Abstract
Specific-pathogen free (SPF) animals were introduced in the 1960s to minimize disease and infection as variables in biomedical research. Our aim was to examine differences in physiological response in rat colonies bred and housed in a conventional versus SPF facility, and implications for research. Sprague-Dawley rats were anesthetized and catheterized for blood and pressure monitoring, and electrocardiogram (ECG) leads implanted. Hematology was assessed, and coagulation profile using rotational thromboelastometry. Health screening was outsourced to Cerberus Sciences. SPF rats had significantly lower pulse pressure (38% decrease), arrhythmias and prolonged QTc (27% increase) compared to conventional rats. No arrhythmias were found in conventional rats. SPF rats had significantly higher white cell, monocyte, neutrophil and lymphocyte counts, and were hyperfibrinolytic, indicated by EXTEM maximum lysis >15%. Independent assessment revealed similar pathogen exclusion between colonies, with the exception of Proteus in SPF animals. Returning to a conventional facility restored normal host physiology. We conclude that SPF animals displayed an abnormal hemodynamic, hematological and hemostatic phenotype in response to anesthesia and surgery, and provide a number of recommendations to help standardize research outcomes and translation.
Subject terms: Cardiovascular biology, Experimental models of disease
Introduction
In the 1950s researchers were increasingly frustrated with the presence of disease or infection as an unwanted variable affecting their experiments1. As a result of an international mandate for better quality animals2, the National Institutes of Health (NIH) issued guidelines for animal housing and husbandry in 19623. Since that time a great deal of attention has been paid to standardizing facilities, light/day cycles, diets, techniques and equipment4. The overall strategy has been to breed animals free of infectious agents, from germ-free stock or caesarean aseptic techniques, then expose the colony to an environment free of selected infectious pathogens (not all) that would otherwise interfere with research objectives2,4. Today, most animal husbandry facilities adopt this specific-pathogen free (SPF) approach and provide certification that a colony or breeding pair is free from common infectious agents that a species is customarily exposed to in the wild. This approach also has additional benefits of cost-saving and ethical benchmarks as it reduces the number of animals used within individual studies. It also aims to improve standardization of animals and the scale of reproducibility (and replicability) among different laboratories around the world working on similar problems and preclinical questions1,4,5.
However, over the past decade, there have been a number of anomalies creeping into the literature and growing concerns about the clinical relevance of SPF laboratory rodents. Recent studies have reported broad and unexpected changes in the immune system of SPF animals, which have been linked to changes in the gut microbiome5,6. Changes in the gut microbiome influences animal models for metabolic disorders, obesity, hematopoiesis, osteoarthritis, inflammatory bowel diseases, allergies, autoimmunity, cancer and neuropsychiatric diseases7–10. More recently, changes in the microbiome were shown to lead to numerous physiological disorders by altering an animal’s circadian rhythm11.
We report that animal breeding and housing changes at James Cook University from a conventional facility to a new SPF facility resulted in profound alterations in the physiology of Sprague Dawley rats, and their response to minor trauma. The physiological variables included decreased tolerance to anesthesia, hemodynamic instability, aberrant hematology, traumatic bleeding and reduced physiological reserve in SPF animals. This altered phenotype to the stress of surgical trauma was completely reversed when animals were returned to the original conventional facility.
Results
Hemodynamics and electrocardiograph
Arterial blood pressure trace in spontaneous breathing SPF animals showed significantly reduced pulse pressure (systolic pressure minus diastolic pressure) despite similar mean arterial pressure (MAP) and heart rate (HR) compared to conventional rats (Fig. 1a,b). Timing of oscillations in the blood pressure waveform during spontaneous breathing were similar between the two colonies. The ECG of SPF animals showed a range of abnormal cardiac rhythms including premature ventricular contractions (skipped beats), salvos and bigeminy episodes (Fig. 2a,b). Arrhythmias were found in 85% of SPF animals (17/20) compared to no arrhythmias in the conventional rats (p < 0.001) (Table 1). There were no differences in RR or QRS complex duration, however SPF rats had significantly prolonged QTc intervals (0.14 vs. 0.11 sec; p = 0.033) (Table 1).
Figure 1.
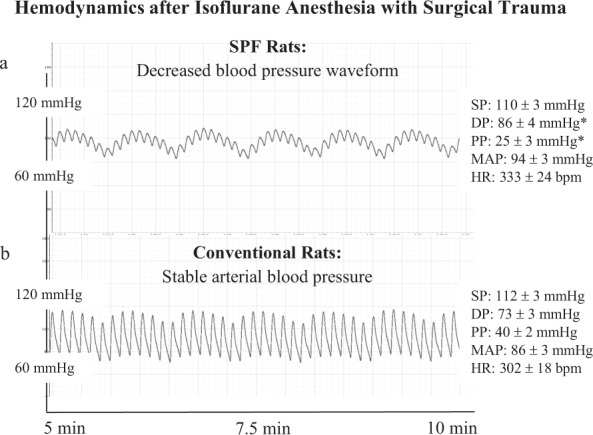
Representative blood pressure traces from SPF (a) and Conventional (b) male Sprague Dawley rats taken under isoflurane anesthesia following surgical instrumentation. Values represent mean ± SEM of n = 5 rats from each colony. SP = systolic pressure; DP = diastolic pressure; PP = pulse pressure calculated from SP – DP; MAP = mean arterial pressure; HR = heart rate. *p < 0.05 compared to Conventional rats. Power (1-β err prob) = 0.97 (Critical t = 1.86, Df = 8, α = 0.05, outcome measure = PP).
Figure 2.
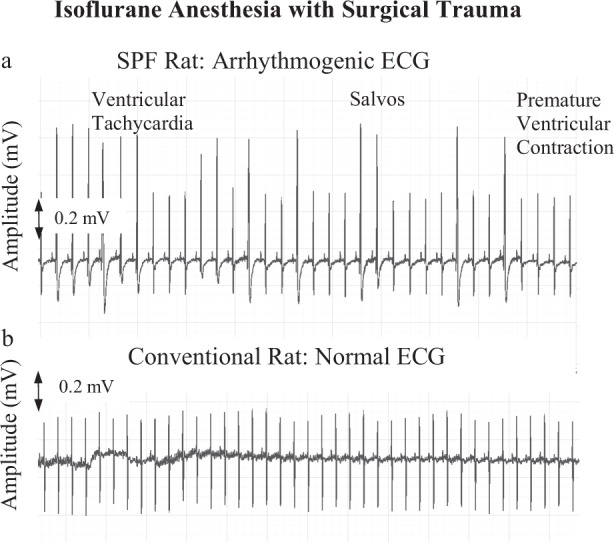
Representative lead II electrocardiograms (ECG) from SPF (a) and Conventional (b) male Sprague Dawley rats taken under isoflurane anesthesia following surgical instrumentation (n = 20 each group). SPF rats were arrhythmogenic with incidences of ventricular tachycardia, Salvos, bigeminy, and premature ventricular contractions. See Table 1 for data.
Table 1.
Episodes of arrhythmias and ECG parameters of male Sprague-Dawley Conventional and SPF rats after 7 days acclimation in the laboratory prior to experimentation.
| Parameter | Conventional (n = 20) |
SPF (n = 20) |
p value |
|---|---|---|---|
| Total Arrhythmias | 0 ± 0 |
21.55 ± 5.91 (0–87) |
< 0.001 |
| PVCs | 0 ± 0 |
16.15 ± 4.48 (0–74) |
< 0.001 |
| Salvos | 0 ± 0 |
3.10 ± 1.40 (0–25) |
0.014 |
| Bigeminy | 0 ± 0 |
1.65 ± 0.60 (0–8) |
0.014 |
| VT | 0 ± 0 |
0.65 ± 0.33 (0–4) |
0.289 |
| RR (sec) | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.458 |
| QRS (sec) | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.994 |
| QT (sec) | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.114 |
| QTc (sec) | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.033 |
Values are mean ± SEM with range in parentheses. Episodes of arrhythmias were counted over a 20-min period following surgical instrumentation. SPF = specific-pathogen free; PVCs = premature ventricular contractions; VT = ventricular tachycardia; QTc = Bazett-corrected QT interval. Power (1-β err prob) = 1.00 (Critical t = 1.69, Df = 38, α = 0.05, outcome measure = total arrhythmias).
Coagulation profile
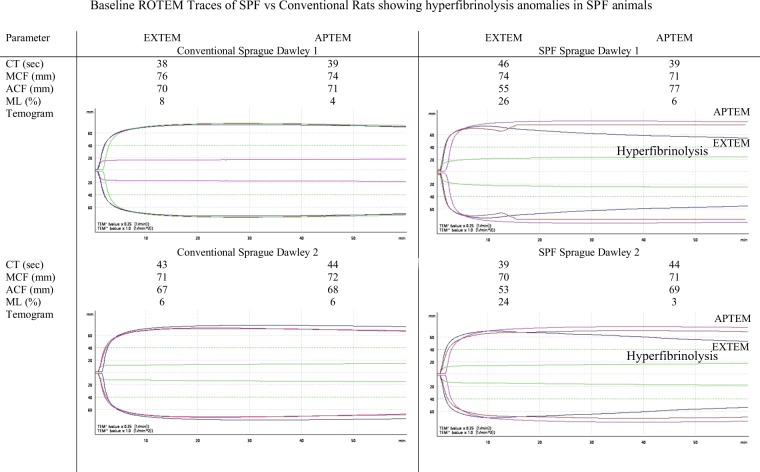
ROTEM analysis showed no differences in EXTEM clot times or clot amplitude (maximum clot firmness) between SPF and conventional rats. However, both SPF animals were hyperfibrinolytic, indicated by EXTEM maximum lysis (ML) percentages >15 (26% and 24% vs. 8% and 6% in conventional rats) (Fig. 3). Hyperfibrinolysis in SPF rats was confirmed with APTEM test which showed correction of ML to 6% and 3% with the addition of aprotonin which inhibits plasmin activation of fibrinolysis.
Figure 3.
ROTEM parameters and Temograms from n = 2 Conventional and n = 2 SPF male Sprague Dawley rats demonstrating hyperfibrinolysis in SPF rats. EXTEM = extrinsically-activated test using tissue factor; APTEM = activation as for EXTEM with aprotinin; CT = clot time; MCF = maximum clot firmness; ACF = actual clot firmness (clot amplitude 60 min following clot initiation); ML = maximum lysis.
Blood counts
The baseline total blood counts for conventional and SPF rats is shown in Table 2. The data indicate that the SPF rats had an overall aberrant hematology, a lower haemoglobin level and a 1.5-fold increase in platelet count compared to conventional rats. The number of white cells and lymphocytes were 1.4-fold higher than in conventional rats (p < 0.05), and monocyte counts 2.7-fold higher. Neutrophils were also 1.6-fold higher in SPF rats (p < 0.05). The hematological data of Said and Abiola12 highlight the differences in the SPF and conventional animals, supporting the notion of an aberrant hematology (Table 2).
Table 2.
Baseline blood counts for male Sprague Dawley Rats.
| Parameter | Conventional (n = 100) | JCU SPF (n = 34) |
*Said and Abiola (12) |
|---|---|---|---|
| WBC (x109/l) | 9.62 ± 0.25 | 13.29 ± 0.86* | 9.04 ± 2.29 |
| LYM (x109/l) | 7.01 ± 0.17 | 9.57 ± 0.85* | 7.50 ± 1.91 |
| MON (x109/l) | 0.51 ± 0.03 | 1.39 ± 0.47 | 0.55 ± 0.25 |
| NEU (x109/l) | 2.09 ± 0.10 | 3.25 ± 0.33* | NR |
| RBC (x1012/l) | 7.17 ± 0.07 | 7.36 ± 0.22* | 7.61 ± 0.27 |
| PLT (109/l) | 168 ± 16 | 247 ± 33* | NR |
| HgB (g/dL) | 15.58 ± 0.14 | 13.91 ± 0.45* | 15.63 ± 0.71 |
| HCT (%) | 38.17 ± 0.40 | 39.82 ± 1.41* | NR |
Values are mean ± SEM with number of animals in parentheses. *p < 0.05 compared to JCU conventional colony. Power (1-β err prob) = 0.999 (Critical t = 1.66, Df = 132, α = 0.05, outcome measure = WBC). SPF = specific-pathogen free; NR = not reported; WBC = white blood cells; LYM = lymphocytes; MON = monocytes; NEU = neutrophils; RBC = red blood cells; HgB = hemoglobin; HCT = hematocrit; PLT = platelets. Data from conventional animals were from a trauma study carried out before transition to SPF animals (see Materials and Methods). Said & Abiola (2014) housed male SD rats (117–264 g) in RC1 propylene cages with metal grill tops (conventional) and collected whole blood for hematological analysis following cervical dislocation and decapitation.
*Said and Abiola12.
Health screening
The externally commissioned health screening for randomly selected rats from the SPF, conventional and SPF animals acclimated for 7 days in the laboratory are shown in Table 3. The standout result is the presence of Proteus spp. in the two SPF groups and not the conventional rats. No animals had pinworms. One out of two animals from all groups had the presence of Entamoeba muris. One SPF animal had Staphylococcus aureus present, however this bacterium was not found in the 7-day laboratory acclimated SPF or conventional rats (Table 3). Viral screening using enzyme immunoassay (EIA) and indirect fluorescent antibody (IFA) techniques confirmed all rats were negative for Kilham Rat virus, Parvovirus (rNS1), Pneumonia Virus of Mice, Rat Coronavirus, Rat Minute Virus, Rat Parvovirus Rat Theilovirus, and Toolans H-1.
Table 3.
Fecal microbiology of male Sprague-Dawley Conventional, SPF rats and SPF rats after 7 days acclimation in the laboratory prior to experimentation.
| Bacteria and Fungi | Test Method | SPF Rats (AITHM Facility) (n = 2) |
Laboratory Acclimated SPF Rats (n = 2) |
Conventional Rats (Bush House) (n = 2) |
|---|---|---|---|---|
| Bordetella bronchiseptica | Culture | 0/2 | 0/2 | 0/2 |
| Bordetella spp | Culture | 0/2 | 0/2 | 0/2 |
| Corynebacterium kutscheri | Culture | 0/2 | 0/2 | 0/2 |
| Klebsiella oxytoca | Culture | 0/2 | 0/2 | 0/2 |
| # Klebsiella pneumoniae | Culture | 0/2 | 1/2# | 0/2 |
| Other significant organisms | Culture | 0/2 | 0/2 | 0/2 |
| Pasteurella pneumotropica | Culture | 0/2 | 0/2 | 0/2 |
| Pasteurella spp | Culture | 0/2 | 0/2 | 0/2 |
| ^ Proteus spp | Culture | 2/2^ | 2/2^ | 0/2 |
| Pseudomonas aeruginosa | Culture | 0/2 | 0/2 | 0/2 |
| Salmonella spp | Culture | 0/2 | 0/2 | 0/2 |
| Staphylococcus aureus | Culture | 1/2 | 0/2 | 0/2 |
| Streptococcus pneumoniae (alpha haem) | Culture | 0/2 | 0/2 | 0/2 |
| Streptococcus spp (beta haem) | Culture | 0/2 | 0/2 | 0/2 |
| Ectoparasite | ||||
| Mycoptes musculinis | Exam | 0/2 | 0/2 | 0/2 |
| Myobia musculi | Exam | 0/2 | 0/2 | 0/2 |
| Polyplax spinulosa | Exam | 0/2 | 0/2 | 0/2 |
| Radfordia spp | Exam | 0/2 | 0/2 | 0/2 |
| Endoparasite | ||||
| Giardia muris | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
| Pinworm – Aspiculuris spp | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
| Pinworm – Aspiculuris tetraptera | PCR | 0/2 | 0/2 | 0/2 |
| Pinworm – Syphacia muris | PCR | 0/2 | 0/2 | 0/2 |
| Pinworm – Syphacia spp | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
| Spironucleus muris | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
| Non-Pathogenic Protozoa | ||||
| Chilomastix bettencourti | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
| *Entamoeba muris | Wet prep/Motility | 1/2* | 1/2* | 1/2* |
| Tritrichomonas muris | Wet prep/Motility | 0/2 | 0/2 | 0/2 |
Microbiology results obtained from Cerberus Sciences on two SPF animals, two SPF animals acclimated for seven days in a conventional facility, and two conventional animals. Results presented as number of positive samples per number tested (2).
#Klebsiella pneumoniae has been associated with bacteraemic disease and widespread abscesses. They inhabit the gastrointestinal tract and are opportunistic agents waiting for the correct conditions to cause infections.
^Proteus species are gram-negative, rod-shaped, and facultatively anaerobes. They are ubiquitous in the environment and can be cultured from the upper respiratory tract and feces. They are commonly the cause of urinary tract infections in immunocompromised animals. The most common species is Proteus mirabilis.
*Entamoeba muris are considered non-pathogenic and frequently found in intestinal contents of normal rodents.
Discussion
Choosing an appropriate animal model for basic research and preclinical studies for translation is extremely complex1,13,14. In the process of shifting animal housing and breeding from a conventional to a new SPF facility at the AITHM, James Cook University, we were unable to reproduce basic baseline physiological data that have been routine in our laboratory for over 10 years, and compatible with published data from other laboratories. We report that SPF animals were hemodynamically-compromised, arrhythmogenic with prolonged QTc, coagulopathic, and had significantly higher white blood cell, lymphocyte and monocyte counts, and lower hemoglobin, compared to conventional rats. This new SPF-phenotype led to a lower physiological reserve in response to anesthesia and surgical stress.
Following isoflurane anesthesia and femoral artery and vein cannulation, arterial blood pressure in SPF animals had a decreased waveform accompanying a significant 38% fall in pulse pressure compared to conventional rats (p < 0.05) (Fig. 1a,b). Since pulse pressure depends largely on stroke volume and arterial stiffness15, our data suggest that the significantly lower pulse pressure in SPF rats was the result of a lower stroke volume and impaired left ventricular-arterial coupling relationship. This was further supported by a prolonged QTc interval in SPF animals (Table 1). A prolonged QTc in animal models and humans have been associated with lower stroke volume, compromised left ventricular function and increased mortality16,17. Our interpretation of a failing cardiovascular system in SPF animals was further supported by the presence of ventricular arrhythmias (Table 1, Fig. 2a,b), and a mild cardiomyopathy indicated from a histopathological multifocal myofiber degeneration, replacement fibrosis and macrophage infiltration (data not shown). Blood pressure and heart rate oscillations (timings of peaks and valleys) were similar between SPF and conventional animals (Fig. 1a,b), indicating there was little or no differences in the baroreflex input contributions to blood pressure regulation in the two colonies18.
Assessment of coagulation properties also revealed the presence of a significant whole blood hyperfibrinolysis in SPF compared to conventional rats. Hyperfibrinolysis is a common bleeding phenotype following severe traumatic injury, shock or major surgery, and believed to be initiated by activation of protein C on the endothelium-thrombomodulin-thrombin complex19–21. However, in our study, SPF animals showed hyperfibrinolysis after minor surgical cut-downs and femoral artery cannulations and instrumentation, a time when baseline data is normally collected prior to experiment. Based on hemodynamic and coagulation status, we conclude that SPF animals possessed a lower physiological reserve to stress of surgery than conventional rats.
The 1.4- to 2.7-fold higher baseline counts of white blood cells, monocytes, lymphocytes, neutrophils and platelet counts, and lower hemoglobin levels, compared to the blood of conventional rats are suggestive of an activated immune system and/or low-grade systemic inflammation. However, when we reverted back to using animals from the conventional facility, a normal hematological profile was restored. Our study supports the work of Beura and colleagues who reported that their ‘standard’ SPF mice were infection-prone as a result of an immature immune system, which was reversed when wild mice were introduced into the colony6. They traced the anomaly to alterations in the animal’s gut microbiome.
Although we do not know the reasons for the abnormal hematology in SPF rats, independent external assessment revealed the exclusion of a number of infectious agents (Table 3), with the exception of Proteus spp. bacteria. Like other gram-negative bacteria, Proteus can release lipopolysaccharide endotoxins, and other reactive substances (e.g. thiobarbituric acid, superoxide radicals) that can translocate across the gut wall and trigger a host’s inflammatory response and possible autoinfection22. Thus, the presence of Proteus bacteria may have contributed to the SPF rats’ hematological profile and reduced stress response. However, we cannot exclude the possibility of effects of other infectious agents that were not investigated (see Table 3). Proteus may have increased in numbers in our SPF colony from the selected exclusion of pathogens in Table 3 that may normally keep Proteus spp. numbers in check22.
This study was an observational sequential study, with limited animal numbers for some of the outcome measures. Despite these limitations, our findings have a number of important implications for basic, goal-directed and translational research involving animals. Given the influence of the microbiome on the host’s physiology such as altering the immune system, the gut-brain axis, circadian rhythm and the cardiovascular system23,24, slight differences have the potential to significantly alter experimental outcomes. This consideration of the gut microbiome on data reproducibility (and replicability) in animal models of disease and injury have been largely ignored or grossly under-reported in the literature25. Pathogen status may be an unwanted variable and contribute to subtle differences in experimental outcomes within a single laboratory or between laboratories around the world10, and a factor in the failure to translate new therapies from animal studies to humans26. On the other hand, the gut microbiome could be manipulated and used as a tool to investigate specific diseases in animal models within defined boundaries1. In an attempt to address this variability in animal studies, we propose the following recommendation, which could accompany a Data Availability Statement requested by many journals. At the end of a scientific publication involving animals, the author(s) could be mandated to write the following1.
Animal pathogen status availability
A list of infectious agents in animals supporting the conclusions of this study are available by contacting the author(s) and/or institutional data hub (with an appropriate URL).
We conclude that SPF animals displayed an abnormal hemodynamic, hematological and bleeding phenotype in response to anesthesia and minor surgery. Returning to a conventional facility restored normal host physiology. Our results highlight the importance of understanding the effect of infectious agents present (and excluded) in SPF animals on experimental design and research outcomes. Further studies are warranted examining gut microbiome differences, and their implications, to basic and translational research.
Materials and Methods
Ethical considerations
This study conforms to the National Health and Medical Research Council Australian Code for the Care and Use of Animals for Scientific Purposes, 8th Edition, 2013, and the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (8th Edition, revised 2011), and complies with the Queensland Animal Care and Protection Act, 2001 (Act No.64 of 2001) and James Cook University guidelines. The study was approved by James Cook University Animal Ethics Committee (IACUC approval #A2296) and US Animal Care and Review Use Office (ACURO).
Conventional sprague dawley colony
Male Sprague-Dawley rats (300–400 g) bred in the conventional James Cook University Breeding Colony were housed in open top caging in a 14–10 hr light-dark cycle with free access to standard rat chow (Norco rural stock) and water ad libitum. Cages were polypropylene with high-top wire mesh cage tops (1640 cm2 floor space). Bedding was Andersons Bed-o’Cobs® ¼” and large Aspen chew blocks (40 × 40 × 200 mm), PVC tubing, and shredded paper were provided for environmental enrichment. The conventional facility has no barrier or containment restrictions, and the rats were used for a traumatic hemorrhage study under the same ethics approval number. These experiments were carried out before the transition to SPF animals.
SPF sprague dawley colony
Male Sprague-Dawley rats (300–400 g) bred in the specific-pathogen free (SPF) Australian Institute of Tropical Health and Medicine (AITHM) Small Animal Facility were maintained in individually ventilated double decker Tecniplast cages (1862 cm2 floor space) in a 14–10 hr light-dark cycle and fed irradiated rat cubes from Speciality Feeds (Glen Forrest, Western Australia). Nutritional content of feed for both colonies was equivalent and exceeded National Research Council (NRC) guidelines. All bedding and materials for environmental enrichment (shredded paper, tubes, chew blocks, tissues) and for nesting were identical to those used in the Conventional colony with the exception of being autoclaved before use. The AITHM Small Animal Facility has strict containment procedures.
Animal acclimation
Seven days prior to surgery, animals from both facilities were transferred to the Medical Research Laboratory and housed in individually ventilated double decker Tecniplast cages containing non-sterilised Andersons Bed-o’Cobs® ¼” corncob bedding with Aspen chew blocks, tunnels and shredded paper to allow for enrichment and natural behaviours.
Animal preparation and instrumentation
Animals were anesthetised in a 2 L induction chamber (9.5 × 22.3 × 9.5 cm) with isoflurane 5% (in 100% oxygen) during the induction phase and 2.5% during any surgical instrumentation, with animals breathing spontaneously. Animals were placed supine in a custom-designed cradle with 2.5% isoflurane and 100% oxygen supplied via nose cone. Aseptic techniques were used for surgery and all surgical equipment was autoclaved prior to use. Sterile chronic catheters (Access Technologies, USA) were inserted in the left femoral artery and vein of anesthetised animals for blood sampling and blood pressure monitoring20. Animals were euthanized using pentobarbitone sodium, 325 mg/ml (Lethabarb (100 mg/kg) injectable.
Blood pressure (n = 5 SPF animals, n = 5 conventional animals)
The femoral artery catheter was connected to a pressure transducer and Bridge Amplifier/Powerlab (AD Instruments) for blood pressure (BP) monitoring. In contrast to hemotology (n = 34, see below), only 5 SPF animals were used for blood pressure analysis and 20 for electrocardiogram (ECG) measurements (see below), because the study was terminated after the adverse events were reported to the Institutional Animal Welfare Officer and Animal Ethics Committee. Blood pressure and ECG data were compared to the hemodynamics of conventional animals measured just prior to the SPF animal experiments.
Electrocardiograph (ECG) (n = 20 SPF animals, n = 20 conventional animals)
ECG leads were implanted subcutaneously in a lead II configuration (right and left forepaws and right hind paw) to measure heart rate20. Electrocardiograms were analysed using the LabChart Pro ECG Analysis module (AD Instruments) with rat beat detection settings and Bazett correction for QT. Arrhythmias were identified using the Lambeth convention as previously described by Canyon and Dobson27. Premature ventricular contractions (PVCs) were defined as discrete and identifiable premature QRS complexes. An episode of bigeminy was recognized as a variant of PVCs and characterized by the minimum sequence: P, QRS, PVC, P, QRS, PVC28. Salvos were defined as two or three consecutive PVCs, and ventricular tachycardia (VT) was defined as a run of four or more consecutive ventricular premature beats28.
Hematology (n = 34 SPF animals, n = 100 conventional animals)
Whole blood (0.5 ml) was collected from the femoral artery catheter of SPF animals (n = 34) to measure complete blood counts using the VetScan HM5 analyser (REM Systems, Macquarie Park, New South Wales). The hematology results reported for conventional animals (n = 100) were from a study immediately prior to transitioning to SPF animals.
Coagulation parameters (n = 2 SPF animals; n = 2 conventional animals)
Rotational thromboelastometry (ROTEM®, Tem International, Munich, Germany) analysis was conducted on citrated whole blood samples according to manufacturer’s instructions to measure clotting time (CT; sec), maximum clot firmness (MCF; mm), actual clot firmness 60 min after clot initiation (ACF; mm), and maximum clot lysis (ML; %)29,30. Assays included EXTEM (extrinsically-activated test using tissue factor) and APTEM (activation as for EXTEM with aprotonin). Aprotinin at low levels (10–50 KIU/mL) inhibits plasmin activation of fibrinolysis and plasmin-induced platelet activation31. Hyperfibrinolysis is defined as ML ≥ 15%32,33 and confirmed with APTEM test.
Health screening, macropathology and histopathology (n = 2 SPF animals; n = 2 conventional animals)
Two male rats from the James Cook University conventional Sprague-Dawley colony, two male SPF rats from the AITHM Small Animal Facility, and two male SPF rats acclimated for seven days in a conventional facility were sent to Cerberus Sciences (Adelaide, South Australia) for investigation by an independent Specialist Veterinary Pathologist. Enteric and respiratory bacteriology and endo- and ecto-parisitology assessments were performed using direct examination, culture, and PCR methods.
Statistical analysis
SPSS Statistical Package 25 was used for statistical analysis (IBM). All values are expressed as mean ± SEM. Complete blood count, hemodynamic and ECG data were evaluated using one-way analysis of variance (ANOVA). Statistical significance was defined as P < 0.05. Post-hoc power analysis was performed using G*Power 3 program.
Acknowledgements
The authors would like to thank Dr. Craig Godfrey, Animal Welfare Officer, James Cook University, and Dr. L. Rasmussen, Specialist Veterinary Pathologist, who performed the health screening. The authors would particularly like to thank the US Department of Defense for continued support of our work, and also the College of Medicine and Dentistry, James Cook University. This work was supported by USSOCOM, IACUC protocol A2296, USAMRMC proposal SO150053 under Award No. W81XWH-USSOCOM-BAA-15-1. The opinions, interpretations, conclusions are those of the authors and are not necessarily endorsed by the US Department of Defense.
Author Contributions
H.L. and G.P.D. performed literature search, H.L. and J.M. performed data collection, all authors contributed equally to data interpretation and manuscript writing.
Data Availability
The datasets supporting the conclusions of this article can be made available by emailing the corresponding author.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dobson GP, Letson HL, Biros E, Morris JL. Specific pathogen-free (SPF) animal status as a variable in biomedical research: Have we come full circle? EBioMedicine (Lancet) 2019;41:42–43. doi: 10.1016/j.ebiom.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane-Petter W. Provision of pathogen-free animals. Proc R Soc Med. 1962;55:253–256. [PubMed] [Google Scholar]
- 3.Prager, E. M., Bergstrom, H. C., Grunberg, N. E. & Johnson, L. R. The importance of reporting housing and husbandry in rat research. Front Behav Neurosci. 5 (2011). [DOI] [PMC free article] [PubMed]
- 4.Masopust D, Sivula CP, Jameson SC. Of mice, dirty mice and men: using mice to understand human immunology. J Immunol. 2017;199:383–388. doi: 10.4049/jimmunol.1700453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao L, Reese TA. Making mouse models that reflect human immune responses. Trends Immunol. 2017;38:181–193. doi: 10.1016/j.it.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Beura LK, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen AK, Krych Ł, Nielsen DS, Hansen CH. A Review of Applied Aspects of Dealing with Gut Microbiota Impact on Rodent Models. ILAR J. 2015;56:250–264. doi: 10.1093/ilar/ilv010. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 9.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY) 2017;46:114–122. doi: 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nature Medicine. 2018;24:1795–1803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said NM, Abiola O. Haematological profile shows that Inbred Sprague Dawley rats have exceptional promise for use in biomedical and pharmacological studies. Asian J Biomed Pharm Sci. 2014;4:33–37. doi: 10.15272/ajbps.v4i37.597. [DOI] [Google Scholar]
- 13.Osuchowski MF, et al. Abandon the Mouse Research Ship? Not just Yet! Shock. 2014;41:463–475. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobson GP. The August Krogh Principle: Seeking Unity in Diversity. Shock. 2014;42:480. doi: 10.1097/SHK.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 15.Lamia B, Chemla D, Richard C, Teboul JL. Clinical review: interpretation of arterial pressure wave in shock states. Crit Care. 2005;9:601–606. doi: 10.1186/cc3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am J Cardiol. 2011;108:1760–1766. doi: 10.1016/j.amjcard.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahou A, et al. Relationship Between QT Interval and Outcome in Low‐Flow Low‐Gradient Aortic Stenosis With Low Left Ventricular Ejection Fraction. J Am Heart Assoc. 2016;5:e003980. doi: 10.1161/JAHA.116.003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Rienzo M, Parati G, Radaelli A, Castiglioni P. Baroreflex contribution to blood pressure and heart rate oscillations: time scales, time-variant characteristics and nonlinearities. Phil. Trans. R. Soc. A. 2009;367:1301–1318. doi: 10.1098/rsta.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobson GP, Letson HL, Sharma R, Sheppard F, Cap AP. Mechanisms of Early Traumatic-Induced Coagulopathy (TIC): The Clot Thickens or Not? J Trauma Acute Care Surg. 2015;79:301–309. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 20.Letson HL, Dobson GP. 3.0% NaCl Adenosine, Lidocaine, Mg2 + (ALM) bolus and 4 hours ‘drip’ infusion reduces non-compressible hemorrhage by 60% in a rat model. J Trauma Acute Care Surg. 2017;82:1063–1072. doi: 10.1097/TA.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 21.Letson HL, Dobson GP. Adenosine, Lidocaine and Mg2 + (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage. PLos One. 2017;12:e0188144. doi: 10.1371/journal.pone.0188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drzewiecka D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb Ecol. 2016;72:741–758. doi: 10.1007/s00248-015-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, FitzGerald GA. Timing of Microbes: the circadian rhythm of the gut microbiome. J Biol Rhythm. 2017;32:505–515. doi: 10.1177/0748730417729066. [DOI] [PubMed] [Google Scholar]
- 25.McCoy KD, Geuking MB, Ronchi F. Gut Microbiome Standardization in Control and Experimental Mice. Curr Protoc Immunol. 2017;117:23.21.21–23.21.13. doi: 10.1002/cpim.25. [DOI] [PubMed] [Google Scholar]
- 26.Vatner SF. Why so few new cardiovascular drugs translate to the clinics. Circ Res. 2016;119:714–717. doi: 10.1161/CIRCRESAHA.116.309512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canyon SJ, Dobson GP. Protection against ventricular arrhythmias and cardiac death using adenosine and lidocaine during regional ischemia in the in vivo rat. Amer. J. Physiol (Heart Circ Physiol) 2004;287:H1286–H1295. doi: 10.1152/ajpheart.00273.2004. [DOI] [PubMed] [Google Scholar]
- 28.Walker MJA, et al. The Lambeth Convention: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovascular Research. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 29.Letson HL, Dobson GP. Correction of Acute Traumatic Coagulopathy with small-volume 7.5% NaCl Adenosine, Lidocaine and Mg2+ (ALM) occurs within 5 min: A ROTEM Analysis. J Trauma Acute Care Surg. 2015;78:773–783. doi: 10.1097/TA.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 30.Davenport L, Letson HL, Dobson GP. Immune-inflammatory activation after a single laparotomy in a rat model: effect of adenosine, lidocaine and Mg2+ infusion to dampen the stress response. Innate Immun. 2017;23:482–494. doi: 10.1177/1753425917718921. [DOI] [PubMed] [Google Scholar]
- 31.Shigeta O, et al. Aprotinin inhibits plasmin-induced platelet activation during cardiopulmonary bypass. Circulation. 1997;96:569–574. doi: 10.1161/01.CIR.96.2.569. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational Thromboelastometry (ROTEM)-Based Coagulation Management in Cardiac Surgery and Major Trauma. J Cardiothorac Vasc Anesth. 2012;26:1083–1093. doi: 10.1053/j.jvca.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Letson HL, Dobson GP. Differential contributions of platelets and fibrinogen to early coagulopathy in a rat model of hemorrhagic shock. Thromb Res. 2016;141:58–61. doi: 10.1016/j.thromres.2016.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article can be made available by emailing the corresponding author.