Abstract
The human gut contains trillions of symbiotic bacteria that play a key role in programming different aspects of host physiology in health and disease. Psychotropic medications act on the central nervous system (CNS) and are used in the treatment of various psychiatric disorders. There is increasing emphasis on the bidirectional interaction between drugs and the gut microbiome. An expanding body of evidence supports the notion that microbes can metabolise drugs and vice versa drugs can modify the gut microbiota composition. In this review, we will first give a comprehensive introduction about this bidirectional interaction, then we will take into consideration different classes of psychotropics including antipsychotics, antidepressants, antianxiety drugs, anticonvulsants/mood stabilisers, opioid analgesics, drugs of abuse, alcohol, nicotine and xanthines. The varying effects of these widely used medications on microorganisms are becoming apparent from in vivo and in vitro studies. This has important implications for the future of psychopharmacology pipelines that will routinely need to consider the host microbiome during drug discovery and development.
Keywords: Psychotropic, Antipsychotic, Antidepressant, Antimicrobial, Gut microbiome
Introduction
In the second instalment of J.K. Rowling’s Harry Potter book series, the trainee wizards encounter a magical Chamber of Secrets deep within the Hogwarts School. In some ways, this story parallels the human body, with the gastrointestinal tract hidden within and guarding many secrets.
The human gastrointestinal tract harbours trillions of microbes, the gut microbiota, which help modulate developmental, immunological and nutritional function in the host (Bengmark 2013; Sampson and Mazmanian 2015; Soto et al. 2018; Valdes et al. 2018; Wang et al. 2016). The colonisation of the gut is generally believed to begin at birth with the infant initially receiving microbial colonisation from the mother as it passes through the birth canal, although this notion has been challenged by a limited number of studies in which microbes were detected in the placenta (Aagaard et al. 2014; Collado et al. 2016; DiGiulio 2012). In a recent review paper, a critical assessment of the evidence supporting these two opposing hypotheses has been carried out and the authors argue that the evidence in support of the “in utero colonization hypothesis” is conceptually and materially flawed (Perez-Muñoz et al. 2017). Events such as illness, antibiotic treatment and changes in diet cause shifts in the microbiota (Codagnone et al. 2018; Conlon and Bird 2015; De Filippo et al. 2010; Rodrigues et al. 2017; Wang et al. 2017). Mode of delivery at birth also affects the microbiota composition, with vaginally delivered infants containing a high abundance of lactobacilli during the first few days, a reflection of the high load of lactobacilli in the vaginal flora (Aagaard et al. 2012; Avershina et al. 2014). In early stages of development, the microbiota is generally low in diversity and is dominated by two main phyla, Actinobacteria and Proteobacteria (Rodriguez et al. 2015). During the first year of life, the microbial diversity increases and by around 2.5 years of age, the composition, diversity and functional capabilities of the infant microbiota resemble those of an adult microbiota (Koenig et al. 2011; Rodriguez et al. 2015). In individuals over the age of 65, the microbial community changes, with an increased abundance of Bacteroidetes phyla and Clostridium cluster IV, in contrast with younger subjects where the cluster XIVa is more prevalent (Claesson et al. 2011). It has been demonstrated that the microbiota of young adults and 70-year-old people is highly similar but differs significantly from that of centenarians (Biagi et al. 2010).
The role of the microbiota in health and disease has stretched to all disciplines of medicine and this now includes pharmacology and therapeutics (Walsh et al. 2018). The field of pharmacomicrobiomics has emerged over the past decade (ElRakaiby et al. 2014; Saad et al. 2012) and has predominantly focused on the impact that the gut microbiota exerts on drug metabolism. Also, a growing body of research has demonstrated that several pharmaceutical compounds, including paracetamol, digoxin, metformin and cancer drugs among others, influence the human gut microbiota and/or microbial isolated strains. As bacteria can, in turn, modulate drug efficacy and toxicity (Alexander et al. 2017; Currò 2018; Li et al. 2016), the emerging drug-microbe bidirectional interaction might be crucial for future drug development and clinical practice. Moreover, this suggests that drug-related confounding effects should be taken into consideration in future microbiome association studies.
In this review, we will focus on psychotropic compounds (from the Greek root psychè = mind and tropòs = turning), which modulate brain and behaviour, and we will explore the scientific evidence on the interaction between psychotropic compounds and the gut microbiome in vivo or in isolated strains (in vitro) (Fig. 1). For each class of psychotropic compound taken into consideration, sub-sections will be based on the experimental approach used (observations in vitro, in vivo or in humans). Regarding in vitro experiments, some attempts have been made to try and find the best dose translational to the human gut setting. Maier and colleagues have deduced colon concentrations on the basis of drug excretion patterns from published work and small intestine concentrations on the basis of daily doses of individual drugs. Based on their approximations, a threshold of 20 μM was below the median small intestine and colon concentration of the majority of human-targeted drugs (Maier et al. 2018). It is important to keep this in mind when considering data generated from in vitro isolated microbial strains.
Fig. 1.
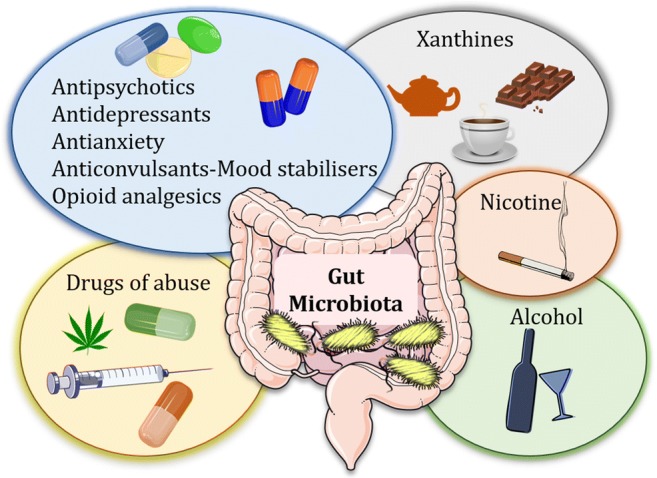
Psychotropic compounds affect the gut microbiota composition
The literature search (PubMed) was conducted using the following terms as inclusion criteria: the chemical name of each drug belonging to either of the following classes: antipsychotics, antidepressants, antianxiety drugs, anticonvulsants/mood stabilisers, opioid analgesics, drugs of abuse OR alcohol/ethanol OR nicotine OR xanthines (caffeine, theobromine, theophylline) AND (gut microbiome OR gut microbiota OR antimicrobial) up to 20 January 2019. Reviews, meta-analyses and systematic reviews were omitted from the search strategy.
Drugs affect the gut microbiota
In Rowling’s book, the trainee wizards eventually find the gate to the Chamber of Secrets; similarly, xenobiotic compounds belonging to several therapeutic classes can reach the Chamber and affect what is hidden within, our gut microbiota.
Antibiotics represent the most direct and effective way of targeting intestinal microbes. Evidence gathered from in vitro and in vivo studies suggests that a course of short-term antibiotics can substantially change the gut microbiota composition (Jakobsson et al. 2010; Maurice et al. 2013). Several host-targeting non-antibiotic drugs have also been shown to influence the gut microbiota. In a population-based cohort, deep sequencing of gut microbiomes of 1135 participants showed relations between the microbiota and 19 drug groups (Zhernakova et al. 2016). Other studies have pointed out an association between drug consumption and microbiome. Analysis of two independent population-level cohorts revealed that, among different factors, the use of medications was responsible for the largest total variance and interacted with other covariate-microbiota associations (Falony et al. 2016). The composition of the gut microbiota can change in relation to the number and type of medications consumed. Differences in the relative abundance of specific bacteria were detected in individuals taking a single drug, a combination or none. In particular, there were differences in the gut microbiota of individuals taking NSAIDs (non-steroidal anti-inflammatory drugs) with PPIs (proton-pump inhibitors) versus those taking NSAIDs without PPIs (Rogers and Aronoff 2016). Regarding polypharmacy, in elderly hospitalised patients, there was a significant negative correlation between the number of drugs and microbial alpha-diversity (Chao1 index). Moreover, the number of drugs was associated with the average relative abundance of 15 different taxa, with PPIs, antidepressants and antipsychotics exhibiting the strongest association with single bacteria abundance (Ticinesi et al. 2017).
The gut microbiota affects the pharmacokinetics of drugs
According to the novel, the Chamber of Secrets is home of a giant serpent, the basilisk. Similar to the basilisk, which has the ability to kill by looking people in the eyes, the microbes harboured in our guts can influence to a certain extent the pharmacokinetics of drugs.
Pharmacokinetics (from the Greek root pharmakon = drug and kinetikos = moving, “putting in motion”) is a branch of pharmacology dedicated to determining the fate of xenobiotics administered to a living organism. Absorption is one of the four compartments of the pharmacokinetics multi-compartmental model (Arundel 1997), together with distribution, metabolism and excretion (ADME) (Pacey et al. 2011).
In the next two sections, we provide some of the most compelling evidence on the interaction between gut microbiome and drug absorption/metabolism prior to discussing the relevance to psychotropic compounds.
The gut microbiota affects drug absorption
In pharmacology, absorption is the movement of a substance from the site of administration to the bloodstream (Doogue and Polasek 2013). Very little is currently known about the role played by the gut microbiota in drug absorption but a few reports on the topic exist. It is interesting to note that all the three studies mentioned in this section use the same experimental approach: manipulation of the gut microbiota through administration of probiotics.
The action of gliclazide, a sulfonylurea used to treat diabetes, may be enhanced by administering probiotics. In diabetic rats, the blood levels of gliclazide are higher following a 3-day pre-treatment with probiotics (at the dose of 75 mg/kg) compared to non-treated rats, suggesting that the gut microbiota might mediate the extent of the drug absorption (Al-Salami et al. 2008). In a recent study, a 3-day administration of Lactobacillus reuteri K8 reduced the absorption of orally administered acetaminophen in mice, whereas administration of Lactobacillus reuteri K9 did not have an effect (Kim et al. 2018). This effect was probably mediated by probiotic-induced modulation of gut microbial enzyme activity given that the probiotic significantly increased both sulfatase and arylsulfate transferase and significantly decreased β-glucuronidase, which are the bacterial enzymes involved in acetaminophen metabolism. Finally, the antiarrhythmic drug amiodarone shows elevated blood levels following administration of probiotics in rats. In details, the probiotic E. coli strain Nissile 1917 was administered to rats for 7 days, followed by a single dose of amiodarone per os. The probiotic increased amiodarone plasmatic levels by 43% compared to saline-treated controls, suggesting a microbiota-mediated increase in drug absorption (Matuskova et al. 2014).
The gut microbiota affects drug metabolism
The fate of xenobiotics depends not only by the host but also by the bacteria harbouring our gastrointestinal tract and it has become more investigated, over the past decades, the role of gut microbiome in xenobiotic metabolism. The whole field has been termed “pharmacomicrobiomics” (Rizkallah et al. 2010). In this paragraph, we offer a glimpse into the known effects of the gut microbiota on drug metabolism.
Digoxin, a cardiac glycoside that has been widely used for hundreds of years to treat heart failure and arrhythmias, is a striking example. This drug is inactivated in the gut by the Actinobacterium Eggerthella lenta (Haiser et al. 2013). Moreover, increased consumption of dietary protein in germ-free mice inhibited the reduction of digoxin by E. lenta (Haiser et al. 2013). The microbial biotransformation of orally administered lovastatin, a drug used for lowering cholesterol levels and reduce the risk of cardiovascular disease, was reduced by concomitant administration of antibiotics in rats (Yoo et al. 2014). This could result in altered systemic concentrations of either the intact drug and/or its metabolites (Yoo et al. 2014). Amlodipine, a medication used to treat high blood pressure and coronary artery disease, undergoes clearance when incubated with a faecal suspension, suggesting that the gut microbiota metabolises this drug (Yoo et al. 2016). As a confirmation, a 2-day treatment with the antibiotic ampicillin in rats increases the plasma levels of amlodipine, possibly because of the decreased microbial biotransformation in the gastrointestinal tract (Yoo et al. 2016). Mesalazine, also known as 5-aminosalicylic acid (5-ASA), is an anti-inflammatory drug used to treat inflammatory bowel disease, including ulcerative colitis or to maintain remission in Crohn’s disease (Rachmilewitz 1989). The faecal microbiota plays a key role in acetylating 5-ASA, with 44% of anaerobic bacteria tested in incubation with the drug exhibiting this property (van Hogezand et al. 1992). The metabolism of sulfasalazine, a drug used for the treatment of rheumatoid arthritis, ulcerative colitis and Crohn’s disease, is also likely to be mediated by intestinal bacteria. When sulfasalazine was fed to conventional rats, none of the drug was recovered in the urine, faeces or caecum; however, if administered in combination with the antibiotic neomycin, the drug was recovered in faeces and the caecum (Peppercorn and Goldman 1972). In addition, when sulfasalazine was given to germ-free rats, recovery of drug in the faeces was over 50% whereas the urine contained an additional 1–2%. In germ-free rats infected with four specific bacteria normally found in the intestinal tract of rodents, sulfasalazine was metabolised as in conventional rats (Peppercorn and Goldman 1972). Sulfasalazine is metabolised by azoreductases in the gut. The probiotic strains Lactobacillus acidophilus L10, Bifidobacterium lactis B94 and Streptococcus salivarius K12 given to rats for three consecutive days increased azoreductase activity in ex vivo colon contents with a corresponding increase in sulfasalazine metabolism (Lee et al. 2012). Interestingly, however, the same probiotic treatment in rats, followed by an oral 100 mg/kg dose of sulfasalazine, did not alter the pharmacokinetic parameters (Lee et al. 2012). Administration of diclofenac, a nonsteroidal anti-inflammatory drug (NSAID), induced enteropathy in mice; however, oral pre-treatment with a bacteria-specific β-glucuronidase inhibitor was able to protect against diclofenac-induced enteropathy (LoGuidice et al. 2012), suggesting that the gut microbiota might play a crucial role in the metabolism of this medication. The antithrombotic effect of aspirin seems to be affected by the gut microbiota. In rats, administration of the antibiotic ampicillin significantly prolongs the bleeding time in aspirin-dosed rats (Kim et al. 2016). Moreover, oral administration of ampicillin reduces the aspirin-metabolising activity of the microbiota by 67% (Kim et al. 2016).
Intestinal microbial azoreductases play a key role in the reduction of azo dyes (Chung et al. 1992). A wide variety of anaerobic bacteria isolated from caecal or faecal contents from experimental animals and humans have the ability to cleave the azo linkages to produce aromatic amines (Chung et al. 1992). Moreover, the azoreductase activity in a variety of intestinal preparations is affected by various dietary factors including antibiotics and supplementation with live cultures of lactobacilli (Chung et al. 1992).
Choline and carnitine are dietary amines that have wide-ranging roles in human metabolism (Zeisel and da Costa 2009) and are precursors of trimethylamine (TMA), a compound that can cause trimethylaminuria when not appropriately metabolised by the host (Mackay et al. 2011). In a recent study, the quantification and detailed characterisation of the TMA-producing bacteria in human faecal samples have resulted particularly in Clostridium XIVa strains and Eubacterium sp. strain AB3007 (Rath et al. 2017). In a different study, carnitine metabolism was mediated by Rieske-type oxygenases present in the human microbiota (Zhu et al. 2014).
Chemotherapeutic drugs have also been shown to be metabolised by the gut microbiota (Alexander et al. 2017). Of 30 chemotherapeutic drugs examined in vitro, the efficacy of 10 was found to be significantly inhibited by certain bacteria, while the same bacteria improved the efficacy of six others (Lehouritis et al. 2015). As further corroboration of these findings, the chemoresistance or increased cytotoxicity observed in vitro with sample drugs (gemcitabine and CB1954) was replicated in in vivo murine subcutaneous tumour models (Lehouritis et al. 2015). The dose-limiting side effect of the common colon cancer chemotherapeutic irinotecan is severe diarrhoea that arises following reactivation of the drug by symbiotic bacterial β-glucuronidases in the gut (Ma and McLeod 2003; Mathijssen et al. 2001). Oral administration of a bacterial β-glucuronidase inhibitor protected mice from irinotecan-induced toxicity, suggesting that such inhibitors may be designed to prevent undesirable enzyme activities in the intestine (Wallace et al. 2010). The gut microbiota also plays a crucial role in the metabolism of 5-fluorouracil, another chemotherapeutic compound (Nakayama et al. 1997). The antineoplastic drug doxorubicin is effectively metabolised by Raoultella planticola in vitro, as demonstrated by Yan and colleagues (Yan et al. 2018). Specifically, R. planticola was shown to deglycosylate doxorubicin into its metabolites 7-deoxydoxorubicinol and 7-deoxydoxorubicinolone via a reductive deglycosylation mechanism. Moreover, doxorubicin was degraded anaerobically by Klebsiella pneumoniae and E. coli BW25113 in vitro (Yan et al. 2018). In a recent study, 5-fluorouracil (5-FU) and 5-fluoro-2′-deoxyuridine (FUDR) were found to act through bacterial ribonucleotide metabolism to elicit their cytotoxic effects in Caenorhabditis elegans (Garcia-Gonzalez et al. 2017), suggesting that bacteria in the host play an important role in the response to chemotherapeutics. Similar findings were also obtained in a different study (Scott et al. 2017). Finally, a recent study found that the anticancer immune effects of cyclophosphamide are modulated by the gut microbiota. Indeed, the changes induced by this chemotherapeutic on the gut microbiota stimulate the generation of a specific subset of “pathogenic” T helper 17 cells and immune responses typically associated to this medication (Viaud et al. 2013).
Interestingly, two studies have also highlighted a role for the microbiome in patients undergoing anti-programmed cell death 1 protein (PD-1) immunotherapy (Gopalakrishnan et al. 2018; Matson et al. 2018; Routy et al. 2018). The diversity and composition of the microbial community differed between responders and non-responders, accompanied by functional differences in gut bacteria in responders (including enrichment of anabolic pathways) (Gopalakrishnan et al. 2018). In the same study, immune profiling suggested enhanced systemic and antitumor immunity in responding patients with a favourable gut microbiome as well as in germ-free mice receiving faecal transplants from responding patients (Gopalakrishnan et al. 2018). Resistance to immunotherapy can be attributed to abnormal gut microbiome composition, according to a different study. Antibiotics administration inhibited the clinical benefit of immunotherapy in patients with cancer; moreover, faecal microbiota transplantation (FMT) from cancer patients into germ-free mice ameliorated the antitumor effect only when the donor was a responder, whereas FMT from non-responding patients failed to do so (Routy et al. 2018).
While a scarce knowledge exists on the link between the microbiome and drug absorption/metabolism, this topic assumes high clinical relevance, considering that changes in absorption and metabolism can correspond to alterations in drug efficacy and toxicity. There are no studies so far exploring the effects of microbial perturbations on psychotropic drug pharmacokinetics and more research is warranted, especially considered that several psychotropics have been shown to alter the gut microbiota composition (see Table 1). Overall, the growing evidence underlies a fascinating interaction between intestinal bacteria and drug efficacy, suggesting that precision medicine strategies should include the intestinal microbiota as a potential treatment modifier (Jobin 2018).
Table 1.
Correlations between psychotropic compounds and microbes
| Psychotropic class | Psychotropic compound | Experimental approach | Details | Reference(s) |
|---|---|---|---|---|
| Antipsychotics | Aripiprazole | In vivo | 4-week administration in rats increase the relative abundances of Clostridium, Ruminiclostridium, Intestinibacter and Eubacterium coprostanoligens | Cussotto et al. 2018 |
| In humans | The microbiota communities of AAP-treated (including aripiprazole) and non-treated patients are significantly separated. The genera Lachnospiraceae, Akkermansia and Sutterella are differentially abundant in the two groups | Flowers et al. 2017 | ||
| Chlorpromazine | In vitro | Antimycobacterial properties | Kristiansen and Vergmann 1986; Molnar et al. 1977 | |
| Synergistic effect in combination with certain antibiotics | Amaral et al. 1992 | |||
| Inhibits significantly the growth of S. aureus and E. coli | Ordway et al. 2002a; Amaral and Lorian 1991; Csiszar and Molnar 1992 | |||
| Fluphenazine | In vitro | Pronounced action against both Gram-positive and Gram-negative bacteria at concentrations of 20–100 μg/mL | Dastidar et al. 1995 | |
| Olanzapine | In vitro | Completely inhibits the growth of E. coli NC101 | Morgan et al. 2014 | |
| In vivo | 3-week administration in rats alters the microbiota profile in both males and females | Davey et al. 2012 | ||
| 4-week administration in mice accelerates weight gain resulting from high-fat diet. The effect is absent under GF conditions but emerges quickly upon microbial colonisation of the gut | Morgan et al. 2014 | |||
| Coadministration with an antibiotic cocktail in female rats attenuates body weight gain, uterine fat deposition, macrophage infiltration of adipose tissue and plasma free fatty acid levels, all of which are increased by olanzapine alone | Davey et al. 2013 | |||
| Coadministration with the prebiotic B-GOS in female rats attenuates olanzapine-induced weight gain | Kao et al. 2018 | |||
| In humans | The microbiota communities of AAP-treated (including olanzapine) and non-treated patients are significantly separated. The genera Lachnospiraceae, Akkermansia and Sutterella are differentially abundant in the two groups | Flowers et al. 2017 | ||
| Cross-sectional study on psychiatric patients. No significant differences in microbiota composition at baseline between AAP users and non-users. Non-AAP users have increase in Alistipes. AAP-treated females have decreased diversity compared with non-treated females | Flowers et al. 2019 | |||
| Prochlorperazine | In vitro | Strongly inhibits Bacillus spp. and Staphylococcus spp. | Rani Basu et al. 2005 | |
| Risperidone | In vivo | 80 μg/day in female mice induces weight gain which correlates with an altered gut microbiota. Faecal transplant from risperidone-treated mice causes a 16% reduction in total resting metabolic rate in naïve recipients, attributable to suppression of non-aerobic metabolism | Bahr et al. 2015b | |
| In humans | The microbiota communities of AAP-treated (including risperidone) and non-treated patients are significantly separated. The genera Lachnospiraceae, Akkermansia and Sutterella are differentially abundant in the two groups | Flowers et al. 2017 | ||
| Chronic treatment in psychiatrically ill children increases the BMI and reduces the ratio of Bacteroidetes/Firmicutes. There is a gradual decrease in the Bacteroidetes/Firmicutes ratio over the ensuing months of treatment | Bahr et al. 2015a | |||
| Thioridazine | In vitro | Antimicrobial activity against methicillin-susceptible S. aureus, vancomycin-resistant pathogenic strains of Enterococcus species, Mycobacterium tuberculosis, Pseudomonas aeruginosa and Mycobacterium avium | Hahn and Sohnle 2014; Ordway et al. 2002b; Wainwright et al. 1999; Amaral et al. 1996; Bettencourt et al. 2000; Ordway et al. 2003; Viveiros and Amaral 2001; Viveiros et al. 2005 | |
| Trifluoperazine | In vitro | Antimicrobial activity against 46 of 55 strains of S. aureus at doses of 10–50 μg/mL. Antimicrobial against Shigella spp., Vibrio cholerae and V. parahaemolyticus at concentrations of 10–100 μg/mL | Mazumder et al. 2001 | |
| Antidepressants | Amitriptyline | In vitro | Out of 254 bacterial strains, 185 are inhibited at different doses, with Staphylococcus spp., Bacillus spp. and Vibrio cholerae being the most affected bacteria. Amitriptyline also inhibits both Cryptococcus spp. and Candida albicans | Mandal et al. 2010 |
| In vivo | At doses of 25 μg/g and 30 μg/g significantly protects mice from Salmonella typhimurium | Mandal et al. 2010 | ||
| Clomipramine | In vitro | Cytotoxic effects against both human protozoan parasites Leishmania donovani and Leishmania major | Zilberstein and Dwyer 1984 | |
| Desipramine | In vitro | Effective against Plasmodium falciparum | Basco and Le Bras 1990; Salama and Facer 1990 | |
| Escitalopram | In vitro | Antimicrobial effect on E. coli, but no effect on L. rhamnosus | Cussotto et al. 2018 | |
| Fluoxetine | In vitro | Strong dose-dependent antimicrobial activity against L. rhamnosus and E. coli | Cussotto et al. 2018 | |
| In vivo | 4-week administration in rats completely inhibits the growth of Succinivibrio and Prevotella caecal taxa | Cussotto et al. 2018 | ||
| Imipramine | In vitro | Cytotoxic effects against both human protozoan parasites Leishmania donovani and Leishmania major | Zilberstein and Dwyer 1984 | |
| Inhibits the growth of E. coli and Yersinia enterocolitica through interference with plasmid replication. It also inhibits the parasite Giardia lamblia | Csiszar and Molnar 1992; Molnar 1988; Weinbach et al. 1992 | |||
| Ketamine | In vitro | Antimicrobial activity against: S. aureus, S. epidermidis, E. faecalis, S. pyogenes, P. aeruginosa and E. coli, with S. aureus and S. pyogenes being the most sensitive strains | Begec et al. 2013; Gocmen et al. 2008 | |
| Sustained antimicrobial activity in a dose-dependent manner against microorganisms in propofol, which is a strong growth-promoting factor | Begec et al. 2013 | |||
| Promethazine | In vitro | Inhibits the growth of E. coli and Yersinia enterocolitica through interference with plasmid replication | Csiszar and Molnar 1992; Molnar 1988 | |
| Sertraline | In vitro | Potent antimicrobial against E. coli | Bohnert et al. 2011 | |
| Inhibits the growth of S. aureus, E. coli and P. aeruginosa and also shows synergy in combination with antibiotics | Ayaz et al. 2015 | |||
| Potent antifungal activity against Cryptococcus neoformans, Coccidioides immitis and Candida spp. | Rossato et al. 2016; Trevino-Rangel Rde et al. 2016; Zhai et al. 2012; Paul et al. 2016, Lass-Florl et al. 2003 | |||
| Kills 97.5% of the promastigotes of Leishmania donovani at a dose of 30 mg/L. At the lowest concentration (3 mg/L), it induces significant loss of viability in the promastigotes (61%) | Palit and Ali 2008 | |||
| Antianxiety drugs | Propranolol | In vitro | Inhibits the growth of S. aureus and E. coli | Kruszewska et al. 2004; Hadera et al. 2018 |
| Does not inhibit the growth of S. aureus | Jerwood and Cohen 2008 | |||
| Anticonvulsants/mood stabilisers | Lamotrigine | In vitro | Good antibacterial activity against Gram-positive bacteria B. subtilis, S. aureus and S. faecalis. Inhibition of bacterial ribosome biogenesis | Qian et al. 2009; Stokes et al. 2014 |
| Lithium | In vivo | 4-week administration in rats changes the caecal microbiome, with many genera being affected | Cussotto et al. 2018 | |
| Valproate | In vitro | Inhibits Mycobacterium smegmatis | Esiobu and Hoosein 2003 | |
| In vivo | 4-week administration in rats changes the caecal microbiome, with many genera being affected | Cussotto et al. 2018 | ||
| Opioid analgesics | Methadone | In vitro | Antimicrobial activity against S. aureus, P. aeruginosa and S. marcescens | Sheagren et al. 1977 |
| In humans | Chronic opioid use (methadone N = 1) in cirrhotic patients induces changes in microbiome composition, with lower relative abundance of Bacteroidaceae | Acharya et al. 2017 | ||
| Morphine | In vitro | Does not possess antimicrobial activity against any of the 10 microbial strains studied with the agar dilution method | Rosenberg and Renkonen 1985 | |
| In vivo | Induces dysbiosis in a morphine-dependent murine model. The dysbiosis is associated to an increase in pathogenic bacteria and a decrease in communities associated with stress | Wang et al. 2018 | ||
| Intermittent or sustained opioid regimen in mice influences the gut microbiome and this is causally related to behaviours associated with opioid dependence | Lee et al. 2018 | |||
| In humans | Chronic opioid use (morphine sulphate N = 1) in cirrhotic patients induces changes in microbiome composition, with lower relative abundance of Bacteroidaceae | Acharya et al. 2017 | ||
| Tramadol | In vitro | Strong bactericidal activity against E. coli and S. epidermidis. Weak antimicrobial activity against S. aureus and P. aeruginosa | Tamanai-Shacoori et al. 2007 | |
| In vivo | Subcutaneous injection in BALB/c-sensitive mice reduces the growth of S. aureus through enhancing phagocytes and tissue inflammation. It does not help eliminate P. aeruginosa | Farzam et al. 2018 | ||
| In humans | Chronic opioid use (tramadol N = 23) in cirrhotic patients induces changes in microbiome composition, with lower relative abundance of Bacteroidaceae | Acharya et al. 2017 | ||
| Drugs of abuse | Cannabis | In vitro | Strong antimicrobial activity against a wide range of microorganisms | Appendino et al. 2008; Ali et al. 2018; Nissen et al. 2010 |
| In vivo | Modifications in the gut microbiota consequential to diet-induced obesity are prevented in mice treated chronically with THC | Cluny et al. 2015 | ||
| In humans | The microbiome of chronic marijuana users displays a Prevotella/Bacteroides ratio that is 13-fold lower than non-users | Panee et al. 2018 | ||
| A combination of THC and CBD mitigates experimental autoimmune encephalomyelitis by altering the gut microbiome | Al-Ghezi et al. 2017 | |||
| Cocaine | In vivo | Administration of antibiotics in mice induces an enhanced sensitivity to cocaine reward and an enhanced sensitivity to the locomotor-sensitising effects of repeated cocaine administration | Kiraly et al. 2016 | |
| In humans | Cocaine users display a higher relative abundance of Bacteroidetes than non-users | Volpe et al. 2014 | ||
| Heroin | In humans | The composition and diversity of intestinal microbiota in a cohort of 50 patients with SUD (of which 52% on heroin) is significantly different from those of healthy controls. The relative abundance of Thauera, Paracoccus and Prevotella is significantly higher in SUDs compared to healthy participants | Xu et al. 2017 | |
| Methamphetamine | In vivo | The gut microbiota of methamphetamine-treated rats differs from that of control rats. The faecal microbial diversity is higher in methamphetamine-treated rats. The genus Phascolarctobacterium is reduced and the family Ruminococcaceae is increased in metamphetamine-treated rats | Ning et al. 2017 | |
| In humans | The composition and diversity of intestinal microbiota in a cohort of 50 patients with SUD (of which 30% on methamphetamine) is significantly different from those of healthy controls. The relative abundance of Thauera, Paracoccus and Prevotella is significantly higher in SUDs compared to healthy participants | Xu et al. 2017 | ||
| Alcohol | NA | In vivo | 4-week intermittent vaporised ethanol in mice alters the gut microbiota, increasing the levels of Alistipes and decreasing Clostridium IV, Dorea and Coprococcus | Peterson et al. 2017 |
| In a mouse model of alcoholic liver disease, Bacteroidetes and Verrucomicrobia are increased in mice fed alcohol | Yan et al. 2011 | |||
| In humans | Human alcoholics with dysbiosis have lower abundances of Bacteroidetes and higher ones of Proteobacteria | Mutlu et al. 2012 | ||
| Alcohol-dependent subjects have an increased intestinal permeability which is linked to significant microbiome alterations | de Timary et al. 2015; Keshavarzian et al. 2009; Leclercq et al. 2014 | |||
| In cirrhotic patients, the proportion of phylum Bacteroidetes is significantly reduced, whereas Proteobacteria and Fusobacteria are highly enriched compared to healthy controls. Enterobacteriaceae, Veillonellaceae and Streptococcaceae are prevalent in patients with cirrhosis at the family level | Chen et al. 2011 | |||
| Nicotine | NA | In vitro | Active against E. coli, P. aeruginosa and S. faecalis at a dose of 2 μg/μL; and against Listeria monocytogenes and viridans streptococci at a dose of 10 μg/mL | Idrees Zaidi et al. 2012; Pavia et al. 2000 |
| In vivo | Influences the gut microbiota composition in a sex-specific manner in mice | Chi et al. 2017 | ||
| In humans | Induces profound changes in the gut microbiome, with an increase of Firmicutes and Actinobacteria and a decrease of Bacteroidetes and Proteobacteria at the phylum level. Smoking cessation induces an increase in microbial diversity | Biedermann et al. 2013 | ||
| Tobacco smokers display a higher relative abundance of Prevotella, lowered Bacteroides and lower Shannon diversity compared to controls | Stewart et al. 2018 | |||
| Xanthines | Caffeine | In vitro | Inhibits the growth of E. coli and E. faecalis | Tatsuya and Kazunori 2013; Daglia et al. 2007 |
| In vivo | Consumption of 500 μL/day of coffee for three consecutive days in specific-pathogen-free mice decreases the levels of E. coli and Clostridium spp. | Tatsuya and Kazunori 2013 | ||
| Caffeine-rich Pu-erh tea remodels the intestinal dysbiosis in mice with metabolic syndrome. Akkermansia muciniphila and Faecalibacterium prausnitzii are the key gut bacterial links between the Pu-erh tea treatment and metabolic syndrome | Gao et al. 2018 | |||
| Chronic coffee consumption in diet-induced obese rats decreases the abundance of Clostridium Cluster XI and increases Enterobacteriaceae. SCFAs are largely increased in the coffee-fed rats | Cowan et al. 2014 | |||
| 8 weeks of coffee consumption in rats does not alter the gut microbiota composition | Cowan et al. 2013 | |||
| Oral administration of 0.7 mg/kg/day caffeine for 21 days in mice decreases Lactobacillus | Kleber Silveira et al. 2018 | |||
| In humans | Consumption of 3 cups of coffee daily for 3 weeks in healthy volunteers increases the population of Bifidobacterium spp. In some subjects, there is a specific increase in the metabolic activity of Bifidobacterium spp. | Jaquet et al. 2009 | ||
| Theobromine | In vivo | 2-week administration of cocoa’s theobromine in rats induces marked changes in gut microbiota. Rats that received a 10% cocoa-containing diet have lower counts of E. coli. Rats that received a 0.25% theobromine-containing diet have lower counts of Bifidobacterium spp., Streptococcus spp. and Clostridium histolyticum-C. perfingens group | Martín-Peláez et al. 2017 | |
| Theophylline | In vivo | Consumption of fermented green tea, containing theophylline, is able to restore the changes in gut microbiota composition associated to diet-induced obesity in mice | Seo et al. 2015 |
Compounds are listed in alphabetic order
AAP atypical antipsychotic, B-GOS bimuno galactooligosaccharide, BMI body mass index, CBD cannabidiol, GF germ-free, NA not addressed, SCFAs short-chain fatty acids, SUD substance use disorders, THC Δ9 tetrahydrocannabinol
Antipsychotics and gut microbiota: a general overview
Antipsychotics are drugs used for the prophylaxis and acute treatment of psychotic illnesses including schizophrenia and psychosis associated with depression and mania (Gardner et al. 2005). They also have an important role as an alternative or adjunct to benzodiazepines in the management of the acutely disturbed patient, for both tranquillisation and sedation. The common mechanism of action of all antipsychotics is to decrease brain dopamine function by blocking the dopamine D2 receptors (Laruelle et al. 2005).
Analysis of faecal microbiota from 76 elderly hospitalised patients showed that, among several therapeutic classes, the use of antipsychotics had a strong association with gut microbiota composition (Ticinesi et al. 2017). In a recent study, differences in faecal microbiota between patients with first-episode psychosis and healthy controls, were associated with response after up to 12 months of treatment (Schwarz et al. 2018), suggesting that the gut microbiota might be involved in treatment response. Specifically, Lactobacillaceae and Bifidobacteria were highly abundant in patients with first-episode psychosis and correlated positively with severity of psychotic symptoms and negatively with global functioning (Schwarz et al. 2018). In a tour de force in vitro screening study of more than 1000 drugs against 40 representative gut bacterial strains, it was found that 24% of human-targeting drugs inhibited the growth of at least one strain (Maier et al. 2018). Provocatively, nearly all subclasses of the chemically diverse antipsychotics targeted a significantly more similar pattern of species than expected from their chemical similarity, raising the possibility that antimicrobial action may not only manifest as side effect of antipsychotics, but also be part of their mechanism of action (Maier et al. 2018). This hypothesis should be ideally verified by assessing whether microbiome manipulations (i.e. antibiotic administration) have an effect on the efficacy of antipsychotics. Remarkably, the Maier et al.’s study provides an exhaustive justification for the dose used in the in vitro screening. The authors argue that, based on drug excretion patterns from published work, the chosen concentration of 20 μM is below the median colon concentration of the human-targeted drugs tested (Maier et al. 2018) and therefore has translational validity. Notably, in their experiment, human-targeted drugs that showed anticommensal activity had lower plasma and estimated small intestinal concentrations than ones with no such activity, suggesting that more human-targeted drugs would inhibit bacterial growth if probed at higher doses, closer to physiological concentrations. In a recent study in vitro, the antibacterial activity of antipsychotics against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii was investigated. Phenothiazines and thioxanthenes showed differential antibacterial activity at concentrations ranging from 64 to 1024 μg/mL, which was independent of antibiotic-resistance patterns (Nehme et al. 2018). How these concentrations translate to those found in the colon following an oral administration of antipsychotics in humans is not clear and is not mentioned in the study; thus, these findings might lack translational relevance.
Typical antipsychotics and gut microbiota
Typical (first-generation) antipsychotics were first developed in the 1950s and the first compounds to come into medical use were the phenothiazines, such as chlorpromazine. Typical antipsychotics are characterised by extrapyramidal adverse effects such as dystonia, Parkinsonian symptoms (bradykinesia, rigidity and tremor), akathisia, tardive dyskinesia, cardiovascular effects such as postural hypotension, prolactin increase and sedation (Leucht et al. 2009).
Evidence from in vitro studies
Studies on the interaction between typical antipsychotics and gut bacteria have been only carried out in vitro. Other approaches, such as in vivo or human observations, are missing from literature, and one reason for this gap might be more and more consideration is directed towards the new class of antipsychotics, the atypical (Skonieczna-Zydecka et al. 2018).
Thioridazine, a phenothiazine antipsychotic, has been shown to possess antimicrobial activity in vitro against methicillin-susceptible S. aureus (Hahn and Sohnle 2014; Ordway et al. 2002b), vancomycin-resistant pathogenic strains of Enterococcus species (Wainwright et al. 1999), Mycobacterium tuberculosis (Amaral et al. 1996; Bettencourt et al. 2000; Ordway et al. 2003; Viveiros and Amaral 2001), Pseudomonas aeruginosa and Mycobacterium avium (Viveiros et al. 2005). Fluphenazine, another typical antipsychotic, possesses pronounced action against both Gram-positive and Gram-negative bacteria at concentrations of 20–100 μg/mL (Dastidar et al. 1995). Upon investigation of the antimicrobial activity of trifluoperazine against 293 strains (from two Gram-positive and eight Gram-negative genera), 46 of 55 strains of S. aureus were inhibited by doses of 10–50 μg/mL. This drug also inhibited strains of Shigella spp., Vibrio cholerae and V. parahaemolyticus at concentrations of 10–100 μg/mL (Mazumder et al. 2001). Bacillus spp. and Staphylococcus spp. were strongly inhibited by the antipsychotic prochlorperazine; while E. coli, Salmonella, Klebsiella and Pseudomonas were only moderately sensitive or resistant to the drug (Rani Basu et al. 2005). Chlorpromazine, another typical antipsychotic, had in vitro antimycobacterial properties (Kristiansen and Vergmann 1986; Molnar et al. 1977) and also exerted an inhibitory synergistic effect in combination with certain antibiotics (Amaral et al. 1992). Moreover, this medication has been shown to inhibit significantly the growth of S. aureus (Ordway et al. 2002a) and E. coli (Amaral and Lorian 1991; Csiszar and Molnar 1992). Keeping in mind that these data come from in vitro bacterial cultures, it is important to remark that a direct extrapolation and translation into the gut microbiome scenario is not always possible.
Atypical antipsychotics and gut microbiota
Atypical antipsychotics act on numerous receptors and modulate several interacting transmitter systems. All atypicals (except amisulpride) exhibit greater antagonism of 5-HT2 receptors than of D2 receptors, compared with the typical agents. Atypical drugs that do antagonise dopamine D2 receptors appear to have affinity for those in the mesolimbic system rather than the nigrostriatal system, producing side effects of lesser degree. Clozapine and risperidone exert substantial antagonism of α2-adrenoceptors, while aripiprazole is a unique drug because it is a partial dopamine D2-receptor agonist that acts conversely as an antagonist in regions where dopamine is overactive, such as the limbic system (Bennett and Brown 2008).
Evidence from in vitro studies
The effect of olanzapine on growth of two commensal bacterial strains, E. coli NC101 and Enterococcus faecalis OGIRF was assessed in vitro across a range of supraphysiologic concentrations (280 to 560 μg/mL). Olanzapine completely inhibited the growth of E. coli at concentrations above 537 μg/mL, while it did not affect the growth of E. faecalis (Morgan et al. 2014).
Evidence from in vivo studies (rodents)
Most of the studies performed in vivo have been focusing on two atypical antipsychotics, olanzapine and risperidone. Administration of olanzapine for 3 weeks in rats was able to induce specific alterations of the microbiota profile in both males and females (Davey et al. 2012). Moreover, administration of olanzapine in mice exacerbated the weight gain induced by high-fat diet (Morgan et al. 2014). Interestingly, this effect was absent under germ-free conditions but emerged quickly upon microbial colonisation of the gut, suggesting that gut microorganisms might be necessary for the common adverse effect of olanzapine, weight gain (Morgan et al. 2014). As a proof of concept, the impact of antibiotics on olanzapine-induced weight gain was also demonstrated. Coadministration of an antibiotic cocktail in female rats treated with 2 mg/kg of olanzapine for 21 days, attenuated body weight gain, uterine fat deposition, macrophage infiltration of adipose tissue and plasma free fatty acid levels, all of which were increased by olanzapine alone (Davey et al. 2013). More recently, one last experiment has looked at microbiota changes and olanzapine administration. In this case, the prebiotic B-GOS (bimuno galactooligosaccharide) was administered to adult female Sprague-Dawley rats in coadministration with olanzapine (2-week, daily intraperitoneal injection at a dose of 10 mg/kg) and the intake of B-GOS significantly attenuated olanzapine-induced weight gain (Kao et al. 2018). Although B-GOS alone increased Bifidobacteria spp., and reduced species within the Firmicutes (Coprococcus, Oscillibacter, C. coccoides, Roseburia intestinalis cluster, Clostridium XVIII cluster) and Proteobacteria (Escherichia/Shigella spp.) phyla, no effects of olanzapine were observed. This is a discrepancy with other studies, maybe due to the duration and dose of olanzapine administration and/or the method of bacterial analysis. Importantly, additional studies are required to test whether the bacteria affected by B-GOS would proliferate beyond control levels with a longer duration of olanzapine administration at a clinically relevant dose. It is important to note that sex differences might play a key role in response to atypical antipsychotics and thus many studies to date have been performed in females; however, more investigations are warranted in male counterparts. The impact of risperidone on the gut microbiota has also been investigated in vivo. Female mice treated with risperidone at a dose of 80 μg/day over 2 months exhibited significant excess weight gain, due to reduced energy expenditure, which correlated with an altered gut microbiota (Bahr et al. 2015b). Interestingly, faecal transplant from risperidone-treated mice into naïve recipients caused a 16% reduction in total resting metabolic rate, attributable to suppression of non-aerobic metabolism (Bahr et al. 2015b). Aripiprazole, an atypical antipsychotic with a mode of action that is distinct from most currently available antipsychotic drugs, was able to induce marked changes in microbiota composition in rats following a 4-week treatment at 20 mg/kg/day. The relative abundance of various taxa including Clostridium, Ruminiclostridium, Intestinibacter and Eubacterium coprostanoligens was increased by aripiprazole administration (Cussotto et al. 2018).
Evidence from studies in humans
A recent study has looked at the association between intake of atypical antipsychotics (AAP) and gut microbiota. In a cross-sectional design study, faecal samples of more than 100 bipolar patients were collected and analysed through 16S ribosomal sequencing. Participants were divided in two groups: one group AAP-treated and one group drug-free at the time of faecal sample collection. Atypical antipsychotics included in the AAP cohort were clozapine, olanzapine, risperidone, quetiapine, asenipine, ziprasodone, lurasidone, aripiprazole, paliperidone and iloperidone. The microbiota communities of AAP-treated and non-treated patients were significantly separated, with AAP-treated females showing decreased species diversity compared to non-AAP-treated females, while males did not show significant diversity. Three specific genera, Lachnospiraceae, Akkermansia and Sutterella were differentially abundant in the two groups (Flowers et al. 2017). While this study provides critical insight into the AAP-mediated changes in gut microbiota, the report included no information regarding diet, which is an important environmental factor that drives the composition of gut microbiota. Moreover, the authors observed medication-specific microbiota differences, but it is not known how these translate into functional differences. In a cross-sectional cohort study on psychiatric patients, the effect of AAPs on the gut microbiota was examined. Although no significant differences in microbiota composition were detected at baseline between AAP users and non-users, non-AAP users showed an increase in the bacterial genus Alistipes. AAP-treated females also had decreased diversity compared with non-treated females (Flowers et al. 2019). One more study in humans has investigated the impact of risperidone on gut microbiota composition. In psychiatrically ill children, chronic treatment with risperidone was associated with an increase in body mass index (BMI) and a significantly lower ratio of Bacteroidetes/Firmicutes as compared with antipsychotic-naïve psychiatric controls. Moreover, a longitudinal observation revealed a gradual decrease in the Bacteroidetes/Firmicutes ratio over the ensuing months of treatment with risperidone (Bahr et al. 2015a). Although the small sample size and the fact that polypharmacy was not taken into account, this study offers preliminary evidence that the human gut microbiome is altered in patients treated chronically with risperidone.
Antidepressants and gut microbiota: a general overview
Antidepressants are medications used to treat symptoms of depression, social anxiety disorder, seasonal affective disorder and mild chronic depression, as well as other conditions (Delgado 2004). Antidepressants can be broadly divided into four main classes: tricyclics (TCAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs) and novel compounds, some of which are related to TCAs or SSRIs (see SNRIs, serotonin-noradrenaline reuptake inhibitors) (Bennett and Brown 2008). The mechanism of action is based on the “monoamine hypothesis”, which proposes that the main cause of depression is a deficiency of the neurotransmitters noradrenaline (NA) and serotonin (5-HT, 5-hydroxytryptamine) in the brain. However, different classes present different mechanisms of action: SSRIs prevent 5-HT reuptake, TCAs inhibit NA reuptake but effects on 5-HT reuptake vary widely and MAOIs increase the availability of NA and 5-HT by preventing their degradation in the presynaptic terminal (Fiedorowicz and Swartz 2004).
In a cohort of elderly subjects, intake of antidepressants was strongly associated with changes in gut microbiota composition (Ticinesi et al. 2017). A different population-level analysis of gut microbiome composition found that antidepressants were significantly correlated to microbiome composition (Falony et al. 2016). The field of antidepressants and gut microbiota is in constant expansion, but there is currently not sufficient knowledge on the effect that these drugs exert on the ecology of the gut microbiota. On the other side, a consistent amount of research has examined the antimicrobial activity that these compounds have against various bacterial strains in vitro. In the following sections, evidence related to all subclasses of antidepressant compounds will be taken into consideration.
Tricyclic antidepressants and gut microbiota
Evidence from in vitro studies
TCAs in general inhibit NA reuptake and some compounds can also block 5-HT reuptake to a certain extent (Horn 1980). All studies to date looking at TCAs and microbiota have been performed in vitro.
Clomipramine and imipramine have been shown to possess cytotoxic effects against both human protozoan parasites Leishmania donovani and Leishmania major (Zilberstein and Dwyer 1984). Mandal and colleagues have analysed the antimicrobial activity of Amitriptyline hydrochloride against 253 bacterial strains (72 Gram-positive and 181 Gram-negative) and 5 fungal strains in vitro. Moreover, they carried out a mortality experiment with or without amitriptyline in mice challenged with a virulent strain of Salmonella typhimurium (Mandal et al. 2010). Out of 254 bacterial strains, 185 were inhibited at different doses of amitriptyline, with Staphylococcus spp., Bacillus spp. and Vibrio cholerae being the most affected bacteria (Mandal et al. 2010). Regarding fungal strains, amitriptyline inhibited both Cryptococcus spp. and Candida albicans. Finally, in the in vivo experiment, amitriptyline at 25 μg/g and 30 μg/g body weight significantly protected the mice from Salmonella typhimurium (Mandal et al. 2010). Promethazine and imipramine have been demonstrated to inhibit the growth of E. coli and Yersinia enterocolitica through interference with plasmid replication (Csiszar and Molnar 1992; Molnar 1988), and imipramine was also able to inhibit the parasite Giardia lamblia (Weinbach et al. 1992). Desipramine has been shown to be effective against Plasmodium falciparum (Basco and Le Bras 1990; Salama and Facer 1990).
Selective serotonin reuptake inhibitors and gut microbiota
Evidence from in vitro studies
SSRIs act, as their name indicates, predominantly by preventing 5-HT reuptake, with little or no effect on NA reuptake (Stahl 1998).
SSRIs have excellent activity against Brucellae (Muñoz-Criado et al. 1996) and they have been shown to be synergistic in combination with antibiotics against some microorganisms such as Corynebacterium urealyticum (Garcia-Rodriguez et al. 1991; Munoz-Bellido et al. 1996). Interestingly, SSRIs also affect the normal physiology of some bacteria, for example they inhibit slime production in coagulase-negative staphylococci (Munoz Criado et al. 1997) and inhibit swarming in swarming species in Proteus (Muñoz-Criado et al. 1998). Upon analysis of the antimicrobial activity of four SSRIs against E. coli, sertraline was the most potent antimicrobial compound (Bohnert et al. 2011). Since the discovery of sertraline as a strong antimicrobial, the research has been focused mainly on this compound. Sertraline inhibits the growth of S. aureus, E. coli and P. aeruginosa, and it also has synergy in combination with antibiotics (Ayaz et al. 2015). Moreover, sertraline has potent antifungal activity against Cryptococcus neoformans (Rossato et al. 2016; Trevino-Rangel Rde et al. 2016; Zhai et al. 2012), Coccidioides immitis (Paul et al. 2016) and Candida spp. (Lass-Florl et al. 2003). In a different study, it was shown that sertraline was able to kill 97.5% of the promastigotes of Leishmania donovani at a dose of 30 mg/L while, at the lowest concentration (3 mg/L), it induced significant loss of viability in the promastigotes (61%) (Palit and Ali 2008). Fluoxetine had a strong dose-dependent antimicrobial activity in vitro against L. rhamnosus and E. coli, while escitalopram only exerted a minor antimicrobial effect on E. coli, without affecting the growth of L. rhamnosus (Cussotto et al. 2018).
Evidence from in vivo studies (rodents)
Evidence from our laboratory has recently shown that 4 weeks of fluoxetine administration in drinking water in rats at a translationally relevant dose of 10 mg/kg/day completely inhibited the growth of Succinivibrio and Prevotella caecal taxa (Cussotto et al. 2018). Whether the microbiome changes influence the efficacy and/or toxicity of fluoxetine still needs to be teased apart.
Other antidepressants and gut microbiota
Evidence from in vitro studies
Interestingly, the era of antidepressants started with isoniazid, a compound that also has antimicrobial activity against Mycobacterium tuberculosis and is currently used to treat tuberculosis (Jena et al. 2014; Lei et al. 2000). Ketamine is a non-competitive NMDA (N-methyl-d-aspartate) antagonist that acts at the PCP (phencyclidine) binding site in the NMDA receptor and possess a fast onset of action as antidepressant (Bennett and Brown 2008). Ketamine showed antimicrobial activity in vitro against six different strains of bacteria: S. aureus, S. epidermidis, E. faecalis, S. pyogenes, P. aeruginosa and E. coli, with S. aureus and S. pyogenes being the most sensitive strains (Begec et al. 2013; Gocmen et al. 2008). There is currently little known regarding the effects of ketamine on gut microbiota and other classes of antidepressants, such as MAOIs (monoamine oxidase inhibitors) and SNRIs (serotonin-norepinephrine reuptake inhibitors), have not been investigated. Given the wide range of antimicrobial effects that most antidepressants show against different strains, it is perhaps not surprising to speculate that SNRIs or MAOIs might exert a microbial effect. This represents a future direction for research.
Antianxiety drugs and gut microbiota
Evidence from in vitro studies
The literature to date lacks comprehensive studies investigating the effects of antianxiety agents on the gut microbiome; however, some studies in vitro have been carried out to assess whether these compounds possess antimicrobial activity. Propranolol is a beta-receptor blocker that is commonly used to overcome the somatic symptoms of anxiety such as tachycardia and palpitations (Whitlock and Price 1974). In vitro, this compound was able to inhibit the growth of S. aureus (Kruszewska et al. 2004) and E. coli (Hadera et al. 2018). However, the data are divergent, as a different study showed that propranolol did not inhibit the growth of S. aureus (Jerwood and Cohen 2008). More research in vivo and in humans is warranted to investigate the microbial effects of antianxiety drugs.
Anticonvulsants/mood stabilisers and gut microbiota
Mood stabilisers are used to treat mood disorders, characterised by intense and sustained mood shifts, typically bipolar disorder, borderline personality disorder and schizoaffective disorder. Many agents described as mood stabilisers are also categorised as anticonvulsants (Rapoport et al. 2009). No population-based studies have been carried out to date looking at the influence of anticonvulsants on the microbiome, but some preclinical data exist and are examined in the following sections.
Evidence from in vitro studies
We have recently screened the antimicrobial activity of lithium and valproate against E. coli and L. rhamnosus in vitro and these two medications did not inhibit the growth of the two bacteria (Cussotto et al. 2018). Interestingly, however, valproate has previously been shown to inhibit Mycobacterium smegmatis but not to affect E. coli (Esiobu and Hoosein 2003). Lamotrigine showed good antibacterial activity against Gram-positive bacteria B. subtilis, S. aureus and S. faecalis (Qian et al. 2009) and inhibition of bacterial ribosome biogenesis (Stokes et al. 2014). Finally, some evidence also showed that gabapentin and topiramate possess differential antimicrobial activity in vitro (Kruszewska et al. 2004).
Evidence from in vivo studies (rodents)
A 4-week administration of lithium and valproate in the chow of Sprague-Dawley rats was able to change markedly the caecal microbiome (Cussotto et al. 2018). Bacterial richness was increased in both treatments compared to vehicle-treated animals; moreover, at the genus level, lithium increased the relative abundance of Ruminococcaceae and decreased Bacteroides, while valproate decreased the relative abundance of S24-7 uncultbact and increased Ruminococcaceae (Cussotto et al. 2018). Valproate, but not lithium, also affected the levels of SCFAs (short-chain fatty acids) in the caecum. How these microbial changes relate to drug efficacy is not clear. Moreover, it is also not clarified whether these drugs affect directly the gut microbiota (i.e. they reach the caecum) or indirectly (i.e. through gut-brain signalling).
Opioid analgesics and gut microbiota
Opioid analgesics act to reduce the intensity and unpleasantness of pain. They produce their effects by activating specific G protein-coupled receptors in the brain, spinal cord and peripheral nervous system (Trang et al. 2015). Acting as agonists at opioid receptors, these compounds reduce neuronal excitability and inhibit the release of pain neurotransmitters (Conlon and Bird 2015).
Evidence from in vitro studies
Morphine did not possess antimicrobial activity against any of the 10 microbial strains studied with the agar dilution method (Rosenberg and Renkonen 1985). Another opioid analgesic, tramadol, had strong bactericidal activity in vitro against E. coli and S. epidermidis and weak antimicrobial activity against S. aureus and P. aeruginosa (Tamanai-Shacoori et al. 2007). Methadone exerted antimicrobial activity in vitro against S. aureus, P. aeruginosa and S. marcescens (Sheagren et al. 1977).
Evidence from in vivo studies (rodents)
In a morphine-dependent murine model, significant shifts in the gut microbiome and metabolome within 1 day following morphine treatment were detected. Morphine was administered through the pellet implantation method, so that plasma levels of morphine were maintained in the 0.6–2.0 μg/mL range (range observed in opioid abusers and patients on opioids for moderate to severe pain). Morphine-induced alterations in gut microbial composition were associated to a significant increase in pathogenic bacteria and a decrease in communities associated with stress tolerance (Wang et al. 2018). In a different study in mice, both intermittent and sustained morphine administration influenced the gut microbiome in a way that was causally related to behaviours associated with opioid dependence (Lee et al. 2018). Interestingly, subcutaneous injections of tramadol reduce the growth of S. aureus through enhancing phagocytes and tissue inflammation; however, it does not eliminate P. aeruginosa (Farzam et al. 2018).
Evidence from studies in humans
One study has examined the effect that opioids might have on the gut microbiota in humans. In a cohort of cirrhotic patients, chronic opioid use (hydromorphone N = 7, fentanyl N = 1, methadone N = 1, morphine sulphate N = 1, oxycodone N = 23, Percocet N = 3, tramadol N = 23 and combinations of the drugs N = 3) was associated to significant changes in microbiome composition, with lower relative abundance of Bacteroidaceae and Ruminococcaceae (Acharya et al. 2017). This analysis was carried out at drug class level, and it was not possible to discriminate between the effects induced by each single compound.
Drugs of abuse, alcohol, nicotine and gut microbiota
Considering that accumulating evidence supports the role of the gut microbiota in central nervous system (CNS) function, the interaction between the gut microbiome and drugs of abuse, as well as alcohol and nicotine, represents an expanding field.
Evidence from in vitro studies
Ketamine was antimicrobial in vitro in a dose-dependent manner against some microorganisms in propofol, which is a strong growth-promoting factor (Begec et al. 2013). The ketamine MIC (minimal inhibitory concentration) was 19.5 μg/mL for S. aureus and 312.5 μg/mL for E. coli and P. aeruginosa. As ketamine has antidepressant potential, some of its microbial effects have already been described in the section “Other antidepressants and gut microbiota”.
Cannabis is obtained from the annual plant Cannabis sativa and its varieties Cannabis indica and Cannabis americana. Psychological reactions to cannabis vary widely, depending on the predisposition of the individual and can include euphoria, memory impairments and time-spatial sense impairments. In vitro assays have shown that cannabis exerts a strong antimicrobial activity against a wide range of microorganisms (Appendino et al. 2008; M M Ali et al. 2018; Nissen et al. 2010).
Nicotine, one of the main components of tobacco, possesses all the characteristics of a drug of dependence. It modulates dopamine activity in the midbrain, particularly in the mesolimbic system, which promotes the development and maintenance of reward behaviour (Rice and Cragg 2004). Two studies in vitro have evaluated the antimicrobial activity of nicotine. The psychotropic compound was active against E. coli, P. aeruginosa and S. faecalis at a dose of 2 μg/μL (Idrees Zaidi et al. 2012) and against Listeria monocytogenes and Viridans streptococci at a dose of 10 μg/mL (Pavia et al. 2000).
Evidence from in vivo studies (rodents)
The gut microbiota of rats that undergoing methamphetamine-induced conditioned place preference is different from that of control animals. Moreover, the faecal microbial diversity is slightly higher in methamphetamine-treated rats. The propionate-producing genus Phascolarctobacterium is attenuated in methamphetamine-treated rats and the family Ruminococcaceae is increased in the same group (Ning et al. 2017). In addition, the short-chain fatty acid propionate was decreased in the faecal matter of rats that received methamphetamine (Ning et al. 2017). The microbiome might play a role also in cocaine addiction: administration of antibiotics in mice induced an enhanced sensitivity to cocaine reward and an enhanced sensitivity to the locomotor-sensitising effects of repeated cocaine administration (Kiraly et al. 2016). Regarding cannabis, recent evidence has shown that modifications in the gut microbiota consequential to diet-induced obesity are prevented in mice treated chronically with Δ9tetrahydrocannabinol (THC), the major psychoactive constituent of cannabis (Cluny et al. 2015).
Alcohol generally exerts on cells in the CNS a depressant effect that is probably mediated by particular membrane ion channels and receptors (Whitlock and Price 1974). Alcohol enhances inhibitory GABAA-stimulated flux of chloride through receptor-gated membrane ion channels, a receptor subtype effect that might be involved in the motor impairment caused by alcohol (Abrahao et al. 2017). Exposure to 4 weeks of chronic intermittent vaporised ethanol in mice markedly altered the gut microbiota, increasing the levels of Alistipes and decreasing Clostridium IV, Dorea and Coprococcus (Peterson et al. 2017). In a mouse model of alcoholic liver disease, Bacteroidetes and Verrucomicrobia were increased in mice fed alcohol compared with a relative predominance of Firmicutes in control mice (Yan et al. 2011). Several other studies in rodents have highlighted a correlation between chronic alcohol consumption, leading to liver disease, and microbiome composition (Fouts et al. 2012; Guarner et al. 1997; Yan and Schnabl 2012). Interestingly, corroborating the idea that the gut microbiome might play a role in alcohol consumption, two dietary means have been used as modulators of the gut microbiome during alcohol consumption. Saturated and unsaturated dietary fats (Kirpich et al. 2016), for example, as well as rhubarb extract (Neyrinck et al. 2017), have been shown to modulate the changes in gut microbiota induced by alcohol intake.
Finally, the psychotropic nicotine administered in drinking water influenced the gut microbiota composition in a sex-specific manner in mice. In treated females, Christensenellaceae, Anaeroplasmataceae and unassigned families in the orders Bacillales were significantly reduced. Families such as Turicibacteraceae and Peptococcaceae were largely increased in male counterparts (Chi et al. 2017).
Evidence from studies in humans
In a human cohort, cocaine users displayed a higher relative abundance of Bacteroidetes than non-users (Volpe et al. 2014). The composition and diversity of intestinal microbiota in a cohort of 50 patients with substance use disorders (SUD; of which 52% on heroin and 30% on methamphetamine) was significantly different from those of healthy controls. The relative abundance of Thauera, Paracoccus and Prevotella was significantly higher in SUD patients compared to healthy participants (Xu et al. 2017). The intestinal microbiota of SUD people would change independently of the type of substance abused, suggesting that the global switch of lifestyle due to SUD in general could be responsible for the changes in microbiome. Importantly, almost all patients with SUDs are involved in alcohol and tobacco addiction, which may also account for the microbiome effects (Xu et al. 2017). The microbiome of chronic marijuana users displayed a Prevotella/Bacteroides ratio that was 13-fold lower than the one of non-users (Panee et al. 2018). A combination of THC and cannabidiol (CBD) has been shown to mitigate experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome (Al-Ghezi et al. 2017).
Regarding alcohol, the mucosa-associated colonic microbiome was altered in alcoholics compared to control participants. Specifically, the alcoholics with dysbiosis had lower median abundances of Bacteroidetes and higher ones of Proteobacteria. Moreover, these alterations were correlated with high levels of serum endotoxin in a subset of the samples (Mutlu et al. 2012). Two similar studies have demonstrated that alcohol-dependent subjects have an increased intestinal permeability which is linked to significant microbiome alterations (de Timary et al. 2015; Keshavarzian et al. 2009; Leclercq et al. 2014). Bacterial overgrowth was found in the jejunum of patients with chronic alcohol abuse (Bode et al. 1984). In cirrhotic patients, the proportion of phylum Bacteroidetes was significantly reduced, whereas Proteobacteria and Fusobacteria were highly enriched compared to healthy controls. Moreover, Enterobacteriaceae, Veillonellaceae and Streptococcaceae were prevalent in patients with cirrhosis at the family level (Chen et al. 2011).
Nicotine consumption and also smoking cessation induced profound changes in the gut microbiome in humans, with an increase of Firmicutes and Actinobacteria and decrease of Bacteroidetes and Proteobacteria at the phylum level. In addition, smoking cessation induced an increase in microbial diversity (Biedermann et al. 2013). The effect of tobacco smoke on the oral and gut microbiome has been recently investigated in a human cohort, where tobacco smokers displayed a higher relative abundance of Prevotella, lowered Bacteroides and lower Shannon diversity in tobacco smokers compared to controls (Stewart et al. 2018).
Xanthines and gut microbiota
The three xanthines caffeine, theophylline and theobromine occur naturally in plants. These compounds have complex and incompletely elucidated actions, which include inhibition of phosphodiesterase (the enzyme that breaks down cyclic AMP), effects on intracellular calcium distribution and noradrenergic function (Bennett and Brown 2008). All xanthines stimulate mental activity to different extents and their effects vary according to the mental state and personality of the subject (Bennett and Brown 2008).
Evidence from in vitro studies
Xanthines were screened against several microbial strain and all compounds displayed antimicrobial activity, with caffeine being the most effective compound (Raj and Dhala 1965). The morphology of Aerobacter aerogenes and A. cloacae was affected by caffeine (Raj and Dhala 1965). Coffee also inhibited the growth of E. coli and E. faecalis in vitro (Tatsuya and Kazunori 2013). However, this is not the first study showing that caffeine has antimicrobial activity in vitro, as a previous experiment had already demonstrated this concept (Daglia et al. 2007).
Evidence from in vivo studies (rodents)
A two-week administration of cocoa’s theobromine to healthy adult rats was shown to induce marked changes in gut microbiota composition. Specifically, rats that received a 10% cocoa-containing diet had lower intestinal counts of E. coli, whereas rat that received a 0.25% theobromine-containing diet had lower counts of Bifidobacterium spp., Streptococcus spp. and Clostridium histolyticum-C. perfingens group compared to normal-fed rats (Martín-Peláez et al. 2017). Consumption of fermented green tea, containing theophylline, was able to restore the changes in gut microbiota composition associated to diet-induced obesity in mice (Seo et al. 2015). In a different study, consumption of 500 μL/day of coffee for three consecutive days in specific-pathogen-free mice induced E. coli and Clostridium spp. counts to decrease significantly (Tatsuya and Kazunori 2013). Caffeine-rich Pu-erh tea remodelled the intestinal dysbiosis in mice with metabolic syndrome (Gao et al. 2018). Specifically, Akkermansia muciniphila and Faecalibacterium prausnitzii were speculated to be the key gut bacterial links between the Pu-erh tea treatment and metabolic syndrome at the genus and species levels (Gao et al. 2018). Chronic coffee consumption in diet-induced obese rats was accompanied by decreased abundance of Clostridium Cluster XI and increased levels of Enterobacteriaceae. Moreover, SCFAs (short-chain fatty acids) were largely increased in the coffee-fed rats (Cowan et al. 2014). It is important to note that studies on the effects of caffeine on gut microbiota are not always consistent, for example in a different experiment on rats, 8 weeks of coffee consumption did not alter the gut microbiota composition (Cowan et al. 2013). A 3-week regimen with oral administration of 0.7 mg/kg/day in mice decreased Lactobacillus ratios compared to controls, but none of the other taxa were affected (Kleber Silveira et al. 2018).
Evidence from studies in humans
Caffeine consumption has received much attention in recent years in relation to microbiome alterations often associated to metabolic disorders. Consumption of 3 cups of coffee daily for 3 weeks in healthy volunteers did not alter faecal profiles of the dominant microbiota, but increased the population of Bifidobacterium spp. (Jaquet et al. 2009). Moreover, in some subjects, there was a specific increase in the metabolic activity of Bifidobacterium spp. (Jaquet et al. 2009).
Conclusion
In Harry Potter, the basilisk hidden in the Chamber has the power to petrify people and poses a threat for Hogwarts School: what happens in the Chamber will affect the entire school and vice versa. Similarly, increasing evidence suggests that the gut microbiome affects and can be affected by various chemical compounds. This bidirectional influence is more and more studied in relation to psychotropic compounds. Initially thought to work only in the brain, in recent years, psychotropic compounds have shown antimicrobial activity in vitro and/or the ability to affect the gut microbiome in vivo. It is evident from the data reviewed here that different microbial effects have been attributed to psychotropic compounds, ranging from medications to drugs of abuse, caffeine and alcohol. The challenge now is to assess the functional role of these microbial changes. It is important to note that the results up to now come mainly from in vitro experiments on isolated strains and further clinical/preclinical experimentation is required to understand whether, for example drug-mediated microbial changes are complementary mechanisms of action or are responsible for the side effects associated to these compounds. Moreover, in terms of polypharmacy, more work is needed to investigate the impact of combinations of drugs on the microbiome. As our knowledge of the gut microbiome increases, the major lesson is that the Chamber of Secrets should be taken into account in future pharmacokinetic and pharmacodynamics analysis of known drugs and become part of the safety pharmacology of drugs in development. It is not bizarre to think that in the future microbiome measures will form part of clinical practice to investigate either the efficacy or side effects of psychotropic compounds. This field of research may also influence selection of individuals for clinical trials. Clearly it will need to be integrated, in a larger systems biology approach, to other -omics and biomarker measures in psychiatric patients.
Compliance with ethical standards
Conflicts of interest
JFC and TGD have research funding from Mead Johnson Nutrition, Cremo, Dupont, Suntory Wellness, Danone-Nutricia and 4D Pharma; JFC, TGD and GC have spoken at meetings sponsored by food and pharmaceutical companies; SC reports no financial interests or potential conflicts of interest.
Footnotes
This article belongs to a Special Issue on Microbiome in Psychiatry & Psychopharmacology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237–265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–1238. doi: 10.1016/j.neuron.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:319–331. doi: 10.1111/apt.13858. [DOI] [PubMed] [Google Scholar]
- Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- Al-Ghezi ZZ, Alghetaa HF, Nagarkatti M, Nagarkatti P. Combination of cannabinoids, Δ9- tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigate experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. J Immunol. 2017;198:219.220. doi: 10.1016/j.bbi.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali E, Almagboul A, Khogali S, Gergeir U (2018) Antimicrobial activity of Cannabis sativa L.
- Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet. 2008;33:101–106. doi: 10.1007/BF03191026. [DOI] [PubMed] [Google Scholar]
- Amaral L, Lorian V. Effects of chlorpromazine on the cell envelope proteins of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1923–1924. doi: 10.1128/AAC.35.9.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral L, Kristiansen J, Lorian V. Synergic effect of chlorpromazine on the activity of some antibiotics. J Antimicrob Chemother. 1992;30:556–558. doi: 10.1093/jac/30.4.556. [DOI] [PubMed] [Google Scholar]
- Amaral L, Kristiansen JE, Abebe LS, Millett W. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: potential use for initial therapy of freshly diagnosed tuberculosis. J Antimicrob Chemother. 1996;38:1049–1053. doi: 10.1093/jac/38.6.1049. [DOI] [PubMed] [Google Scholar]
- Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
- Arundel PA. A multi-compartmental model generally applicable to physiologically-based pharmacokinetics. IFAC Proceed. 1997;30:129–133. doi: 10.1016/S1474-6670(17)44557-5. [DOI] [Google Scholar]
- Avershina E, Storro O, Oien T, Johnsen R, Pope P, Rudi K. Major faecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol Ecol. 2014;87:280–290. doi: 10.1111/1574-6941.12223. [DOI] [PubMed] [Google Scholar]
- Ayaz M, Subhan F, Ahmed J, Khan A-U, Ullah F, Ullah I, Ali G, Syed N-I-H, Hussain S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J Biol Res. 2015;22:4. doi: 10.1186/s40709-015-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate-Peril MA, Kirby JR, Calarge CA. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CML, Pearson NA, Murry DJ, Grobe JL, Kirby JR. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2:1725–1734. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco LK, Le Bras J. Reversal of chloroquine resistance with desipramine in isolates of Plasmodium falciparum from Central and West Africa. Trans R Soc Trop Med Hyg. 1990;84:479–481. doi: 10.1016/0035-9203(90)90006-Z. [DOI] [PubMed] [Google Scholar]
- Begec Z, Yucel A, Yakupogullari Y, Erdogan MA, Duman Y, Durmus M, Ersoy MO. The antimicrobial effects of ketamine combined with propofol: an in vitro study. Braz J Anesthesiol. 2013;63:461–465. doi: 10.1016/j.bjan.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bengmark S. Gut microbiota, immune development and function. Pharmacol Res. 2013;69:87–113. doi: 10.1016/j.phrs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Bennett PN, Brown MJ 2008 Clinical pharmacology. Elsevier
- Bettencourt MV, Bosne-David S, Amaral L. Comparative in vitro activity of phenothiazines against multidrug-resistant Mycobacterium tuberculosis. Int J Antimicrob Agents. 2000;16:69–71. doi: 10.1016/S0924-8579(00)00199-0. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M, Loessner MJ, Vavricka SR, Fried M, Schreiber S, Schuppler M, Rogler G. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- Bohnert JA, Szymaniak-Vits M, Schuster S, Kern WV. Efflux inhibition by selective serotonin reuptake inhibitors in Escherichia coli. J Antimicrob Chemother. 2011;66:2057–2060. doi: 10.1093/jac/dkr258. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- Chi L, Mahbub R, Gao B, Bian X, Tu P, Ru H, Lu K. Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem Res Toxicol. 2017;30:2110–2119. doi: 10.1021/acs.chemrestox.7b00162. [DOI] [PubMed] [Google Scholar]
- Chung KT, Stevens SE, Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18:175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Keenan CM, Reimer RA, Le Foll B, Sharkey KA. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ(9)-tetrahydrocannabinol. PLoS One. 2015;10:e0144270. doi: 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone MG, Spichak S, O’Mahony SM, O’Leary OF, Clarke G, Stanton C, Dinan TG, Cryan JF (2018) Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry [DOI] [PubMed]
- Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan TE, Palmnas M, Ardell K, Yang JJ, Reimer R, Vogel H, Shearer J. Chronic coffee consumption alters gut microbiome: potential mechanism to explain the protective effects of coffee on type 2 diabetes? FASEB J. 2013;27:951.951–951.951. [Google Scholar]
- Cowan TE, Palmnäs MSA, Yang J, Bomhof MR, Ardell KL, Reimer RA, Vogel HJ, Shearer J. Chronic coffee consumption in the diet-induced obese rat: impact on gut microbiota and serum metabolomics. J Nutr Biochem. 2014;25:489–495. doi: 10.1016/j.jnutbio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Csiszar K, Molnar J. Mechanism of action of tricyclic drugs on Escherichia coli and Yersinia enterocolitica plasmid maintenance and replication. Anticancer Res. 1992;12:2267–2272. [PubMed] [Google Scholar]
- Currò D. The role of gut microbiota in the modulation of drug action: a focus on some clinically significant issues. Expert Rev Clin Pharmacol. 2018;11:171–183. doi: 10.1080/17512433.2018.1414598. [DOI] [PubMed] [Google Scholar]
- Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, Stanton C, Dinan TG, Cryan JF (2018) Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology [DOI] [PubMed]
- Daglia M, Papetti A, Grisoli P, Aceti C, Spini V, Dacarro C, Gazzani G. Isolation, identification, and quantification of roasted coffee antibacterial compounds. J Agric Food Chem. 2007;55:10208–10213. doi: 10.1021/jf0722607. [DOI] [PubMed] [Google Scholar]
- Dastidar SG, Chaudhury A, Annadurai S, Roy S, Mookerjee M, Chakrabarty AN. In vitro and in vivo antimicrobial action of fluphenazine. J Chemother. 1995;7:201–206. doi: 10.1179/joc.1995.7.3.201. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O'Sullivan O, Crispie F, Dinan TG, Cryan JF, O'Mahony SM. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Timary P, Leclercq S, Starkel P, Delzenne N. A dysbiotic subpopulation of alcohol-dependent subjects. Gut Microbes. 2015;6:388–391. doi: 10.1080/19490976.2015.1107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL. How antidepressants help depression: mechanisms of action and clinical response. J Clin Psychiatry. 2004;65:25–30. [PubMed] [Google Scholar]
- DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Doogue MP, Polasek TM. The ABCD of clinical pharmacokinetics. Ther Adv Drug Saf. 2013;4:5–7. doi: 10.1177/2042098612469335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElRakaiby M, Dutilh BE, Rizkallah MR, Boleij A, Cole JN, Aziz RK. Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics. OMICS: J Integr Biol. 2014;18:402–414. doi: 10.1089/omi.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiobu N, Hoosein N. An assessment of the in vitro antimicrobial effects of two antiepileptic drugs—sodium valproate and phenytoin. Antonie Van Leeuwenhoek. 2003;83:63–68. doi: 10.1023/A:1022992224594. [DOI] [PubMed] [Google Scholar]
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- Farzam H, Farahani A, Tafkik A, Gorgin Karaji A, Mohajeri P, Rezaei M, Jalalvandi F. Antibacterial effect of tramadol against Staphylococcus aureus and Pseudomonas aeruginosa: an in vivo study. New Microbes New Infect. 2018;24:42–46. doi: 10.1016/j.nmni.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10:239–248. doi: 10.1097/00131746-200407000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37:261–267. doi: 10.1002/phar.1890. [DOI] [PubMed] [Google Scholar]
- Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM, Ellingrod VL (2019) Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacotherapy [DOI] [PMC free article] [PubMed]
- Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Xie Q, Kong P, Liu L, Sun S, Xiong B, Huang B, Yan L, Sheng J, Xiang H (2018) Polyphenol- and caffeine-rich postfermented pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect Immun 86 [DOI] [PMC free article] [PubMed]
- Garcia-Gonzalez AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169:431–441.e438. doi: 10.1016/j.cell.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez J, Sanchez JEG, Munoz Bellido JL. In vitro activity of 79 antimicrobial agents against Corynebacterium group D2. Antimicrob Agents Chemother. 1991;35:2140–2143. doi: 10.1128/AAC.35.10.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Can Med Assoc J. 2005;172:1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocmen S, Buyukkocak U, Caglayan O. In vitro investigation of the antibacterial effect of ketamine. Ups J Med Sci. 2008;113:39–46. doi: 10.3109/2000-1967-211. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–1378. doi: 10.1016/S0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- Hadera M, Mehari S, Basha N, Amha N, Berhane Y (2018) Study on antimicrobial potential of selected non-antibiotics and its interaction with conventional antibiotics UKJPB 6, 01-07
- Hahn BL, Sohnle PG. Effect of thioridazine on experimental cutaneous staphylococcal infections. In vivo (Athens, Greece) 2014;28:33–38. [PMC free article] [PubMed] [Google Scholar]
- Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta() Science. 2013;341:295–298. doi: 10.1126/science.1235872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AS. The mode of action of tricyclic antidepressants: a brief review of recent progress. Postgrad Med J. 1980;56(Suppl 1):9–12. [PubMed] [Google Scholar]
- Idrees Zaidi M, Wattoo F, Hamid M, Wattoo S, Ahmed Tirmizi S, Salman S (2012) Antibacterial activities of nicotine and its zinc complex
- Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol. 2009;130:117–121. doi: 10.1016/j.ijfoodmicro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Jena L, Waghmare P, Kashikar S, Kumar S, Harinath BC. Computational approach to understanding the mechanism of action of isoniazid, an anti-TB drug. Int J Mycobacteriol. 2014;3:276–282. doi: 10.1016/j.ijmyco.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–364. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- Jobin C. Precision medicine using microbiota. Science. 2018;359:32–34. doi: 10.1126/science.aar2946. [DOI] [PubMed] [Google Scholar]
- Kao AC-C, Spitzer S, Anthony DC, Lennox B, Burnet PWJ. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry. 2018;8:66. doi: 10.1038/s41398-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Yoo DH, Jung IH, Lim S, Jeong JJ, Kim KA, Bae ON, Yoo HH, Kim DH. Reduced metabolic activity of gut microbiota by antibiotics can potentiate the antithrombotic effect of aspirin. Biochem Pharmacol. 2016;122:72–79. doi: 10.1016/j.bcp.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Kim JK, Choi MS, Jeong JJ, Lim SM, Kim IS, Yoo HH, Kim DH. Effect of probiotics on pharmacokinetics of orally administered acetaminophen in mice. Drug Metab Dispos. 2018;46:122–130. doi: 10.1124/dmd.117.077222. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ. Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep. 2016;6:35455. doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Petrosino J, Ajami N, Feng W, Wang Y, Liu Y, Beier JI, Barve SS, Yin X, Wei X, Zhang X, McClain CJ. Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am J Pathol. 2016;186:765–776. doi: 10.1016/j.ajpath.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber Silveira A, Moresco KS, Mautone Gomes H, da Silva Morrone M, Kich Grun L, Pens Gelain D, de Mattos Pereira L, Giongo A, Rodrigues De Oliveira R, Fonseca Moreira JC. Guarana (Paullinia cupana Mart.) alters gut microbiota and modulates redox status, partially via caffeine in Wistar rats. Phytother Res. 2018;32:2466–2474. doi: 10.1002/ptr.6185. [DOI] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen JE, Vergmann B. The antibacterial effect of selected phenothiazines and thioxanthenes on slow-growing mycobacteria. Acta Pathol Microbiol Immunol Scand B. 1986;94:393–398. doi: 10.1111/j.1699-0463.1986.tb03073.x. [DOI] [PubMed] [Google Scholar]
- Kruszewska H, Zareba T, Tyski S. Examination of antimicrobial activity of selected non-antibiotic drugs. Acta Pol Pharm. 2004;61(Suppl):18–21. [PubMed] [Google Scholar]
- Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A. Mechanism of action of antipsychotic drugs: from dopamine D2 receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2005;27:S16–S24. doi: 10.1016/j.clinthera.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Lass-Florl C, Ledochowski M, Fuchs D, Speth C, Kacani L, Dierich MP, Fuchs A, Wurzner R. Interaction of sertraline with Candida species selectively attenuates fungal virulence in vitro. FEMS Immunol Med Microbiol. 2003;35:11–15. doi: 10.1111/j.1574-695X.2003.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Zhang H, Orlovich DA, Fawcett JP. The influence of probiotic treatment on sulfasalazine metabolism in rat. Xenobiotica. 2012;42:791–797. doi: 10.3109/00498254.2012.660508. [DOI] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW (2018) The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, Urbaniak C, Byrne WL, Tangney M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5:14554. doi: 10.1038/srep14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Wei CJ, Tu SC. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of inha inhibitor. J Biol Chem. 2000;275:2520–2526. doi: 10.1074/jbc.275.4.2520. [DOI] [PubMed] [Google Scholar]
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- Li H, He J, Jia W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2016;12:31–40. doi: 10.1517/17425255.2016.1121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGuidice A, Wallace BD, Bendel L, Redinbo MR, Boelsterli UA. Pharmacologic targeting of bacterial beta-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J Pharmacol Exp Ther. 2012;341:447–454. doi: 10.1124/jpet.111.191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem. 2003;10:41–49. doi: 10.2174/0929867033368619. [DOI] [PubMed] [Google Scholar]
- Mackay RJ, McEntyre CJ, Henderson C, Lever M, George PM. Trimethylaminuria: causes and diagnosis of a socially distressing condition. Clin Biochem Rev. 2011;32:33–43. [PMC free article] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Sinha C, Kumar Jena A, Ghosh S, Samanta A. An investigation on in vitro and in vivo antimicrobial properties of the antidepressant: amitriptyline hydrochloride. Braz J Microbiol. 2010;41:635–645. doi: 10.1590/S1517-83822010000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Peláez S, Camps-Bossacoma M, Massot-Cladera M, Rigo-Adrover M, Franch À, Pérez-Cano FJ, Castell M. Effect of cocoa’s theobromine on intestinal microbiota of rats. Mol Nutr Food Res. 2017;61:1700238. doi: 10.1002/mnfr.201700238. [DOI] [PubMed] [Google Scholar]
- Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskova Z, Anzenbacherova E, Vecera R, Tlaskalova-Hogenova H, Kolar M, Anzenbacher P. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PLoS One. 2014;9:e87150. doi: 10.1371/journal.pone.0087150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder R, Ganguly K, Dastidar SG, Chakrabarty AN. Trifluoperazine: a broad spectrum bactericide especially active on staphylococci and vibrios. Int J Antimicrob Agents. 2001;18:403–406. doi: 10.1016/S0924-8579(01)00324-7. [DOI] [PubMed] [Google Scholar]
- Molnar J. Antiplasmid activity of tricyclic compounds. Methods Find Exp Clin Pharmacol. 1988;10:467–474. [PubMed] [Google Scholar]
- Molnar J, Beladi I, Foldes I. Studies on antituberculotic action of some phenothiazine derivatives in vitro. Zentralbl Bakteriol Orig A. 1977;239:521–526. [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. 2014;9:e115225. doi: 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz Criado S, Fajardo M, Gutierrez MN, Munoz-Bellido JL, Garcia Rodriguez JA (1997) Psychiatric drugs inhibit slime production in Staphylococcus epidermidis. International Congress of Chemotherapy
- Munoz-Bellido JL, Munoz-Criado S, Garcia-Rodriguez JA. In-vitro activity of psychiatric drugs against Corynebacterium urealyticum (Corynebacterium group D2) J Antimicrob Chemother. 1996;37:1005–1009. doi: 10.1093/jac/37.5.1005. [DOI] [PubMed] [Google Scholar]
- Muñoz-Criado S, Muñoz-Bellido XL, García-Rodríguez JA. In vitro activity of nonsteroidal antiinflammatory agents, phenotiazines, and antidepressants against Brucella species. Eur J Clin Microbiol Infect Dis. 1996;15:418–420. doi: 10.1007/BF01690103. [DOI] [PubMed] [Google Scholar]
- Muñoz-Criado S, Muñoz-Bellido JL, Alonso-Manzanares MA, Gutiérrez-Zufiaurre MN, García-Rodríguez JA. Psychotropic drugs inhibit swarming in Proteus spp. and related genera. Clin Microbiol Infect. 1998;4:447–449. doi: 10.1111/j.1469-0691.1998.tb00393.x. [DOI] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Kinouchi T, Kataoka K, Akimoto S, Matsuda Y, Ohnishi Y. Intestinal anaerobic bacteria hydrolyse sorivudine, producing the high blood concentration of 5-(E)-(2-bromovinyl) uracil that increases the level and toxicity of 5-fluorouracil. Pharmacogenetics. 1997;7:35–43. doi: 10.1097/00008571-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Nehme H, Saulnier P, Ramadan AA, Cassisa V, Guillet C, Eveillard M, Umerska A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS One. 2018;13:e0189950. doi: 10.1371/journal.pone.0189950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrinck AM, Etxeberria U, Taminiau B, Daube G, Van Hul M, Everard A, Cani PD, Bindels LB, Delzenne NM (2017) Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res 61 [DOI] [PubMed]
- Ning T, Gong X, Xie L, Ma B. Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front Microbiol. 2017;8:1620. doi: 10.3389/fmicb.2017.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, Biavati B, Monti A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.) Fitoterapia. 2010;81:413–419. doi: 10.1016/j.fitote.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Ordway D, Viveiros M, Leandro C, Amaral L, Arroz MJ, Molnar J, Kristiansen JE. Chlorpromazine has intracellular killing activity against phagocytosed Staphylococcus aureus at clinical concentrations. J Infect Chemother. 2002;8:227–231. doi: 10.1007/s10156-002-0188-4. [DOI] [PubMed] [Google Scholar]
- Ordway D, Viveiros M, Leandro C, Arroz MJ, Amaral L. Intracellular activity of clinical concentrations of phenothiazines including thioridiazine against phagocytosed Staphylococcus aureus. Int J Antimicrob Agents. 2002;20:34–43. doi: 10.1016/S0924-8579(02)00110-3. [DOI] [PubMed] [Google Scholar]
- Ordway D, Viveiros M, Leandro C, Bettencourt R, Almeida J, Martins M, Kristiansen JE, Molnar J, Amaral L. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:917–922. doi: 10.1128/AAC.47.3.917-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey S, Workman P, Sarker D. Pharmacokinetics and pharmacodynamics in drug development. In: Schwab M, editor. Encyclopedia of cancer. Berlin Heidelberg: Springer; 2011. pp. 2845–2848. [Google Scholar]
- Palit P, Ali N. Oral therapy with sertraline, a selective serotonin reuptake inhibitor, shows activity against Leishmania donovani. J Antimicrob Chemother. 2008;61:1120–1124. doi: 10.1093/jac/dkn046. [DOI] [PubMed] [Google Scholar]
- Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J NeuroImmune Pharmacol. 2018;13:113–122. doi: 10.1007/s11481-017-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Mortimer RB, Mitchell M. Sertraline demonstrates fungicidal activity in vitro for Coccidioides immitis. Mycology. 2016;7:99–101. doi: 10.1080/21501203.2016.1204368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia CS, Pierre A, Nowakowski J. Antimicrobial activity of nicotine against a spectrum of bacterial and fungal pathogens. J Med Microbiol. 2000;49:675–676. doi: 10.1099/0022-1317-49-7-675. [DOI] [PubMed] [Google Scholar]
- Peppercorn MA, Goldman P. The role of intetstinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181:555–562. [PubMed] [Google Scholar]
- Perez-Muñoz ME, Arrieta M-C, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson VL, Jury NJ, Cabrera-Rubio R, Draper LA, Crispie F, Cotter PD, Dinan TG, Holmes A, Cryan JF. Drunk bugs: chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav Brain Res. 2017;323:172–176. doi: 10.1016/j.bbr.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Lv P-C, Shi L, Fang R-Q, Song Z-C, Zhu H-L (2009) Synthesis, antimicrobial activity of lamotrigine and its ammonium derivatives
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. Br Med J. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj CV, Dhala S. Effect of naturally occurring xanthines on bacteria. I. Antimicrobial action and potentiating effect on antibiotic spectra. Appl Microbiol. 1965;13:432–436. doi: 10.1128/am.13.3.432-436.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani Basu L, Mazumdar K, Dutta NK, Karak P, Dastidar SG. Antibacterial property of the antipsychotic agent prochlorperazine, and its synergism with methdilazine. Microbiol Res. 2005;160:95–100. doi: 10.1016/j.micres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Basselin M, Kim H-W, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rizkallah M, Saad R, Aziz R (2010) The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics
- Rodrigues RR, Greer RL, Dong X, Dsouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;22:178.e171–178.e179. doi: 10.1016/j.cmi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PH, Renkonen OV. Antimicrobial activity of bupivacaine and morphine. Anesthesiology. 1985;62:178–179. doi: 10.1097/00000542-198502000-00015. [DOI] [PubMed] [Google Scholar]
- Rossato L, Loreto ES, Zanette RA, Chassot F, Santurio JM, Alves SH. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol (Praha) 2016;61:399–403. doi: 10.1007/s12223-016-0449-8. [DOI] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Saad R, Rizkallah MR, Aziz RK. Gut pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathogens. 2012;4:16. doi: 10.1186/1757-4749-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A, Facer CA. Desipramine reversal of chloroquine resistance in wild isolates of Plasmodium falciparum. Lancet. 1990;335:164–165. doi: 10.1016/0140-6736(90)90034-3. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung K-Y, Herrera-Dominguez L, Sudiwala S, Pessia A, Clayton PT, Bryson K, Velagapudi V, Mills PB, Typas A, Greene NDE, Cabreiro F. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169:442–456.e418. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DB, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY, Bae IH, Lee SJ. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food. 2015;18:549–556. doi: 10.1089/jmf.2014.3265. [DOI] [PubMed] [Google Scholar]
- Sheagren JN, Barsoum IS, Lin MY. Methadone: antimicrobial activity and interaction with antibiotics. Antimicrob Agents Chemother. 1977;12:748–750. doi: 10.1128/AAC.12.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Zydecka K, Loniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, Galling B (2018) Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology [DOI] [PMC free article] [PubMed]
- Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, Kahn CR (2018) Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry [DOI] [PMC free article] [PubMed]
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors: serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/S0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Stewart CJ, Auchtung TA, Ajami NJ, Velasquez K, Smith DP, De La Garza R, II, Salas R, Petrosino JF. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ. 2018;6:e4693. doi: 10.7717/peerj.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JM, Davis JH, Mangat CS, Williamson JR, Brown ED. Discovery of a small molecule that inhibits bacterial ribosome biogenesis. Elife. 2014;3:e03574. doi: 10.7554/eLife.03574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanai-Shacoori Z, Shacoori V, Jolivet-Gougeon A, Vo Van JM, Repere M, Donnio PY, Bonnaure-Mallet M. The antibacterial activity of tramadol against bacteria associated with infectious complications after local or regional anesthesia. Anesth Analg. 2007;105:524–527. doi: 10.1213/01.ane.0000267525.51017.b8. [DOI] [PubMed] [Google Scholar]
- Tatsuya N, Kazunori O. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol Lett. 2013;343:161–168. doi: 10.1111/1574-6968.12142. [DOI] [PubMed] [Google Scholar]
- Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7:11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and poppies: the good, the bad, and the ugly of opioid analgesics. J Neurosci. 2015;35:13879–13888. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino-Rangel Rde J, Villanueva-Lozano H, Hernandez-Rodriguez P, Martinez-Resendez MF, Garcia-Juarez J, Rodriguez-Rocha H, Gonzalez GM. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol. 2016;54:280–286. doi: 10.1093/mmy/myv109. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361 [DOI] [PMC free article] [PubMed]
- van Hogezand RA, Kennis HM, van Schaik A, Koopman JP, van Hees PA, van Tongeren JH. Bacterial acetylation of 5-aminosalicylic acid in faecal suspensions cultured under aerobic and anaerobic conditions. Eur J Clin Pharmacol. 1992;43:189–192. doi: 10.1007/BF01740669. [DOI] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros M, Amaral L. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int J Antimicrob Agents. 2001;17:225–228. doi: 10.1016/S0924-8579(00)00343-5. [DOI] [PubMed] [Google Scholar]
- Viveiros M, Martins M, Couto I, Kristiansen JE, Molnar J, Amaral L. The in vitro activity of phenothiazines against Mycobacterium avium: potential of thioridazine for therapy of the co-infected AIDS patient. In Vivo. 2005;19:733–736. [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, Kane AV. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs. 2014;75:347–357. doi: 10.15288/jsad.2014.75.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright M, Phoenix DA, Gaskell M, Marshall B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother. 1999;44:823–825. doi: 10.1093/jac/44.6.823. [DOI] [PubMed] [Google Scholar]
- Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S, Redinbo MR. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Griffin BT, Clarke G, Hyland NP (2018) Drug–gut microbiota interactions: implications for neuropharmacology. Br J Pharmacol [DOI] [PMC free article] [PubMed]
- Wang M, Monaco MH, Donovan SM. Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonatal Med. 2016;21:380–387. doi: 10.1016/j.siny.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering. 2017;3:71–82. doi: 10.1016/J.ENG.2017.01.008. [DOI] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep. 2018;8:3596. doi: 10.1038/s41598-018-21915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach EC, Levenbook L, Alling DW. Binding of tricyclic antidepressant drugs to trophozoites of Giardia lamblia. Comp Biochem Physiol C. 1992;102:391–396. doi: 10.1016/0742-8413(92)90131-P. [DOI] [PubMed] [Google Scholar]
- Whitlock FA, Price J. Use of beta-adrenergic receptor blocking drugs in psychiatry. Drugs. 1974;8:109–124. doi: 10.2165/00003495-197408020-00004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K. Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep. 2017;7:3628. doi: 10.1038/s41598-017-03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4:110–118. doi: 10.4254/wjh.v4.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A, Culp E, Perry J, Lau JT, MacNeil LT, Surette MG, Wright GD. Transformation of the anticancer drug doxorubicin in the human gut microbiome. ACS Infect Dis. 2018;4:68–76. doi: 10.1021/acsinfecdis.7b00166. [DOI] [PubMed] [Google Scholar]
- Yoo DH, Kim IS, Van Le TK, Jung IH, Yoo HH, Kim DH. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42:1508–1513. doi: 10.1124/dmd.114.058354. [DOI] [PubMed] [Google Scholar]
- Yoo HH, Kim IS, Yoo DH, Kim DH. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J Hypertens. 2016;34:156–162. doi: 10.1097/HJH.0000000000000773. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA, LifeLines cohort, S. Weersma RK, Feskens EJM, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Jameson E, Crosatti M, Schafer H, Rajakumar K, Bugg TD, Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D, Dwyer D. Antidepressants cause lethal disruption of membrane function in the human protozoan parasite Leishmania. Science. 1984;226:977–979. doi: 10.1126/science.6505677. [DOI] [PubMed] [Google Scholar]


