Abstract
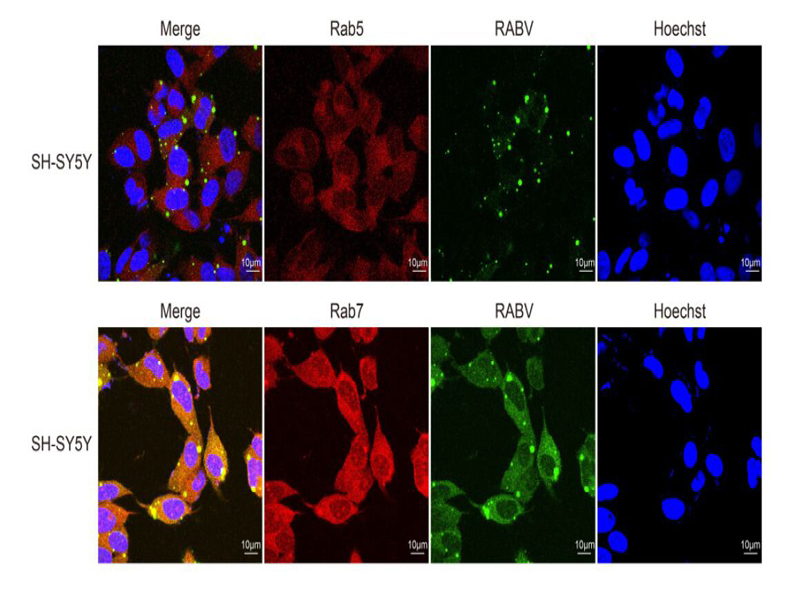
Rabies virus (RABV) is a highly neurotropic virus that follows clathrin-mediated endocytosis and pH-dependent pathway for trafficking and invasion into endothelial cells. Early (Rab5, EEA1) and late (Rab7, LAMP1) endosomal proteins play critical roles in endosomal sorting, maturity and targeting various molecular cargoes, but their precise functions in the early stage of RABV neuronal infection remain elusive. In this study, the relationship between enigmatic entry of RABV with these endosomal proteins into neuronal and SH-SY5Y cells was investigated. Immunofluorescence, TCID50 titers, electron microscopy and western blotting were carried out to determine the molecular interaction of the nucleoprotein (N) of RABV with early or late endosomal proteins in these cell lines. The expression of N was also determined by down-regulating Rab5 and Rab7 in both cell lines through RNA interference. The results were indicative that N proficiently colocalized with Rab5/EEA1 and Rab7/LAMP1 in both cell lines at 24 and 48 h post-infection, while N titers significantly decreased in early infection of RABV. Down-regulation of Rab5 and Rab7 did not inhibit N expression, but it prevented productive infection via blocking the normal trafficking of RABV in a low pH environment. Ultrathin sections of cells studied by electron microscope also verified the close association of RABV with Rab5 and Rab7 in neurons. From the data it was concluded that primary entry of RABV strongly correlates with the kinetics of Rab-proteins present on early and late vesicles, which provides helpful clues to explain the early events of RABV in nerve cells.
Keywords: Rab5, Rab7, rabies virus(RABV), endosomes, colocalization
Acknowledgments
The study was supported by the National Key Research and Development Program of China (Grant No. 216YFD 0500402) and Natural Science Foundation of China (Grants No. 31272579 and 31472208).
Footnotes
These authors contributed equally to this work.
References
- Barbieri MA, Roberts RL, Gumusboga A, Highfield H A-, Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5. J Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-W. [DOI] [PubMed] [Google Scholar]
- Callaghan J, Simonsen A G, Ban-Hock TO, Stenmark H. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 1999;338:539–543. doi: 10.1042/bj3380539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Bahr SJ. Rab7 activity affects epidermal growth factor: epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J BiolChem. 2006;281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- Cook NR, Row PE, Davidson HW. Lysosome Associated Membrane Protein 1 (Lamp1)Traffics Directly from the TGN to Early Endosomes. Traffic. 2004;5:685–699. doi: 10.1111/j.1600-0854.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- Colpitts TM, Moore AC, Kolokoltsov AA, Davey RA. Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins Rab5 and Rab7. Virology. 2007;369:78–91. doi: 10.1016/j.virol.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway of Neuron. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Flannigan DJ, Zewail AH. 4D electron microscopy: principles and applications. AccChem Res. 2012;45:1828–1839. doi: 10.1021/ar3001684. [DOI] [PubMed] [Google Scholar]
- Hislop JN, Islam TA, Eleftheriadou I, Carpentier DC, Trabalza A, Parkinson M, Schiavo G, Mazarakis ND. Rabies virus envelope glycoprotein targets lentiviral vectors to the axonal retrograde pathway in motor neurons. J BiolChem. 2014;289:16148–16163. doi: 10.1074/jbc.M114.549980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome Maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC. Human Rabies: a 2016 Update. Curr Infect Dis Rep. 2016;18:1–6. doi: 10.1007/s11908-016-0540-y. [DOI] [PubMed] [Google Scholar]
- Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GMCSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940–4948. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol, 81: 4881–4885. [DOI] [PMC free article] [PubMed]
- Lewis P, Lentz TL. Rabies virus entry into cultured rat hippo campalneurons. J Neurocytol. 1998;27:559–573. doi: 10.1023/A:1006912610044. [DOI] [PubMed] [Google Scholar]
- Liu SL, Wu QM, Zhang LJ, Wang ZJ, Sun EZ, Zhang ZL, Pang DW. Three-Dimensional Tracking of Rab5 and Rab7 Associated Infection Process of Influenza Virus. Small. 2014;22:4746–4753. doi: 10.1002/smll.201400944. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;24:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Petrareanu C, Lazar C, Florian P, Branza-Nichita N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J Virol. 2013;11:6415–6427. doi: 10.1128/JVI.00393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J Virol. 2001;75:1387–1400. doi: 10.1128/JVI.75.3.1387-1400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. CurrBiol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal J, Seabra M. Evolution of the Rab family of small GTP-binding proteins. J. MolBiol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Piccinotti S, Kirchhausen T, Whelana SPJ. Uptake of Rabies Virus into Epithelial Cells by Clathrin-Mediated Endocytosis Depends upon Actin. J Virol. 2011;87:11637–11647. doi: 10.1128/JVI.01648-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrea CAS, Leonardb D, Corverab S, Kurt-Jonesa EA, Finberga RW. Antibodies to cell surface proteins redirect intracellular trafficking pathways. ExpMolPathol. 2011;91:723–732. doi: 10.1016/j.yexmp.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, YannisKalaidzidis Y, Zerial M. Rab Conversion as a Mechanism of Progression from Early to Late Endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rubino M, Miaczynska M, Lippé R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol- Chem. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- Schmieg N, Menendez G, Schiavo G, Terenzioc M. Semin Cell Dev Biol. 2013. Signalling endosomes in axonal transport: Travel updates on the molecular highway; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Seabra M, Mules E, Hume A. RabGTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/S1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Song Y, Jinli H, Bin Q, Yanchao L, Ye X, Ming D, Zhenhong G, Maolin Z, Liankun S. Street Rabies Virus Causes Dendritic Injury and F-actin depolymerization in the Hippocampus. J Gen Virol. 2013;94:276–283. doi: 10.1099/vir.0.047480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. The EMBO J. 1994;13:1287. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. RabGTPases as coordinators of vesicle traffic. 2009. Nat Rev Mol Cell Biol, 10: 513–525. [DOI] [PubMed]
- Vanlandingham PA, Ceresa BP. Rab7 Regulates Late Endocytic Trafficking Downstream of Multivesicular Body Biogenesis and Cargo Sequestration. J BiolChem. 2009;284:12110–12124. doi: 10.1074/jbc.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela EM, Colpitts T M, Zhang LH, Davey RA, Aronson JF. Pichinde virus is trafficked through a dynamin 2 endocytic pathway that is dependent on cellular Rab5- and Rab7-mediated endosomes. Arch Virol. 2008;153:1391–1396. doi: 10.1007/s00705-008-0129-3. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidricaire G, Tremblay MJ. Rab5 and Rab7, but Not ARF6, Govern the Early Events of HIV-1 Infection in Polarized Human Placental Cells. J Immunol. 2005;175:6517–6530. doi: 10.4049/jimmunol.175.10.6517. [DOI] [PubMed] [Google Scholar]
- Weir DL, Laing ED, Smith IL, Wang L, Broder CC. Host cell virus entry mediated by asutralian bat lyssavirus G envelop glycoprotein occurs through a clathrin-mediated endocytosic pathway that required actin and Rab5. Virol J. 2014;11:1–10. doi: 10.1186/1743-422X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a Tethering Protein of the Early Sorting Endosome, Shows a Polarized Distribution in Hippocampal Neurons, Epithelial Cells, and Fibroblasts. MolBiol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]


