Abstract Abstract
Erythrophylloporus is a lamellate genus in the family Boletaceae that has been recently described from China based on E.cinnabarinus, the only known species. Typical characters of Erythrophylloporus are reddish-orange to yellowish-red basidiomata, including lamellae, bright yellow basal mycelium and smooth, broadly ellipsoid, ellipsoid to nearly ovoid basidiospores. During our survey on diversity of Boletaceae in Thailand, several yellowish-orange to reddish- or brownish-orange lamellate boletes were collected. Based on both morphological evidence and molecular analyses of a four-gene dataset (atp6, tef1, rpb2 and cox3), they were recognised as belonging in Erythrophylloporus and different from the already known species. Two new species, E.paucicarpus and E.suthepensis are therefore introduced from Thailand with detailed descriptions and illustrations. Moreover, two previously described Phylloporus species, P.aurantiacus and P.fagicola, were also revised and recombined in Erythrophylloporus. A key to all known Erythrophylloporus species is provided.
Keywords: atp6, cox3, Taxonomy, Phylloporus , Pulveroboletus group, multigene phylogeny, Boletales , Southeast Asia
Introduction
Most fungi in the family Boletaceae are pileate-stipitate with poroid hymenophore, but some have a lamellate hymenophore. Lamellate Boletaceae are currently classified in four genera, Phylloporus Quél, which contains about 84 species worldwide, Phylloboletellus Singer from South America and Mexico, the two recently described genera Phylloporopsis Angelini et al., from the New World and Erythrophylloporus Ming Zhang & T.H. Li from Asia, each of which circumscribes only one species (http://www.indexfungorum.org, Farid et al. 2018; Zhang and Li 2018).
The genus Erythrophylloporus was recently described from China, with E.cinnabarinus Ming Zhang & T.H. Li as the type species. According to Zhang & Li (2018), the typical characters of the genus are orange to reddish-orange basidiomes, reddish-orange to yellowish-red lamellae turning greyish-green when bruised, bright yellow to orange yellow context staining blackish-blue to dark blue when exposed, bright yellow basal mycelium, smooth and broadly ellipsoid to nearly ovoid basidiospores and yellowish-brown pigmented cystidia. During our survey on the diversity of Boletaceae in Thailand, several collections of lamellate boletes were discovered. Some collections were recognised to belong to Erythrophylloporus by possessing yellowish-orange to deep orange to reddish-orange basidiomata with bright yellow basal mycelium and smooth basidiospores. We also found that two described Phylloporus species, P.aurantiacus Halling & G.M. Mueller from Costa Rica and P.fagicola Montoya & Bandala from Mexico (Halling et al. 1999, Montoya and Bandala 2011), share similar morphological characters with the genus Erythrophylloporus, but until now, have not been included in a molecular phylogeny. In this study, a combination of phylogenetic and morphological evidence indicated that our Thai collections were new species, that, together with the two aforementioned American Phylloporus species, belong in Erythrophylloporus. Therefore, we introduce two new species with detailed descriptions and illustrations and propose two new combinations. As some of the species we studied have some characters that do not fit with the protologue of the genus, we emend its description.
Materials and methods
Specimen collecting
Specimens were obtained and photographed from community forests and Doi Suthep-Pui National Park, Chiang Mai Province, northern Thailand during the rainy season in 2015 to 2016. The specimens were wrapped in aluminium foil and taken to the laboratory. After description of macroscopic characters, all specimens were dried in an electric drier at 45–50 °C. Examined specimens were deposited in the herbaria CMUB, MFLU, BKF or BR (Index Herbariorum; Thiers, continuously updated).
Morphological studies
Macroscopic descriptions were made based on detailed field notes and photos of fresh basidiomata. Colour codes follow Kornerup and Wanscher (1978). Macrochemical reactions (colour reactions) of fresh basidiomata were determined using 10% potassium hydroxide (KOH) and 28–30% ammonium hydroxide (NH4OH) in water. Microscopic structures were observed from dried specimens mounted in 5% KOH, NH4OH, Melzer’s reagent or 1% ammoniacal Congo red. A minimum of 50 basidiospores, 20 basidia and 20 cystidia were randomly measured at 1000× with a calibrated ocular micrometer using an Olympus CX51 microscope. The notation ‘[m/n/p]’ represents the number of basidiospores m measured from n basidiomata of p collections. Dimensions of microscopic structures are presented in the following format: (a–)b–c–d (–e), in which c represents the average, b the 5th percentile, d the 95th percentile and a and e the minimum and maximum values, respectively. Q, the length/width ratio, is presented in the same format. A section of the pileus surface was radially and perpendicularly cut at a point halfway between the centre and margin of the pileus. Sections of stipitipellis were taken from halfway up the stipe and longitudinally cut, perpendicularly to the surface. All microscopic features were drawn by free hand using an Olympus Camera Lucida model U−DA, fitted to the microscope cited above. For scanning electron microscopy (SEM), a spore print was mounted on to a SEM stub with double-sided tape. The sample was coated with gold, examined and photographed with a JEOL JSM–5910 LV SEM (JEOL, Japan).
DNA isolation, PCR amplification and DNA sequencing
Genomic DNA was extracted from fresh tissue preserved in CTAB or about 10–15 mg of dried specimens using a CTAB isolation procedure adapted from Doyle and Doyle (1990). Portions of the genes atp6, tef1, rpb2 and cox3 were amplified by the polymerase chain reaction (PCR) technique. The tailed primers ATP6-1M40F and ATP6-2M (Raspé et al. 2016) and the primer pairs EF1-983F/EF1-2218R (Rehner and Buckley 2005) and bRPB2-6F/bRPB2-7.1R (Matheny 2005) were used to amplify atp6, tef1 and rpb2, respectively. PCR conditions were the same as in Raspé et al. (2016). Part of the mitochondrial gene cox3 was amplified with the primers COX3M1-F and COX3M1-R (Vadthanarat et al. 2019), using KAPA2G™ Robust HotStart polymerase (Kapa Biosystems, Wilmington, MA, USA) and the following PCR programme: 2 min 30 s at 95 °C; 35 cycles of 25 s at 95 °C, 30 s at 48 °C, 30 s at 72 °C; 3 min at 72 °C. PCR products were purified by adding 1 U of Exonuclease I and 0.5 U FastAP Alkaline Phosphatase (Thermo Scientific, St. Leon-Rot, Germany) and incubated at 37 °C for 1 h, followed by inactivation at 80 °C for 15 min. Sequencing was performed by Macrogen Inc. (Korea and The Netherlands) with PCR primers, except for atp6, for which universal primers M13F-pUC(-40) and M13F(-20) were used; for tef1, additional sequencing was performed with two internal primers, EF1-1577F and EF1-1567R (Rehner and Buckley 2005).
Alignment and phylogeny inference
The sequences were assembled in GENEIOUS Pro v. 6.0.6 (Biomatters) and introns were removed prior to alignment, based on the amino acid sequence of previously published sequences. All sequences, including sequences from GenBank, were aligned using MAFFT version 7 (Katoh and Standley 2013) on the server accessed at http://mafft.cbrE.jp/alignment/server/.
Maximum Likelihood (ML) phylogenetic tree inference was performed using RAxML-HPC2 version 8.2.10 (Stamatakis 2006) on the CIPRES web portal (Miller et al. 2009). The phylogenetic tree was inferred from a four-partitions combined dataset, using the GTRCAT model with 25 categories. Two Buchwaldoboletus and nine Chalciporus species from subfamily Chalciporoideae were used as the outgroup. Statistical support of clades was obtained with 1,000 rapid bootstrap replicates.
For Bayesian Inference (BI), the best-fit model of substitution amongst those implementable in MrBayes was estimated separately for each gene using jModeltest (Darriba et al. 2012) on the CIPRES portal, based on the Bayesian Information Criterion (BIC). The selected models were GTR+I+G for atp6 and cox3, SYM+I+G for tef1 and K80+I+G for rpb2. Partitioned Bayesian analysis was performed with MrBayes 3.2 (Ronquist et al. 2012) on the CIPRES portal. Two runs of five chains were run for 15,000,000 generations and sampled every 1,000 generations. The chain temperature was decreased to 0.02 to improve convergence. At the end of the run, the average deviation of split frequencies was 0.007058 and the Potential Scale Reduction Factor (PSRF) values of all parameters were close to 1. The burn-in phase (25%) was estimated by checking the stationarity in the plot generated by the sump command.
Results
Phylogenetic analysis
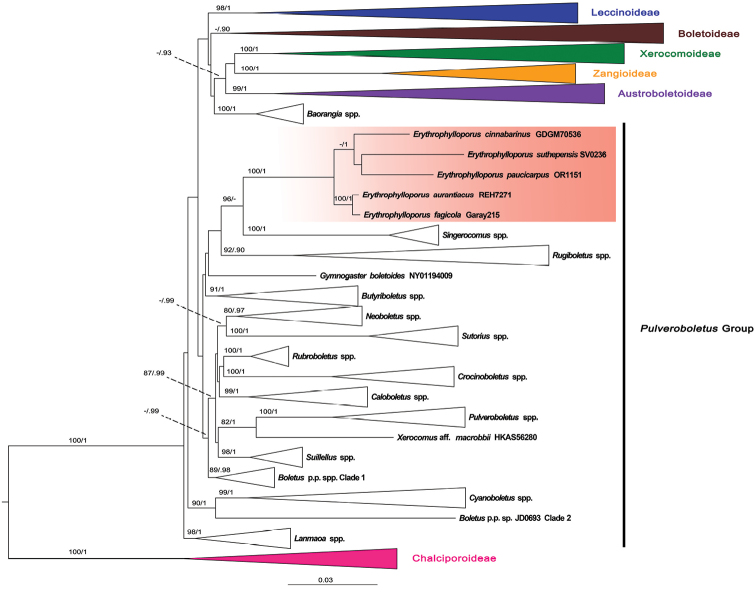
Twenty-five sequences were newly generated and deposited in GenBank (Table 1). The sequences from three specimens, OR0689, OR1135 (E.paucicarpus) and OR0615B (E.suthepensis), were not included in our phylogenic analyses because they were identical to the sequences of the type specimens of E.paucicarpus and E.suthepensis. The alignment contained 906 sequences (179 for atp6, 313 for tef1, 279 for rpb2, 135 for cox3) from 315 voucher specimens and was 2946 characters long (TreeBase number 24078). ML and BI trees showed similar topologies without any supported conflict (Bootstrap Support values, BS ≥ 70% and posterior probabilities, PP ≥ 0.90; Fig. 1). The four-gene phylogram indicated that the included taxa formed seven major clades, representing the Austroboletoideae, Boletoideae, Chalciporoideae, Leccinoideae, Xerocomoideae, Zangioideae and the Pulveroboletus group. Erythrophylloporuscinnabarinus (typus generis) grouped with the two new Erythrophylloporus species, E.paucicarpus and E.suthepensis, in a highly supported clade (BS = 100% and PP = 1). The two New World Phylloporus species (P.aurantiacus voucher REH7271 and P.fagicola voucher Garay215) also clustered in the Erythrophylloporus clade indicating that they are close relatives. Erythrophylloporus formed a clade sister to the genus Singerocomus T.W. Henkel & M.E. Sm. with high Bootstrap support (96%) but low posterior probability support (0.86) within the Pulveroboletus group. Some undescribed species formed two different generic clades in the Pulveroboletus group. Boletus p.p. spp. clade 1 contains two specimens, HKAS63598 and HKAS9660, named “Boletus sp.” in Wu et al. (2016), as well as two of our specimens, OR0832 and OR1002. Boletus p.p. sp. clade 2 contains a single African specimen, JD0693, sister to and morphologically different from Cyanoboletus.
Table 1.
List of collections used in this study, with origin and GenBank accession numbers. Newly generated sequences are presented in bold.
Figure 1.
Phylogenetic tree inferred from the four-gene dataset (atp6, rpb2, tef1 and cox3), including Erythrophylloporus species and selected Boletaceae using Maximum Likelihood and Bayesian Inference methods (ML tree is presented). The two Buchwaldoboletus and nine Chalciporus species in subfamily Chalciporoideae were used as outgroup. Most of the taxa not belonging to the Pulveroboletus group were collapsed into subfamilies. All generic clades, including one undescribed generic clade in Pulveroboletus group that were highly supported, were also collapsed. Bootstrap support values (BS ≥ 70%) and posterior probabilities (PP ≥ 0.90) are shown above the supported branches.
Taxonomy
Erythrophylloporus
Ming Zhang & T.H. Li, Mycosystema 37(9): 1111–1126 (2018)
Description.
Basidiomata stipitate-pileate with lamellate hymenophore, small to medium-sized; Pileus subhemispheric to convex when young becoming convex to plano-convex to plano-subdepressed when old, dry, pruinose or velutinous, subtomentose to tomentose, yellowish-orange to red; pileus context vivid yellow to yellowish-orange. Hymenophore lamellae, slightly thick, decurrent, deeply yellowish-orange to deep orange or reddish-orange to orange red or brownish-orange to red. Stipe central to slightly excentric, cylindrical or clavate, yellowish- to reddish-orange to yellowish red, with scattered yellowish- to reddish-orange to red scales on surface, with bright yellow basal mycelium; stipe context solid, yellow to reddish-yellow or yellow with olivaceous brown. Staining none or slightly reddening or greening or gradually bluing or dark violet, greyish to blackish-blue when bruised on the basidiomata or context or lamellae. Spore print olivaceous brown. Basidiospores ovoid or ellipsoid to broadly ellipsoid to subovoid, thin-walled, with non-bacillate surface. Basidia clavate to narrowly clavate. Cheilocystidia and pleurocystidia present, subcylindrical or narrowly conical to narrowly fusiform to ventricose with slightly or obtuse apex, thin-walled, sometimes thick-walled, originating more or less deeply in the sub hymenium or from hymenophoral trama, hyaline or sometimes containing yellowish-brown pigments. Pileipellis a subcutis to cutis to trichoderm to palisadoderm, composed of thin to slightly thick-walled hyphae. Clamp connection absent in all tissues.
Typus species.
Erythrophylloporuscinnabarinus Ming Zhang & T.H. Li.
Known Distribution.
Asia (China and Thailand), North America (Mexico) and Central America (Costa Rica).
Remarks.
Erythrophylloporus is easily distinguished from other lamellate Boletaceae genera by a combination of the following characters: the intense orange to red colour of the pileus and lamellae; bright yellow basal mycelium; ovoid or ellipsoid to broadly ellipsoid to subovoid basidiospores with non-bacillate surface; pleurocystidia originating more or less deeply in the subhymenium or from hymenophoral trama.
Erythrophylloporus paucicarpus
Raspé, Vadthanarat & Lumyong sp. nov.
823605
Figure 2.
Habits of Thai Erythrophylloporus species AE.paucicarpusBE.suthepensis. Scale bars: 1 cm.
Figure 3.
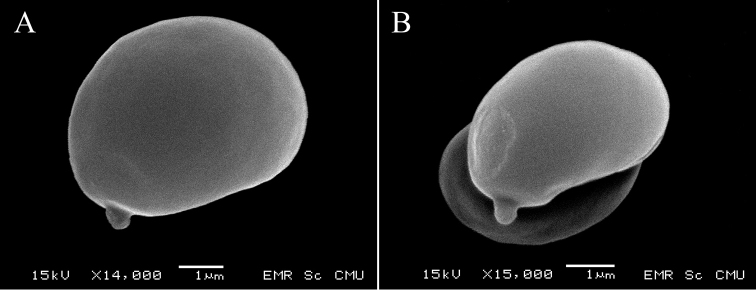
Scanning electron micrographs of basidiospores from Thai Erythrophylloporus show smooth surfaces AE.paucicarpusBE.suthepensis. Scale bars: 1 µm.
Figure 4.
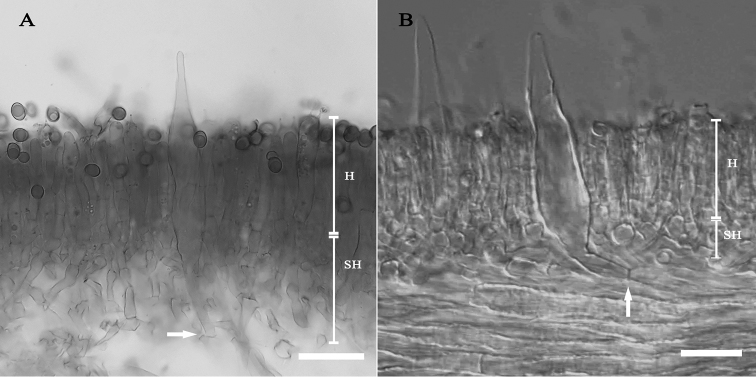
Origin of pleurocystidia (white arrow), more or less deep in the subhymenium or from hymenophoral trama AE.paucicarpusBE.suthepensis – hymenium (H), subhymenium (SH), Scale bars: 25 µm (A–B).
Figure 5.
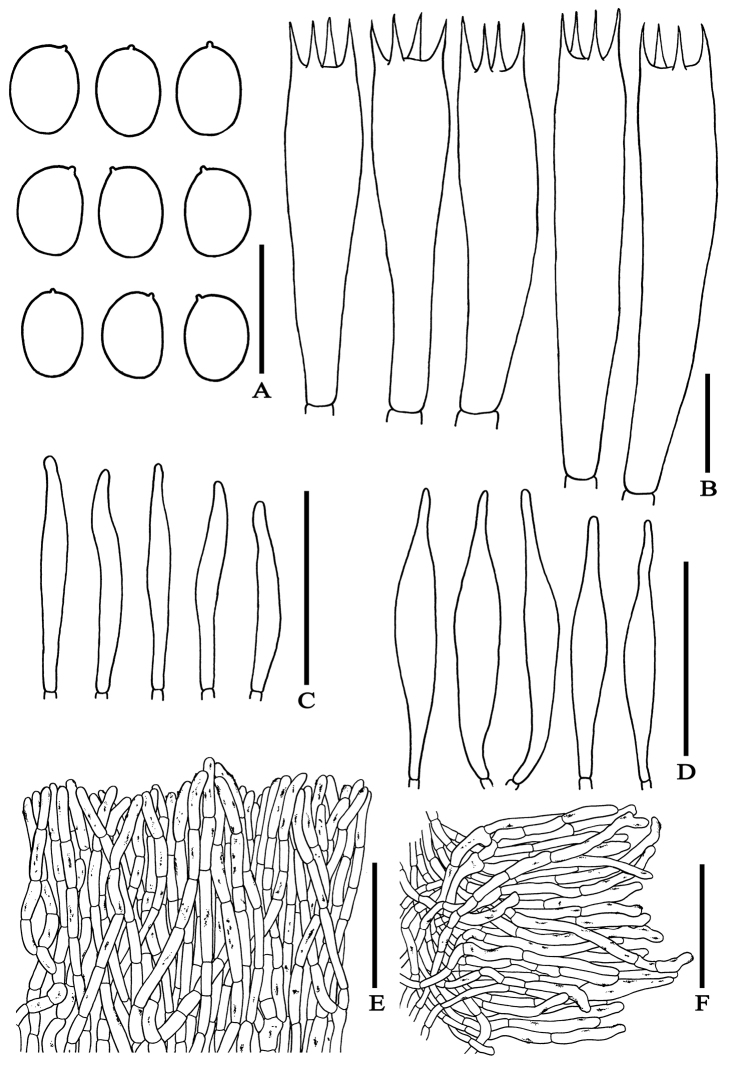
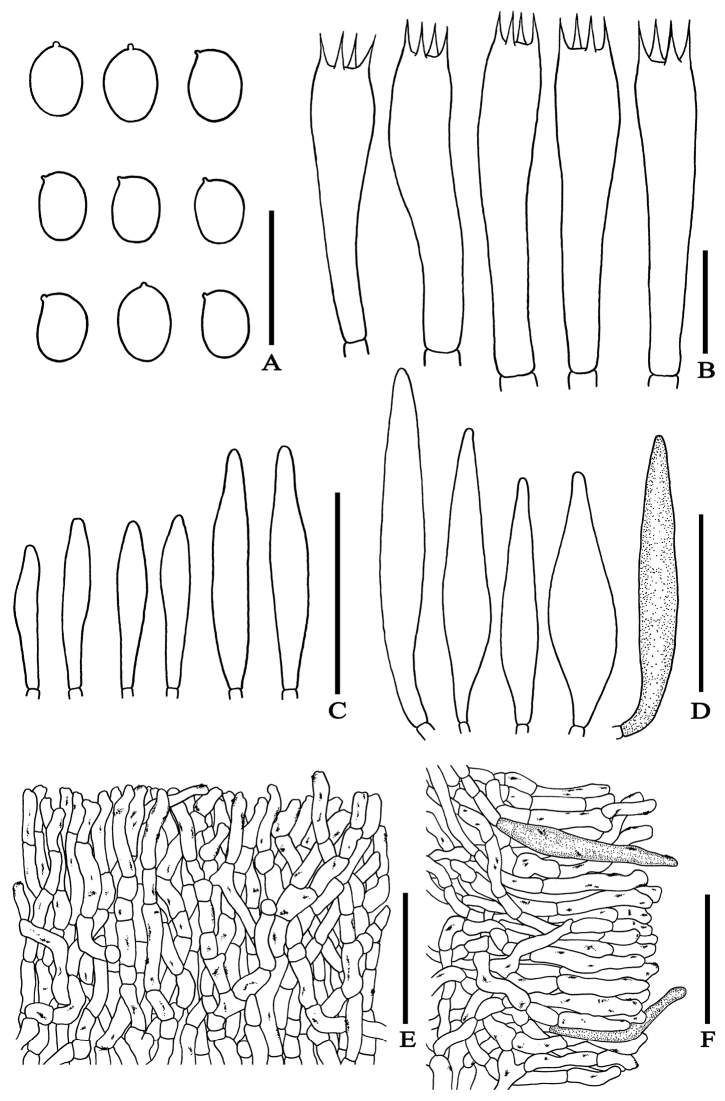
Microscopic features of ErythrophylloporuspaucicarpusA basidiospores B basidia C cheilocystidia D pleurocystidia E pileipellis F stipitipellis. – Scale bars: 10 µm (A–B); 50 µm (C–F). All drawings were made from the type (OR1151).
Holotype.
THAILAND, Chiang Mai Province, Mae On District, Huay Kaew, 18°52'0"N, 99°17'30"E, elev. 700 m, 16 August 2016, O. Raspé & S. Vadthanarat, OR1151, (holotype: CMUB, isotype: BR).
Etymology.
from Latin “pauci-” meaning few and “carpus” meaning fruits or what is harvested, refers to the low number of basidiomata produced.
Description.
Basidiomata stipitate-pileate with lamellate hymenophore, small to medium-sized. Pileus 2.3–5.5 cm in diameter, plano-convex with involute margin at first becoming almost plane to slightly depressed with inflexed to straight margin, irregularly and coarsely crenate in age, sometimes with low and broad umbo and a few to several verrucae, especially when young; surface more or less even, tomentose, dull, slightly moist, colour distribution patchy with red to brownish-orange (9B8 to 9C8), brownish-red (10E8 to 10D8) becoming orange-red to orange (8B/C8 to 6B7) at the margin when old, abruptly paler at the margin. Pileus context 3–4 mm thick half-way to the margin, tough, colour distribution even, yellow (3A6) to yellowish-orange (4A5), slowly reddening when exposed, especially at the centre and above lamellae. Stipe 2.4–4.5 × 0.7–1.3 cm, central or sometimes slightly eccentric, clavate with strigose base, straight to curved, terete, even, dull, dry, tomentose, yellowish-orange (4–5A7–8) to orange (6–7A7–8) with orange to yellowish-orange (7B/C7–8 to 4A7–8) coarse scales, with bright yellow (2A6–7) basal mycelium. Stipe context solid, fleshy fibrous, yellow marbled with olivaceous brown (4D5, 5D5). Hymenophore lamellate; lamellae decurrent, close, thick, 40–42 lamellae, with 4–6 different lengths of lamellullae, 2–4.5 mm wide half-way to margin, somewhat anastomosing, especially near the stipe, yellowish-orange (4-5A6-7) with orange to red tinge, slightly reddening when bruised. Odour rubbery; Taste not recorded. Spore print olive-brown (4E7).
Macrochemical reactions. KOH on pileus and stipe surface deep red at first, then red-brown to brown, with pale orange aura on the pileus; brown on pileus context, dark red-brown on stipe context; brownish-orange on hymenophore. NH4OH on pileus first red, then orange; on pileus context bluing at first then with a greenish tinge; on stipe surface and context briefly bluing; no reaction on hymenophore.
Basidiospores [208/4/4] (5.9–)6.1–6.8–7.5(–8) × (4.1–)4.6–5.1–5.5(–6) µm, Q = (1.2–)1.23–1.33–1.48(–1.56); from the type (OR1151) (6–)6.3–6.8–7.5(–7.8) × (4.6–)4.8–5.2–5.5(–6) µm, Q = (1.2–)1.22–1.31–1.48(–1.56), N = 88, broadly ellipsoid to ellipsoid, smooth under light microscope and SEM, yellowish to pale brown in water, hyaline in 5% KOH, thin-walled, inamyloid. Basidia 4–spored, (37.8–)38–45.6–54.7(–54.8) × (6.2–)–6.3–8–9.5(–9.6) µm, narrowly clavate to subcylindrical, attenuated towards the base, clampless, hyaline to yellowish hyaline in water, Melzer’s reagent and 5% KOH; sterigmata up to 5.5 µm long. Cheilocystidia (35.4–)35.5–49.9–61.8(–61.9) × (3.9–)3.9–6–7.7(–7.7) µm, narrowly fusiform with obtuse apex, projecting up to 30 µm, thin-walled, smooth, yellowish hyaline in water, in 5% KOH and NH4OH, inamyloid. Pleurocystidia (66.9–)67.4–80.3–93.5(–94.7) × (8.8–)8.9–11.7–16.1(–16.2) µm, abundant, narrowly conical with obtuse, somewhat prolonged apex, projecting up to 32 µm, thin-walled, smooth, yellowish hyaline in water, in 5% KOH and NH4OH, arising more or less deeply in the subhymenium or from hymenophoral trama, inamyloid. Hymenophoral trama subregular near the pileus context becoming slightly divergent near the edge, 87–238 µm wide, widest near the pileus context then getting narrower when close to the edge, composed of clampless hyphae 4.5–8 µm wide, yellowish hyaline in water, hyaline in 5% KOH and NH4OH. Pileipellis a palisadoderm to trichoderm 83–165 µm thick, composed of slightly thick-walled, cylindrical hyphae, terminal cells 16–46 × 4–6.5 µm with rounded apex, hyaline or yellowish hyaline to yellowish-orange hyaline hyphae ornamented with scattered fine epiparietal encrustation when observed in water, hyaline to yellowish hyaline in 5% KOH and NH4OH, inamyloid. Pileus trama composed of slightly thick-walled, strongly interwoven hyphae, 4.5–8.5 µm wide, inamyloid. Stipitipellis a disrupted palisadoderm perpendicular to the stipe axis, 63–145 µm thick, composed of slightly thick-walled, slightly rough, cylindrical, yellow to yellowish-orange in water, yellowish hyaline hyphae in 5% KOH and NH4OH, terminal cells 13–57 × 3–8 µm, cylindrical to irregular hyphae with rounded to notched apex; wall covered by dispersed fine encrustations when observed in water. Caulocystidia not seen. Stipe trama composed of parallel hyphae, densely packed, 4–8.5 µm wide; hyphae wall covered by dispersed encrustations when observed in water. Clamp connections not seen in any tissue.
Habit and habitat.
On soil, mostly solitary in dipterocarp forest dominated by Dipterocarpustuberculatus, D.obtusifolius, Shoreaobtusa, S.siamensis, Quercus spp. and Lithocarpus spp.
Known distribution.
Currently known only from Chiang Mai Province, northern Thailand.
Additional specimens examined.
– THAILAND, Chiang Mai Province, Muang District, Doi Suthep-Pui National Park, 18°48'05"N–98°55'40"E, elev. 800 m, 17 May 2015, O. Raspé, OR0615A (CMUB, BKF, BR); Mae Taeng District, Baan Tapa, 19°08'29"N, 98°45'47"E, elev. 1035 m, 4 August 2015, O. Raspé & A. Thawthong, OR0689 (MFLU, BR); Mae On District, Huay Kaew, 18°52'12"N, 99°18'12"E, elev. 780 m, 15 August 2016, O. Raspé & S. Vadthanarat, OR1135 (CMUB, BR).
Remarks.
E.paucicarpus is characterised by the following combination of features: orange to brownish- to orange-red basidiomata, yellowish-orange lamellae that turn slightly red when bruised; pileus context yellow to yellowish-orange that slowly reddens when exposed and mostly occurring as solitary basidiomata.
In the inferred molecular phylogeny, E.paucicarpus clustered close to E.suthepensis and E.cinnabarinus (65% BS and 1 PP), but the two species are different from E.paucicarpus in that they have darker lamellae which are orange to orange red or brownish-orange. Moreover, spores of E.paucicarpus are wider and longer (5.9–8 × 4.1–6 µm) than those of E.suthepensis (4.6–5.9 × 3.5–4.5 µm) and, on average, longer than those of E.cinnabarinus (5.5–7 × 4.5–5.5 µm) (Zhang and Li 2018). Erythrophylloporuspaucicarpus also differs from both species by the slight reddening of the context and lamellae when exposed or bruised, whereas E.suthepensis context seems unchanging when exposed and lamellae turn blue when bruised. In E.cinnabarinus, the context slowly turns dark violet, blackish-blue to dark blue when exposed and lamellae turn greyish-blue, or greyish-green when bruised (Zhang and Li 2018).
Erythrophylloporuspaucicarpus is different from the two New World species by the reddening of the context, whereas in E.fagicola, it turns blue and, in E.aurantiacus, the colour remains unchanged when exposed. Moreover, E.fagicola has somewhat thick-walled (0.8–3.5 µm) pleurocystidia (Montoya and Bandala 2011), which are not found in E.paucicarpus. Although the basidiospores of E.paucicarpus and E.aurantiacus are similar in size (E.aurantiacus = 6.0–7.5 × 4–5.5 µm), they differ in shape, being more ovoid in E.aurantiacus than in E.paucicarpus. Erythrophylloporuspaucicarpus also differs from E.aurantiacus by macro-chemical reactions. In the latter, the pileus surface and pileus context are unchanging with NH4OH (Halling et al. 1999), while in E.paucicarpus, the pileus becomes orange to red and the pileus context initially turns blue then with a greenish tinge.
Erythrophylloporus suthepensis
Vadthanarat, Raspé & Lumyong sp. nov.
823606
Figure 6.
Microscopic features of ErythrophylloporussuthepensisA basidiospores B basidia C cheilocystidia D pleurocystidia E pileipellis F stipitipellis showing some dark caulocystidia mixed with slightly rough, cylindrical to irregular hyphae. – Scale bars: 10 µm (A–B); 50 µm (C–F). All drawings were made from the type (SV0236).
Holotype.
THAILAND, Chiang Mai Province, Muang District, Doi Suthep-Pui National Park, 18°48'47"N, 98°55'56"E, elev. 645 m, 25 August 2015, S. Vadthanarat, SV0236, (holotype CMUB, isotype BKF, BR).
Etymology.
Refers to the type locality Doi Suthep.
Description.
Basidiomata stipitate-pileate with lamellate hymenophore, small-sized. Pileus (1.0–)2.5– 3.5 cm in diameter, subumbonate with involute margin at first, becoming convex to plano-convex with inflexed margin; surface even with some small pustules, tomentose, dull, slightly moist, yellow (3–4A4– 5) becoming light orange to orange-red (5–6A5–7 to 7–8A–B7–8) with patches of light yellow to light orange (4–5A5–6) becoming brownish-orange to dull red (7B–C8 to 8B–D8) with age, the colour of the margin when young clearly paler than the rest of the pileus, bluing when bruised. Pileus context 2–3 mm thick half-way to the margin, tough, yellowish-orange (4A5), unchanging when bruised. Stipe 2.5– 4.5 × 0.3– 0.8 cm, central, slightly curved, terete, dull, dry, yellowish-orange (2A6–7) with greyish-orange (5–6 B 7–8) coarse scales at first, then light yellow or reddish-yellow to brownish-orange (4A/B5–6 to 7C6) with brownish-red to reddish-dark brown (7F4–5, 8C7–8, 8F5–7) scales, sub-bulbous, with bright yellow to greyish-yellow (2A6–7 to 3A/B5–6) sparse basal mycelium that extends half-way up the stipe. Stipe context solid, tough, reddish-yellow (4A6) near the pileus then paler to light yellow (4A5) near the base, unchanging when bruised. Hymenophore lamellate; lamellae decurrent, subdistant, slightly thick, with sinuate edge, of varying lengths, 26–34 lamellae, with 4–6 different lengths of lamellullae, 4–5 mm wide half-way to margin, brownish-orange (7C7–8) with deep yellow to orange (4–5A7–8) edge, bluish-grey when looking tangentially to the surface, bluing when bruised. Odour rubbery. Taste mild with rubbery texture. Spore print olivaceous brown (4F5).
Macrochemical reactions. KOH orange-brown on pileus and stipe surface; yellowish-brown on pileus and stipe context and hymenophore. NH4OH yellowish-brown on pileus and stipe surface and hymenophore; yellowish on pileus and stipe context.
Basidiospores [218/4/2] (4.6–)4.8–5.2–5.7(–5.9) × (3.5–)3.6–4–4.3(–4.5) µm, Q = (1.15–)1.21–1.32–1.44(–1.57); from the type (SV0236) (4.6–)4.8–5.2–5.7(–5.9) × (3.5–)3.6–3.9–4.4(–4.5) µm, Q = (1.15–)1.21–1.32–1.43(–1.57), N = 80, broadly ellipsoid to subglobose, smooth under light microscope and SEM, yellowish to pale brown in water, hyaline in 5% KOH, thin-walled, inamyloid. Basidia 4-spored, (24.7–)25.3–31.1–35.8(–35.9) × (5.3–)5.3–6.6–7.5(–7.5) µm, narrowly clavate to subcylindrical, attenuated towards the base, clampless, hyaline to yellowish hyaline in water, Melzer’s reagent and 5% KOH; sterigmata up to 4.5 µm long. Cheilocystidia (37.3–)37.9–51–63.8(–64.1) × (5.3–)5.4–8.5–12.4(–13.7) µm, narrowly conical to narrowly fusiform with obtuse apex, projecting up to 25 µm, thin-walled, smooth, yellowish-hyaline in water, hyaline in 5% KOH and NH4OH, inamyloid, more or less forming a sterile edge . Pleurocystidia (46.5–)49.2–68.9–95.2(–99.3) × (9.3–)9.6–12.6–18.9(–20) µm, abundant, narrowly conical with obtuse apex, projecting up to 28 µm, thin-walled, mostly yellowish hyaline in water and hyaline in 5% KOH and NH4OH, some containing yellowish-brown to dark brown pigments in water and yellowish-pale brown in 5% KOH and NH4OH, inamyloid, arising more or less deeply in the subhymenium or from hymenophoral trama. Hymenophoral trama subregular near the pileus context becoming slightly divergent near the edge, 46–192 µm wide, widest near the pileus context then getting narrower when close to the edge, composed of clampless hyphae 2.5–7.5 µm wide, pinkish-red hyaline in water, especially at the centre of the trama, yellowish hyaline to hyaline in 5% KOH and NH4OH. Pileipellis a palisadoderm to trichoderm 71–119 µm thick, composed of slightly thick-walled, cylindrical to irregular hyphae with fine encrustation on the wall, terminal cells 12–46 × 3.5–9 µm with pointed to notched apex or sometimes truncated apex, with 6–15(–28) µm short cells at the base, hyaline or yellowish-orange hyaline to orange hyaline hyphae with scattered fine encrustation on the wall when observed in water, hyaline to yellowish hyaline in 5% KOH and NH4OH, inamyloid. Pileus context composed of slightly thick-walled, strongly interwoven hyphae, 5–8.5 µm wide, inamyloid. Stipitipellis a disrupted palisadoderm perpendicular to the stipe axis, 47–123 µm thick, composed of slightly thick-walled, cylindrical to irregular hyphae with fine encrustations on the wall, yellow to yellowish-orange, intermixed with mostly yellowish hyaline to yellowish-brown hyphae in 5% KOH and NH4OH, terminal cells 14–47 × 4–8.5 µm with variously notched apex. Caulocystidia mixed in a group with the stipitipellis hyphae, same shape and size as the pleurocystidia, dark brown in water, paler in 5% KOH and NH4OH. Stipe context composed of parallel, densely packed, 4–9.5 µm wide hyphae, hyphae wall with scattered fine encrustations when observed in water. Clamp connections not seen in any tissue.
Habit and habitat.
On soil, gregarious (up to 10 basidiomata) in dipterocarp forest dominated by Dipterocarpustuberculatus, D.obtusifolius, Shoreaobtusa and S.siamensis, mixed with scattered fagaceous trees.
Known distribution.
Currently known only from Doi Suthep-Pui National Park, Chiang Mai Province, northern Thailand.
Additional specimens examined.
– THAILAND, Chiang Mai Province, Meuang District, Doi Suthep-Pui National Park, 18°48'05"N, 98°55'40"E, elev. 800 m, 17 May 2015, O. Raspé, OR0615B (CMUB, BKF, BR).
Remarks.
Erythrophylloporussuthepensis is characterised by the following combination of features: yellow to light orange to orange red to brownish-orange to dull red pileus; brownish-orange lamellae with deep yellow to orange edge; the colour of the lamellae appears more bluish-grey when observed from an oblique angle to the surface; pileus surface and lamellae turning blue when bruised; some pleurocystidia containing yellowish-brown to dark brown pigments in water; basidiospores that are smaller or shorter (4.6–5.9 × 3.5–4.5 µm) than the other Erythrophylloporus species (E.aurantiacus = 6.0–7.5 × 4–5.5µm; E.cinnabarinus = 5.5–7 × 4.5–5.5 µm; E.fagicola = 6.5–11 × 4–7.5 µm; E.paucicarpus = 5.9–8 × 4.1–6 µm) (Halling et al. 1999, Montoya and Bandala 2011, Zhang and Li 2018).
Morphologically, E.suthepensis is quite similar to E.cinnabarinus in that they have similar colours in pileus and lamellae; the lamellae in both species also turn more or less blue to dark blue when bruised. Erythrophylloporussuthepensis and E.cinnabarinus are also similar, based on some pleurocystidia containing yellowish-brown to dark brown pigments, but those features are not found in E.paucicarpus and in the two New World Erythrophylloporus species (Halling et al. 1999, Montoya and Bandala 2011). However, the pleurocystidia containing brown pigments seem to be more frequent in E.cinnabarinus, which also has, on average, larger basidiospores than E.suthepensis (Zhang and Li 2018).
The pinkish-red hymenophoral trama of E.suthepensis was not found in either E.paucicarpus or in the two American Erythrophylloporus species. In our observation of the two American specimens (E.aurantiacus voucher REH7271 and E.fagicola voucher Garay215), we found that the hymenophoral trama was yellowish hyaline when observed in water. The original description of E.cinnabarinus does not mention the colour of the hymenophoral trama and we could not obtain a specimen to observe this character. However, other morphological characters and phylogenetic evidence are enough to differentiate E.suthepensis from E.cinnabarinus.
Our phylogenetic analyses of a four-gene dataset revealed that Phylloporusaurantiacus from Costa Rica and P.fagicola from Mexico clustered in the Erythrophylloporus clade with high support (BS = 100% and PP = 1). Both species possess the distinctive morphological characters of Erythrophylloporus, which include yellowish-orange to reddish-orange basidiomata, orange to orange brown lamellae, bright yellow basal mycelium, ovoid or ellipsoid to broadly ellipsoid basidiospores with smooth surface and subcylindrical to subfusoid to ventricose cheilocystidia and pleurocystidia (Halling et al. 1999, Montoya and Bandala 2011). Therefore, the following two new combinations are proposed:
Erythrophylloporus aurantiacus
(Halling & G.M. Muell.) Raspé & Vadthanarat comb. nov.
823607
Basionym.
Phylloporusaurantiacus Halling & G.M. Mueller, Mycotaxon 73: 64 (1999)
Specimen examined.
– COSTA RICA. Near town of Palo Verde, elev. 1600 m, 11 June 1994, Halling 7271 (NY).
Erythrophylloporus fagicola
(Montoya & Bandala) Raspé & Vadthanarat comb. nov.
823608
Basionym.
Phylloporusfagicola Montoya & Bandala, Mycotaxon 117: 10 (2011)
Specimen examined.
– MEXICO. Veracruz: Mpio. Acatlán, Acatlán Volcano, 29 September 2009, Garay 215 (XAL).
Key to the species in Erythrophylloporus
| 1 | Growing in North or Central America | 2 |
| – | Growing in Southeast Asia or in tropical to subtropical China | 3 |
| 2 | Bluing of the context when exposed; basidiospores ellipsoid to oblong, obtuse, 6.5–11 × 4–7.5 µm; pleurocystidia somewhat thick-walled (0.8–3.5 µm thick) | E. fagicola |
| – | Context unchanging when exposed; basidiospores ovoid to subellipsoid, 6.0–7.5 × 4–5.5 µm; pleurocystidia thin-walled | E. aurantiacus |
| 3 | Yellowish-orange lamellae slightly reddening when bruised; context slowly or slightly reddening when exposed | E. paucicarpus |
| – | Brownish-orange or orange, deep orange, reddish-orange to orange red lamellae bluing to greyish-green when bruised; context unchanging to gradually turning dark violet, blackish to dark blue | 4 |
| 4 | Basidiospores 4.6–5.9 × 3.5–4.5 µm, broadly ellipsoid to subglobose; cystidia mostly hyaline, only some containing yellowish-brown to dark brown pigments. | E. suthepensis |
| – | Basidiospores 5.5–7 × 4.5–5.5 µm, broadly ellipsoid, ellipsoid to nearly ovoid; cystidia usually containing yellowish-brown pigments | E. cinnabarinus |
Discussion
Both phylogeny and morphology support the placement of the two new species from Thailand, E.paucicarpus and E.suthepensis in the genus Erythrophylloporus. Phylogenetically, both species were highly supported in the Erythrophylloporus clade close to E.cinnabarinus (typus generis). Morphologically, they are characterised by having yellowish-orange to reddish- to brownish-orange basidiomata with bright yellow basal mycelium and smooth, ellipsoid, broadly ellipsoid to subglobose basidiospores. The other lamellate Boletaceae in Phylloporus, Phylloboletellus and Phylloporopsis are solely similar to the new species by having a lamellate hymenophore instead of a poroid hymenophore. However, Phylloporus differs from Erythrophylloporus species by having whitish- to yellowish-pale brown basidiomata with yellow to golden-yellow lamellae, with off-white to whitish to yellow basal mycelium and most species in the genus have basidiospores with more or less bacillate ornamentation under SEM (Neves & Halling 2010, Neves et al. 2012, Zeng et al. 2013). The single Phylloboletellus species, Ph.chloephorus Singer differs from Erythrophylloporus by having longitudinally ridged basidiospores (Bandala et al. 2004). The sole species of Phylloporopsis, Phy.boletinoides, differs by having beige to olive-cream or olive buff lamellate to subporoid hymenophore, with anastomosing and interveined gills and basal mycelium whitish to yellowish (Farid et al. 2018). Moreover, those genera are phylogenetically distant from Erythrophylloporus. (Bandala et al. 2004, Neves & Halling 2010, Neves et al. 2012, Zeng et al. 2013, Farid et al. 2018).
Interestingly, Phylloporuscoccineus Corner, described from Singapore (Corner 1970), is similar to Erythrophylloporus species, in that it produces crimson to scarlet, lamellate basidiomata with orange to orange-red lamellae and yellow basal mycelium, broadly ellipsoid to subglobose and smooth basidiospores. It probably should also be transferred to Erythrophylloporus, but we refrain from doing so until specimens become available for molecular study. According to the protologue of P.coccineus, it differs from the newly described Asian species of Erythrophylloporus by having larger basidiospores (7.5–10 × 6.5–8 µm), larger cheilocystidia (70–120 × 10–18 µm) and larger caulocystidia (up to 200 × 10–16 µm) (Corner 1970).
Erythrophylloporus species formed two clades, an Asian species clade (BS = 65% and PP = 1) and a New World species clade (BS = 100% and PP = 1) (Fig. 1). The Asian one contains three species, E.cinnabarinus, E.paucicarpus and E.suthepensis, while the American clade contains the remaining two species E.aurantiacus and E.fagicola. Erythrophylloporusaurantiacus and E.fagicola seem to be genetically very close to each other, much closer than the species in the Asian clade. Only morphological differences between the two species were used to separate them from each other. Erythrophylloporusfagicola produces larger basidiospores than E.aurantiacus and pleurocystidia are somewhat thick-walled (0.8–3.5 µm thick) in E.fagicola, whereas they are thin-walled in E.aurantiacus and the latter has non-staining context, whereas the former has a cyanescent context. However, the descriptions were based on a limited number of collections and more samples are desirable to verify whether the morphological traits observed are good characters differentiating the two species or merely extremes of a continuum in morphological variation within a single species.
Regarding the phylogenetic affinities of Erythrophylloporus, Zhang and Li (2018) reported that it was likely close to the genus Rugiboletus G. Wu & Zhu L. Yang and Lanmaoa G. Wu & Zhu L. Yang, based on a multilocus dataset of nrLSU, tef1, rpb1 and rpb2, although this relationship was not supported in their phylogram. In our phylogeny, based on a multilocus dataset of atp6, tef1, rpb2 and cox3, with wider taxon sampling, Erythrophylloporus also clustered within the Pulveroboletus group, but was sister to Singerocomus with high bootstrap support (96%) but relatively weak posterior probability support (0.86). Singerocomus contains three species, S.atlanticus A.C. Magnago, S.inundabilis (Singer) T.W. Henkel and S.rubriflavus T.W. Henkel & Husbands that have some similar morphological characters to Erythrophylloporus, including red-orange to red pileus and light yellow basal mycelium. The three existing Singerocomus species are clearly different from all known Erythrophylloporus species by having a poroid, non-cyanescent hymenophore (Henkel et. al. 2016, Magnago et al. 2018). However, the hymenophore structure (lamellate vs. poroid) is not sufficient to separate genera in Boletaceae. Phylloporus currently contains both lamellate and poroid species, although some poroid species have already been transferred to another genus, Hourangia (Zhu et al. 2015). Phylogenetic analyses, including the remaining poroid Phylloporus species, are needed to verify their taxonomic position.
Erythrophylloporus putatively forms ectomycorrhizal associations with trees in family Fagaceae, including the genera Fagus, Lithocarpus and Quercus (Neves and Halling 2010, Montoya and Bandala 2011, Zhang and Li 2018). The two Thai Erythrophylloporus species were found in forests dominated by Dipterocarpaceae trees, mainly Dipterocarpus, including D.tuberculatus, D.obtusifolius and Shorea, including S.obtusa and S.siamensis. However, some Quercus and Lithocarpus trees (Fagaceae) were also observed in the vicinity and could also be the ectomycorrhizal partners. Further study is needed to confirm the ectomycorrhizal relationships of Erythrophylloporus.
Supplementary Material
Acknowledgements
Financial support from the Graduate School, Chiang Mai University, is appreciated. The work was partly supported by a TRF Research Team Association Grant (RTA5880006) to SL and OR. OR is grateful to the Fonds National de la Recherche Scientifique (Belgium) for travel grants. Authors are grateful for the permit number 0907.4/4769 granted by the Department of National Parks, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment for collecting in Doi Suthep-Pui National Park.
Citation
Vadthanarat S, Amalfi M, Halling RE, Bandala V, Lumyong S, Raspé O (2019) Two new Erythrophylloporus species (Boletaceae) from Thailand, with two new combinations of American species. MycoKeys 55: 29–57. https://doi.org/10.3897/mycokeys.55.34570
Funding Statement
1. Fonds National de la Recherche Scientifique (Belgium) 2. TRF Research Team Association Grant (RTA5880006) 3. Graduate School, Chiang Mai University
References
- Bandala VM, Montoya L, Jarvio D. (2004) Two interesting records of boletes found in coffee plantations in eastern Mexico. Persoonia 18: 365–380. [Google Scholar]
- Binder M, Hibbett DS. (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981. 10.1080/15572536.2006.11832626 [DOI] [PubMed] [Google Scholar]
- Binder M, Larsson KH, Matheny PB, Hibbett DS. (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of agaricomycetidae dominated by corticioid forms. Mycologia 102: 865–880. 10.3852/09-288 [DOI] [PubMed] [Google Scholar]
- Chuankid B, Vadthanarat S, Hyde KD, Thongklang N, Zhao R, Lumyong S, Raspé O. (2019) Three new Phylloporus species from tropical China and Thailand. Mycological Progress 18(5): 603–614. 10.1007/s11557-019-01474-6 [DOI] [Google Scholar]
- Corner EJH. (1970) Phylloporus Quél. and Paxillus Fr. in Malaya and Borneo. Nova Hedwigia 20: 793–822. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- Doyle JJ, Doyle JL. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. 10.2307/2419362 [DOI] [Google Scholar]
- Farid A, Gelardi M, Angelini C, Franck AR, Costanzo F, Kaminsky L, Ercole E, Baroni TJ, White AL, Garey JR, Smith ME, Vizzini A. (2018) Phylloporus and Phylloboletellus are no longer alone: Phylloporopsis gen. nov. (Boletaceae), a new smooth-spored lamellate genus to accommodate the American species Phylloporusboletinoides. Fungal Systematics and Evolution 2: 341–359. 10.3114/fuse.2018.02.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling RE, Mueller GM, Dallwitz MJ. (1999) A new Phylloporus (Basidiomycetes, Boletaceae) with a key to species in Colombia and Costa Rica. Mycotaxon 73: 63–67. [Google Scholar]
- Halling RE, Nuhn M, Fechner NA, Osmundson TW, Soytong K, Arora D, Hibbett DS, Binder M. (2012a) Sutorius: a new genus for Boletuseximius. Mycologia 104(4): 951–961. 10.3852/11-376 [DOI] [PubMed] [Google Scholar]
- Halling RE, Nuhn M, Osmundson T, Fechner N, Trappe JM, Soytong K, Arora D, Hibbett DS, Binder M. (2012b) Affinities of the Boletuschromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Australian Systematic Botany 25: 418–431. 10.1071/SB12028 [DOI] [Google Scholar]
- Henkel TW, Obase K, Husbands D, Uehling JK, Bonito G, Aime MC, Smith ME. (2016) New Boletaceae taxa from Guyana: Binderoboletussegoi gen. and sp. nov., Guyanaporusalbipodus gen. and sp. nov., Singerocomusrubriflavus gen. and sp. nov., and a new combination for Xerocomusinundabilis. Mycologia 108(1): 157–173. 10.3852/15-075 [DOI] [PubMed] [Google Scholar]
- Hosen MI, Feng B, Wu G, Zhu XT, Li YC, Yang ZL. (2013) Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Diversity 58: 215–226. 10.1007/s13225-012-0211-8 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerp A, Wanscher JH. (1978) Methuen Handbook of Colour (3rd edn). Eyre Methuen Ltd, London, 252 pp. [Google Scholar]
- Kretzer AM, Bruns TD. (1999) Use of atp6 in fungal phylogenetics: an example from the Boletales. Molecular Phylogenetics and Evolution 13: 483–492. 10.1006/mpev.1999.0680 [DOI] [PubMed] [Google Scholar]
- Li YC, Feng B, Yang ZL. (2011) Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Diversity 49: 125–143. 10.1007/s13225-011-0096-y [DOI] [Google Scholar]
- Li YC, Li F, Zeng NK, Cui YY, Yang ZL. (2014) A new genus Pseudoaustroboletus (Boletaceae, Boletales) from Asia as inferred from molecular and morphological data. Mycological Progress 13: 1207–1216. 10.1007/s11557-014-1011-1 [DOI] [Google Scholar]
- Magnago AC, Henkel T, Neves MA, Borges da Silveira RM. (2018) Singerocomusatlanticus sp. nov., and a first record of Singerocomusrubriflavus (Boletaceae, Boletales) for Brazil. Acta Botanica Brasilica 32(2): 222–231. 10.1590/0102-33062017abb0320 [DOI] [Google Scholar]
- Matheny PB. (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. (2009) The CIPRES portals. CIPRES. http://www.phylo.org/portal2/home
- Montoya L, Bandala VM. (2011) A new Phylloporus from two relict Fagusgrandifoliavar.mexicana populations in a montane cloud forest. Mycotaxon 117: 9–18. 10.5248/117.9 [DOI] [Google Scholar]
- Neves MA, Binder M, Halling R, Hibbett D, Soytong K. (2012) The phylogeny of selected Phylloporus species inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Diversity 55(1): 109–123. 10.1007/s13225-012-0154-0 [DOI] [Google Scholar]
- Neves MA, Halling RE. (2010) Study on species of Phylloporus I: Neotropics and North America. Mycologia 102(4): 923–943. 10.3852/09-215 [DOI] [PubMed] [Google Scholar]
- Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett DS. (2013) Phylogenetic overview of the Boletineae. Fungal Biology 117: 479–511. 10.1016/j.funbio.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Orihara T, Lebel T, Ge Z-W, Smith ME, Maekawa N. (2016) Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite-like insertions for molecular identification. Persoonia 37: 173–198. 10.3767/003158516X691212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihara T, Smith ME, Shimomura N, Iwase K, Maekawa N. (2012) Diversity and systematics of the sequestrate genus Octaviania in Japan: two new subgenera and eleven new species. Persoonia 28: 85–112. 10.3767/003158512X650121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Jones EBG, Maharachchikumbura SSN et al. (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Diversity. 10.1007/s13225-019-00421-w [DOI]
- Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S. (2016) Pulveroboletusfragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycological Progress 15: 38. 10.1007/s11557-016-1179-7 [DOI]
- Rehner SA, Buckley E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. 10.3852/mycologia.97.1.84 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006) Raxml-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Thiers B. (continuously updated) Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum. nybg.org/science/ih/
- Vadthanarat S, Lumyong S, Raspé O. (2019) Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 54: 1–29. https://doi: 10.3897/mycokeys.54.35018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadthanarat S, Raspé O, Lumyong S. (2018) Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R.rostratispora from Thailand. MycoKeys 29: 63–80. 10.3897/mycokeys.29.22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Feng B, Xu J, Zhu XT, Li YC, Zeng NK, Hosen MI, Yang ZL. (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. 10.1007/s13225-014-0283-8 [DOI] [Google Scholar]
- Wu G, Li YC, Zhu XT, Zhao K, Han LH, Cui YY, Li F, Xu JP, Yang ZL. (2016) One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. 10.1007/s13225-016-0375-8 [DOI] [Google Scholar]
- Wu G, Zhao K, Li YC, Zeng NK, Feng B, Halling RE, Yang ZL. (2015) Four new genera of the fungal family Boletaceae. Fungal Diversity 81: 1–24. 10.1007/s13225-015-0322-0 [DOI] [Google Scholar]
- Zhang M, Li TH. (2018) Erythrophylloporus (Boletaceae, Boletales), a new genus inferred from morphological and molecular data from subtropical and tropical China. Mycosystema 37(9): 1111–1126. [Google Scholar]
- Zhao K, Wu G, Feng B, Yang ZL. (2014a) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycological Progress 13: 1127–1136. 10.1007/s11557-014-1001-3 [DOI] [Google Scholar]
- Zhao K, Wu G, Yang ZL. (2014b) A new genus, Rubroboletus, to accommodate Boletussinicus and its allies. Phytotaxa 188: 61–77. 10.11646/phytotaxa.188.2.1 [DOI] [Google Scholar]
- Zeng NK, Tang LP, Li YC, Tolgor B, Zhu XT, Zhao Q, Yang ZL. (2013) The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Diversity 58: 73–101. 10.1007/s13225-012-0184-7 [DOI]
- Zhu XT, Wu G, Zhao K, Halling RE, Yang ZL. (2015) Hourangia, a new genus of Boletaceae to accommodate Xerocomuscheoi and its allied species. Mycological Progress 14: 37. 10.1007/s11557-015-1060-0 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.