells and plasma cells remain undefined and future investigations must describe if mitochondria anatomy can define the fate of these populations.
Fine-tuning the regulation of oxidant production is also required to coordinate plasma cell fate. Higher mtROS levels are important for sustaining both mitochondrial activity and mass and activating the CSR (Fig. 4A). On the other hand, lower mtROS levels were directly associated with plasma cell differentiation [254]. Interestingly, increased oxidant production inhibits the synthesis of the heme group by preventing the addition of ferrous ion to the protoporphyrin IX group. Heme is iron-prophyrin complexes that modify protein function through conformational modifications and have been shown to regulate both the CSR and differentiation of B cells into plasma cells by directly and indirectly modulating the transcription factors associated with these processes (Bach2 and Blimp-1, respectively) [261].
Altogether, these results highlight the paucity of studies describing both B cell metabolism and the impact of redox status and mitochondrial milieu on B cell development and function. Future studies are needed to reveal whether mitochondrial dynamics can dictate B cell fate as is observed in T lymphocytes.
12. Future perspectives
The concept of mitochondria as being more than the powerhouse of immune cells is relatively new. Recent studies have shown that mitochondrial metabolites and mtROS are important regulators of signaling pathways and cell fate in both innate and adaptive immune cells. Furthermore, mitochondrial dynamics modulate not only cell metabolism, but also cell fate and function. The many roles of mitochondria in different immune cells have been uncovered, but this is only a small portion of the different aspects that mitochondria can orchestrate in immune cells. Understanding cell metabolism in vivo is one of the challenges in immunometabolism, but great progress has been made in recent studies. Thus, modulating mitochondria in immune cells may be a future approach to treat a wide spectrum of diseases, such as inflammatory diseases and cancer.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We gratefully acknowledge Dr. Catherine A. Reardon from the Committee on Molecular Metabolism and Nutrition, University of Chicago and from Ben May Department for Cancer Research, University of Chicago, Chicago, IL, USA for the manuscript editing. The authors acknowledge the support of the São Paulo Research Foundation (FAPESP Grants No. 2014/10910-7, no. 2015/15626-8, no. 2015/26682-6, no. 2016/18031-8 and no. 2017/05264-7), National Council for Scientific and Technological Development (CNPq) and FAEPEX. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The authors also acknowledge the website https://smart.servier.com used to generate the manuscript figures.
Contributor Information
Niels Olsen Saraiva Câmara, Email: niels.camara@gmail.com.
Pedro Manoel Mendes Moraes-Vieira, Email: pmvieira@unicamp.br.
References
- 1.Alwarawrah Y., Kiernan K., MacIver N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016;7(1):66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C.H., Qiu J., O'Sullivan D., Buck M.D., Noguchi T., Curtis J.D. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi B.S., Martinez-Falero I.C., Corset C., Munder M., Modolell M., Müller I. Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages. J. Leukoc. Biol. 2009;85(2):268–277. doi: 10.1189/jlb.0508310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Rourke B. From bioblasts to mitochondria: ever expanding roles of mitochondria in cell physiology. Front. Physiol. 2010;1:7. doi: 10.3389/fphys.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angajala A., Lim S., Phillips J.B., Kim J.H., Yates C., You Z. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metabol. 2016;27(2):105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35(9):505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta. 2006;1757(5–6):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 13.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashida Gnanaprakasam J.N., Wu R., Wang R. Metabolic reprogramming in modulating T cell reactive oxygen species generation and antioxidant capacity. Front. Immunol. 2018;9:1075. doi: 10.3389/fimmu.2018.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiński M.M., Röth D., Krammer P.H., Gülow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Arch Immunol Ther Exp (Warsz) 2013;61(5):367–384. doi: 10.1007/s00005-013-0235-0. [DOI] [PubMed] [Google Scholar]
- 17.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 18.Mookerjee S.A., Goncalves R.L.S., Gerencser A.A., Nicholls D.G., Brand M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta. 2015;1847(2):171–181. doi: 10.1016/j.bbabio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Gladden L.B. Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 2004;558(Pt 1):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.W O. Versuche an überlebendem carcinomgewebe. In: M S., editor. Klin Wochenschr. 1923. pp. 776–777. [Google Scholar]
- 21.Love D.C., Hanover J.A. The hexosamine signaling pathway: deciphering the "O-GlcNAc code. Sci. STKE. 2005;2005(312):re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 22.Chiaradonna F., Ricciardiello F., Palorini R. The nutrient-sensing hexosamine biosynthetic pathway as the hub of cancer metabolic rewiring. Cells. 2018;7(6) doi: 10.3390/cells7060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jesus T., Shukla S., Ramakrishnan P. Too sweet to resist: control of immune cell function by O-GlcNAcylation. Cell. Immunol. 2018;333:85–92. doi: 10.1016/j.cellimm.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan G., Walther D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol. 2015;11(2) doi: 10.1371/journal.pcbi.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017;18(7):452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons J.J., Milner J.D., Rosenzweig S.D. Glycans instructing immunity: the emerging role of altered glycosylation in clinical immunology. Front Pediatr. 2015;3:54. doi: 10.3389/fped.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres C.R., Hart G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259(5):3308–3317. [PubMed] [Google Scholar]
- 30.Hanover J.A. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J. 2001;15(11):1865–1876. doi: 10.1096/fj.01-0094rev. [DOI] [PubMed] [Google Scholar]
- 31.Hart G.W., Housley M.P., Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre T., Dehennaut V., Guinez C., Olivier S., Drougat L., Mir A.M. Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes and Alzheimer's disease. Biochim. Biophys. Acta. 2010;1800(2):67–79. doi: 10.1016/j.bbagen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Chen L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 34.Schultz B.E., Chan S.I. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu. Rev. Biophys. Biomol. Struct. 2001;30:23–65. doi: 10.1146/annurev.biophys.30.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Mills E., O'Neill L.A. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24(5):313–320. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Williams N.C., O'Neill L.A.J. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 2018;9:141. doi: 10.3389/fimmu.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy M.P., O'Neill L.A.J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174(4):780–784. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce E.J., Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015;15(1):18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everts B., Amiel E., Huang S.C., Smith A.M., Chang C.H., Lam W.Y. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15(4):323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabol. 2016;24(1):158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tol V.A. Aspects of long-chain acyl-COA metabolism. Mol. Cell. Biochem. 1975;7(1):19–31. doi: 10.1007/BF01732160. [DOI] [PubMed] [Google Scholar]
- 44.McGarry J.D., Brown N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244(1):1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 45.Nomura M., Liu J., Rovira, Gonzalez-Hurtado E., Lee J., Wolfgang M.J. Fatty acid oxidation in macrophage polarization. Nat. Immunol. 2016;17(3):216–217. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patsoukis N., Bardhan K., Chatterjee P., Sari D., Liu B., Bell L.N. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S. Enhancing CD8 T cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Divakaruni A.S., Hsieh W.Y., Minarrieta L., Duong T.N., Kim K.K.O., Desousa B.R. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metabol. 2018;28(3):490–503. doi: 10.1016/j.cmet.2018.06.001. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raud B., Roy D.G., Divakaruni A.S., Tarasenko T.N., Franke R., Ma E.H. Etomoxir actions on regulatory and memory T cells are independent of cpt1a-mediated fatty acid oxidation. Cell Metabol. 2018;28(3):504–515. doi: 10.1016/j.cmet.2018.06.002. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao C.H., Liu G.Y., Wang R., Moon S.H., Gross R.W., Patti G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLoS Biol. 2018;16(3) doi: 10.1371/journal.pbio.2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curi R., Newsholme P., Pithon-Curi T.C., Pires-de-Melo M., Garcia C., Homem-de-Bittencourt Júnior P.I. Metabolic fate of glutamine in lymphocytes, macrophages and neutrophils. Braz. J. Med. Biol. Res. 1999;32(1):15–21. doi: 10.1590/s0100-879x1999000100002. [DOI] [PubMed] [Google Scholar]
- 53.Wood T. Physiological functions of the pentose phosphate pathway. Cell Biochem. Funct. 1986;4(4):241–247. doi: 10.1002/cbf.290040403. [DOI] [PubMed] [Google Scholar]
- 54.Perner A., Nielsen S.E., Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003;29(4):642–645. doi: 10.1007/s00134-002-1628-4. [DOI] [PubMed] [Google Scholar]
- 55.Gray G.R., Stamatoyannopoulos G., Naiman S.C., Kliman M.R., Klebanoff S.J., Austin T. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet. 1973;2(7828):530–534. doi: 10.1016/s0140-6736(73)92350-7. [DOI] [PubMed] [Google Scholar]
- 56.Stanton R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64(5):362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCommis K.S., Baines C.P. The role of VDAC in cell death: friend or foe? Biochim. Biophys. Acta. 2012;1818(6):1444–1450. doi: 10.1016/j.bbamem.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Rojo M., Legros F., Chateau D., Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 2002;115(Pt 8):1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 60.Fritz S., Rapaport D., Klanner E., Neupert W., Westermann B. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J. Cell Biol. 2001;152(4):683–692. doi: 10.1083/jcb.152.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galonek H.L., Hardwick J.M. Upgrading the BCL-2 network. Nat. Cell Biol. 2006;8(12):1317–1319. doi: 10.1038/ncb1206-1317. [DOI] [PubMed] [Google Scholar]
- 62.Youle R.J. Cell biology. Cellular demolition and the rules of engagement. Science. 2007;315(5813):776–777. doi: 10.1126/science.1138870. [DOI] [PubMed] [Google Scholar]
- 63.Chan D.C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Walther D.M., Rapaport D. Biogenesis of mitochondrial outer membrane proteins. Biochim. Biophys. Acta. 2009;1793(1):42–51. doi: 10.1016/j.bbamcr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Mazunin I.O., Levitskii S.A., Patrushev M.V., Kamenski P.A. Mitochondrial matrix processes. Biochemistry (Mosc.) 2015;80(11):1418–1428. doi: 10.1134/S0006297915110036. [DOI] [PubMed] [Google Scholar]
- 66.Gurung P., Lukens J.R., Kanneganti T.D. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 2015;21(3):193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 68.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 69.Artyomov M., Sergushichev A., Schilling J.D. Integrating immunometabolism and macrophage diversity. Semin. Immunol. 2016;28(5):417–424. doi: 10.1016/j.smim.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingelsson B., Soderberg D., Strid T., Soderberg A., Bergh A.C., Loitto V. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc. Natl. Acad. Sci. U. S. A. 2018;115(3):E478–E487. doi: 10.1073/pnas.1711950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 72.Dorn G.W. Evolving concepts of mitochondrial dynamics. Annu. Rev. Physiol. 2018;81:1–17. doi: 10.1146/annurev-physiol-020518-114358. [DOI] [PubMed] [Google Scholar]
- 73.Gao Z., Li Y., Wang F., Huang T., Fan K., Zhang Y. Mitochondrial dynamics controls anti-tumour innate immunity by regulating CHIP-IRF1 axis stability. Nat. Commun. 2017;8(1):1805. doi: 10.1038/s41467-017-01919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buck M.D., O'Sullivan D., Klein Geltink R.I., Curtis J.D., Chang C.H., Sanin D.E. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisner V., Picard M., Hajnoczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018;20(7):755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62(3):341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garaude J., Acín-Pérez R., Martínez-Cano S., Enamorado M., Ugolini M., Nistal-Villán E. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016;17(9):1037–1045. doi: 10.1038/ni.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam G.Y., Huang J., Brumell J.H. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin. Immunopathol. 2010;32(4):415–430. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 79.DeLeo F.R., Allen L.A., Apicella M., Nauseef W.M. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 1999;163(12):6732–6740. [PubMed] [Google Scholar]
- 80.Nauseef W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004;122(4):277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 81.Schrader M., Costello J., Godinho L.F., Islinger M. Peroxisome-mitochondria interplay and disease. J. Inherit. Metab. Dis. 2015;38(4):681–702. doi: 10.1007/s10545-015-9819-7. [DOI] [PubMed] [Google Scholar]
- 82.Pascual-Ahuir A., Manzanares-Estreder S., Proft M. Pro- and antioxidant functions of the peroxisome-mitochondria connection and its impact on aging and disease. Oxid Med Cell Longev. 2017;2017:9860841. doi: 10.1155/2017/9860841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles) Physiol. Rev. 1966;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 84.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta. 2012;1822(9):1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc.) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 86.Muller F. The nature and mechanism of superoxide production by the electron transport chain: its relevance to aging. J Am Aging Assoc. 2000;23(4):227–253. doi: 10.1007/s11357-000-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller F.L., Liu Y., Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 89.Thomas C., Mackey M.M., Diaz A.A., Cox D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 2009;14(3):102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 90.Liochev S.I. The mechanism of "Fenton-like" reactions and their importance for biological systems. A biologist's view. Met. Ions Biol. Syst. 1999;36:1–39. [PubMed] [Google Scholar]
- 91.Hayyan M., Hashim M.A., AlNashef I.M. Superoxide ion: generation and chemical implications. Chem. Rev. 2016;116(5):3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 92.Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 93.Paiva C.N., Bozza M.T. Are reactive oxygen species always detrimental to pathogens? Antioxidants Redox Signal. 2014;20(6):1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurst J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012;53(3):508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pattison D.I., Davies M.J., Hawkins C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012;46(8):975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 96.Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Marí M., Morales A., Colell A., García-Ruiz C., Fernández-Checa J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxidants Redox Signal. 2009;11(11):2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orrenius S., Gogvadze V., Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 99.Hwang C., Sinskey A.J., Lodish H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 100.Griffith O.W., Meister A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. U. S. A. 1985;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mak T.W., Grusdat M., Duncan G.S., Dostert C., Nonnenmacher Y., Cox M. Glutathione primes T cell metabolism for inflammation. Immunity. 2017;46(6):1089–1090. doi: 10.1016/j.immuni.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 102.Marí M., Colell A., Morales A., von Montfort C., Garcia-Ruiz C., Fernández-Checa J.C. Redox control of liver function in health and disease. Antioxidants Redox Signal. 2010;12(11):1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 105.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 108.Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 109.Radi R., Cassina A., Hodara R., Quijano C., Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 110.Knoops B., Goemaere J., Van der Eecken V., Declercq J.P. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxidants Redox Signal. 2011;15(3):817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 111.Park J., Choi H., Kim B., Chae U., Lee D.G., Lee S.R. Peroxiredoxin 5 (Prx5) decreases LPS-induced microglial activation through regulation of Ca. Free Radic. Biol. Med. 2016;99:392–404. doi: 10.1016/j.freeradbiomed.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 112.Knoops B., Argyropoulou V., Becker S., Ferté L., Kuznetsova O. Multiple roles of peroxiredoxins in inflammation. Mol. Cells. 2016;39(1):60–64. doi: 10.14348/molcells.2016.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tal M.C., Sasai M., Lee H.K., Yordy B., Shadel G.S., Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106(8):2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sadatomi D., Nakashioya K., Mamiya S., Honda S., Kameyama Y., Yamamura Y. Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J. Biochem. 2017;161(6):503–512. doi: 10.1093/jb/mvw098. [DOI] [PubMed] [Google Scholar]
- 115.Sandhir R., Halder A., Sunkaria A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1090–1097. doi: 10.1016/j.bbadis.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 116.van Horssen J., van Schaik P., Witte M. In press: Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 117.Hsu C.C., Tseng L.M., Lee H.C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016;241:1281–1295. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ježek J., Cooper K.F., Strich R. Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants (Basel) 2018;7(1) doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin S.M., Youle R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012;125(Pt 4):795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poole A.C., Thomas R.E., Yu S., Vincow E.S., Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka A., Cleland M.M., Xu S., Narendra D.P., Suen D.F., Karbowski M. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010;191(7):1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ziviani E., Tao R.N., Whitworth A.J. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. U. S. A. 2010;107(11):5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan N.C., Salazar A.M., Pham A.H., Sweredoski M.J., Kolawa N.J., Graham R.L. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 2011;20(9):1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soubannier V., McLelland G.L., Zunino R., Braschi E., Rippstein P., Fon E.A. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 2012;22(2):135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 127.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 128.Rosales C., Demaurex N., Lowell C.A., Uribe-Querol E. Neutrophils: their role in innate and adaptive immunity. J Immunol Res. 2016;2016:1469780. doi: 10.1155/2016/1469780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sbarra A.J., Karnovsky M.L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 1959;234(6):1355–1362. [PubMed] [Google Scholar]
- 131.Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Investig. 1982;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valentine W.N., Beck W.S. Biochemical studies on leucocytes. I. Phosphatase activity in health, leucocytosis, and myelocytic leucemia. J. Lab. Clin. Med. 1951;38(1):39–55. [PubMed] [Google Scholar]
- 133.Maianski N.A., Geissler J., Srinivasula S.M., Alnemri E.S., Roos D., Kuijpers T.W. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. 2004;11(2):143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 134.Riffelmacher T., Clarke A., Richter F.C., Stranks A., Pandey S., Danielli S. Autophagy-dependent generation of free fatty acids is critical for normal neutrophil differentiation. Immunity. 2017;47(3):466–480. doi: 10.1016/j.immuni.2017.08.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rožman S., Yousefi S., Oberson K., Kaufmann T., Benarafa C., Simon H.U. The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Differ. 2015;22(3):445–456. doi: 10.1038/cdd.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Skendros P., Mitroulis I., Ritis K. Autophagy in neutrophils: from granulopoiesis to neutrophil extracellular traps. Front Cell Dev Biol. 2018;6:109. doi: 10.3389/fcell.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rice C.M., Davies L.C., Subleski J.J., Maio N., Gonzalez-Cotto M., Andrews C. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat. Commun. 2018;9(1):5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Winterbourn C.C., Hampton M.B., Livesey J.H., Kettle A.J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 2006;281(52):39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 139.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 140.Steinberg B.E., Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci. STKE. 2007;2007(379):pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 141.Douda D.N., Yip L., Khan M.A., Grasemann H., Palaniyar N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood. 2014;123(4):597–600. doi: 10.1182/blood-2013-09-526707. [DOI] [PubMed] [Google Scholar]
- 142.Douda D.N., Khan M.A., Grasemann H., Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. U. S. A. 2015;112(9):2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 144.Pilsczek F.H., Salina D., Poon K.K., Fahey C., Yipp B.G., Sibley C.D. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 145.Parker H., Dragunow M., Hampton M.B., Kettle A.J., Winterbourn C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012;92(4):841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 146.Papayannopoulos V., Metzler K.D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wynn J.L., Scumpia P.O., Delano M.J., O'Malley K.A., Ungaro R., Abouhamze A. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 148.Adam-Vizi V., Starkov A.A. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimer's Dis. 2010;20(Suppl 2):S413–S426. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Amini P., Stojkov D., Felser A., Jackson C.B., Courage C., Schaller A. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 2018;9(1):2958. doi: 10.1038/s41467-018-05387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Behnen M., Möller S., Brozek A., Klinger M., Laskay T. Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Front. Immunol. 2017;8:184. doi: 10.3389/fimmu.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodríguez-Espinosa O., Rojas-Espinosa O., Moreno-Altamirano M.M., López-Villegas E.O., Sánchez-García F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145(2):213–224. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bao Y., Ledderose C., Seier T., Graf A.F., Brix B., Chong E. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J. Biol. Chem. 2014;289(39):26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bao Y., Ledderose C., Graf A.F., Brix B., Birsak T., Lee A. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J. Cell Biol. 2015;210(7):1153–1164. doi: 10.1083/jcb.201503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou W., Cao L., Jeffries J., Zhu X., Staiger C.J., Deng Q. Neutrophil-specific knockout demonstrates a role for mitochondria in regulating neutrophil motility in zebrafish. Dis Model Mech. 2018;11(3) doi: 10.1242/dmm.033027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng X., Chen M., Meng X., Chu X., Cai C., Zou F. Phosphorylation of dynamin-related protein 1 at Ser616 regulates mitochondrial fission and is involved in mitochondrial calcium uniporter-mediated neutrophil polarization and chemotaxis. Mol. Immunol. 2017;87:23–32. doi: 10.1016/j.molimm.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 156.Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Correa-da-Silva F., Pereira J.A.S., de Aguiar C.F., de Moraes-Vieira P.M.M. Mitoimmunity-when mitochondria dictates macrophage function. Cell Biol. Int. 2018;42(6):651–655. doi: 10.1002/cbin.10921. [DOI] [PubMed] [Google Scholar]
- 159.Castoldi A., Naffah de Souza C., Camara N.O., Moraes-Vieira P.M. The macrophage switch in obesity development. Front. Immunol. 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Braga T.T., Agudelo J.S., Camara N.O. Macrophages during the fibrotic process: M2 as friend and foe. Front. Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Freemerman A.J., Johnson A.R., Sacks G.N., Milner J.J., Kirk E.L., Troester M.A. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014;289(11):7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fukuzumi M., Shinomiya H., Shimizu Y., Ohishi K., Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect. Immun. 1996;64(1):108–112. doi: 10.1128/iai.64.1.108-112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diskin C., Pålsson-McDermott E.M. Metabolic modulation in macrophage effector function. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jha A.K., Huang S.C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 167.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McFadden B.A., Purohit S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J. Bacteriol. 1977;131(1):136–144. doi: 10.1128/jb.131.1.136-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–470. doi: 10.1016/j.cell.2016.08.064. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van den Bossche J., Baardman J., Otto N.A., van der Velden S., Neele A.E., van den Berg S.M. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 172.Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang S.C., Smith A.M., Everts B., Colonna M., Pearce E.L., Schilling J.D. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling Axis is essential for macrophage alternative activation. Immunity. 2016;45(4):817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vats D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metabol. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang S.C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A.M. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014;15(9):846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Namgaladze D., Brune B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim. Biophys. Acta. 2014;1841(9):1329–1335. doi: 10.1016/j.bbalip.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 177.Covarrubias A.J., Aksoylar H.I., Yu J., Snyder N.W., Worth A.J., Iyer S.S. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5 doi: 10.7554/eLife.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang F., Zhang S., Vuckovic I., Jeon R., Lerman A., Folmes C.D. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metabol. 2018;28(3):463–475. doi: 10.1016/j.cmet.2018.08.012. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y., Subramanian M., Yurdagul A., Jr., Barbosa-Lorenzi V.C., Cai B., de Juan-Sanz J. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171(2):331–345. doi: 10.1016/j.cell.2017.08.041. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Escoll P., Song O.R., Viana F., Steiner B., Lagache T., Olivo-Marin J.C. Legionella pneumophila modulates mitochondrial dynamics to trigger metabolic repurposing of infected macrophages. Cell Host Microbe. 2017;22(3):302–316. doi: 10.1016/j.chom.2017.07.020. e7. [DOI] [PubMed] [Google Scholar]
- 181.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 183.Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metabol. 2014;20(4):614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Serbulea V., Upchurch C.M., Schappe M.S., Voigt P., DeWeese D.E., Desai B.N. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc. Natl. Acad. Sci. U. S. A. 2018;115(27) doi: 10.1073/pnas.1800544115. E6254-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Biswas S.K., Chittezhath M., Shalova I.N., Lim J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012;53(1–3):11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 186.Moraes-Vieira P.M., Yore M.M., Dwyer P.M., Syed I., Aryal P., Kahn B.B. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metabol. 2014;19(3):512–526. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jantsch J., Chakravortty D., Turza N., Prechtel A.T., Buchholz B., Gerlach R.G. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J. Immunol. 2008;180(7):4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 189.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Archiv. 2004;447(5):689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- 190.Everts B., Amiel E., Huang S.C., Smith A.M., Chang C.H., Lam W.Y. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKϵ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15(4):323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Everts B., Amiel E., van der Windt G.J., Freitas T.C., Chott R., Yarasheski K.E. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thwe P.M., Pelgrom L., Cooper R., Beauchamp S., Reisz J.A., D'Alessandro A. Cell-intrinsic glycogen metabolism supports early glycolytic reprogramming required for dendritic cell immune responses. Cell Metabol. 2017;26(3):558–567. doi: 10.1016/j.cmet.2017.08.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zaccagnino P., Saltarella M., Maiorano S., Gaballo A., Santoro G., Nico B. An active mitochondrial biogenesis occurs during dendritic cell differentiation. Int. J. Biochem. Cell Biol. 2012;44(11):1962–1969. doi: 10.1016/j.biocel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 194.Del Prete A., Zaccagnino P., Di Paola M., Saltarella M., Oliveros Celis C., Nico B. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic. Biol. Med. 2008;44(7):1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 195.Sheng K.C., Pietersz G.A., Tang C.K., Ramsland P.A., Apostolopoulos V. Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J. Immunol. 2010;184(6):2863–2872. doi: 10.4049/jimmunol.0903458. [DOI] [PubMed] [Google Scholar]
- 196.Svajger U., Obermajer N., Jeras M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology. 2010;129(4):525–535. doi: 10.1111/j.1365-2567.2009.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malinarich F., Duan K., Hamid R.A., Bijin A., Lin W.X., Poidinger M. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J. Immunol. 2015;194(11):5174–5186. doi: 10.4049/jimmunol.1303316. [DOI] [PubMed] [Google Scholar]
- 198.Oberkampf M., Guillerey C., Mouries J., Rosenbaum P., Fayolle C., Bobard A. Mitochondrial reactive oxygen species regulate the induction of CD8(+) T cells by plasmacytoid dendritic cells. Nat. Commun. 2018;9(1):2241. doi: 10.1038/s41467-018-04686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ryu S.W., Han E.C., Yoon J., Choi C. The mitochondrial fusion-related proteins Mfn2 and OPA1 are transcriptionally induced during differentiation of bone marrow progenitors to immature dendritic cells. Mol. Cells. 2015;38:89–94. doi: 10.14348/molcells.2015.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Swainson L., Kinet S., Manel N., Battini J.L., Sitbon M., Taylor N. Glucose transporter 1 expression identifies a population of cycling CD4+CD8+ human thymocytes with high CXCR4-induced chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12867–12872. doi: 10.1073/pnas.0503603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.MacIver N.J., Michalek R.D., Rathmell J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menk A.V., Scharping N.E., Moreci R.S., Zeng X., Guy C., Salvatore S. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 2018;22(6):1509–1521. doi: 10.1016/j.celrep.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 205.Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Johnson M.O., Wolf M.M., Madden M.Z., Andrejeva G., Sugiura A., Contreras D.C. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. 2018;175(7):1780–1795. doi: 10.1016/j.cell.2018.10.001. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kuwahara M., Izumoto M., Honda H., Inoue K., Imai Y., Suzuki J. 2017. Glutamine Metabolism Regulates Th2 Cell Differentiation via the α-ketoglutarate-dependent Demethylation of Histone H3K27. [Google Scholar]
- 209.Chang C.H., Curtis J.D., Maggi L.B., Jr., Faubert B., Villarino A.V., O'Sullivan D. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sena L.A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D.A. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Contento R.L., Campello S., Trovato A.E., Magrini E., Anselmi F., Viola A. Adhesion shapes T cells for prompt and sustained T-cell receptor signalling. EMBO J. 2010;29(23):4035–4047. doi: 10.1038/emboj.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Quintana A., Schwindling C., Wenning A.S., Becherer U., Rettig J., Schwarz E.C. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl. Acad. Sci. U. S. A. 2007;104(36):14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20(11):1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 214.Endo Y., Asou H.K., Matsugae N., Hirahara K., Shinoda K., Tumes D.J. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase. ACC1. Cell Rep. 2015;12(6):1042–1055. doi: 10.1016/j.celrep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 215.Raha S., Raud B., Oberdorfer L., Castro C.N., Schreder A., Freitag J. Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur. J. Immunol. 2016;46(9):2233–2238. doi: 10.1002/eji.201546152. [DOI] [PubMed] [Google Scholar]
- 216.Lee J., Walsh M.C., Hoehn K.L., James D.E., Wherry E.J., Choi Y. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J. Immunol. 2014;192(7):3190–3199. doi: 10.4049/jimmunol.1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Devadas S., Zaritskaya L., Rhee S.G., Oberley L., Williams M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 2002;195(1):59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaminski M., Kiessling M., Suss D., Krammer P.H., Gulow K. Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol. Cell. Biol. 2007;27(10):3625–3639. doi: 10.1128/MCB.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kaminski M.M., Roth D., Sass S., Sauer S.W., Krammer P.H., Gulow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim. Biophys. Acta. 2012;1823(5):1041–1052. doi: 10.1016/j.bbamcr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Kaminski M.M., Sauer S.W., Klemke C.D., Suss D., Okun J.G., Krammer P.H. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 2010;184(9):4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 221.Yi J.S., Holbrook B.C., Michalek R.D., Laniewski N.G., Grayson J.M. Electron transport complex I is required for CD8+ T cell function. J. Immunol. 2006;177(2):852–862. doi: 10.4049/jimmunol.177.2.852. [DOI] [PubMed] [Google Scholar]
- 222.Los M., Schenk H., Hexel K., Baeuerle P.A., Droge W., Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14(15):3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jackson S.H., Devadas S., Kwon J., Pinto L.A., Williams M.S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 224.Kwon J., Shatynski K.E., Chen H., Morand S., de Deken X., Miot F. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 2010;3(133):ra59. doi: 10.1126/scisignal.2000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kaminski M.M., Sauer S.W., Kaminski M., Opp S., Ruppert T., Grigaravicius P. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2(5):1300–1315. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 226.McKinstry K.K., Strutt T.M., Swain S.L. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol. Rev. 2010;236:110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Taub D.D., Hesdorffer C.S., Ferrucci L., Madara K., Schwartz J.B., Goetzl E.J. Distinct energy requirements for human memory CD4 T-cell homeostatic functions. FASEB J. 2013;27(1):342–349. doi: 10.1096/fj.12-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.O'Sullivan D., van der Windt G.J., Huang S.C., Curtis J.D., Chang C.H., Buck M.D. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41(1):75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.D'Souza A.D., Parikh N., Kaech S.M., Shadel G.S. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion. 2007;7(6):374–385. doi: 10.1016/j.mito.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Boothby M.R., Hodges E., Thomas J.W. Molecular regulation of peripheral B cells and their progeny in immunity. Genes Dev. 2019;33(1–2):26–48. doi: 10.1101/gad.320192.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Browne E.P. Regulation of B-cell responses by Toll-like receptors. Immunology. 2012;136(4):370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen X., Jensen P.E. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz) 2008;56(2):77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 234.Carter M.J., Mitchell R.M., Meyer Sauteur P.M., Kelly D.F., Truck J. The antibody-secreting cell response to infection: kinetics and clinical applications. Front. Immunol. 2017;8:630. doi: 10.3389/fimmu.2017.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schroeder H.W., Jr., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125(2 Suppl 2):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tsui C., Martinez-Martin N., Gaya M., Maldonado P., Llorian M., Legrave N.M. Protein kinase C-beta dictates B cell fate by regulating mitochondrial remodeling, metabolic reprogramming, and heme biosynthesis. Immunity. 2018;48(6):1144–11459 e5. doi: 10.1016/j.immuni.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mendoza P., Martinez-Martin N., Bovolenta E.R., Reyes-Garau D., Hernansanz-Agustin P., Delgado P. R-Ras2 is required for germinal center formation to aid B cells during energetically demanding processes. Sci. Signal. 2018;11(532) doi: 10.1126/scisignal.aal1506. [DOI] [PubMed] [Google Scholar]
- 238.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 239.Montecino-Rodriguez E., Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36(1):13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Clarke A.J., Riffelmacher T., Braas D., Cornall R.J., Simon A.K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 2018;215(2):399–413. doi: 10.1084/jem.20170771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pillai S., Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9(11):767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 242.Stein M., Dutting S., Mougiakakos D., Bosl M., Fritsch K., Reimer D. A defined metabolic state in pre B cells governs B-cell development and is counterbalanced by Swiprosin-2/EFhd1. Cell Death Differ. 2017;24(7):1239–1252. doi: 10.1038/cdd.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zouali M., Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity. Front. Immunol. 2011;2:63. doi: 10.3389/fimmu.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015;15(3):160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 245.Jellusova J., Cato M.H., Apgar J.R., Ramezani-Rad P., Leung C.R., Chen C. Gsk3 is a metabolic checkpoint regulator in B cells. Nat. Immunol. 2017;18(3):303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cho S.H., Raybuck A.L., Stengel K., Wei M., Beck T.C., Volanakis E. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537(7619):234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jang K.J., Mano H., Aoki K., Hayashi T., Muto A., Nambu Y. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat. Commun. 2015;6:6750. doi: 10.1038/ncomms7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Caro-Maldonado A., Wang R., Nichols A.G., Kuraoka M., Milasta S., Sun L.D. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J. Immunol. 2014;192(8):3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dufort F.J., Gumina M.R., Ta N.L., Tao Y., Heyse S.A., Scott D.A. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J. Biol. Chem. 2014;289(10):7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Waters L.R., Ahsan F.M., Wolf D.M., Shirihai O., Teitell M.A. Initial B cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience. 2018;5:99–109. doi: 10.1016/j.isci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kunisawa J., Sugiura Y., Wake T., Nagatake T., Suzuki H., Nagasawa R. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015;13(1):122–131. doi: 10.1016/j.celrep.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 252.Irish J.M., Czerwinski D.K., Nolan G.P., Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J. Immunol. 2006;177(3):1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 253.Polikowsky H.G., Wogsland C.E., Diggins K.E., Huse K., Irish J.M. Cutting edge: redox signaling hypersensitivity distinguishes human germinal center B cells. J. Immunol. 2015;195(4):1364–1367. doi: 10.4049/jimmunol.1500904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wheeler M.L., Defranco A.L. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J. Immunol. 2012;189(9):4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Richards S.M., Clark E.A. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur. J. Immunol. 2009;39(12):3395–3403. doi: 10.1002/eji.200939587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sugamata R., Donko A., Murakami Y., Boudreau H.E., Qi C.F., Kwon J. Duox1 regulates primary B cell function under the influence of IL-4 through BCR-mediated generation of hydrogen peroxide. J. Immunol. 2019;202(2):428–440. doi: 10.4049/jimmunol.1601395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vene R., Delfino L., Castellani P., Balza E., Bertolotti M., Sitia R. Redox remodeling allows and controls B-cell activation and differentiation. Antioxidants Redox Signal. 2010;13(8):1145–1155. doi: 10.1089/ars.2009.3078. [DOI] [PubMed] [Google Scholar]
- 258.Price M.J., Patterson D.G., Scharer C.D., Boss J.M. Progressive upregulation of oxidative metabolism facilitates plasmablast differentiation to a T-independent antigen. Cell Rep. 2018;23(11):3152–3159. doi: 10.1016/j.celrep.2018.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lam W.Y., Becker A.M., Kennerly K.M., Wong R., Curtis J.D., Llufrio E.M. Mitochondrial pyruvate import promotes long-term survival of antibody-secreting plasma cells. Immunity. 2016;45(1):60–73. doi: 10.1016/j.immuni.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lam W.Y., Jash A., Yao C.H., D'Souza L., Wong R., Nunley R.M. Metabolic and transcriptional modules independently diversify plasma cell lifespan and function. Cell Rep. 2018;24(9):2479–24792 e6. doi: 10.1016/j.celrep.2018.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Watanabe-Matsui M., Muto A., Matsui T., Itoh-Nakadai A., Nakajima O., Murayama K. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood. 2011;117(20):5438–5448. doi: 10.1182/blood-2010-07-296483. [DOI] [PubMed] [Google Scholar]