Abstract
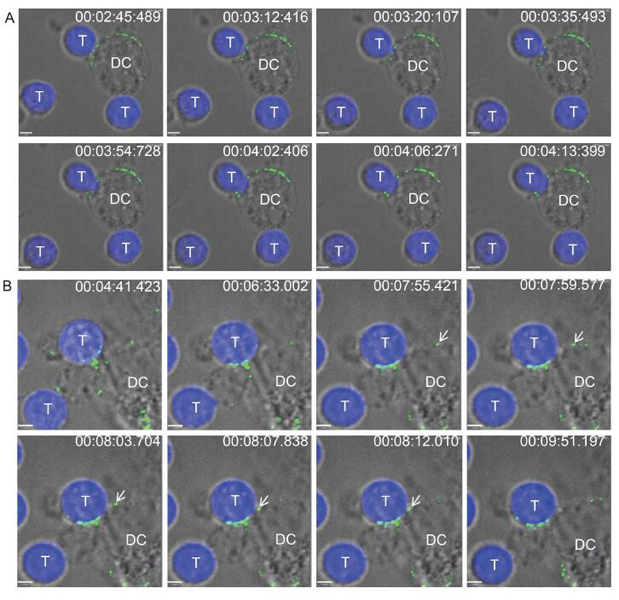
Skin-resident dendritic cells (DCs) likely encounter incoming viruses in the first place, and their migration to lymph nodes following virus capture may promote viral replication. However, the molecular mechanisms underlying these processes remain unclear. In the present study, we found that compared to cell-free viruses, DC-bound viruses showed enhanced capture of JEV by T cells. Additionally, JEV infection was increased by co-culturing DCs and T cells. Blocking the C-type lectin receptor DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) with neutralizing antibodies or antagonists blocked JEV transmission to T cells. Live-cell imaging revealed that DCs captured and transferred JEV viral particles to T cells via virological synapses formed at DC-T cell junctions. These findings indicate that DC-SIGN plays an important role in JEV transmission from DCs to T cells and provide insight into how JEV exploits the migratory and antigen-presenting capabilities of DCs to gain access to lymph nodes for dissemination and persistence in the host.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s12250-017-4034-3 and is accessible for authorized users.
Keywords: Japanese encephalitis virus (JEV), DC-SIGN, T lymphocytes, in trans
Electronic supplementary material
DC-SIGN promotes Japanese encephalitis virus transmission from dendritic cells to T cells via virological synapses
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFC1200400), the National Natural Science Foundation of China Grants (81572009 and 31570165) and the National High Technology Research and Development Program of China (2014AA021406). We thank the Core Facility and Technical Support at Wuhan Institute of Virology for technique supports of Confocal Microscopy (Dr. Ding Gao) and Flow Cytometry (Ms. Juan Min).
Contributor Information
Qinxue Hu, Email: qhu@wh.iov.cn.
Yalan Liu, Email: liuyl@wh.iov.cn.
References
- Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK. Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol. 2009;183:2462–2474. doi: 10.4049/jimmunol.0801952. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BM, McNerney GP, Thompson DL, Hubner W, de Los Reyes K, Chuang FY, Huser T, Chen BK. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe. 2011;10:551–562. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, de Vries RD, van der Vlist M, Yuksel S, Litjens M, de Swart RL, Geijtenbeek TB. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 2008;4:e1000049. doi: 10.1371/journal.ppat.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, Bess JW, Bavari S, Lowekamp BC, Bliss D, Lifson JD, Subramaniam S. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A. 2010;107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. 2000a. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell, 100: 575–585. [DOI] [PubMed]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet H S, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Halary F, Amara A, Lortat-Jacob H, Messerle M, Delaunay T, Houles C, Fieschi F, Arenzana-Seisdedos F, Moreau JF, Dechanet-Merville J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/S1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Zhao JX, Yamamoto T, Konno H. Immunohistochemical demonstration of viral antigens in Japanese encepha-litis. Acta Neuropathol. 1986;70:79–81. doi: 10.1007/BF00689518. [DOI] [PubMed] [Google Scholar]
- Jin W, Li C, Du T, Hu K, Huang X, Hu Q. DC-SIGN plays a stronger role than DCIR in mediating HIV-1 capture and transfer. Virology. 2014;458-459:83–92. doi: 10.1016/j.virol.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Li C, Jin W, Du T, Wu B, Liu Y, Shattock RJ, Hu Q. Binding of HIV-1 virions to alpha4beta 7 expressing cells and impact of antagonizing alpha4beta 7 on HIV-1 infection of primary CD4+T cells. Virol Sin. 2014;29:381–392. doi: 10.1007/s12250-014-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- Mathur A, Kulshreshtha R, Chaturvedi UC. Evidence for latency of Japanese encephalitis virus in T lymphocytes. J Gen Virol. 1989;70:461–465. doi: 10.1099/0022-1317-70-2-461. [DOI] [PubMed] [Google Scholar]
- Mathur A, Arora KL, Rawat S, Chaturvedi UC. Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol. 1986;67:381–385. doi: 10.1099/0022-1317-67-2-381. [DOI] [PubMed] [Google Scholar]
- Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol (Paris) 1984;135D:113–134. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE, Brown M, Hornbeck P, Mizuguchi J, Ohara J, Rabin E, Snapper C, Tsang W. Regulation of B-lymphocyte activation, proliferation, and differentiation. Ann N Y Acad Sci. 1987;505:82–89. doi: 10.1111/j.1749-6632.1987.tb51284.x. [DOI] [PubMed] [Google Scholar]
- Pierson T C, Kielian M. Flaviviruses: braking the entering. Curr Opin Virol. 2013;3:3–12. doi: 10.1016/j.coviro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X X, Ma L, Liu Q W, Li C, Huang Z, Wu L, Xiong SD, Wang JH, Wang HB. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virol J. 2014;11:47. doi: 10.1186/1743-422X-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mathur A, Prakash V, Kulshreshtha R, Kumar R, Chaturvedi UC. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol. 1991;85:85–89. doi: 10.1111/j.1365-2249.1991.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimauchi T, Piguet V. DC-T cell virological synapses and the skin: novel perspectives in dermatology. Exp Dermatol. 2015;24:1–4. doi: 10.1111/exd.12511. [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- Wang P, Hu K, Luo S, Zhang M, Deng X, Li C, Jin W, Hu B, He S, Li M, Du T, Xiao G, Zhang B, Liu Y, Hu Q. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology. 2016;488:108–119. doi: 10.1016/j.virol.2015.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DC-SIGN promotes Japanese encephalitis virus transmission from dendritic cells to T cells via virological synapses


