Abstract
This research shows that high-performance biosensors can be produced by modification of screen-printed electrodes with enzymes and conducting hydrogel based on sol–gel matrix and single-walled carbon nanotubes. Tetraethoxysilane, dimethyldiethoxysilane and polyvinyl alcohol were used as the sol–gel matrix basis. Modified SWCNT provide direct electron transfer during glucose oxidation, as confirmed by cyclic voltammetry. The developed conducting sol–gel screen-printed electrodes can determine glucose within the concentration range 0.045–1.04 mM. The developed biosensor is not only in pace with its world analogues but even exceeds them by some analytical and metrological properties. The developed conducting sol–gel biosensor was used to measure the concentration of glucose in blood. The test results differed only insignificantly from those received with the help of standard glucose meter.
Keywords: Organic–inorganic hybrid sol–gel, ORMOSIL, Biocatalyst, Amperometric glucose biosensor
Introduction
Biosensor analysis has proven to be efficient for a variety of practical tasks in the recent decade. Statistics has it that biosensors were especially effective in industrial biotechnology, food industry, ecology and clinical diagnostics (Depagne et al. 2011; Yang et al. 2015; Mehrotra 2016). No sample preparation required, cheap cost, availability and user-friendliness are the advantages of biosensor analysis compared to other analytical methods. Development of conducting solid matrix capable to maintain long-term stability of biomaterial without additional reactive chemicals is now a promising direction for research (Ansari and Qayyum 2012).
Screen-printed electrodes are a convenient research platform for biocatalysts. Screen-printed electrodes are made by covering a plastic matrix with a layer of conductive paste. They are small and multipurpose, while modern developments allow launching a low-cost commercial production of single-use electrodes (Ahmed et al. 2016). Biosensors based on functionalized carbon nanotubes (FCNT) and screen-printed electrodes have been more frequently studied in recent times. Due to unique physical and chemical properties of FCNT electrochemical biosensors could be improved to give rise to amperometric enzyme electrodes, immunosensors and nucleic acid sensors (Desimone et al. 2009). In this research we describe the feasibility of creation of a biosensor based on glucose oxidase enzyme and single-walled carbon nanotubes for sensing glucose in alcoholic products (Arlyapov et al. 2017).
Biosensor efficiency largely depends on the method used to immobilize biomaterial. Application of sol–gel for synthesis of nonorganic materials and biomolecules has been developed in the past two decades (Niu et al. 2013; Wang et al. 2015). Nowadays, researchers are working on new silica gels and glasslike materials featuring higher biocompatibility based on several high-reactive precursors that can be used to create a variety of materials. Sol–gel materials can be effectively used in biosensors thanks to their high biocompatibility, protective properties, high mechanical, heat and biological stability (Kang et al. 2008; Tan et al. 2011; Yildirim et al. 2011). To produce bioactive materials, reactive chemical in a hybrid material must remain intact, maintain high activity, long-term stability and high resistance.
Sol–gel method is most often used to immobilize enzymes (Sarma et al. 2009; Yang et al. 2009; Gupta et al. 2016). For example, sol–gel encapsulation of creatine kinase will protect it from thermal denaturation. Encapsulated enzyme remains active by 50% 10 times longer (125 h) at 47 °C compared to a free one (13 h) (Nguyen and Zink 2002). On top of this, sol–gel encapsulation also ensures good protection in aggressive high pH medium. This became evident as phosphotase was immobilized in a silica sol–gel matrix (Frenkel-Mullerad 2005). Alkaline phosphatase is most active at pH 9.5, while when immobilized it remains active at pH close to 1, presumably, because proteins are surrounded by only one or two layers of water molecules in a restricted space in pores.
However, only some critical features and conditions have been discovered for the time being to produce such structures purposefully. Thus, alkyl–alkoxy silane (a hydrophobic agent with hydrolysis resistant bonds (C–Si) is an important part of sol–gel synthesis of biohybrids. Earlier (Kamanin et al. 2015; Kamanina et al. 2016), we studied addition of hydrolysis resistant single bond compound (Ponamoreva et al. 2015)—methyltriethoxysilane—to tetraethoxysilane (TEOS) as a hydrophobic agent. Dimethyldiethoxysilane (DMDES) has two hydrolysis resistant bonds and is a starter compound for synthesis of silicones. Supposedly, this will make a less tight but stronger matrix for enzyme immobilization and generate more reaction groups to build covalent bonds with CNT. As carbon nanomaterials are functionalized by carboxyl groups, covalent bonds between CNT and sol–gel matrix are built.
Diagnosis and monitoring of various diseases require too much effort for routine tests of blood samples and other associated tests. Now chemical methods for the analysis of glucose active develop. They based on fluorescence analysis (Ngo et al. 2019) and various electrochemical approaches (Hwang et al. 2018). Such sensors allow the analysis of glucose for low limits, but do not have absolute selectivity, like enzyme biosensors based on glucose oxidase. Enzymatic amperometric glucose biosensors are the most common commercially available devices and have been widely studied over the past decades (Bahadır and Sezgintürk 2015). However, it is necessary to develop biosensors with more accessible test strips using new methods that are being actively published at the present time (Sabu et al. 2019).
The objective of this research was to develop a screen-printed electrode modified with carbon nanotubes and sol–gel encapsulated enzyme to be used in a non-mediated biosensor, which is a user-friendly and reliable tool for instant sensing of glucose level in blood.
Materials and methods
Modification of carbon screen printed electrodes
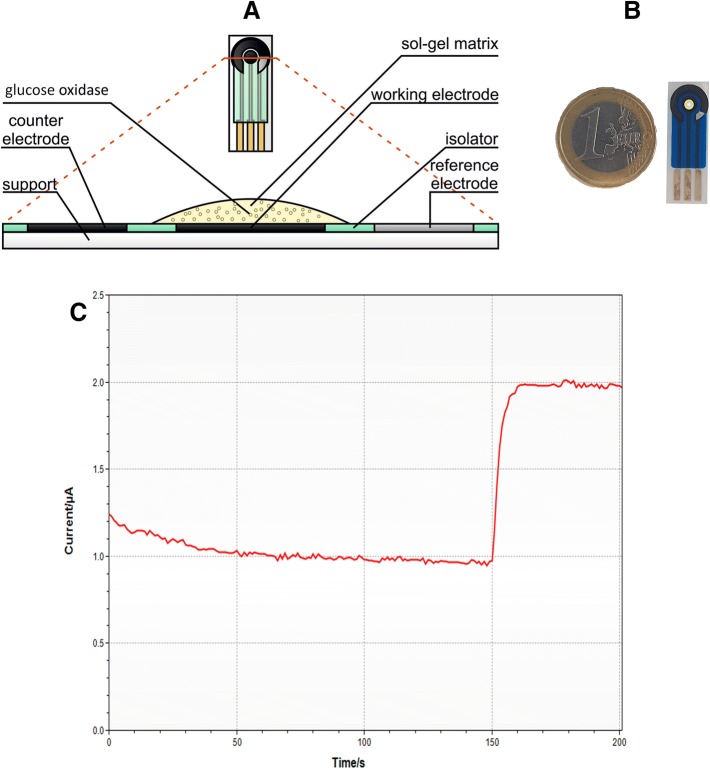
To modify electrodes, tetraethoxysilane (TEOS, Sigma-Aldrich, United States) and dimethyldiethoxysilane (DMDES, Acros Organics, Belgium) were mixed in the following proportions: 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90 in microtubes, the total volume of the mixture being 100 μl. 20 μl of 5% polyvinyl alcohol (PVA), 60 μl of water, 10 μl of nanotubes (Uglerod Chg LLC, Russia), 50 μl of glucose oxidase (GOx, Sigma-Aldrich, United States), 5 μl of 1.2 M NaF (Sigma-Aldrich, United States) were added to each mixture. After being mixed twice, the received mixture was quickly transferred to the electrode. Polymerization took 15–30 min (Fig. 1).
Fig. 1.
Screen-printed electrode a structure, b real sample, c the biosensor response on glucose
The EmStat potentiostat (PalmSens, Netherlands) was used to record the signal. The working potential for the electrode with SWCNTs was − 0.4 V relative to the silver chloride reference electrode. The measurements were carried out in a cuvette of 4 ml in sodium potassium phosphate buffer solution with pH 6.8 upon stirring with a magnetic stirrer (Ekros, Russia) with the rate of 200 rpm. Different glucose concentrations from 0.25 to 3.75 mmol/dm3 have added to determine the calibration curve. The analytical signal (the response of the biosensor) upon the addition of glucose was the amplitude of the change in the current, passed through the working electrode from the moment of the sample injection until the maximum level of current was reached. The biosensor response on glucose has shown in Fig. 1c. After each measurement, the electrode was washed with buffer solution for 1–2 min.
Investigation of electron transfer processes by cyclic voltammetry
The voltammetric measurements were carried out in sodium–potassium phosphate buffer solution with pH 6.8 in anoxic medium. Oxygen was removed from the buffer by bubbling argon in the cuvette before measurement for 10 min and during the measurements. The regime of cyclic voltammograms was obtained with the use of galvanopotentiostat Ekotest-VA (Ekoniks-Expert, Russia). The potential range was − 0.8 to 0.0 V; the scan rate of the potential was varied from 10 to 100 mV/s.
Scanning electron microscopy (SEM)
The samples were applied to the surface of the metal plating and were coated with platinum–carbon mixture in a vacuum sputtering equipment JEE-4X (JEOL, Japan). Electron microscopic analysis of the samples was performed on a scanning electron microscope JSM-6510 LV (JEOL, Japan). The operating voltage for SEM analysis was 10 kV.
IR spectroscopy
The IR spectra of sol–gel systems were recorded with an FMS 1201 Fourier IR spectrometer (OOO Monitoring, Russia) using a multiple attenuated total internal reflection (MATIR) attachment of horizontal type with a zinc selenide prism (resolution 4 cm−1). When studying the kinetics of formation of the sol–gel matrix, the IR spectra of the samples were recorded during a 35-min period at 5-min intervals in the wavenumber range 4000–500 cm−1.
Results and discussion
Development of conducting hydrogel based on sol–gel modified with CNT
Being widely used to encapsulate and stabilize all classes of biological materials, sol–gel method has become an attractive basis for biosensor matrices. Silane sol–gel matrix is created as a result of hydrolysis and polycondensation reaction with oxo-alkoxides as side products. We have a vast experience in production of biocatalysts based on microorganisms immobilized in different sol–gel matrices (Niu et al. 2013; Ponamoreva et al. 2015). The latter can be employed to immobilize an enzyme tightly fixing biomaterial but the matrix are not conductors, i.e. cannot transfer electrons. This research showed that conducting materials for immobilization of enzymes can be produced by integration of CNT into organosilicone sol–gel matrices. Previously, the nanotubes have shown their effectiveness when used in combination with other organic matrices (Arlyapov et al. 2019).
In this research, enzyme was immobilized in a sol–gel matrix based on tetraethoxysilane, dimethyldiethoxysilane and polyvinyl alcohol. DMDES served as a hydrophobic agent, which increased mechanical stability of the matrix, made its components less toxic to the biomaterial and allowed changing the size of pores (Barczak et al. 2016). Proportion of TEOS and DMDES can be optimized in each and every case to produce a sol–gel matrix with micropores of the right size for the encapsulated enzyme to remain highly active, while retained in matrix pores.
Silane sol–gel matrix is created as a result of hydrolysis and polycondensation reaction with oxo-alkoxides as side products. Hydrolysis happens simultaneously with polycondensation as soon as the former is initiated. Hydrolysis generates silanol (
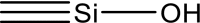 ) groups, and condensation—siloxane (
) groups, and condensation—siloxane (
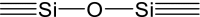 ) groups. Reactions end in release of water and alcohol (Brinker and Scherer 1990).
) groups. Reactions end in release of water and alcohol (Brinker and Scherer 1990).
Sol–gel matrix formation process was studied by means of 30-min IR spectroscopy, with data being registered every 5 min. Figure 2 shows IR spectra recorded for varied proportions of silane precursors, PVA and base catalyst. Normalized bands were applied to measure matrix formation time and the number of Si–O–Si bonds. IR spectroscopy helped to determine the most suitable proportion of precursors in the matrix.
Fig. 2.
a Exemplary IR spectroscopy of organosilicone sol–gel matrix consisting of DMDES, TEOS and PVA. b Dependency of absorption band intensity 1100 cm−1/1270 cm−1 on DMDES concentration in the matrix
The ratio of band intensities 1100–1270 cm−1 expresses the amount by which Si–O–Si bonds prevail over Si–CH3 ones. The more Si–CH3 bonds a matrix contains, the larger and more porous it is. Consequently, the minimum graph value corresponds with the least dense matrix, which allows easiest diffusion. Hence, TEOS and DMDES were taken in proportion 70 to 30 for further research.
At the next stage of sol–gel matrix formation, optimization continued according to the amount of carbon nanotubes and enzyme on the surface of the operational electrode. A criterion for optimization was the maximum current amplitude value on addition of analyte to cuvette. A few screen-printed electrodes were prepared and modified with varied quantities of SWCNT and glucose oxidase encapsulated in a sol–gel matrix. Contour diagram in Fig. 3 shows SWCNT and glucose oxidase concentration in a modifying mixture and responses of a biosensor based on these electrodes.
Fig. 3.
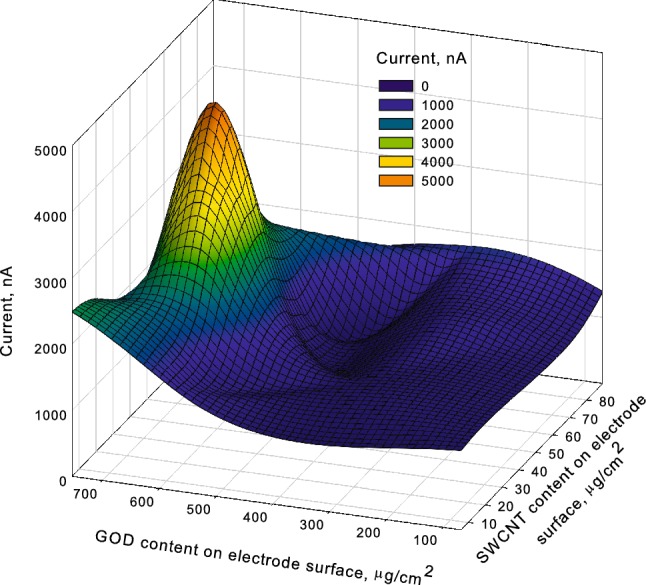
Biosensor responses for series of electrodes
A biosensor based on a screen-printed electrode with 52 μg/cm2 of SWCNT and 645 μg/cm2 of glucose oxidase demonstrated the strongest response and was selected for a more detailed study.
Pictures of resulting structures were captured by an electronic microscope (Fig. 4).
Fig. 4.
a Selected SWCNT; b sol–gel matrix without SWCNT; c conducting sol–gel with SWCNT and glucose-oxidase
The picture features a typical sol–gel matrix structure. An added enzyme is immobilized on the matrix surface (small balls are presumably the enzyme capsules adsorbed by a sol–gel structure fragment). When carbon nanotubes are introduced, they form a conducting network connecting sol–gel capsules with entrapped enzyme.
Electron transfer pattern in the formed matrix was studied by means of cyclic voltammetry. This method analyzes dependency of potential or peak current on potential scan rate. It identifies important characteristics of oxidation–reduction reaction for different electrochemical systems: heterogeneous electron transfer rate constant and quantity of electroactive substance on the electrode surface.
A cyclic voltammogram was recorded for a modified screen-printed graphite electrode coated with glucose oxidase immobilized in organosilicone sol–gel with CNT at a scan rate 0.14–0.32 V/s (Fig. 5) to determine the main characteristics of the electrochemical process.
Fig. 5.
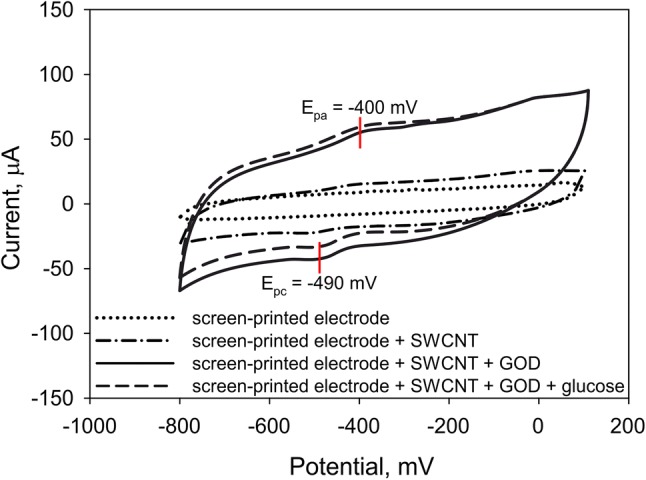
A cyclic voltammogram for a screen-printed graphite electrode coated with glucose oxidase immobilized in organosilicone sol–gel with CNT. Silane proportion 70 to 30 (at scan rate 0.2 V/s)
Anodic peak was reached at potential Epa = − 400 mV, cathodic peak at Epc = − 490 mV; difference of peaks (ΔEp) amounted to 90 mV, which corresponds with the electrochemical behavior of glucose oxidase (Ponamoreva et al. 2018). This is suggestive of glucose oxidase coenzyme’s (FAD) capability to reduce to FADH2 and oxidize in such conditions. The cyclic voltammogram recorded at different scan rates revealed that the process is limited by diffusion, if an enzyme is entrapped in a sol–gel matrix modified with CNT (transfer of electrons from CNT). The electrode attracts electrons both as an anode and cathode, but more often as an anode, which means that it can be used for a wider variety of applications (transfer ratio in the anodic and cathodic processes amounted to 0.78 ± 0.04 and 0.12 ± 0.01, respectively). Heterogenic electron transfer constant equaling 0.0098 ± 0.0005 cm/s confirms quite a high efficiency of the studied matrix as compared to the matrices based on organic materials and CNT described in Nguyen and Zink (2002)and Ngo et al. (2019). Although the received constants are slightly lower than the constants of matrices based on multi-walled CNT and high-oriented polyaniline nanotubes (Ponamoreva et al. 2018), generally it may be still suggested that the developed electrodes have high analytical properties.
Determination of properties of the biosensor system based on the developed printed electrodes
A calibration curve method reflecting the relationship between the biosensor responses and the concentration of analyte in a cuvette was employed to determine sensitivity and detection range for the developed screen-printed electrodes. Figure 6 shows calibration curve for GOD enzyme electrodes coated with conducting gel based on sol–gel matrix encapsulated CNT. Other calibration curves looked the same and are not presented in this research.
Fig. 6.
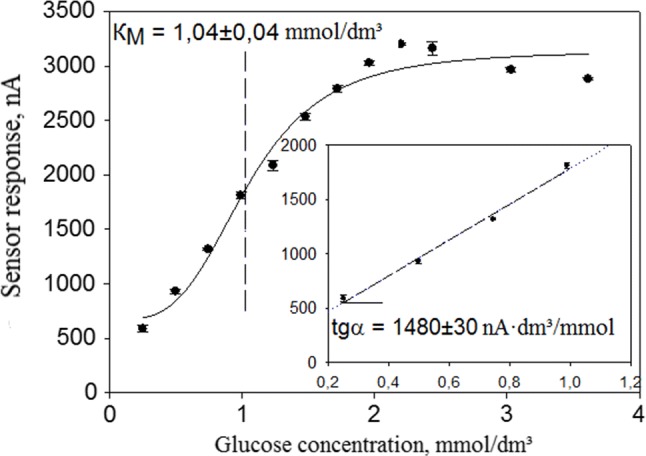
Calibration curve featuring dependency of sensor responses on glucose concentration in cuvette
Enzyme based receptors are catalytic bioreceptors, i.e. biological response in such systems is a result of enzyme reactions. The calibration curve on Fig. 6 was characterized by Michaelis–Menten equation:
where Rmax represents the maximum biosensor response, when all the bioreceptor enzyme molecules are engaged in formation of an enzyme–substrate complex; KM is an effective Michaelis constant, numerically equal to substrate concentration at which the level of biosensor response reaches half of maximum value; [S]—substrate concentration.
See basic feature of biosensors based on modified screen-printed electrodes in Table 1.
Table 1.
Basic characteristics of biosensor based on modified screen-printed electrode
| Characteristics | TEOS + DMDES | Selenium nanoparticle-mesoporous silica composite matrix and carbon paste electrode (Sarma et al. 2009) | Reduced graphene oxide covalently conjugated to magnetic nanoparticles (Fe3O4) (Tan et al. 2011) | Hybrid sol–gel film consisting of TEOS, bovine serum albumin (BSA) and chitosan (Arlyapov et al. 2017) |
|---|---|---|---|---|
| Reproducibility, % | 1.9 | 2.8 | 4 | – |
| Sensitivity coefficient, nA mM−1 | 1480 ± 30 | 3400 | 5900 | 1840 |
| Lower border of detection range, mM | 0.045 | 0.1 | 0.5 | 0.032 |
| Upper border of detection range, mM | 1.04 ± 0.04 | 0.02 | 1 | 0.1 |
| Duration of single measurement, min | 1–3 | – | 3 | 3 |
The developed biosensor based on the screen-printed electrode modified with GOD and SWCNT immobilized in TEOS and DMDES sol–gel matrix was compared to several electrodes: one modified with GOD and gold nanoparticles immobilized in TEOS matrix; the second one based on GOD, reduced iron oxide and Fe3O4 particles; the third one based on GOD immobilized in an organosilicone matrix with BSA and chitosan. The developed sensor has set the lowest detection limit (0.015 mM), its analogues, however, have higher sensitivity coefficient (1480 ± 30 nA mM−1 for the developed biosensor).
Testing of developed biosensor
A comparative test of human blood samples was done using the standard method and conducting hydrogel biosensor. Certified test strips One Touch Select [LifeSkan Inc. (Johnson and Johnson, USA)] were applied as a reference. Test results are shown in Fig. 7.
Fig. 7.
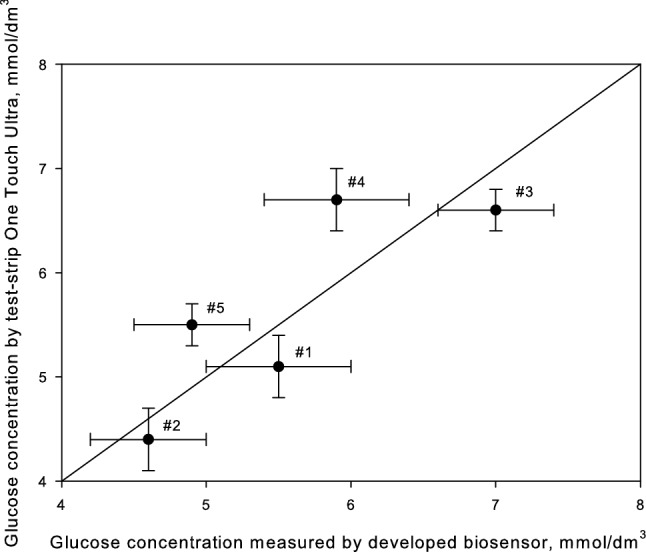
Measurement of glucose concentration in blood and blood plasma
The blood sample #1 was taken 2 h after breakfast, #2: 3 h after breakfast, #3: 20 min after abundant meals rich in carbohydrates, #4: 2 h after meals, #5: 3 h after meals.
Statistical analysis (modified Student test) revealed insignificant difference between the results of the two methods. Our method of screen-printed electrode modification with conducting CNT based hydrogel allows creation of biosensors featuring high analytical and metrological properties, which can serve as an alternative to standard analysis.
Conclusions
This research shows that high-performance biosensors can be produced by modification of screen-printed electrodes with enzymes and conducting hydrogel based on sol–gel matrix and single-walled carbon nanotubes. Modified SWCNT caused direct electron transfer during glucose oxidation.
The developed conducting sol–gel screen-printed electrodes can determine glucose within the concentration range 0.045–1.04 mM, without considering sample dilution. Our biosensor is not only in pace with its world analogues but even exceeds them by some analytical and metrological properties (reproducibility, lower and upper border of detection range and duration of single measurement).
The developed conducting sol–gel biosensor was used to measure the concentration of glucose in blood. The test results differed only insignificantly from those received with the help of standard glucose meter. These conclusions allow expanding the applications of biosensor and will give an impulse to the development of modern analytical biotechnology.
Acknowledgements
The study was performed within the framework of the State Task of the Ministry of Education and Science of the Russian Federation (No. 0824_2019-0007).
References
- Ahmed MU, Hossain MM, Safavieh M, et al. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit Rev Biotechnol. 2016;36:495–505. doi: 10.3109/07388551.2014.992387. [DOI] [PubMed] [Google Scholar]
- Ansari SA, Qayyum H. Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol Adv. 2012;30:512–523. doi: 10.1016/j.biotechadv.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Arlyapov VA, Kamanin SS, Kamanina OA, Reshetilov AN. Biosensor based on screen-printed electrode and glucose-oxidase modified with the addition of single-walled carbon nanotubes and thermoexpanded graphite. Nanotechnol Russ. 2017;12:658–666. doi: 10.1134/S1995078017060039. [DOI] [Google Scholar]
- Arlyapov VA, Kamanina OA, Kamanin SS, et al. Monitoring of biotechnological processes by enzyme electrodes modified with carbon nanotubes. Appl Biochem Microbiol. 2019;55:313–321. doi: 10.1134/S0003683819030037. [DOI] [Google Scholar]
- Bahadır EB, Sezgintürk MK. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal Biochem. 2015;478:107–120. doi: 10.1016/j.ab.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Barczak M, McDonagh C, Wencel D. Micro- and nanostructured sol–gel-based materials for optical chemical sensing (2005–2015) Microchim Acta. 2016;183(7):2085–2109. doi: 10.1007/s00604-016-1863-y. [DOI] [Google Scholar]
- Brinker CJ, Scherer GW. Sol–gel science. The physics and chemistry of sol–gel process. San Diego: Academic Press; 1990. [Google Scholar]
- Depagne C, Roux C, Coradin T. How to design cell-based biosensors using the sol–gel process. Anal Bioanal Chem. 2011;400:965–976. doi: 10.1007/s00216-010-4351-y. [DOI] [PubMed] [Google Scholar]
- Desimone MF, Alvarez GS, Foglia ML, Diaz LE. Development of sol–gel hybrid materials for whole cell immobilization. Recent Pat Biotechnol. 2009;3:55–60. doi: 10.2174/187220809787172605. [DOI] [PubMed] [Google Scholar]
- Frenkel-Mullerad H. Sol–gel materials as efficient enzyme protectors: preserving the activity of phosphatases under extreme pH conditions. J Am Chem Soc. 2005;127:8077–8081. doi: 10.1021/ja0507719. [DOI] [PubMed] [Google Scholar]
- Gupta S, Prabha CR, Murthy CN. Functionalized multi-walled carbon nanotubes/polyvinyl alcohol membrane coated glassy carbon electrode for efficient enzyme immobilization and glucose sensing. J Environ Chem Eng. 2016;4:3734–3740. doi: 10.1016/j.jece.2016.08.021. [DOI] [Google Scholar]
- Hwang D-W, Lee S, Seo M, Chung TD. Recent advances in electrochemical non-enzymatic glucose sensors—a review. Anal Chim Acta. 2018;1033:1–34. doi: 10.1016/j.aca.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Kamanin SS, Arlyapov VA, Machulin AV, et al. Biosensors based on modified screen-printed enzyme electrodes for monitoring of fermentation processes. Russ J Appl Chem. 2015 doi: 10.1134/s1070427215030167. [DOI] [Google Scholar]
- Kamanina OA, Lavrova DG, Arlyapov VA, et al. Silica sol–gel encapsulated methylotrophic yeast as filling of biofilters for the removal of methanol from industrial wastewater. Enzyme Microb Technol. 2016;92:94–98. doi: 10.1016/j.enzmictec.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Kang X, Mai Z, Zou X, et al. Glucose biosensors based on platinum nanoparticles-deposited carbon nanotubes in sol–gel chitosan/silica hybrid. Talanta. 2008;74:879–886. doi: 10.1016/j.talanta.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Mehrotra P. Biosensors and their applications—a review. J Oral Biol Craniofac Res. 2016;6:153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo Y-LT, Choi WM, Chung JS, Hur SH. Highly biocompatible phenylboronic acid-functionalized graphitic carbon nitride quantum dots for the selective glucose sensor. Sens Actuators B Chem. 2019;282:36–44. doi: 10.1016/j.snb.2018.11.031. [DOI] [Google Scholar]
- Nguyen DT, Zink JI. Stabilization of creatine kinase encapsulated in silicate sol–gel materials and unusual temperature effects on its activity. Chem Mater. 2002;14:4300–4306. doi: 10.1021/cm020398t. [DOI] [Google Scholar]
- Niu X, Wang Z, Li Y, et al. “Fish-in-net”, a novel method for cell immobilization of zymomonas mobilis. PLoS One. 2013;8:769–772. doi: 10.1371/journal.pone.0079569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponamoreva ON, Kamanina OA, Alferov VA, et al. Yeast-based self-organized hybrid bio-silica sol–gels for the design of biosensors. Biosens Bioelectron. 2015;67:321–326. doi: 10.1016/j.bios.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Ponamoreva O, Afonina E, Kamanina O, et al. Yeast Debaryomyces hansenii within ORMOSIL shells as a heterogeneous biocatalyst. Appl Biochem Microbiol. 2018;54:736–742. doi: 10.1134/S0003683818070062. [DOI] [Google Scholar]
- Sabu C, Henna TK, Raphey VR, et al. Advanced biosensors for glucose and insulin. Biosens Bioelectron. 2019;10:15. doi: 10.1016/j.bios.2019.03.034. [DOI] [PubMed] [Google Scholar]
- Sarma AK, Vatsyayan P, Goswami P, Minteer SD. Recent advances in material science for developing enzyme electrodes. Biosens Bioelectron. 2009;24:2313–2322. doi: 10.1016/j.bios.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Tan SN, Wang W, Ge L. Comprehensive biomaterials. Oxford: Elsevier; 2011. Biosensors based on sol–gel-derived materials; pp. 471–489. [Google Scholar]
- Wang X, Ben Ahmed N, Alvarez GS, et al. Sol–gel encapsulation of biomolecules and cells for medicinal applications. Curr Top Med Chem. 2015 doi: 10.2174/1568026614666141229112734. [DOI] [PubMed] [Google Scholar]
- Yang S, Jia WZ, Qian QY, et al. Simple approach for efficient encapsulation of enzyme in silica matrix with retained bioactivity. Anal Chem. 2009;81:3478–3484. doi: 10.1021/ac802739h. [DOI] [PubMed] [Google Scholar]
- Yang N, Chen X, Ren T, et al. Carbon nanotube based biosensors. Sens Actuators B Chem. 2015;207:690–715. doi: 10.1016/j.snb.2014.10.040. [DOI] [Google Scholar]
- Yildirim N, Odaci D, Ozturk G, et al. Sol–gel encapsulated glucose oxidase arrays based on a pH sensitive fluorescent dye. Dye Pigment. 2011;89:144–148. doi: 10.1016/j.dyepig.2010.10.003. [DOI] [Google Scholar]