Abstract
Rationale
Multiple drugs are known to induce metabolic malfunctions, among them second-generation antipsychotics (SGAs). The pathogenesis of such adverse effects is of multifactorial origin.
Objectives
We investigated whether SGAs drive dysbiosis, assessed whether gut microbiota alterations affect body weight and metabolic outcomes, and looked for the possible mechanism of metabolic disturbances secondary to SGA treatment in animal and human studies.
Methods
A systematic literature search (PubMed/Medline/Embase/ClinicalTrials.gov/PsychInfo) was conducted from database inception until 03 July 2018 for studies that reported the microbiome and weight alterations in SGA-treated subjects.
Results
Seven articles reporting studies in mice (experiments = 8) and rats (experiments = 3) were included. Olanzapine was used in five and risperidone in six experiments. Only three articles (experiments = 4) in humans fit our criteria of using risperidone and mixed SGAs. The results confirmed microbiome alterations directly (rodent experiments = 5, human experiments = 4) or indirectly (rodent experiments = 4) with predominantly increased Firmicutes abundance relative to Bacteroidetes, as well as weight gain in rodents (experiments = 8) and humans (experiments = 4). Additionally, olanzapine administration was found to induce both metabolic alterations (adiposity, lipogenesis, plasma free fatty acid, and acetate levels increase) (experiments = 3) and inflammation (experiments = 2) in rodents, whereas risperidone suppressed the resting metabolic rate in rodents (experiments = 5) and elevated fasting blood glucose, triglycerides, LDL, hs-CRP, antioxidant superoxide dismutase, and HOMA-IR in humans (experiment = 1). One rodent study suggested a gender-dependent effect of dysbiosis on body weight.
Conclusions
Antipsychotic treatment-related microbiome alterations potentially result in body weight gain and metabolic disturbances. Inflammation and resting metabolic rate suppression seem to play crucial roles in the development of metabolic disorders.
Electronic supplementary material
The online version of this article (10.1007/s00213-018-5102-6) contains supplementary material, which is available to authorized users.
Keywords: Microbiota, Second-generation antipsychotics, Metabolism, Dysbiosis
Introduction
Second-generation antipsychotics (SGAs) have been used successfully for the treatment of schizophrenia, bipolar disorders, autism spectrum disorders, major depressive disorders, tic disorder, agitation, sleeping problems, and dementia, among others (Vasan and Abdijadid 2018). The number of prescriptions for SGAs has increased worldwide for both youths and adults (Ilies et al. 2017), with the most recent cross-sectional study of 14 countries finding that quetiapine, risperidone (RIS), and olanzapine (OLZ) are the most frequently prescribed atypical SGAs (Hálfdánarson et al. 2017). An alarming increase in prescriptions, particularly in youth between 15 and 19 years of age, forces us to turn our attention to the health consequences of long-term SGA treatment (Kalverdijk et al. 2017). These consequences include various cardiometabolic adverse effects, such as significant weight gain, hypertriglyceridaemia, hypercholesterolaemia, hypertension, and impaired glucose metabolism (De Hert et al. 2011; Galling and Correll 2015; Galling et al. 2016; Vancampfort et al. 2016), which are all related to metabolic syndrome and cardiovascular disease (Sjo et al. 2017). These changes emerge even after short exposure and increase with cumulative dosages, and differ between agents (Bak et al. 2014).
Overall, in people with severe mental illness, life expectancy is shortened by 10–20 years (Chang et al. 2011), predominantly due to an imbalance in the cardiometabolic system. The prevalence of metabolic syndrome was observed in approximately 30% of patients treated with SGAs (Sanchez-Martinez et al. 2017). Therefore, the American Diabetes Association and the American Psychiatric Association released consensus guidelines to monitor weight and other metabolic parameters in patients treated with SGAs. Moreover, the use of olanzapine in children is discouraged by the Food and Drug Administration because of its association with obesity (American Diabetes Association et al. 2004).
The mechanism of metabolic disruptions, including obesity, hypertension, diabetes, and atherosclerosis (Weiss and Hennet 2017), secondary to SGAs is not fully understood. However, several hypotheses have been proposed, referring to (i) illness- and lifestyle-related factors on metabolism (unhealthy diet, low physical activity, smoking) (Alvarez-Jiménez et al. 2008; Lau et al. 2016; Dayabandara et al. 2017), (ii) SGAs increasing energy intake via neurotransmitter binding in the hypothalamus (Lu et al. 2015), (iii) decreased energy expenditure due to the sedative effect of SGAs (Zimmermann et al. 2003), and (iv) genetic risk associated with the primary illness (Zhang et al. 2016). Other potentially related findings from previous research include (v) diminished insulin synthesis due to the affinity of SGAs for serotonin receptors in the pancreas, leading to diabetic-like metabolic changes (Zhang et al. 2013; Ballon et al. 2014); (vi) elevated muscle, adipose tissue, and liver insulin resistance and glucose transporter efficiency via inhibition of glucose uptake (Dwyer and Donohoe 2003; Verhaegen and Van Gaal 2017); and (vii) accelerated adipose tissue lipogenesis and elevated liver fat content in SGA-treated subjects (Chintoh et al. 2009).
A new approach discussed recently is mediation of SGA-induced adverse effects via the gut microbiota. Maier et al. (Maier et al. 2018) reported that almost one quarter of non-antibiotic drugs used in humans, predominantly antipsychotics, possess antimicrobial activity with potential to imbalance the gut ecosystem. Recently, the inhibition of Escherichia coli APC105 growth in vitro with escitalopram was shown as well as its modulatory effects toward other intestinal bacteria in animals (Cussotto et al. 2018). This might mean that the administration of psychopharmacologic drugs may mimic the effect of low-dose antibiotics and thereby be at least partly responsible for antimicrobial resistance of gut microbiota. On the other hand, Nehme et al. reported that atypical antipsychotics, including RIS, OLZ, aripiprazole, clozapine, and quetiapine, would not possess antimicrobial activity, while phenothiazines and thioxanthenes would inhibit the growth of tested bacteria at various minimum concentrations (Nehme et al. 2018).
As dysbiosis may contribute to body weight alterations and cardiometabolic outcomes (Angelakis et al. 2013; Omer and Atassi 2017; Heiss and Olofsson 2017), SGA-induced dysbiosis has been hypothesized to cause adverse metabolic effects (Kanji et al. 2018). In spite of reports linking specific changes in microbiota to weight gain and metabolic disturbances, the subject has not been comprehensively and systematically reviewed, and the mechanism underlying the potential influence of the microbiota on metabolic processes have not been discussed in detail, taking into account limitations regarding study quality.
Therefore, we prepared the first systematic review (SR) investigating the following aims: (1) whether SGAs drive dysbiosis, (2) assessing whether alterations of gut microbiota composition and function affect body weight and metabolic outcome, and (3) examining the possible mechanisms of metabolic disturbances secondary to SGA treatment in rodent and human studies.
Material and methods
Search strategy and selection criteria
This study was conducted according to the requirements established in the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocols (Shamseer et al. 2015). Two independent authors (AM and KSZ) systematically searched PubMed/Medline/Embase/PsycInfo/Clinicaltrials.gov from database inception until 03 July 2018. The search was conducted using the following terms identified as medical subject headings (MeSH bold font), Supplementary Concept Record terms (SCR italic font), and free text terms: (Microbiota OR Gastrointestinal Microbiome OR microbiome OR microbio*) AND (antipsych* OR neurolept* OR SGA* OR Antipsychotic agents OR Anti-Anxiety agents OR Anti-depressive Agents OR Anti-depressive Agents, Second-Generation OR Hypnotics and Sedatives OR Antimanic Agents OR Olanzapine OR Risperidone OR Atypical Antipsychotics) AND (Body Weight OR Body Weight Changes OR Body Weights and Measures OR Body Mass Index OR BMI OR Metabolism OR metabolic* OR Lipids OR tRMR OR Cholesterol OR Triglycerides OR Cholesterol, LDL OR LDL OR Fatty Acids OR Fatty Acids, Volatile OR Acetates OR Butyrates OR Butyric Acid OR Propionates OR hepatic* OR SCFA OR Toxins, Biological OR Bacterial Toxins OR Endotoxins OR Lipopolysaccharides OR Lipid A OR O Antigens OR LPS OR Glucose OR Insulin OR HOMA-IR OR Inflammation OR Cytokines OR Interleukins). Reviews, meta-analyses, and systematic reviews were omitted from the search strategy. The electronic search was supplemented by a manual review of the reference lists from eligible publications and relevant reviews.
Inclusion criteria for animal/human studies were as follows:
Treatment with SGAs.
An in vivo study.
A study reporting on metabolic as well as body weight changes and alterations of microbiome composition and function (measured by direct and indirect methods). When an animal or human study consisted of only a few experiments, only experiments fulfilling the above criteria were included and described.
Data extraction and analysis
At least two authors (AM, KSZ, IŁ) independently extracted information from each study, including details on study characteristics (e.g., study design, treatment protocol, duration, number of subjects, outcome parameters, gut microbiota analysis technique), treatment characteristics (e.g., psychopharmacological agent, dosage, duration of treatment), and subject/patient characteristics (e.g., age, sex, comorbidities, metabolic outcomes). When abstracting data from figures, WebPlot digitizer software was used (https://automeris.io/WebPlotDigitizer/).
The significance of the analysed studies was arbitrarily assigned according to the following scheme: strong—germ-free study, faecal transplantation, statistical significance; middle—conflicting data, lack of relevance due to small group size, data difficult to explain; weak—only co-incidence.
Risk of bias assessment
Two authors (KSŻ and IŁ) independently assessed the risk of bias using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) Risk of Bias tool for animal studies (Hooijmans et al. 2014), except for item 9 (selective outcome reporting), as this was not assessed in any of the surveyed studies. The STROBE assessment (Vandenbroucke et al. 2014) was used for studies in humans, except for item 16 (main results: unadjusted estimates, confounder-adjusted estimates, category boundaries, translating estimates of relative risk into absolute risk for a meaningful period), as it was not applicable. Outcomes were expressed as the percentage of low-risk judgements (i.e., by dividing the low-risk score by the total number of judgements). When the number was below 16 points (50%), we arbitrarily defined the quality as low. When the results represented up to 60% of the maximum number of points, we treated the study as of moderate quality. Results up to and over 75% were considered high or very high quality, respectively. When a discrepancy occurred, a third author (WM) was involved (Supplementary Figs. S1 and S2).
Results
Descriptive data
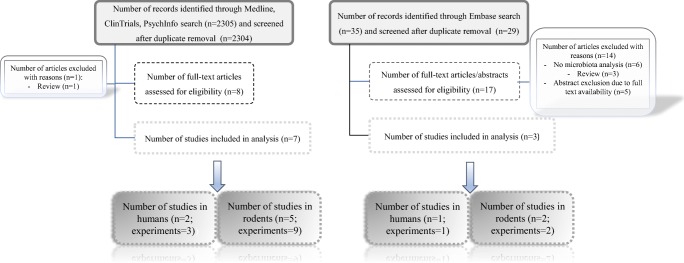
The initial search yielded 2340 hits; 2315 articles were excluded as duplicates or after evaluation at the title or abstract level. Out of 25 full-text articles that were reviewed, 15 were excluded for not fulfilling the inclusion criteria. Reasons for exclusion were review (n = 4), no microbiota analysis (n = 6), medications other than SGAs (n = 1), and full-text unavailability (n = 4), resulting in 10 articles that included 15 experiments in the systematic review (Fig. 1).
Fig. 1.
Study flow chart
Study and sample characteristics
Rodents
Overall, seven articles (experiments = 11) comprising 282 rodents were included: four articles had conducted the experiments using mice (n = 198; C57BL/6J) and three using rats (n = 84; Sprague–Dawley rats). Rats were of both genders (Davey et al. 2012) or females only (Davey et al. 2013; Kao et al. 2018), aged 6–8 weeks and weighing approximately 200–250 g. Female mice were either 4–8 weeks (Morgan et al. 2014) or 6–7 weeks old (Bahr et al. 2015b), and no information regarding age and gender were found in the other two mouse articles (Grobe et al. 2015; Riedl et al. 2017). Agents tested were RIS (experiments = 6, n = 150 rodents) (Grobe et al. 2015; Bahr et al. 2015b; Riedl et al. 2017) and OLZ (experiments = 5, n = 132 rodents) (Davey et al. 2012, 2013; Morgan et al. 2014) administered orally (experiments = 7, n = 185 rodents) (Morgan et al. 2014; Grobe et al. 2015; Bahr et al. 2015b) or intraperitoneally (experiments = 3, n = 84 rodents) (Davey et al. 2012, 2013).
The influence of the microbiota on the metabolic outcome was analysed using the following experimental models: antibiotic usage (experiments = 2) (Davey et al. 2013; Bahr et al. 2015b), high-fat diet (HFD) (Morgan et al. 2014), germ-free model (Morgan et al. 2014), microbiota transfer (Grobe et al. 2015), or SGA treatment prior to cecectomy (Riedl et al. 2017) (one study each). Five rodent protocols were placebo-controlled (Davey et al. 2012, 2013; Grobe et al. 2015; Bahr et al. 2015b; Kao et al. 2018), including one with a faecal transfer trial (Grobe et al. 2015), one study had a cross-sectional design (Morgan et al. 2014), and one described SGA treatment prior to sham operation (Riedl et al. 2017) (for more details, see Table 1).
Table 1.
Summary of rodent studies
| Reference | Subjects: - Age - % Females |
Aim, design, and procedure | Number of subjects; duration of intervention | Groups + used substances - Dosage - Administration |
Outcome and conclusions |
|---|---|---|---|---|---|
| Davey et al. (2012) (Ireland) |
Sprague–Dawley rats: - 6 wks - NR |
Aim: analysis of the influence of OLZ administration on body weight, behaviour, gut microbiota, and inflammatory and metabolic markers in both gender rats. Design: placebo-controlled (OLZ or VEH). Procedure: Rats were treated with vehicle and two doses of OLZ for 21 days. |
N = 24; 21 d |
OLZ (n = 8) - 2 mg/kg twice daily - Intraperitoneal injection B.I.D. OLZ (n = 8) - 4 mg/kg twice daily - Intraperitoneal injection B.I.D. VEH (n = 8) Distilled water acidified with glacial acetic acid - Twice daily - Intraperitoneal injection |
Metabolic: (OLZ vs VEH): 1. ↑ body weight (only in females, higher for lower dose); 2. ↑ food and water intake (mostly in females); 3. ↓ locomotor activity; 4. adipose tissue: ↑ visceral fat, ↓ gene expression of SREBP-1 (in females), ↑ inflammation markers (IL-6 mRNA expression in females and 4-fold increase (insignificant) in males, CD68 expression in females and males); 5. plasma cytokines: ↑ IL-8 and IL-1ß in females, ↓ IL-6 and TNFα in males; 6. ↓ circulating levels of ghrelin in females, ↑ hypothalamic expression of ghrelin 1a receptor mRNA in males. Microbiota: females: 1. ↓ diversity, 2. phyla abundance: ↑ Firmicutes OLZ 2 mg/kg, 4 mg/kg vs VEH (84.06%, 88.12% vs 72.11%, respectively); ↓ Actinobacteria OLZ 2 mg/kg, 4 mg/kg vs VEH (0.34% and 0.15% vs 3.72%, respectively); ↓ Proteobacteria OLZ 2 mg/kg, 4 mg/kg vs VEH (0.15% and 0.77% vs 1.60%, respectively); ↓ Bacteroidetes OLZ 4 mg/kg 10.88% vs VEH 17.57%. Males: minimal impact of treatment affecting phyla abundance: ↑ Firmicutes OLZ 4 mg/kg 91.63% vs VEH 82.66%; ↓ Proteobacteria OLZ 2 mg/kg 0.94 vs VEH 3.15%; ↓ Bacteroidetes OLZ 4 mg/kg 7.97% vs VEH 14.08%. Conclusion: OLZ treatment is related with weight gain, metabolic disturbances, inflammation and microbiota alteration in gender dependent matter. |
| Davey et al. (2013) (Ireland) |
Sprague–Dawley rats: - 6 wks - 100% |
Aim: evaluation if alteration of gut microbiota can play a role in metabolic complications caused by OLZ administration. Design: placebo-controlled. Procedure: After 5 days of lead-in phase with VEH or ABX (to reduce bacteria population in the gastrointestinal tract), rats were randomized to OLZ or VEH treatment lasting 21 days. |
N = 36/40; 21 d |
VEH + OLZ (n = 9/10) OLZ: - 2 mg/kg twice daily - Intraperitoneal injection VEH: - Water acidified with glacial acetic acid - Intraperitoneal injection ABX + OLZ (n = 9/10) OLZ: - 2 mg/kg twice daily - Intraperitoneal injection ABX: - Neomycin (250 mg/kg/day), metronidazole (50 mg/ kg/day), polymyxin B (9 mg/kg/day) - Total vol. 4 mg/kg - Per os VEH + VEH (n = 9/10) - Water acidified with glacial acetic acid - Intraperitoneal injection ABX + VEH (n = 9/10) ABX: - Neomycin (250 mg/day), metronidazole (50 mg/kg/day), polymyxin B (9 mg/kg/day) - Total vol. 4 mg/kg - Per os VEH: - Water acidified with glacial acetic acid - Intraperitoneal injection |
Metabolic: (OLZ + VEH): 1. ↑ weight gain; 2. ↑ fat mass; 3. ↑macrophage infiltration of adipose tissue; 4. ↑ free fatty acid release; 5. ↑ hepatic expression of lipogenic enzyme fatty acid synthase (FAS) (effects 1–5 were attenuated by ABX); 6. ↓ insulin sensitivity (direct OLZ effect); 7. ↑ expression of sterol-regulatory element binding protein-1c (SREBP-1c) and acetyl Co-A carboxylase-1 (ACC) (effect of OLZ + ABX treatment). Gut microbiota: 1. OLZ + VEH vs VEH + VEH: A trend toward increased abundance of phylum Firmicutes (82.9% vs 76.5%) and reductions of phylum Bacteroidetes (10.0% vs 14.3%); 2. OLZ + ABX vs OLZ VEH: a trend toward reduced phylum Firmicutes (66.7% vs 82.9%) and increased phylum Bacteroidetes (18.9% vs 10.0%). Conclusion: Gut microbiome plays a role in metabolic disturbances caused by OLZ administration |
| Morgan et al. (2014) (USA) |
C57BL/6J mice: - 6 wks - 100% |
Experiment no. 1—germ-free study: Aim: testing whether weight gain induced by OLZ treatment in mice having “obesogenic” bacterial profile caused by HFD depends on gut microbiota Design: placebo-controlled study Procedure: group A: germ-free mice—HFD for 7 wks → gut colonization with caecal content from conventionally raised mice → HFD for 9 weeks Group B: germ-free mice HFD + OLZ for 7 wks → colonization → HFD for 2 wks → HFD + OLZ for 7 wks |
N = 24; 14 wks (7 wks germ-free + 7 wks conventional). |
HFD (n = 12) - 45% kcal fat - Per os HFD + OLZ (n = 12) HFD: - 45% kcal fat - Per os OLZ: - 50 mg/kg of HFD diet - Per os |
Metabolic: Germ-free phase: no significant difference in body weight; conventional housing conditions: significant weight gain in the OLZ + HFD group compared to HFD group. Conclusion: gut microbiota was necessary to potentiate weight gain caused by OLZ treatment. |
|
Experiment no. 2—cross-over study: Aim: studying the influence of OLZ treatment on the weight gain and gut microbiota composition in mice having “obesogenic” bacterial profile induced by HFD. Design: cross-over study Procedure: Group A: 2 wks chow (14 kcal% fat) → 5 wks HFD → 4 wks HFD + OLZ Group B: 2 wks chow → 1 wk. HFD → 4 wks HFD + OLZ → 4 wks HFD |
N = 24; 4 wks |
HFD + OLZ (n = 12) HFD: - 45% kcal fat - Per os OLZ: - 50 mg/kg of HFD diet - Per os HFD (n = 12) - 45% kcal fat - Per os |
Metabolic: 1. Weight gain is more rapid during OLZ ingestion than the placebo phase; 2. adiposity correlated positively with total body weight in OLZ phase; 3. OLZ increased adiposity even after accounting for weight gain. Gut microbiota: 1. decreased alpha diversity, without adjusting for temporal and cohousing effects; 2. increase in the relative abundance of classes Erysipelotrichi, Actinobacteria, and Gammaproteobacteria; 3. decreased abundance of class Bacteroidia. Erysipelotrichi enrichment due to OLZ treatment was correlated with more rapid weight gain (0.71% increase in weight per 1% increase in abundance). Conclusion: OLZ and HFD have synergistic effect on gut microbiota composition. Relative abundance of some bacteria are associated with more rapid weight gain. |
||
| Kao et al. (2018) (UK) |
Sprague–Dawley rats - 6–8 wks - 100% |
Aim: (1) evaluation of influence of prebiotic [Bimuno™ galactooligosaccharides (B-GOS®)] powder on OLZ-induced weight gain. (2) Testing whether prebiotic can affect mechanism of the action of olanzapine on cortical and hippocampal NMDAR subunit proteins and transcripts. (3) Exploration of the influence of prebiotic and OLZ on the inflammatory as well as metabolic markers and faecal microbiota composition. Design: placebo-controlled. Procedure Group 1: 1 wk water → 2 wks water + saline Group 2: 1 wk B-GOS® → 2 wks B-GOS® + saline Group 3: 1 wk water → 2 wks OLZ + water Group 4: 1 wk B-GOS® → 2 wks B-GOS® + OLZ |
N = 24; 2 wks |
Group 1: Water - Per os Saline - Intraperitoneal injection Group 2: B-GOS®: - 0.5 g/kg/day - Per os Saline - Intraperitoneal injection Group 3: Water - Per os OLZ - 10 mg/kg - Intraperitoneal injection Group 4 (B-GOS®/olanzapine): B-GOS®: - 0.5 g/kg/day - Per os OLZ - 10 mg/kg - Intraperitoneal injection |
Metabolic: 1. OLZ—↑ weight gain; 2. B-GOS® prevented weight gain caused by OLZ; 3. ↑ acetate in OLZ and B-GOS® groups and ↓ acetate in Gr. 4; 4. ↑ TNFα in Gr. 3 and 4; 5. ↑ WAT GPR43 mRNA in Gr. 4. 6. No influence of B-GOS® on frontal cortex 5-HT2AR blockade caused by OLZ; 7. ↑ cortical GluN1 protein level in Gr. 4; 8. ↑ cortical GluN2A mRNA in Gr.2; Gut microbiota: Gr. 2 vs Gr. 1: 1. ↑ genus Bifidobacteria; 2. ↓ genera: Escherichia/Shigella spp., Coprococcus spp., Oscillibacter spp., Clostridium Coccoides spp., Roseuria Intestinalis cluster, and Clostridium XVIII cluster. No influence of short term OLZ treatment on faecal bacteria composition was observed. Conclusion: Supplementation of B-GOS® to OLZ treatment may prevent weight gain and have favourable effect on cognitive function. Elucidation of mechanism of the influence of B-GOS® on the weight gain caused by short term OLZ treatment seems to be independent on faecal bacteria composition and needs further studies. |
| Bahr et al. (2015b) (USA) |
Wild-type C57BL/6J mice: - 6–7 wks - 100% |
Experiment no. 1: Aim: evaluation of the role of the gut microbiota in the development of the weight gain induced by RIS treatment Design: prospective, placebo-controlled. Procedure: Two groups of mice were treated with RIS at two concentrations the third with placebo. |
N = 15; 60 d |
RIS1 (n = 5) - 80 μg/d - Per os RIS2 (n = 5) - 80 ng/d - Per os VEH (n = 5) - Acidified water - Per os |
Metabolic: RIS1 vs VEH: weight gain. Gut microbiota (at 58 d): RIS1 vs VEH: ↓ OTUs, ↑ Phyla: Firmicutes and Actinobacteria, ↑ genera: Bacteroides, Allobaculum, Turicibacter, and Aneroplasma, ↓ phyla: Bacteroidetes and Proteobacteria, ↓ henera: Alistipes, Lactobacillus, and Akkermansia. Conclusion: Higher dose of RIS was associated with weight gain and microbiota alterations. |
|
Experiment no. 2: Aim: evaluation whether administration of antibiotics, which slightly affect gut microbiota composition, together with RIS, will affect weight gain and energy expenditure. Design: prospective, placebo and verum-controlled. Procedure: Mice were randomized to RIS or VEH-treated group for 48 days. ABX treatment started on the 10th day and continued for 10 days. |
N = 48; 48 d |
VEH (n = 8) - Acidified water - Per os Ampicillin (n = 8) - 0.54 mg - Per os Ciprofloxacin (n = 8) - 0.24 mg - Per os RIS (n = 8) - 0.80 μg - Per os RIS + ampicillin (n = 8) - 0.80 μg + 0.54 mg - Per os RIS + ciprofloxacin (n = 8) - 0.80 μg + 0.24 mg - Per os |
Metabolic: Induced weight increase in both RISP and RISP + ABX groups compared to control groups (VEH and both ABX), neither antibiotic significantly changed the influence of RIS on weight gain. No changes concerning food intake, digestive efficiency and energy absorption were observed. Gut microbiota: PcoA of unweight UniFrac distance revealed that ABX had synergistic influence on gut microbiota with RIS. Conclusion: RIS alone is responsible for increased body weight due to decreased energy expenditure. |
||
|
Experiment no. 3: Aim: confirmation that weight gain after RIS treatment is associated with gut microbiota changes and decreased energy expenditure using faecal transfer model Design: prospective, placebo-controlled. Procedure: Donor mice: group 1—water ad libitum; group 2—water with risperidone ad libitum (20 mg/ml, n = 5) for 9 wks. Faecal material from donor mice was transferred by gavage to naive recipients once daily for 2 wks. |
N = 22; NA |
Donors (9 wks) VEH (n = 4): - Water - Per os RIS (n = 5): - 20 mg/ml in drinking water - Per os Recipients (14 d) VEH (n = 6): - Faecal transfer from VEH donors RIS (n = 7): - Faecal transfer from RISP donors (calculated dose of RIS 8.7 ± 1 ng/ml/d) |
Metabolic: faecal transplantation from RIS mice—16% reduction in tRMR for recipients due to a reduction in non-aerobic RMR. Conclusion: Microbiota modification after RIS administration is responsible for reduction of non-aerobic RMR. |
||
|
Experiment no. 4: Aim: evaluation whether RIS may affect weight gain and energy expenditure due to influence on phageom in the mice gut using phage transfer model Design: prospective, placebo-controlled. Procedure: Phage were isolated from the stool of RIS- and VEH-treated mice and transferred by gavage each day to two groups of mice for 24 days |
N = 26; NA |
Donors VEH (n = 6): - NR RIS (n = 7): -NR Recipients VEH (n = 6): - 7 × 109 phage particles from VEH donors RIS (n = 7): - 7 × 109 phage particles from RIS donors |
Metabolic: Transfer of phage from RIS-treated donors vs VEH-treated donors caused weight gain, ↓ energy expenditure. Conclusion: Phageome alterations after RIS treatment are sufficient to cause weight gain and decrease energy expenditure. |
||
| Grobe et al. (2015) (USA) |
C57BL/6J mice: - NR - NR |
Aim: confirmation that weight gain after RIS treatment is associated with gut microbiota changes and decreased energy expenditure using faecal transfer model Design: prospective, placebo-controlled. Procedure: donor mice: group 1—vehicle; group 2—RIS. Faecal material from donor mice was transferred by gavage to naive recipients once daily for 2 wks. |
N = 13; 2 wks |
Donors VEH (n = NR): - NR RIS (n = NR): -NR Recipients VEH (n = 6): - Faecal transfer from VEH donors RIS (n = 7): - Faecal transfer from RISP donors - 84 ng/d |
Metabolic: RIS vs VEH recipients: massive reduction in tRMR. Conclusion: Microbiota modification after RIS administration is responsible for reduction of tRMR. |
| Riedl et al. (2017) (USA) |
C57BL/6J mice: - NR - NR |
Aim: analyse whether lack of cecal microbiota can affect RIS influence on tRMR Procedure: In subset of mice used in different experiments, tRMR was measured twice—first time after pretreatment with RIS and second time after cecetomy. |
N = 26; NR |
RIS (n = 14) - 80 μg/d - Per os Sham operation (n = 12) this group was used also as the control for other experiments |
Metabolic: RIS treatment caused significant decrease of tRMR in comparison to the control group. Cecectomy conducted in RIS pretreated mice did not additionally affect tRMR suppression. Conclusion: modulation of microbiota after RIS treatment alone is sufficient to decrease tRMR. |
ABX antibiotics, BD bipolar disorder, BMI body mass index, d days, GOS galactooligosaccharides, Gr. group, HFD high-fat diet, HOMA-IR homeostasis model assessment-estimated insulin resistance, hs-CRP high-sensitivity C-reactive protein, KEGG Kyoto Encyclopedia of Genes and Genomes, NR not reported, OLZ olanzapine, PCoA principal coordinate analysis, PICRUSt Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, RIS risperidone, SCFA short-chain fatty acid, SCZ schizophrenia, SGA second-generation antipsychotic, SOD superoxide dismutase, tRMR total resting metabolic rate, tx treatment, VEH vehicle, vs versus, wks weeks, wk week, yrs years
Humans
Overall, three observational studies in humans (experiments = 4; n = 232) including two cross-sectional groups and two longitudinal groups were included (Bahr et al. 2015a; Flowers et al. 2017; Yuan et al. 2018), all aiming to assess whether RIS (n = 74) (Bahr et al. 2015a; Yuan et al. 2018) or mixed SGAs (n = 117) (Flowers et al. 2017) would affect the microbiota composition and, consequently, metabolic indices. One article included 33 male children (mean age: cross-sectional group, 12.2 ± 2.5 years; longitudinal group, 11.7 ± 1.1 years; no treatment, 12.0 ± 1.8 years) (Bahr et al. 2015a). In addition to chronic RIS treatment, patients were also administered psychostimulants (100%), α-2 agonists (66%), and selective serotonin reuptake inhibitors (SSRIs, 11%), whereas controls did not receive antipsychotics but were taking psychostimulants (70%), α-2 agonists (30%), and SSRIs (20%). Another study involved 117 adults (study group treated with SGAs, 34 females and 12 males aged 46 ± 12 years; control group, 48 females and 21 males aged 51.7 ± 13.5 years) (Flowers et al. 2017). Co-administration of antidepressants (53%), mood stabilizers (57%), lithium (22%), and benzodiazepines (39%) was identified in SGAs and the control group. The last study evaluated RIS-induced metabolic parameters such as antioxidant superoxide dismutase (SOD) and high-sensitivity C-reactive protein (hs-CRP) in relations to microbiota composition between drug naïve 41 schizophrenia (SCZ) patients (18 females, 23 males; mean age 23.1 ± 8 years) and healthy controls (21 females, 20 males; mean age 24.7 ± 6.7 years) (Yuan et al. 2018). For more details, see Table 2.
Table 2.
Summary of human studies
| Reference | Subjects: - Age - % Females |
Aim, design and procedure | Number of subjects; duration of intervention | Groups + used substances - Dosage - Administration |
Outcome and conclusions |
|---|---|---|---|---|---|
| Bahr et al. (2015a) (USA) |
Cross-sectional group: - 9–15, 12.2 ± 2.5 yrs - 0% |
Aim: evaluation of the impact of chronic and longitudinal RIS treatment on body weight and faecal microbiota composition Design: observational studies: cross-sectional and longitudinal Procedure: observation of the dynamics of body weight and gut microbiota alterations following the onset of RIS treatment. Cross-sectional group: stool sample after mean 3.6 ± 2.4 yrs of RIS treatment |
Cross-sectional group: N = 18/at least 1-yr RIS treatment |
Chronic RIS (n = 18) - NR - Per os No treated group (n = 10) - NA - NA |
Metabolic: BMI Z-score increased by mean 0.31 ± 1.11 points over the course of treatment. Whereas in controls, it seemed to be unchanged (mean ΔBMI Z-score = 0.09 ± 0.61). PICRUSt analysis predicted KEGG orthologues: ↑ pathway levels for butyrate and propionate metabolism in RIS-treated group, ↑ SCFA production, alteration of tryptophan metabolism Gut microbiota: RIS treatment caused ↑ in Shannon diversity (5.9 vs 5.2), PcoA of unweighted UniFrac distances shown ↑ phylogenetic diversity, robust difference between the overall gut microbial profiles but no appreciable difference between significant vs no-significant BMI gain groups during treatment Bacterial abundances (RIS vs control): ↓ Bacteroidetes/Firmicutes ratio, ↑ phyla: Firmicutes*, Proteobacteria, and Tenericutes; families: Erysipelotrichaceae* and Ruminococcaceae; genera: Clostridium*, Lactobacillus*, Ralstonia*, and Eubacterium. (*more abundant in chronic RISP-treated children who had a significant gain in BMI compared to those with no BMI gain). ↑ Phylum Actinobacteria, Tenericutes order Coriobacteriales and species Collinsella aerofaciens in RISP group without weight gain (weight gain protective activity?) ↓ Phylum Bacteroidetes** and Verrucomicrobia**; genera: Prevotella** and Alistipes. (**less abundant in chronic RISP-treated children who had a significant gain in BMI compared to those with no BMI gain) Conclusion: Gut microbiota is altered in patients chronically treated with RIS and may be associated with weight gain and metabolic disturbances |
|
Longitudinal group: - 9–13, 11.7 ± 1.1 - 0% female Control group: - 10–14, 12.0 ± 1.8 |
Longitudinal group: stool sample within few days of starting treatment (mean 3.2 ± 5.2) and then monthly for 10 months. Control group for both groups: 10 to 14-year-olds psychiatrically ill but not treated with SGAs, only with psychostimulants or selective serotonin inhibitors (SSRIs) |
Longitudinal group: N = 5 10-month RIS treatment |
Longitudinal RIS (n = 5) - NR - Per os |
Metabolic: BMI Z-scores increased by mean 0.28 ± 0.23 units. Gut microbiota: ↑ Firmicutes/Bacteroidetes ratio depending on the time of treatment. The percent abundance of Firmicutes and Bacteroidetes did not significantly correlate with the RIS-induced weight gain. Conclusion: Changes of gut microbiota composition starts few months after RIS treatment and correlate with weight gain. Probably due to small group size this correlation is not significant. |
|
| Flowers et al. (2017) (USA) |
Adults with BD: - 46.0 ± 12.0 yrs - 69.6% No SGA treatment: - 51.7 ± 13.5 yrs - 73.9% |
Aim: detection significant clustering of microbial communities between two groups of bipolar disorder patients (treated vs not treated with SGAs). Design: observational, cross-sectional. Procedure: study group: treated with SGAs (clozapine, olanzapine, risperidone, quetiapine, asenipine, ziprasodone, lurasidone, aripiprazole, paliperidone, and iloperidone) and some with antidepressants, mood stabilizers, lithium, benzodiazepines. Control group: no SGAs tx, but some were treated with the same drugs as the study group. |
N = 117/use of SGAs at the time of stool collection |
SGAs (n = 49) - NR - NR No SGA (n = 68) - NR - NR |
SGAs vs No SGAs: Metabolic: ↑ BMI 31 ± 7 vs 27.5 ± 6 (significant after correcting for age and gender). Gut microbiota: ↓ Simpson diversity in females, ↑ family Lachnospiraceae in the whole cohort of patients treated with SGAs and cohort of obese subjects, no significant changes were observed in subgroup of obese and not obese patients despite on treatment); ↓ genera: Akkermansia (the whole cohort of patients treated with SGAs, cohort of obese subjects, subgroup of non-obese patients treated with SGAs), Sutterella. Conclusion: SGA treatment is associated with weight gain, decreased species richness in females and specific gut microbiota changes (which can play difficult to explain role in a weight gain process). |
| Yuan et al. (2018) |
Adults with SCZ - 23.1 ± 8.0 yrs - 43.9% |
Aim: to assess influence of RIS treatment on the metabolic parameters, redox system, inflammation relative to microbiota composition Design: observational, longitudinal. Procedure: the dynamics of metabolic outcome and gut microbiota alterations during 24 wks of RIS treatment (4 time points: baseline, 6, 12, 24 wks) |
N = 82, 24 wks |
RIS (n = 41) - Titrated from 1 mg/day up to 4–6 mg/day as clinically established - NR |
Metabolic: ↑ weight (since 12 wks), ↑ BMI (since 6 wks); ↑ fasting serum glucose level (since 6 wks), ↑ HOMA-IR (since 6 wks); ↑ LDL (since 24 wks), ↑ triglycerides (since 12 wks); ↑ SOD (since 6 wks); ↑ serum levels of hs-CRP (since 12 wks), compared to baseline. At the endpoint serum levels of SOD negatively correlated with serum levels of LDL and HOMA-IR after controlling for potential confounding variables. Gut microbiota: ↓ Clostridium coccoides group (since 6 wks); ↑ Bifidobacterium spp. (since 6 wks), ↑ Escherichia coli; (since 6 wks), ↓ Lactobacillus spp. (since 12 wks) in comparison to baseline values. At baseline, Bifidobacterium spp. count negatively correlated with serum levels of LDL and Escherichia coli count negatively correlated with serum levels of triglycerides and hs-CRP (after controlling for age, gender, smoking status, and disease duration). In the hierarchical multiple linear regression model (adjusted for age, gender, smoking status, and disease duration), the changes in faecal Bifidobacterium spp. significantly correlated with the changes in weight over 24 wks. Conclusion: Body weight increase in SCZ patients treated with RIS are associated with abnormalities in the microbiota composition and the dysbiosis might contribute to the regulation of inflammation and oxidative stress thus metabolic malfunctions. |
ABX antibiotics, BD bipolar disorder, BMI body mass index, d days, GOS galactooligosaccharides, Gr. group, HFD high-fat diet, HOMA-IR homeostasis model assessment-estimated insulin resistance, hs-CRP high-sensitivity C-reactive protein, KEGG Kyoto Encyclopedia of Genes and Genomes, NR not reported, OLZ olanzapine, PCoA principal coordinate analysis, PICRUSt Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, RIS risperidone, SCFA short-chain fatty acid, SCZ schizophrenia, SGA second-generation antipsychotic, SOD superoxide dismutase, tRMR total resting metabolic rate, tx treatment, VEH vehicle, vs versus, wks weeks, wk week, yrs years
Risk of bias
An analysis of the overall risk of bias in rodent studies was limited by restricted information being provided. Results were heterogeneous with randomization in three articles (60%) (Davey et al. 2012, 2013; Bahr et al. 2015b), and no information regarding potential conflicts of interest was reported in one article (20%) (Morgan et al. 2014). Other key study quality indicators were poor, and an unclear risk for most types of SYRCLE’s bias was identified (Fig. S2). The reporting quality of the human studies was low (Bahr et al. 2015a) (score 13; 40.62%) and moderate (score 17; 53.12%) (Flowers et al. 2017), but a study by Yuan et al. (Yuan et al. 2018) was found to be of relatively high quality (score 20; 62.5%). For details, see Supplementary Figs. S1 and S2.
Microbiota evaluation
Rodents
Bacteria in stool were tested in five studies [four OLZ (Davey et al. 2012, 2013; Morgan et al. 2014; Kao et al. 2018) and one RIS study (Bahr et al. 2015b)] using widely applied 16S rRNA sequencing methods. In two OLZ studies (Davey et al. 2012; Morgan et al. 2014) information was provided regarding microbiota diversity. In one RIS study (Bahr et al. 2015b), the number of bacterial operational taxonomic units (OTUs) was reported. The abundance of bacterial phyla was analysed in two OLZ studies (Davey et al. 2012, 2013) and one RIS (Bahr et al. 2015b) study, whereas bacterial classes were studied in two OLZ studies (Morgan et al. 2014; Kao et al. 2018) and bacterial genera in one RIS study (Bahr et al. 2015b). Briefly, a skewed Firmicutes/Bacteroidetes ratio was the most frequent observation of our SR, secondary to OLZ (Davey et al. 2012, 2013) and RIS (Bahr et al. 2015b) treatment. Detailed data are presented in Table 1.
Reduced microbiome diversity was identified in 16 rats following OLZ treatment (both genders) (Davey et al. 2012) and 24 female mice after HFD and OLZ regimen (Morgan et al. 2014). Only one study reported data on fewer OTUs in female mice treated with RIS (Bahr et al. 2015b). OLZ treatment in rats increased the abundance of Firmicutes from 6.40 to 16.01% and decreased the abundance of Bacteroidetes from − 6.69 to − 4.30%. The effect was dose dependent and greater in females (Davey et al. 2012, 2013). In addition, the abundance of Actinobacteria (females 2 mg, − 3.38%; 4 mg, − 3.57%) and Proteobacteria (females 2 mg, − 1.45%; 4 mg, − 0.83%; males 2 mg, − 2.21%) was decreased compared with vehicle-treated rodents (Davey et al. 2012). The other OLZ rodent study found an increase in the relative abundance of classes Erysipelotrichia (up to 3.40%) and Gammaproteobacteria (up to 0.45%), whereas the abundance of class Bacteroidia was reduced (− 5.30%) (Morgan et al. 2014). Only in a single study (Kao et al. 2018) OLZ administration caused no variations within microbiota composition in comparison with vehicle-treated rodents, possibly because of the short treatment duration and the dose of the administered drugs. However, OLZ was administered prior to B-galactooligosaccharide (B-GOS) and attenuated prebiotic mode of action (↑ Bifidobacterium; ↓ Escherichia/Shigella spp., Coprococcus spp., Oscillibacter spp., C. coccoides spp., Roseuria Intestinalis cluster, and Clostridium XVIII cluster) which indirectly suggested that this SGA influenced gut microbiota. Also, acetate concentration in faeces, a by-product of gut microbiota, increased in OLZ-treated rodents, implying that microbiome structure and function could be at least partly changed by SGA (Kao et al. 2018).
Although RIS was implemented in six rodent experiments (from three articles), a microbiota analysis was performed in only one of them. An increase in the relative abundance of Firmicutes (32.6%) was found with a reciprocal decrease in the relative abundance of Bacteroidetes (− 22.40%) in drug-treated subjects (Bahr et al. 2015b). Alistipes spp. and Lactobacillus spp. were more prevalent in control-treated rodents, whereas the population of Allobaculum spp. increased (36.5%) in the RIS group (Bahr et al. 2015b). Furthermore, SGAs were shown to possess antibacterial properties in vitro; OLZ inhibited the growth of anaerobic bacteria (Bahr et al. 2015b), and diminished the growth of Escherichia coli NC101 but not Enterococcus faecalis OGIRF cultures (Morgan et al. 2014).
Humans
In two human studies (Bahr et al. 2015a; Flowers et al. 2017), the bacteria in stools were tested using 16S rRNA sequencing methods, while in a third study (Yuan et al. 2018), the copy numbers of five bacterial genera (Bifidobacterium spp., Clostridium coccoides group, Lactobacillus spp., and Bacteroides spp.) were determined by means of qPCR analysis. The difference in microbial communities between observed groups was calculated using principal coordinate analysis (PCoA). One study assessed Shannon diversity (all sample species) (Bahr et al. 2015a), and another focused on Simpson (dominant species) diversity (Flowers et al. 2017). The abundance of bacterial phyla was analysed in one study (Bahr et al. 2015a), whereas bacterial families were analysed in two human studies (Bahr et al. 2015a; Flowers et al. 2017) and bacterial genera in all human studies (Bahr et al. 2015a; Flowers et al. 2017; Yuan et al. 2018). The Firmicutes/Bacteroidetes ratio was reported in only the RIS study (Bahr et al. 2015a).
Flowers et al. (2017) reported reduced Simpson diversity in females treated with SGAs which remained significant after adjusting for age, BMI, and benzodiazepine treatment (p = 0.002, β = − 4.6, R2 = 0.12). A greater abundance of Lachnospiraceae was observed in obese patients treated with SGAs, whereas Akkermansia and Sutterella abundance was higher in controls, though only the first two differences (Lachnospiraceae and Akkermansia) remained significant (p = 0.001 and p = 0.03) after adjusting for BMI and gender. Lastly, the study found that Akkermansia were less prevalent in non-obese SGA users (p = 0.005) (Flowers et al. 2017).
Bahr et al. (2015a) identified a significantly higher Shannon diversity index (0.7 points) and phylogenetic diversity in 18 male adolescents chronically (> 12 months) treated with RIS compared to psychiatric control participants. The Bacteroidetes/Firmicutes ratio was significantly lowered (0.15 vs 1.24, respectively, p < 0.05) in chronic and short-term (1–3 months) RIS users. The tendency to decrease the Bacteroidetes/Firmicutes ratio observed in short-term RIS users seemed to correlate with the change in BMI Z-score, which is a function of both age and gender and shows the deviation from the population mean. The observed results were not significant, probably because of the small number of patients. Moreover, the authors observed that long-term treatment with RIS and significant weight gain in RIS users were associated with alterations in the gut microbiome: an increased abundance of the phylum Proteobacteria, families Erysipelotrichaceae and Ruminococcaceae, and genera Clostridium, Lactobacillus, Ralstonia, and Eubacterium and decreased abundance of the genera Prevotella and Alistipes. Interestingly, the abundance of phylum Actinobacteria and species Collinsella aerofaciensin was elevated in the RIS group without weight gain, which suggests a protective effect of these bacteria in chronic RIS users (Bahr et al. 2015a).
In a study by Yuan et al. (2018), authors found that 24-week RIS treatment was associated with a significant overall increase in the copy numbers of faecal Bifidobacterium spp. (F(3,160) = 7.298, p < 0.001; week 0, 6.72 ± 1.35 l g copies/g; week 24, 7.24 ± 0.78 l g copies/g) and Escherichia coli (F(3,160) = 8.280, p < 0.001; week 0, 7.58 ± 0.68 l g copies/g; week 24, 8.03 ± 0.66 l g copies/g). Interestingly, the copy numbers of faecal Bacteroides spp. did not change over 24 weeks of RIS treatment (F(3,160) = 2.188, p = 0.092). They also noticed that after 6 weeks of treatment, the copy numbers of Bifidobacterium spp. (at 6 weeks p < 0.05, 12, 24 weeks p < 0.001) and Escherichia coli (at 6 weeks p < 0.05, 12 p < 0.01, 24 p < 0.001) elevated. Copy numbers of Clostridium coccoides group were lower after 6 weeks of treatment (at 6 and 12 weeks p < 0.01, 24 weeks p < 0.001), and Lactobacillus spp. was decreased at 12 and 24 weeks of RIS administration (p < 0.001).
Metabolic outcome
Rodents
The effect of OLZ administration on body weight was measured using different experimental models in four studies (experiments = 5) including C57BL/6J female mice (experiments = 2) (Morgan et al. 2014) or both genders of Sprague–Dawley rats (experiments = 3) (Davey et al. 2012, 2013; Kao et al. 2018). The impact of RIS on body weight was determined in three studies (experiments = 6); one study included C57BL/6J female mice (Bahr et al. 2015b), and two other studies (conference abstracts) did not report mouse gender (Grobe et al. 2015; Riedl et al. 2017). We did not conduct aggregated analysis concerning body weight, due to methodological differences and various ways of expressing measured values.
In general, administration of OLZ increased body weights in female rats (Davey et al. 2013; Kao et al. 2018) and female mice (Morgan et al. 2014). This effect was dose independent in two studies (Davey et al. 2012, 2013). In one study, OLZ administration caused increased adiposity (percentage of body fat), even after correction for weight gain (Morgan et al. 2014). The body weight increases induced by OLZ was counteracted by antibiotic administration (Davey et al. 2013) and lack of bacteria (germ-free mouse model), and potentiated by an HFD (Morgan et al. 2014). The increase in body weight induced by RIS administration was not affected by antibiotics (Bahr et al. 2015b). In rodents receiving RIS, the non-aerobic resting metabolic rate (RMR) was suppressed in mice in three studies (Grobe et al. 2015; Bahr et al. 2015b; Riedl et al. 2017). One study reported no direct information regarding body weight but identified that, in RIS-treated rodents, the suppression of tRMR was not affected by cecectomy (Riedl et al. 2017). Increased fat mass and free fatty acid release and elevated expression of lipogenic enzymes were observed in 12 female rats (Davey et al. 2013). Importantly, in six female rats, administration of SGAs resulted in elevated expression of macrophage marker CD68 in adipose tissue, indicating that body weight gains were associated with recruitment of macrophages into the fat mass (Davey et al. 2013).
Humans
In 18 male children chronically treated with RIS, the BMI Z-score increased by a mean 0.31 ± 1.11 points, and the BMI Z-score increased over 10 months of treatment (mean 0.28 ± 0.23 units) in a longitudinal study arm (Bahr et al. 2015a). Flowers et al. (2017) observed higher BMI in patients receiving SGAs (31 ± 7 vs 27.5 ± 6, p = 0.006; after correcting for age and gender, p = 0.04). Yuan et al. (2018) discovered that RIS treatment caused a significant increase in weight (F(3,160) = 4.331, p = 0.006), BMI (F(3,160) = 5.025, p = 0.002), fasting serum glucose levels (F(3,160) = 5.081, p = 0.002), triglycerides (F(3,160) = 3.428, p = 0.019), LDL (F(3,160) = 3.973, p = 0.009), and HOMA-IR (F(3,160) = 10.187, p < 0.001). At a week 6 of treatment, increases in BMI (week 0, 20.54 ± 4.87 kg/m2; week 6 21.96 ± 5.36 kg/m2; p < 0.05), glucose (week 0, 4.37 ± 1.03 mmol/l; week 6, 4.63 ± 0.81 mmol/l; < 0.01), HOMA-IR (week 0, 0.97 ± 0.67; week 6, 1.39 ± 1.17; p < 0.001), and LDL (week 0, 2.22 ± 1.25 mmol/l; week 6, 2.62 ± 1.53 mmol/l; p < 0.05) were observed. At 12 and 24 weeks, all metabolic parameters mentioned above were also significantly increased (BMI—week 12, 22.54 ± 5.7 kg/m2; p < 0.01; week 24, 22.88 ± 6.97 kg/m2; p < 0.001; LDL—week 12, 2.69 ± 1.36 mmol/l; p < 0.01; week 24, 2.63 ± 1.19 mmol/l; p < 0.01). Additionally, weight increased significantly (week 12, 63.49 ± 18.94 kg, p < 0.01; week 24, 62.85 ± 19.73, p < 0.01), as well as serum triglyceride level (week 0, 0.96 ± 1.33 mmol/l; week 12, 1.28 ± 0.97 mmol/l, p < 0.01; week 24, 1.37 ± 1.37 mmol/l; p < 0.001).
No other metabolic investigations were undertaken; however, Bahr et al. (2015a) performed Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analyses and found that bacterial orthologues enriched in chronic RIS patients compared to controls were responsible for environmental information processing pathways and cellular processes, including short-chain fatty acid and tryptophan metabolism. In persons free of psychiatric treatment, there were more orthologues involved in bacterial metabolic pathways, such as vitamin metabolism. Detailed data extracted from original papers included in our systematic review are provided in Table 2.
The role of microbiota in metabolic outcomes
Rodents
Most studies investigating this hypothesis have been performed in different experimental models. Morgan et al. (2014), in their germ-free experiment in gnotobiotic mice, showed that the microbiota is necessary to induce metabolic changes after OLZ treatment. One experiment assumed that the relative abundance of class Erysipelotrichi, which is increased by OLZ, is linked to rapid weight gain; every 1% increase in abundance resulted in a weight gain of 0.7%. The same pattern, though more pronounced, was identified relative to the class Actinobacteria (for which an OLZ effect was not observed); every 1% increase in abundance resulted in a weight gain of almost 5% (Morgan et al. 2014). In a study by Kao et al. (2018) 2-week intraperitoneal OLZ administration in female rodents elevated body weights, and prebiotic therapy attenuated this effect. The study indicated no OLZ-induced alterations of gut microbiota which means that B-GOS supplementation may prevent weight gain independently of its influence on gut microbiota. In a rodent RIS intervention (Bahr et al. 2015b), the medication caused a significant increase in weight (2.8 g) compared to control mice, and co-administration of antibiotics had no significant effect on the weight gain. When performing microbiota transfer from RIS-treated mice, recipients had a 16% reduction in total resting metabolic rate (tRMR) due to a reduction in non-aerobic RMR. tRMR states for the largest portion of total energy need thus is relevant to describe metabolic outcomes (Astrup et al. 1999) Similarly, transfer of the phageome from RIS mice resulted in a significant weight gain in recipients relative to the vehicle study arm (p < 0.05) (37). Two studies obtained indirect data on the metabolic influence of microbiota changes following RIS treatment (Grobe et al. 2015; Riedl et al. 2017). In the study by Grobe et al. (2015), faecal transplants from RIS-treated rodents resulted in elevated body mass through non-aerobic RMR suppression, which was found to be unaffected by cecectomy (Riedl et al. 2017). Study conclusions are shown in Table 3.
Table 3.
Summary of the rodent studies showing a relationship between metabolic changes and microbiota alteration after SGA treatment
| Study | SGA | Relationship | Comment |
|---|---|---|---|
| Davey et al. (2012)), Ireland | OLZ | Weak | Metabolic disturbances, inflammation, and microbiota alterations were observed only in female mice. In males, impact on microbiota and metabolism was minimal. |
| Davey et al. (2013), Ireland | OLZ | Strong | Metabolic effects of OLZ were associated with gut microbiota changes and were attenuated by antibiotics, which strongly reduced gut microbiota content. |
| Morgan et al. (2014), USA | OLZ | Strong | Results of few experiments shown that gut microbiota was necessary to induce weight gain (germ-free mice model) and that weight gain was related to the relative abundance of the special bacteria (cross-over study design). |
| Kao et al. (2018), UK | OLZ | Not observed | Short-term OLZ treatment did not affect faecal bacterial composition in female rats. |
| Bahr et al. (2015a), USA | RIS | Strong | Faecal and phage transplantation from mice treated with RIS caused weight gain and decreased energy expenditure. |
| Grobe et al. (2015), USA | RIS | Strong | Faecal transplantation from mice treated with RIS caused decreased tRMR. |
| Riedl et al. (2017), USA | RIS | Not clear | Cecectomy does not affect decreased tRMR after RIS treatment. It means that antibacterial properties of RIS are enough to reduce tRMR, and a further reduction of bacteria count via cecectomy is not required or that the mechanism does not depend on intestinal microbiota. |
Humans
In humans, the data are less clear. Bahr et al. found increased BMI Z-scores in humans treated with RIS (mean 0.31 ± 1.11 points over the treatment course), whereas in controls, the scores seemed to be unchanged (Bahr et al. 2015a). Chronic treatment with RIS and a significant gain in BMI resulted in a lower Bacteroidetes/Firmicutes ratio compared with the control group. Moreover, differences in bacterial composition were observed in RIS-treated children who had BMI gains compared to those who did not. Detailed data concerning the association of differences in microbiota abundance depending on body weight gain are presented in Table 2. In the initial phase of RIS treatment lasting 10 months, BMI Z-scores increased a mean 0.28 ± 0.23 units, which seemed to correlate with a decreased Bacteroidetes/Firmicutes ratio starting 1–3 months after treatment initiation (the observed result was not significant, likely due to the small sample size).
In the second human study, SGA treatment resulted in higher BMIs, even after adjusting for patient age and gender followed by significant elevation in the abundance of Lachnospiraceae. Akkermansia counts were significantly lowered in the SGA-treated group, including non-obese patients. Surprisingly higher Lachnospiraceae and lower Akkermansia counts were observed in non-SGA-treated obese individuals (Flowers et al. 2017).
In the last human study, the authors (Yuan et al. 2018) found that at baseline Bifidobacterium spp., counts negatively correlated with serum levels of LDL and Escherichia coli count was negatively correlated with serum levels of triglycerides and hs-CRP, even after controlling for age, gender, smoking status, and disease duration. Following the treatment, a decrease in Clostridium coccoides group (since 6 weeks) and Lactobacillus spp. (since 12 weeks) and elevations in numbers of Bifidobacterium spp. (since 6 weeks) and Escherichia coli (since 6 weeks), in comparison to baseline values, were reported. When they conducted hierarchical multiple linear regression, only the differences in faecal Bifidobacterium spp. count significantly correlated with the weight changes over 24 weeks of RIS treatment (Yuan et al. 2018). A summary of the evidence from human studies is provided in Table 4.
Table 4.
Summary of the human studies showing a relationship between metabolic changes and microbiota alteration after SGA treatment
| Study | SGA | Relationship | Comment |
|---|---|---|---|
| Bahr et al. (2015a), USA | OLZ | Moderate | A specific microbiota alteration is observed in weight-gained children chronically treated with RIS. Longitudinal study showed correlation between changes in gut microbiota and weight gain caused by RIS treatment (result was not significant probably due to small sample size). |
| Flowers et al. (2017), USA | Different SGAs | Weak | Changes of a specific bacteria abundance are associated with lack of weight gain after SGA treatment, but results are difficult to explain. |
| Yuan et al. (2018), China | RIS | Strong | Weight gain in patients treated with RIS is significantly correlated with faecal bacterial abundance. |
Discussion
To the best of our knowledge, this is the first SR investigating the effect of SGAs on intestinal microbiota in relation to frequent metabolic adverse events associated with their use in clinical practice. This SR aimed to find answers to three questions: (1) do SGAs affect intestinal microbiota resulting in dysbiosis, (2) whether SGA-related metabolic disorders are associated with dysbiosis, and finally (3) to elucidate the mechanisms behind SGA treatment and dysbiosis leading to body weight and metabolic disturbances (Fig. 2) (Delzenne et al. 2011). Although numbers of existing rodent and human studies are limited, we found that dysbiosis secondary to SGA treatment can play a role in metabolic alterations, including weight gain.
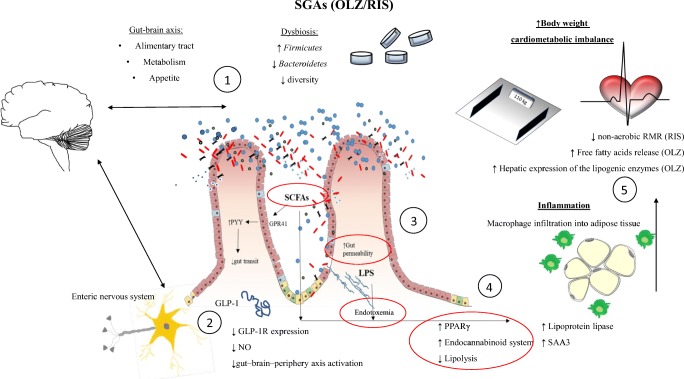
Fig. 2.
Schematic presentation of possible mechanisms of metabolic disturbances secondary to SGA treatment. SGAs affect the gut microbiota, causing shifts in two major phyla: Firmicutes and Bacteroidetes. (1) The gut-brain axis controls metabolism, appetite, and digestive tract functions and may become altered under dysbiosis insult. (2) SCFAs activate G protein binding receptor (GPR) which is followed by secretion of YY peptide (PYY) resulting in lowered gut motility. Dysbiosis also induces GLP-1 resistance, followed by diminished GLP-1 receptor expression and hampered nitric oxide production. Consequently, the gut-brain peripheral axis responsible for insulin secretion and stomach emptying is inhibited. (3) Dysbiosis is associated with loss of integrity of the gastrointestinal barrier and increased permeability of the intestinal mucosa for gut lumen antigens, including bacterial LPS. (4) High-energy SCFAs and endotoxemia affect multiple metabolic pathways. Activation of the differentiation of peroxisomal gamma proliferator-activated receptors (PPARγ) and the pro-inflammatory endocannabinoid system takes place which regulates fatty acid synthase (FAS), enhancing hepatic de novo lipogenesis. LPS exacerbates hepatic steatosis and insulin resistance. Consequently, macrophages infiltrate adipose tissue and (5) body weight increases. The mechanisms include suppression of non-aerobic RMR (OLZ), increased free fatty acid release, and elevated hepatic expression of the lipogenic enzyme fatty acid synthase (RIS). Possible targets counteracted by prebiotics and probiotics are circled in red. FAS fatty acid synthase, GLP-1 glucagon-like peptide 1, GPR G protein binding receptor, LPS lypopolysaccharide, NO nitroic oxide, OLZ olanzapine, PPARγ peroxisomal gamma proliferator-activated receptors, PYY YY peptide, RIS risperidone, RMR resting metabolic rate, SAA3 serum amyloid A3, SCFAs short-chain fatty acids
The human gut is colonized by roughly 39 × 109 bacterial cells (Abbott 2016), with other species, including Archaea, eukaryotes, fungi, and viruses (Consortium et al. 2012). Dysbiosis is an imbalance in the number, composition, or function of bacteria in a given environment. Dysbiosis has been confirmed in all but one of the experimental studies in which the content of bacteria in stools was assessed. The most frequent observation was an increase in the Firmicutes/Bacteroidetes phyla ratio. It should be emphasized that the ratio of Firmicutes/Bacteroidetes was elevated in all experimental studies investigating this parameter. These two phyla are generally dominant in the human intestinal microbiome, comprising approximately 90% of the gut microbiota (Consortium et al. 2012). The Bacteroidetes phylum has been found to synthesize acetate and propionate, while Firmicutes mainly by-product is butyrate (den Besten et al. 2013). Beneficial effects of SCFAs were shown as far as gastrointestinal functions, neuro/immune regulation, and host metabolism were concerned (Maciejewska et al. 2018; van de Wouw et al. 2018). Proper concentration of SCFAs acting via its receptors is crucial for energy homeostasis. When GPR43-deficient mice were fed with normal diet, they started to accumulate fat and SCFA-dependent activation of the receptor resulted in suppressed insulin signalling within the adipose tissue thus inhibited fat storage (Kimura et al. 2013). In 2005, Ley et al. hypothesized that differences in gut microbial ecology may be an important factor affecting energy homeostasis (Ley et al. 2005). Later, an increased Firmicutes/Bacteroidetes ratio was observed in obese rodents and humans (Turnbaugh et al. 2009).
Elevated gut microbiota fermentative metabolism by over-represented Firmicutes may therefore promote more intensive intestinal monosaccharide absorption, energy extraction from non-digestible food components, hepatic de novo lipogenesis, and adipocyte fatty acid storage. The analysis of the SCFA concentration represents an indirect way to analyse microbiota composition (at least a skewed Firmicutes count) and can be viewed as a marker of microbiota metabolic function.
Observations from experimental studies have only been partially confirmed in human studies. Bahr et al. (2015a) observed increased Firmicutes/Bacteroidetes ratios in both cross-sectional and longitudinal studies. In other human studies, bacterial phyla were not reported. Another result of experimental studies confirmed in the human trial was the increase in the abundance of class Erysipelotrichi, which was found to be related to the occurrence of non-alcoholic fatty liver disease (Spencer et al. 2011; Henao-Mejia et al. 2012; Raman et al. 2013). Of note, as recently demonstrated by Schwarz et al. (2018) in first-episode psychosis patients, the abundance of predominantly Lactobacillus from Firmicutes phyla was increased which correlated negatively with different clinical scores of schizophrenia. Also, after 12 months of treatment in patients with smaller alterations within gut microbiota at baseline, remission rate was more frequent. However, patients were not drug-naïve, and received antipsychotics for approximately 20 days, which at least partly confirm the higher abundance of Firmicutes following SGA treatment. We found no more data on how SGA-induced dysbiosis may affect the clinical course of the disease and consequently treatment success. To close the circle, Lactobacillus represents only a single genus within the Firmicutes phyla; thus, the effect of SGAs on Firmicutes/Bacteroidetes ratio in humans could only be speculative and requires further research.
The mechanism of dysbiosis secondary to SGAs has not been fully explained. In two studies included in the present SR, both OLZ (Morgan et al. 2014) and RIS (Bahr et al. 2015b) had an antimicrobial nature. Moreover, the principal coordinate analysis of the uniweighted UniFrac distance showed that antibiotics had a synergistic influence on gut microbiota with RIS. This effect is predominantly typical of drugs subject to enterohepatic circulation and intensely excreted in the bile (Morgan et al. 2014). Bactericidal activity causes dysbiosis via elimination of specific bacteria from the gastrointestinal tract. The observed phenomenon of a greater increase in body mass in naive SGA users (Maayan and Correll 2010) can be caused, among other reasons, by the induction of bacterial resistance in relation to the repeated use of SGAs. It should be emphasized that the observed results are not unambiguous and easy to interpret. The composition of intestinal bacteria varies among individuals and is analogous to fingerprints. Also, in individual studies (also experimental), various taxonomic groups of bacteria were analysed, and their content was analysed only in stools. The composition of bacteria in the stool is more stable and does not depend on external factors compared with the composition of bacteria in the small intestine. Changes in the microbiota of the small intestine have a much greater effect on the metabolic functions of the body. Therefore, in further experimental studies, attention should be paid to this problem.
To address the question of whether SGA-induced dysbiosis may be responsible for metabolic malfunctions, we analysed a few experimental models. Two studies introduced antibiotic cocktails as experimental variables (Davey et al. 2013; Bahr et al. 2015b). Co-administration of antibiotics, which significantly reduced gut bacterial content, prevented dysbiosis and its metabolic consequences. On the other hand, antibiotics used only to slightly modify gut microbiota had antibacterial activity similar to SGAs but did not influence their metabolic effects. These observations confirm a potential causal relationship between dysbiosis caused by the intake of SGAs and metabolic disorders. Similar results were previously reported in mouse models (Mathur et al. 2016). As some antipsychotics have been documented to possess antimicrobial activity (Kristiansen 1979), the administration of these drugs may resemble the mode of action of low-dose antibiotic cocktails, which may be responsible for increased body mass as observed in livestock (Morgan et al. 2014). In contrast, some non-absorbable antibiotics (e.g., rifaximin) have been shown to reduce the abundance of methanogenic bacteria (Mathur et al. 2016), resulting in significant weight loss in obese individuals with diabetes (Riedl et al. 2017). The antibiotic effect on body fat composition is plausibly dose- and age-related (Cox et al. 2014). Additionally, Morgan et al. (2014) implemented HFD, which induces changes in the composition of the gut microbiota toward an obesogenic composition with subsequent metabolic consequences, including metabolic syndrome (Yang et al. 2017). However, HFD had no impact on the anthropometric indices of germ-free mice (Bäckhed et al. 2007), which may indicate that metabolic disturbances during OLZ treatment are sourced from altered gut microbiota. It was observed that both HFD and OLZ have a synergistic effect on gut microbiota composition but weight gain in mice receiving OLZ is more rapid than feeding only with HFD. This means that in the case of metabolic disorders caused by OLZ administration, in addition to the SGA-mediated obesogenic effect on gut microbiota, other factors should also be taken into consideration. Also, it is possible that such treatment is more pronounced in comparison to HFD alone. Morgan et al. (2014) confirmed the relationship between OLZ-induced dysbiosis and metabolic disorders using a germ-free model, in which they showed that the lack of bacteria in the gastrointestinal tract in mice receiving OLZ did not cause weight gain, and their conventional housing leading to the colonization of the digestive tract resulted in induction of weight gain. A similar observation was made by Bäckhed et al. (2007) who found that gnotobiotic mice had less body fat than mice housed under conventional conditions. Morgan et al. (2014) confirmed the relationship between weight gain and OLZ administration using the cross-over model, proving that the relative abundance of bacteria of the Erysipelotrichi class was related to weight gain.
Another argument for the relationship between the occurrence of dysbiosis and metabolic disorders with an increase in body weight caused by the administration of OLZ was provided by Davey et al. (2012) who observed microbiota and metabolic disorders only in female mice. In male mice, metabolic side effects and impact on bacterial abundance were minimal. In human trials included into present SR, no differences in metabolic outcome between males and females were reported (Flowers et al. 2017; Yuan et al. 2018). However, such gender-dependent discrepancies were reported earlier and may be due to drug pharmacokinetic differences (Harris et al. 1995; Beierle et al. 1999) which further support the necessity to conduct more studies. Bahr et al. and Grobe et al. provided the strongest evidence for a link between the occurrence of metabolic disorders and gut microbiota (Grobe et al. 2015; Bahr et al. 2015b). The authors observed that faecal and phage transplantation from mice treated with RIS caused weight gain and decreased rest metabolic rate. The phenotypic effect of faecal transplantation is considered as a very strong evidence of intestinal microbiota, also in terms of its effect on metabolism. For example, lean mice-derived microbiota transferred to germ-free mice resulted in lower body fat increases than the transfer of the ob/ob mice microbiome (Turnbaugh et al. 2006). Riedl et al. did not confirm this observation and found that cecectomy (associated with marked reduction of gut microbiota counts) did not influence the suppression of non-aerobic RMR caused by RIS treatment (Riedl et al. 2017). This observation may indicate that RIS is an antibacterial agent that reduces RMR, and a further reduction of bacteria count via cecectomy is not needed. However, it is also possible that the mechanism does not depend on intestinal microbiota. It should be emphasized that the results of this study come from a conference summary, and may not contain the necessary data for accurate interpretation of these observations (Riedl et al. 2017).
Human studies provide little evidence on the relationship between metabolic changes and microbiota alterations after SGA treatment. Bahr et al. (2015a) found specific microbiota alterations in weight gain of children chronically treated with RIS, although the results were not significant probably due to the small sample size. However, PcoA of unweighted UniFrac distances showed elevated phylogenetic diversity and robust differences between the overall gut microbial profiles, but no appreciable differences between significant versus non-significant BMI gain groups during treatment. Bioinformatic analysis (Bahr et al. 2015a) demonstrated increased butyrate and propionate metabolism in the RIS-treated group, as well as SCFA production and impairments in tryptophan metabolism. Flowers et al. (2017) found that low abundance of bacteria from the genera Akkermansia was associated with a lack of weight gain after SGA treatment. However, these results are difficult to explain. Akkermansia muciniphila may serve as a negative marker of inflammation as it was found that the abundance of this genus is reduced under the regimen of HFD and its decline correlated negatively with lipid synthesis, plasma markers of insulin resistance, cardiovascular risk, and adiposity in rodents (Schneeberger et al. 2015). In a recent study published by Yuan et al. (2018), it was concluded that weight gain in patients treated with RIS was significantly correlated with an increase of faecal Bifidobacterium spp. abundance. Bifidobacterium spp. have an anti-inflammatory effect against systemic inflammation, and an increased abundance could be a compensatory reaction after weight gain and inflammation.
To answer the question whether dysbiosis caused by SGA administration is related to the occurrence of metabolic disorders, prospective clinical trials, and further experimental studies in which the same taxonomic groups of bacteria and their metabolic functions are assessed are necessary.
Based on the results of the analysed studies, we attempted to describe the mechanism of metabolic disorders originating from SGA treatment. Based on the current systematic review, we conclude that inflammation is critical to inducing weight gain and other metabolic alterations secondary to SGA use (Straczkowski et al. 2002; Kim et al. 2006). First, dysbiosis affects energy homeostasis of the body and lipid metabolism (Slyepchenko et al. 2016; Boulangé et al. 2016). Also, dysbiosis alters the structure and function of the intestinal barrier and may cause the translocation of bacterial antigens into the systemic circulation (Küme et al. 2017). Data on the presence of various microorganisms in extracolonic tissues and organs (Nagpal and Yadav 2017) following HFD (Wirostko et al. 1990) in obese individuals are increasing (Schwiertz et al. 2010). Bacterial lipopolysaccharide (LPS) components of gram-negative bacteria and cyanobacteria and high-energy SCFAs play major roles in energy harvesting. These molecules, among others, activate the G protein binding receptor (GPR), followed by secretion of the YY peptide (PYY), resulting in decreased intestinal motility, increased fat storage by reduced expression of the lipoprotein lipase inhibitor (fasting-induced adipose factor (FIAF)), activation of the differentiation of peroxisomal gamma proliferator-activated receptors (PPARγ) and the pro-inflammatory endocannabinoid system, respectively, and the development of adipose mass. These components regulate fatty acid synthase (FAS), enhancing hepatic de novo lipogenesis. LPS exacerbates hepatic steatosis and insulin resistance (Marlicz et al. 2014; Jin et al. 2017; Rorato et al. 2017). Macrophages possess the ability to phagocytose LPS, migrate to peripheral tissues, and release pro-inflammatory cytokines. Consequently, adipokine synthesis is decreased and leptin and ghrelin levels increase. All of these pathways sustain systemic inflammation (Tilg and Kaser 2009, 2011; Park and Scherer 2011) and may contribute to metabolic alterations in patients exposed to HFD with type 2 diabetes mellitus (Burcelin 2012). Unfortunately, LPS and gut barrier function were not measured in the analysed studies.
In immune-related pathogenesis of obesity, bacterial LPS and peptidoglycans, as pro-inflammatory agents, activate pathogen recognition receptors (PRRs) on macrophages and neutrophils, and as part of the non-specific immune response (Burcelin 2012) are responsible for hyperinsulinaemia and insulin resistance (Saberi et al. 2009). Furthermore, immune-related mechanisms may contribute to gut microbial alterations. For example, mice lacking TLR5 develop dysbiosis followed by metabolic syndrome (Tilg and Kaser 2009; Tremaroli and Bäckhed 2012). Obesogenic-type dysbiosis induces inflammation within the gut and affects neurotransmitter levels and the gut-brain axis function (Collins et al. 2012). Skewed production of serotonin in the gut (Clarke et al. 2013) may be at least partly responsible for weight gain secondary to SGA treatment via microbial alterations (Collins et al. 2012). However, none of the studies included in this SR reported such an association. A study by Kao et al. (2018), however, demonstrated that the B-GOS mode of action is independent of the serotonin pathway.
Only one study in this systematic review reported elevated levels of TNF-α when co-administered with B-GOS (Kao et al. 2018). TNF-α was found to act as weight gain suppressant and influence adipocytes lipid metabolism (Langhans and Hrupka 1999; Coppack 2001). Unexpectedly, another study discovered macrophage infiltration of adipose tissue (Davey et al. 2013). However, Xu et al. (2003) discovered that macrophage infiltration of adipose tissue is associated with the development of inflammation and insulin resistance in obese individuals. In one human study (Yuan et al. 2018), elevated concentrations of hs-CRP and decreased levels of SOD in patients with SCZ were found, and these alterations were more pronounced following RIS treatment, proving that both oxidative stress and inflammation may be responsible for metabolic malfunctions. No other research evaluated the role of gut permeability in systemic inflammation. Based on data included in this systematic review, we postulate that the assessment of intestinal permeability may serve as a surrogate marker of both gut dysbiosis and metabolic alterations. This should be verified in well-controlled trials in obese individuals. However, Davey et al. (2012) have shown that OLZ administration was associated with an inflammation in female mice that can suggest that intrinsic properties of this agent may directly alter inflammatory mechanisms.
We conclude that metabolic disturbances during SGA treatment may be the consequence, at least in part, of gut dysbiosis. Numerous trials confirmed a beneficial effect of prebiotics and probiotics on gut microbiota composition, with a lower risk of metabolic and weight disturbances. We found only one study in which B-GOS administration attenuated OLZ-mediated weight gain independently of serotonin pathways and acted positively on gut microbiota composition when utilized alone. Therefore, we suggest further research, considering probiotic/prebiotic/synbiotic therapy with concomitant SGA treatment. Such co-therapy may not only positively prevent or reduce weight gain but also modulate fasting glucose and glycated haemoglobin, dyslipidaemia, total and LDL cholesterol, and hypertension. Moreover, as discovered by Kao et al. (2018), prebiotics may elevate cortical glutamate receptor subunit mRNA expression (GluN1) in contrast to reductions of this receptor density typically seen in chronic SGAs users affecting their cognition (Krzystanek et al. 2015, 2016) negatively. Of particular interest is a search for target probiotic strains, such as Collinsella aerofaciens, which is increased in children treated with RIS without commensurate weight gains (Bahr et al. 2015a). Potential next-generation probiotic bacteria include Akkermansia, Bacteroides spp., and Eubacteriumhalli, as well as bacterial structural elements (cell wall proteins) and metabolites (e.g., SCFAs) (Romaní-Pérez et al. 2017; Muszyńska et al. 2018)
This systematic review has at least four limitations. First, the number of studies identified and included in this review was low. Most of the included studies were conducted in rodent models with an unclear risk of bias. Thus, the results of these studies may not be fully extrapolated to humans. Second, although reliable molecular techniques were used for microbiota analyses in all of the included studies, none of the research used intestinal samples. Therefore, it is impossible to conclude the microbiota composition in various parts of the gastrointestinal tract. Third, none of the human studies were randomized and placebo controlled. Notably, SGAs have also been found to affect food intake habits (Reynolds and McGowan 2017) and, consequently, microbiota composition (Turnbaugh 2017). Although such a relationship was demonstrated in one study, and in female rodents only (Davey et al. 2012), detailed observations should be the subject of further research. Thus, our conclusions need to be cautiously considered.
In conclusion, this systematic review proves that alterations in the gut microbiota composition causing low-level inflammation and decreased energy expenditure can play a role in body weight gain during SGA treatment. Experimental research targeting the gastrointestinal microbiota to discover the exact mechanism of SGA action associated with poor metabolic outcomes and controlled prospective human studies should be initiated and followed. We truly believe that experimental and clinical studies should include an assessment of intestinal barrier integrity, markers of the generalized inflammatory process (e.g., LPS), and the effect of gut microbiota modifications (prebiotics, probiotics, antibiotics) on metabolic side effects of SGAs. Lastly, studies in the field of metabolomics should also be the next step in such experiments to. Due to individual composition and function of gut microbiota, the content of the microbiome metabolites, e.g., SCFAs or secondary bile acids, which play an important role in metabolic and cardiovascular health, would comprehensively decipher the impact of SGA-induced dysbiosis on human metabolism (Alemán et al. 2018; Chambers et al. 2018).
Electronic supplementary material
(DOCX 21 kb)
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
Drs. Skonieczna-Żydecka, Łoniewski, Misera, Stachowska, Maciejewska, and Marlicz declare no conflict of interest. Dr. Galling has received honoraria from Lundbeck and Otsuka.
Contributors
Conception—KSŻ, IŁ, and WM; literature search—KSŻ and AM; data analysis—KSŻ, IŁ, AM, ES, DM, WM, and BG; risk of bias and quality of study assessment—KSŻ, IŁ, and WM; writing the manuscript—all; final revision and approval—all.
Footnotes
This article belongs to a Special Issue on Microbiome in Psychiatry & Psychopharmacology
References
- Abbott A (2016) Scientists bust myth that our bodies have more bacteria than human cells. Nature News. 10.1038/nature.2016.19136
- Alemán JO, Bokulich NA, Swann JR, Walker JM, de Rosa JC, Battaglia T, Costabile A, Pechlivanis A, Liang Y, Breslow JL, Blaser MJ, Holt PR. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J Transl Med. 2018;16:244. doi: 10.1186/s12967-018-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2008;193:101–107. doi: 10.1192/bjp.bp.107.042853. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. American Psychiatric Association. American Association of Clinical Endocrinologists. North American Association for the Study of Obesity Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13:889–899. doi: 10.1016/S1473-3099(13)70179-8. [DOI] [PubMed] [Google Scholar]
- Astrup A, Gøtzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69:1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate-Peril MA, Kirby JR, Calarge CA. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CML, Pearson NA, Murry DJ, Grobe JL, Kirby JR. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2:1725–1734. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9:e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab. 2014;25:593–600. doi: 10.1016/j.tem.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Beierle I, Meibohm B, Derendorf H. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. 1999;37:529–547. [PubMed] [Google Scholar]
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R. Regulation of metabolism: a cross talk between gut microbiota and its human host. Physiology (Bethesda) 2012;27:300–307. doi: 10.1152/physiol.00023.2012. [DOI] [PubMed] [Google Scholar]
- Chambers Edward S., Preston Tom, Frost Gary, Morrison Douglas J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Current Nutrition Reports. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-K, Hayes RD, Perera G, Broadbent MTM, Fernandes AC, Lee WE, Hotopf M, Stewart R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6:e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintoh AF, Mann SW, Lam L, Giacca A, Fletcher P, Nobrega J, Remington G. Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophr Res. 2009;108:127–133. doi: 10.1016/j.schres.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Consortium THMP, Huttenhower C, Gevers D, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/PNS2001110. [DOI] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate Rodriguez JG, Rogers AB, Robine N, Loke P’, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussotto Sofia, Strain Conall R., Fouhy Fiona, Strain Ronan G., Peterson Veronica L., Clarke Gerard, Stanton Catherine, Dinan Timothy G., Cryan John F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. 2018;236(5):1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O’Sullivan O, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva V. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–2241. doi: 10.2147/NDT.S113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99–105. doi: 10.1192/bjp.bp.110.084665. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003;75:255–260. doi: 10.1016/S0091-3057(03)00079-0. [DOI] [PubMed] [Google Scholar]
- Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37:261–267. doi: 10.1002/phar.1890. [DOI] [PubMed] [Google Scholar]
- Galling B, Correll CU. Do antipsychotics increase diabetes risk in children and adolescents? Expert Opin Drug Saf. 2015;14:219–241. doi: 10.1517/14740338.2015.979150. [DOI] [PubMed] [Google Scholar]
- Galling B, Roldán A, Nielsen RE, Nielsen J, Gerhard T, Carbon M, Stubbs B, Vancampfort D, de Hert M, Olfson M, Kahl KG, Martin A, Guo JJ, Lane HY, Sung FC, Liao CH, Arango C, Correll CU. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73:247–259. doi: 10.1001/jamapsychiatry.2015.2923. [DOI] [PubMed] [Google Scholar]
- Grobe J, Bahr S, Weidemann B, et al. Transfer of obesity via the gut microbiome is mediated specifically through suppression of non-aerobic resting metabolism. FASEB J. 2015;29:857.2. doi: 10.1096/fasebj.29.1_supplement.857.2. [DOI] [Google Scholar]
- Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, Furu K, Garuoliené K, Hoffmann F, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, Litchfield M, López SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Pearson SA, Reutfors J, Saastamoinen LK, Sato I, Schuiling-Veninga CCM, Shyu YC, Skurtveit S, Verdoux H, Wang LJ, Yahni CZ, Bachmann CJ. International trends in antipsychotic use: a study in 16 countries, 2005-2014. Eur Neuropsychopharmacol. 2017;27:1064–1076. doi: 10.1016/j.euroneuro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J Innate Immun. 2017;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilies D, Huet A-S, Lacourse E, Roy G, Stip E, Amor LB. Long-term metabolic effects in French-Canadian children and adolescents treated with second-generation antipsychotics in monotherapy or Polytherapy: a 24-month descriptive retrospective study. Can J Psychiatr. 2017;62:827–836. doi: 10.1177/0706743717718166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CJ, Engstler AJ, Ziegenhardt D, Bischoff SC, Trautwein C, Bergheim I. Loss of lipopolysaccharide-binding protein attenuates the development of diet-induced non-alcoholic fatty liver disease in mice. J Gastroenterol Hepatol. 2017;32:708–715. doi: 10.1111/jgh.13488. [DOI] [PubMed] [Google Scholar]
- Kalverdijk LJ, Bachmann CJ, Aagaard L, Burcu M, Glaeske G, Hoffmann F, Petersen I, Schuiling-Veninga CCM, Wijlaars LP, Zito JM. A multi-national comparison of antipsychotic drug use in children and adolescents, 2005-2012. Child Adolesc Psychiatry Ment Health. 2017;11:55. doi: 10.1186/s13034-017-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanji S, Fonseka TM, Marshe VS, Sriretnakumar V, Hahn MK, Müller DJ. The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur Arch Psychiatry Clin Neurosci. 2018;268:3–15. doi: 10.1007/s00406-017-0820-z. [DOI] [PubMed] [Google Scholar]
- Kao AC-C, Spitzer S, Anthony DC, Lennox B, Burnet PWJ (2018) Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry:8. 10.1038/s41398-018-0116-8 [DOI] [PMC free article] [PubMed]
- Kim C-S, Park H-S, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen JE. Experiments to illustrate the effect of chlorpromazine on the permeability of the bacterial cell wall. Acta Pathol Microbiol Scand B. 1979;87:317–319. doi: 10.1111/j.1699-0463.1979.tb02445.x. [DOI] [PubMed] [Google Scholar]
- Krzystanek Marek, Bogus Katarzyna, Pałasz Artur, Krzystanek Ewa, Worthington John J., Wiaderkiewicz Ryszard. Effects of long-term treatment with the neuroleptics haloperidol, clozapine and olanzapine on immunoexpression of NMDA receptor subunits NR1, NR2A and NR2B in the rat hippocampus. Pharmacological Reports. 2015;67(5):965–969. doi: 10.1016/j.pharep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Krzystanek Marek, Bogus Katarzyna, Pałasz Artur, Wiaderkiewicz Anna, Filipczyk Łukasz, Rojczyk Ewa, Worthington John, Wiaderkiewicz Ryszard. Extended neuroleptic administration modulates NMDA-R subunit immunoexpression in the rat neocortex and diencephalon. Pharmacological Reports. 2016;68(5):990–995. doi: 10.1016/j.pharep.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Küme T, Acar S, Tuhan H, Çatlı G, Anık A, Gürsoy Çalan Ö, Böber E, Abacı A. The relationship between serum Zonulin level and clinical and laboratory parameters of childhood obesity. J Clin Res Pediatr Endocrinol. 2017;9:31–38. doi: 10.4274/jcrpe.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W, Hrupka B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides. 1999;33:415–424. doi: 10.1054/npep.1999.0048. [DOI] [PubMed] [Google Scholar]
- Lau SL, Muir C, Assur Y, Beach R, Tran B, Bartrop R, McLean M, Caetano D. Predicting weight gain in patients treated with clozapine: the role of sex, body mass index, and smoking. J Clin Psychopharmacol. 2016;36:120–124. doi: 10.1097/JCP.0000000000000476. [DOI] [PubMed] [Google Scholar]
- Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ML, Wang TN, Lin TY, Shao WC, Chang SH, Chou JY, Ho YF, Liao YT, Chen VCH. Differential effects of olanzapine and clozapine on plasma levels of adipocytokines and total ghrelin. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;58:47–50. doi: 10.1016/j.pnpbp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. 2010;10:1175–1200. doi: 10.1586/ern.10.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewska D, Skonieczna-Zydecka K, Lukomska A, et al (2018) The short chain fatty acids and lipopolysaccharides status in Sprague-Dawley rats fed with high-fat and high-cholesterol diet. J Physiol Pharmacol 69. doi: 10.26402/jpp.2018.2.05 [DOI] [PubMed]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlicz W, Ostrowska L, Łoniewski I. Review paper<br>the role of gut microbiota in weight management by non-invasive interventions and bariatric surgery. Nutrition, Obesity & Metabolic Surgery. 2014;1:20–29. doi: 10.5114/noms.2014.44566. [DOI] [Google Scholar]
- Mathur R, Chua KS, Mamelak M, Morales W, Barlow GM, Thomas R, Stefanovski D, Weitsman S, Marsh Z, Bergman RN, Pimentel M. Metabolic effects of eradicating breath methane using antibiotics in prediabetic subjects with obesity. Obesity (Silver Spring) 2016;24:576–582. doi: 10.1002/oby.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. 2014;9:e115225. doi: 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszyńska B, Grzywacz-Kisielewska A, Kała K, Gdula-Argasińska J. Anti-inflammatory properties of edible mushrooms: a review. Food Chem. 2018;243:373–381. doi: 10.1016/j.foodchem.2017.09.149. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab. 2017;71(Suppl 1):11–16. doi: 10.1159/000479918. [DOI] [PubMed] [Google Scholar]
- Nehme H, Saulnier P, Ramadan AA, Cassisa V, Guillet C, Eveillard M, Umerska A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS One. 2018;13:e0189950. doi: 10.1371/journal.pone.0189950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer E, Atassi H. The microbiome that shapes us: can it cause obesity? Curr Gastroenterol Rep. 2017;19:59. doi: 10.1007/s11894-017-0600-y. [DOI] [PubMed] [Google Scholar]
- Park J, Scherer PE. Leptin and cancer: from cancer stem cells to metastasis. Endocr Relat Cancer. 2011;18:C25–C29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Ahmed I, Gillevet PM, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–875.e1–3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, McGowan OO. Mechanisms underlying metabolic disturbances associated with psychosis and antipsychotic drug treatment. J Psychopharmacol (Oxford) 2017;31:1430–1436. doi: 10.1177/0269881117722987. [DOI] [PubMed] [Google Scholar]
- Riedl RA, Burnett CM, Pearson NA, et al. The biomass and composition of the gut microbiota modify anaerobic metabolism. FASEB J. 2017;31:890.2–890.2. doi: 10.1096/fasebj.31.1_supplement.890.2. [DOI] [Google Scholar]
- Romaní-Pérez M, Agusti A, Sanz Y. Innovation in microbiome-based strategies for promoting metabolic health. Curr Opin Clin Nutr Metab Care. 2017;20:484–491. doi: 10.1097/MCO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- Rorato Rodrigo, Borges Beatriz de Carvalho, Uchoa Ernane Torres, Antunes-Rodrigues José, Elias Carol Fuzeti, Elias Lucila Leico Kagohara. LPS-Induced Low-Grade Inflammation Increases Hypothalamic JNK Expression and Causes Central Insulin Resistance Irrespective of Body Weight Changes. International Journal of Molecular Sciences. 2017;18(7):1431. doi: 10.3390/ijms18071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi M, Woods N-B, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez V, Romero-Rubio D, Abad-Perez MJ, Descalzo-Cabades MA, Alonso-Gutierrez S, Salazar-Fraile J, Montagud V, Facila L. Metabolic syndrome and cardiovascular risk in people treated with long-acting injectable antipsychotics. Endocr Metab Immune Disord Drug Targets. 2017;18:379–387. doi: 10.2174/1871530317666171120151201. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Sjo CP, Stenstrøm AD, Bojesen AB, Frølich JS, Bilenberg N. Development of metabolic syndrome in drug-naive adolescents after 12 months of second-generation antipsychotic treatment. J Child Adolesc Psychopharmacol. 2017;27:884–891. doi: 10.1089/cap.2016.0171. [DOI] [PubMed] [Google Scholar]
- Slyepchenko A, Maes M, Machado-Vieira R, Anderson G, Solmi M, Sanz Y, Berk M, Köhler C, Carvalho A. Intestinal Dysbiosis, gut Hyperpermeability and bacterial translocation: missing links between depression, obesity and type 2 diabetes. Curr Pharm Des. 2016;22:6087–6106. doi: 10.2174/1381612822666160922165706. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straczkowski M, Dzienis-Straczkowska S, Stêpieñ A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87:4602–4606. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Adiponectin and JNK: metabolic/inflammatory pathways affecting gastrointestinal carcinogenesis. Gut. 2009;58:1576–1577. doi: 10.1136/gut.2009.190959. [DOI] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ. Microbes and diet-induced obesity: fast, cheap, and out of control. Cell Host Microbe. 2017;21:278–281. doi: 10.1016/j.chom.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol Lond. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Correll CU, Galling B, Probst M, de Hert M, Ward PB, Rosenbaum S, Gaughran F, Lally J, Stubbs B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–174. doi: 10.1002/wps.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, STROBE Initiative Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Vasan S, Abdijadid S (2018) Atypical antipsychotic agents. In: StatPearls. StatPearls publishing, Treasure Island (FL) [PubMed]
- Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Investig. 2017;40:1165–1174. doi: 10.1007/s40618-017-0719-6. [DOI] [PubMed] [Google Scholar]
- Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirostko E, Johnson L, Wirostko B. Ulcerative colitis associated chronic uveitis. Parasitization of intraocular leucocytes by mollicute-like organisms J Submicrosc Cytol Pathol. 1990;22:231–239. [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BG, Hur KY, Lee MS. Alterations in gut microbiota and immunity by dietary fat. Yonsei Med J. 2017;58:1083–1091. doi: 10.3349/ymj.2017.58.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Xiuxia, Zhang Peifen, Wang Yaping, Liu Yafei, Li Xue, Kumar Bachoo Upshant, Hei Gangrui, Lv Luxian, Huang Xu-Feng, Fan Xiaoduo, Song Xueqin. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophrenia Research. 2018;201:299–306. doi: 10.1016/j.schres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhu Y, Zhou W, Gao L, Yuan L, Han X. Serotonin receptor 2C and insulin secretion. PLoS One. 2013;8:e54250. doi: 10.1371/journal.pone.0054250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-P, Lencz T, Zhang RX, Nitta M, Maayan L, John M, Robinson DG, Fleischhacker WW, Kahn RS, Ophoff RA, Kane JM, Malhotra AK, Correll CU. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42:1418–1437. doi: 10.1093/schbul/sbw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res. 2003;37:193–220. doi: 10.1016/S0022-3956(03)00018-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 21 kb)