Abstract
Background
The management of adrenal incidentaloma is still a challenge with respect to determining its functionality (hormone secretion) and malignancy. In this light, we performed 18F-FDG PET/CT scan to assess the SUVmax values in different adrenal masses including Cushing syndrome, pheochromocytoma, primary hyperaldosteronism and non-functional adrenal adenomas.
Methods
Total 109 (73 F, 36 M) patients with adrenal mass (incidentaloma), mean age of 53.3 ± 10.2 years (range, 24–70) were screened by 18F-FDG PET/CT. Data of 18F-FDG PET/CT imaging of the patients were assessed by the same specialist. Adrenal masses were identified according to the calculated standardized uptake values (SUVs). Clinical examination, 24-h urine cortisol, catecholamine metabolites, 1-mg dexamethasone suppression test, aldosterone/renin ratio and serum electrolytes were analyzed.
Results
Based on the clinical and hormonal evaluations, there were 100 patients with non-functional adrenal mass, four with cortisol-secreting, four with pheochromocytomas and one with aldosterone-secreting adenoma. Mean adrenal mass diameter of 109 patients was 2.1 ± 4.3 (range, 1–6.5 cm). The 18F-FDG PET/CT imaging of the patients revealed that lower SUVmax values were found in non-functional adrenal masses (SUVmax 3.2) when compared to the functional adrenal masses including four with cortisol-secreting adenoma (SUVmax 10.1); four with pheochromcytoma (SUVmax 8.7) and one with aldosterone-secreting adenomas (SUVmax 3.30). Cortisol-secreting (Cushing syndrome) adrenal masses showed the highest SUVmax value (10.1), and a cut-off SUVmax of 4.135 was found with an 84.6% sensitivity and 75.6% specificity cortisol-secreting adrenal adenoma.
Conclusions
Consistent with the similar studies, non-functional adrenal adenomas typically do not show increased FDG uptake and a certain form of functional adenoma could present various FDG uptake in FDG PET/CT. Especially functional adrenal adenomas (cortisol secreting was the highest) showed increased FDG uptake in comparison to the non-functional adrenal masses. Therefore, setting a specific SUVmax value in the differentiation of malignant adrenal lesion from the benign one is risky and further studies, including a high number of functional adrenal mass are needed.
Keywords: 18F-FDG PET-CT, Cushing syndrome(s), pheochromocytoma(s), non-functional adrenal adenomas
Introduction
Since the early 1980s, the prevalence of adrenal masses have been increasing due to the frequent use of radiological imaging such as magnetic resonance imaging (MRI), computerized tomography (CT) in clinical practice. The prevalence of adrenal incidentalomas detected by abdominal CT scan varies between 2.5 and 4% in adult populations. The management of adrenal incidentalomas depends on the lesion’s being benign or malignant and any adrenal hormone secretion related with the Cushing syndrome, primary aldosteronism and pheochromocytoma. Based on the obtained findings, functional adrenal masses and malignant potential tumors are operated on, whereas those with non-functional and benign characteristic of radiological evaluation are only followed up to 5 years (1, 2). According to the current endocrine and surgical guideline (3), every patient elaborately adhered to the endocrine and imaging procedures. Despite performing a cascade of the tests and procedures, identification of some lesions still remains uncertain (4, 5). Especially, bilateral adrenal masses are still a clinical dilemma for clinicians. In the initial diagnosis, CT or MRI are common preferable imaging techniques for adrenal masses, but CT and/or MRI do not separate the functional adenomas from the non-functional adenomas.
18F-FDG positron emission CT was originally developed to evaluate benign and malignant lesions. However, there were limited studies on the usage in determining the functional status of adrenal incidentalomas. In certain studies, it was reported that 18F-FDG PET CT could help in the differentiation of adrenal masses with and without hormone secretion (6, 7).
In this study, we aimed at determining whether 18F-FDG PET/CT scanning of adrenal mass could identify the functional status of the adrenal masses. We also made a comparison between the pathological diagnosis and 18F-FDG PET/CT imaging of functional adrenal masses which mimicked malignant lesion.
Patients and methods
Study populations
During a 24-month period, 109 patients (73 females, 36 males) with adrenal mass who were referred to the university hospital for further investigation by 18F-FDG PET/CT imaging were included. All adrenal masses were incidentally detected on CT or MRI for other reasons. Past medical history, comorbid disorders, drug administration and family history for any genetic disease were questioned.
In the physical examination, body mass index (BMI), blood pressure measurements and routine systematic examination were performed. Especially, attention on stigmas of Cushing syndrome and high blood pressure for primary aldosteronism and pheochromocytoma were taken into consideration.
All patients were screened for hyperaldosteronism, hypercortisolism and pheochromocytoma. Plasma renin/aldosterone ratios, plasma normetanephrine, metanephrines and urinary free cortisol (UFC) were also studied. Autonomous cortisol secretion was described as serum cortisol >1.8 µg/dL following 1 mg of overnight dexamethasone suppression tests or 2 days of low-dose dexamethasone suppression test plus the following additional measurements; 24-h urinary free cortisol excretion (UFC), suppressed serum adrenocorticotrophic hormone (ACTH) levels (<10 IU/mL) and elevated midnight serum or salivary cortisol values (3).
All patients were evaluated by the same multidisciplinary expert team as similar to the international studies and the following criteria were considered (3):
Those that had radiological imaging of adrenal lesions were reported as suspicious for malignancy.
Those who had evidences of hormone hypersecretion (Cushing syndrome, primary aldosteronism, pheochromocytoma),
Those who had hormone hypersecretion with comorbid disorders including diabetes mellitus, hypertension, osteoporosis, obesity, and
Those with adrenal mass diameter above 6 cm or with an increase in the diameter on the 6-month follow-up imaging were taken to adrenal surgery.
Post-operative histopathological diagnosis of the specimens was studied by the same experienced pathologist. Weiss criteria (8) were used for adrenocortical adenomas and PASS criteria (Pheochromocytoma of the Adrenal Gland Scaled Score) (9) for pheochromocytoma.
Inclusion criteria of the subjects were as follows: patients with non-functional or functional adrenal masses (i.e. CS, primary aldosteronism, pheochromocytoma). Exclusion criteria were history of malignancy, any chronic drug use such as cortisol and its analogs.
DHEAS, ACTH and cortisol values were analyzed by using the PCR method, the enzymatic-labeled chemiluminescent immunometric assay method, and chemiluminescence (Beckman DXI 800 auto analyzer; Beckman Coulter Diagnostics), respectively. PRA and PA levels were measured using the radioimmunoassay method. The high performance liquid chromatography (HPLC) method was used to analyze urine cortisol and metanephrine values.
PET-CT imaging: patients were analyzed by a consensus of experienced nuclear medicine physicians who were blind for the patient data, except for the adrenal side (left or right) of the mass. Adrenal mass was objectively analyzed by the measurement of the calculated standardized uptake value (SUV). The activity of the adrenal mass was obtained by drawing a region of interest (ROI) that encompasses the central two-thirds of the mass if relatively homogeneous or the most uniform area if the mass was heterogeneous. In this study, SUVmax cut-off value for adrenal malignant lesions was considered as 4.04 as previously reported by Blake et al. (10).
The study was approved by Cukurova University Ethical Committee in 2017. Written informed consent was obtained from all patients.
Statistical analysis
Data distribution was tested by the Shapiro–Wilk test. Mean and standard deviations were presented for normal distributed variables; for non-normal distributed variables, median and quartile statistics were given. Pearson correlation coefficient was calculated to examine the linear relationship between two continuous variables. Student t and one-way ANOVA tests were performed to compare the mean differences between the subgroups. To assess the performance of the diagnostic tests, ROC curves were performed, sensitivity, specificity, positive predictive and negative predictive values were calculated.
Results
Mean age of the patients was 53.3 ± 10.0 years (range 24–70 years). Mean adrenal mass diameter of the subjects was 2.1 ± 4.3 cm (range 1.0–8.0). Mean adrenal mass diameters of nine functional adenomas were as follows: hypercortisolemia (Cushing syndrome) 2.5 ± 1.6 cm (range, 1.7–4.1 cm); pheochromocytoma 4.1 ± 2.3 cm (range, 2–6.4) and aldosterone-secreting adenoma 1 cm. There were four patients with non-functional and adrenal mass diameter of ≥6 cm (range, 6–8). Of four patients presented with cortisol hypersecretion, two were operated due to the evidences of Cushing syndrome-related comorbid disorders such as type 2 diabetes and obesity. Of 109 patients, 65 (59.6%) had left-sided adrenal mass and the remaining 44 (40.4%) had right-sided adrenal mass.
Post-operative diagnosis
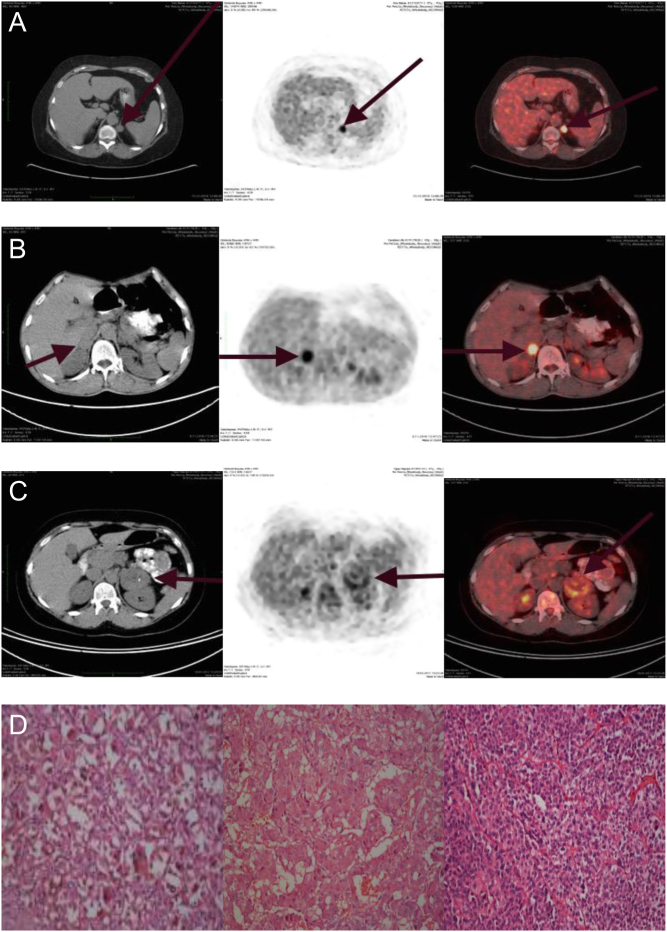
In the post-operative pathological diagnosis of the 13 patients who underwent surgery, 11 subjects were diagnosed as having adrenocortical adenoma (Weiss criteria <4) and two as adrenocortical carcinoma. One of the two patients with adrenocortical carcinoma was diagnosed with pheochromocytoma (Pass criteria >4, SUVmax 8.2). The other patient had a non-secretory adrenocortical carcinoma (Weiss criteria >4, SUVmax 6.9) (Fig. 1D). When we grouped the pathological diagnosis of the patients according to the functional status of adrenal mass, diagnosis of the patients with Cushing syndrome was adrenal adenoma (Weiss criteria <4), three patients with pheochromocytoma also had adenoma (Weiss criteria <4) and the remaining one patient had carcinoma (Weiss criteria >4). The pathological diagnosis of the patients is shown in Table 1.
Figure 1.
(A) Coronal and axial fused 18F-FDG PET/CT images of the patient 1 adrenal glands, which was diagnosed Cushing syndrome. PET/CT showing a 1.4 × 1.7 cm left-cortisol-secreting adenoma with SUVmax 11.38. (B) Coronal and axial fused 18F-FDG PET/CT images of the patient 2 adrenal glands which were diagnosed pheochromocytoma. PET/CT showing a 3.0 × 2.1 cm right-pheochromocytoma with SUVmax 7.86. (C) Coronal and axial fused 18F-FDG PET/CT images of the patient 3 adrenal glands which were diagnosed non-functional adrenal adenoma. PET/CT showing a 52 × 50 cm- non-functional adenoma with SUVmax 4.56. (D) Adenoma cells have abundant intracytoplasmic granular lipofuscin pigment with eosinophilic cytoplasm (patient1, left side, H-E × 400). The cells of pheochromocytoma have a finely granular, basophilic cytoplasm with round-to-oval nuclei (patient 2, middle part, H-E × 200). The cells of the neoplasm have diffuse pattern with lack of clear cytoplasm (patient 3, right side, H-E × 200).
Table 1.
Demonstration of the patients’ hormonal, radiographic and pathological features.
| N | Mass size (cm) | SUVmax (mean ± s.d.) | Pathological diagnosis | P | |
|---|---|---|---|---|---|
| Non-functional-nonoperated adrenal masses | 96 | 2.2 ± 3.1 (range 1–8) | 3.23 ± 1.65 | – | 0.066 |
| Cushing syndrome | 4 | 2.5 ± 1.6 (range 1.7–4.1) | 10.17 ± 3.27 | Adenomaa | 0.001 |
| Pheochromocytoma | 4 | 4.1 ± 2.3 (range 2–6.4) | 8.71 ± 2.72 | Adenomab Carcinomac |
0.004 |
| Primary hyperaldosteronism | 1 | 1 | 3.30 | Adenoma | – |
| Operated with increased size | 4 | 6.1 ± 2.7 (range 6–8) | 5.76 ± 1.26 | Adenomaa Carcinomad |
0.32 |
We used the patients with non-functional nonoperated adrenal mass as control to compare the patients with secreting adrenal masses in the Student t-test. Bold indicates statistical significance.
aWeiss criteria <4 criteria; bPass Criteria <4 criteria; cPass Criteria >4 criteria; dWeiss criteria >4 criteria.
18F-FDG PET/CT imaging
The results of 18F-FDG PET/CT imaging of the patients were that four patients with hypercortisolemia (CS and autonomous cortisol secretion) showed higher SUVmax values of 10.1 ± 3.2 (range, 4.17–13.66) compared to that of the 100 patients with non-functioning adenomas (SUVmax of 3.2 ± 1.6) (range, 1.5–4.9) (P < 0.001) (Fig. 1A, B and C).
A cut-off SUVmax of 4.135 was found as an identifying value for a cortisol-secreting adrenal mass with a sensitivity of 84.6% and specificity of 75.6% (Fig. 2).
Figure 2.
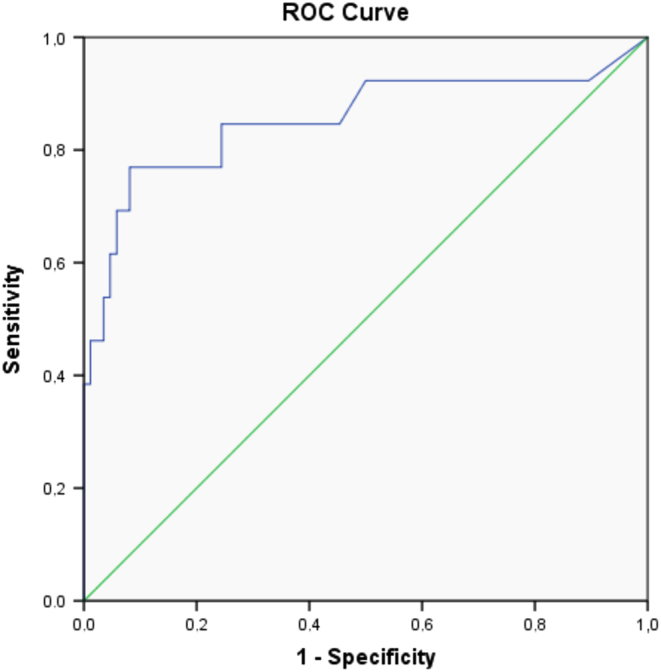
Roc analyses for different cut-off values of SUVmax in patients with adrenal masses to differentiate non-secreting, non-malignant adrenal masses from cortisol-secreting, non-malignant adrenal masses. Sensitivity of 84.6% and specificity of 75.6% for cortisol-secreting masses.
Four patients with pheochromocytoma showed moderate-to-high SUVmax values (8.7 ± 2.7) compared to the patients with non-functioning adenomas (P < 0.005) (Fig. 1B, C and Table 1). Of 109 patients, 13 (11.9%) were operated due to functional adrenal mass (n = 9) and malignancy risk (n = 4) according to the CT findings revealing a tumor size of ≥6 cm. The patients who were operated due to functionality and/or mass size of ≥6 cm (n = 13, 11.9%) revealed higher SUVmax value than the patients without an operation (9.06 ± 7.01 vs 3.23 ± 1.65, P < 0.001). There was no statistically significant correlation between mass size and SUVmax value (P = 0.280).
To determine whether background metabolic activity could be some false-positives among adrenocortical adenomas, we calculated specific criteria called as adrenal to liver SUVmax ratio (uptake of 18F-FDG adrenal/uptake of 18F-FDG liver ratio).
For all patients with adrenal masses, adrenal-to-liver SUVmax ratio was calculated as 0.83 ± 0.72. For patients with carcinoma, adrenal-to-liver SUVmax ratio was calculated as 2.74 ± 1.2. For patients with hypercortisolemia, adrenal-to-liver SUVmax ratio was 4.13 ± 5.83; for patients with pheochromocytoma, it was 1.76 ± 0.86 and for patients with non-functional it was 0.79 ± 0.38.
Discussion
In the management of adrenal masses, CT with/without contrast-enhanced and chemical shift magnetic resonance imaging (MRI) has been used as the initial imaging protocols (11, 12, 13, 14). Attenuation characteristics by Hounsfield Unit (HU) of adrenal masses are generally accepted to differentiate adenomas from non-adenomas masses. Nevertheless, CT and MRI scanning cannot distinguish between adenomas with and without hormone secretion (15, 16, 17). Hence, in addition to the imaging findings, endocrinologic assessment of the adrenal masses is necessary as well as the issue of malignancy.
18F-FDG PET/CT is a nuclear medicine method and based on glucose and deoxyglucose uptake by cancer cells. In this technique, standard uptake value (SUV) has been commonly used as a measurement index (18, 19). Until now, nuclear scintigraphy (iodocholesteoral or 123I/131I-labeled metaiodobenzylguanidine) have been used to determine the functional hormone secretions of adrenal masses (20). However, in some recent studies using 18F-FDG uptake in the adrenal masses, increased uptake was reported in functional adrenal masses (21, 22, 23, 24, 25). Many factors may affect SUV measurements such as weight or blood glucose level, duration of uptake period or the type of ROI. Due to these variables, some authors prefer to use adrenal to liver SUVmax instead of only adrenal SUVmax value. And studies reported that most of the adrenal adenomas had FDG uptake (mean ratio 0.66) less than the liver (26, 27, 28, 29). In 2009, Groussin et al. (30) studied this method in subjects without a previous cancer history to predict the malignancy of adrenal masses. In this study, a cut-off value above 1.45 for adrenal to liver SUVmax ratio was suggested in favor of adrenal adenoma. Nearly almost all of these studies were on whether the adrenal mass was malign or non-malign. In this study, adrenal-to-liver ratio of patients with carcinoma was calculated as 2.74 ± 1.2. While adrenal-to-liver SUVmax value of patients (n = 96) with non-functional adrenal mass was found as 0.79 ± 0.38; adrenal-to-liver SUVmax value of patients with hypercortisolemia was detected as 4.13 ± 5.83. Adrenal-to-liver SUVmax ratio in patients with hypercortisolemia was a false positive compared to patients with carcinoma, but there was not any correlation between malignant masses and secreting but non-malignant tumors because of sample size of the patients.
With respect to the functional adrenal mass, Patel et al. (31) reported that cortisol-secreting adenomas showed higher SUVmax values (5.9), not only compared to non-functional adrenal adenomas (SUVmax 4.2) but also to aldosterone-secreting adenomas (SUVmax 3.3) (P < 0.007). Some of the similar studies also supported these findings (22, 23, 24, 25, 32). Consistent with these studies, we found higher SUVmax (10.1 ± 3.2) in patients with cortisol-secreting adrenal adenoma compared to patients with non-functional adrenal adenomas (SUVmax 3.2 ± 1.6) (P < 0.001). In 2011, Vassiliadi and colleagues (33) reported that patients with adrenal masses who had an odds ratio of 5.50 predicted the autonomous cortisol secretion. In another study, it was also reported an increased 18F-FDG uptake in patients with cortisol-hypersecreting benign adrenal adenoma (34). Hypercortisolemia was suggested as an underlying mechanisms for high SUVmax values in cortisol-secreting adrenal adenomas due to an increased glucose metabolism (21, 31). Similarly, in our study in patients with hypercortisolemia (Cushing syndrome and subclinical cortisol secretion), an increased 18F-FDG uptake was found compared to other patients. Unfortunately, we had a limited case of cortisol-secreting adrenal adenoma (n = 4) with respect to the non-functional adrenal adenomas (n = 100).
Pheochromocytomas, which is another functional adrenal tumor, could accumulate FDG with a relatively high sensitivity (0.90) (35, 36). Although it has been known that malignant pheochromocytoma FDG uptake is greater than that of the benign pheochromocytomas; the intensity of FDG uptake is variable. Among patients with pheochromocytoma, SUVmax was reported to help in the identification of malignant pheochromocytoma with a cut-off mean SUVmax 7.1 (range 5.1–14.6) from the benign pheochromocytoma (37, 38, 39). In our study, mean SUVmax of three patients with benign pheochromocytomas was found to be 8.7 ± 2.7, and there was only one patient with malignant pheochromocytoma (SUVmax 8.2). We also revealed two patients with non-functional malign adrenal mass who had adrenal operation due to large adrenal mass (adenoma diameter >6 cm) and both patients as expected showed high SUVmax values before the operation (5.76 ± 1.26).
Shortcomings of our study were the relatively low number of subjects (n = 9) with functional adrenal adenoma which was not enough to make a comparison in the study and with other studies in terms of functional adrenal mass SUVmax characteristics. In addition, we need more subjects with functional adrenal adenoma who were screened by FDG PET/CT and post-operative period confirmed histopathological diagnosis. Nevertheless, screening by FDG PET/CT in a relatively high number of patients (n = 96) with non-functional adrenal mass would add data on the management of adrenal masses.
In conclusion, as consistent with the similar studies, non-functional adrenal adenomas typically do not show increased FDG uptake and a certain form of functional adenoma could present various FDG uptake in FDG PET/CT. Especially, cortisol-secreting adrenal adenomas were found to reveal markedly increased FDG uptake than the others with and without functional. We consider setting a specific SUVmax value in differentiation of benign adenomas from malignant ones could be deceptive. Nevertheless, 18F-FDG PET CT screening may be helpful in searching for functional characteristics of adenomas especially in patients with cortisol-secreting adrenal adenomas.
Limitations
Because our study was a pilot study, the sample size was relatively small. Lack of a healthy-control subject group and patients with functional adrenal mass to compare with patients with non-functional adrenal incidentaloma may be thought as the deficiency of the study. Besides, our study was mainly focused on the characteristics and SUVmax value of the non-functional adrenal masses. There is a need for further more extensive studies to report conclusive results.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Eldeiry LS, Alfisher MM, Callahan CF, Hanna NN, Garber JR. The impact of an adrenal incidentaloma algorithm on the evaluation of adrenal nodules. Journal of Clinical and Translational Endocrinology 2018. 5 . ( 10.1016/j.jcte.2018.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker J, Woloszyn J, Bold R, Campbell MJ. The adrenal incidentaloma: an opportunity to improve patient care. Journal of General Internal Medicine 2018. 33 . ( 10.1007/s11606-017-4240-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2016. 175 G1–G34. ( 10.1530/EJE-16-0467) [DOI] [PubMed] [Google Scholar]
- 4.Kaltsas G, Chrisoulidou A, Piaditis G, Kassi E, Chrousos G. Current status and controversies in adrenal incidentalomas. Trends in Endocrinology and Metabolism 2012. 23 . ( 10.1016/j.tem.2012.09.001) [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro Cavalari EM, de Paula MP, Arruda M, Carraro N, Martins A, de Souza K, Coelho MC, de Oliveira e Silva de Morais NA, Moraes AB, Vieira Neto L. Nonfunctioning adrenal incidentaloma: a novel predictive factor for metabolic syndrome. Clinical Endocrinology 2018. 89 . ( 10.1111/cen.13822) [DOI] [PubMed] [Google Scholar]
- 6.Montiel A, Gonzales S, Perez MI, Sanchez Gonzalez J, Montesinos P. Using the modified Dixon technique to evaluate incidental adrenal lesions on 3T MRI. Radiologia 2018. 60 . ( 10.1016/j.rx.2018.06.001) [DOI] [PubMed] [Google Scholar]
- 7.Dong A, Cui Y, Wang Y, Zuo C, Bai Y. (18)F-FDG PET/CT of adrenal lesions. American Journal of Roentgenology 2014. 203 . [DOI] [PubMed] [Google Scholar]
- 8.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. American Journal of Surgical Pathology 1984. 8 . ( 10.1097/00000478-198403000-00001) [DOI] [PubMed] [Google Scholar]
- 9.Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. American Journal of Surgical Pathology 2002. 26 . ( 10.1097/00000478-200205000-00002) [DOI] [PubMed] [Google Scholar]
- 10.Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, Boland GW. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy – initial experience. Radiology 2006. 238 . ( 10.1148/radiol.2383042164) [DOI] [PubMed] [Google Scholar]
- 11.Blake MA, Kalra MK, Sweeney AT, Lucey BC, Maher MM, Sahani DV, Halpern EF, Mueller PR, Hahn PF, Boland GW. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology 2006. 238 . ( 10.1148/radiol.2382041514) [DOI] [PubMed] [Google Scholar]
- 12.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Makita O, Kadota M, Takahashi M. Adrenal masses: quantification of fat content with double-echo chemical shift in-phase and opposed-phase Flash MR images for differentiation of adrenal adenomas. Radiology 2001. 218 . ( 10.1148/radiology.218.3.r01mr15642) [DOI] [PubMed] [Google Scholar]
- 13.Korobkin M, Giordano TJ, Brodeur FJ, Francis IR, Siegelman ES, Quint LE, Dunnick NR, Heiken JP, Wang HH. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology 1996. 200 . ( 10.1148/radiology.200.3.8756925) [DOI] [PubMed] [Google Scholar]
- 14.Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, Berland LL, Pandharipande PV. Management of incidental adrenal masses: a white paper of the ACR incidental findings committee. Journal of the American College of Radiology 2017. 14 . ( 10.1016/j.jacr.2017.05.001) [DOI] [PubMed] [Google Scholar]
- 15.Patel J, Davenport MS, Cohan RH, Caoili EM. Can established CT attenuation and washout criteria for adrenal adenoma accurately exclude pheochromocytoma? American Journal of Roentgenology 2013. 201 . ( 10.2214/AJR.12.9620) [DOI] [PubMed] [Google Scholar]
- 16.Platzek I, Sieron D, Plodeck V, Borkowetz A, Laniado M, Hoffmann RT. Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. European Radiology 2019. 29 . ( 10.1007/s00330-018-5626-5) [DOI] [PubMed] [Google Scholar]
- 17.Elsayes KM, Emad Eldin S, Morani AC, Jensen CT. Practical approach to adrenal imaging. Urologic Clinics of North America 2018. 45 . ( 10.1016/j.ucl.2018.03.005) [DOI] [PubMed] [Google Scholar]
- 18.Pavon-Paz I, Rosado-Sierra JA, Balsa-Breton MA, Guijarro-Armas G, Merino-Viveros M. Use of 18F-FDG-positron emission tomography in presurgical evaluation of nonspecific or suspectious adrenal masses in non-oncologic patients. Urologic Clinics of North America 2018. 45 . [DOI] [PubMed] [Google Scholar]
- 19.Wong KK, Arabi M, Bou-Assaly W, Marzola MC, Rubello D, Gross MD. Evaluation of incidentally discovered adrenal masses with PET and PET-CT. European Journal of Radiology 2012. 81 . ( 10.1016/j.ejrad.2010.12.060) [DOI] [PubMed] [Google Scholar]
- 20.Yoh T, Hosono M, Komeya Y, Im SW, Ashikaga R, Shimono T, Tsuchiya N, Okada M, Hanada K, Yagyu Y, et al Quantitative evaluation of norcholesterol scintigraphy, CT attenuation value, and chemical-shift MR imaging for characterizing adrenal adenomas. Annals of Nuclear Medicine 2008. 22 . ( 10.1007/s12149-008-0143-2) [DOI] [PubMed] [Google Scholar]
- 21.Papadakis GZ, Millo C, Stratakis CA. Benign hormone-secreting adenoma within a larger adrenocortical mass showing intensely increased activity on 18F-FDG PET/CT. Endocrine 2016. 54 . ( 10.1007/s12020-016-0969-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu A, Oriuchi N, Tsushima Y, Higuchi T, Aoki J. High [18F] 2-fluoro-2-deoxy-D-glucose (FDG) uptake of adrenocortical adenoma showing subclinical Cushing’s syndrome. Annals of Nuclear Medicine 2003. 17 . [DOI] [PubMed] [Google Scholar]
- 23.Ansquer C, Scigliano S, Mirallie E, Taïeb D, Brunaud L, Sebag F, Leux C, Drui D, Dupas B, Renaudin K, et al 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. European Journal of Nuclear Medicine and Molecular Imaging 2010. 37 . ( 10.1007/s00259-010-1471-8) [DOI] [PubMed] [Google Scholar]
- 24.Çiftçi E, Turgut B, Cakmakcılar A, Ertürk SA. Diagnostic importance of 18F-FDG PET/CT parameters and total lesion glycolysis in differentiating between benign and malignant adrenal lesions. Nuclear Medicine Communications 2017. 38 . ( 10.1097/MNM.0000000000000712) [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Lee SW, Pak K, Kim IJ, Kim K. Diagnostic accuracy of 18F-FDG PET or PET/CT for the characterization of adrenal masses: a systematic review and meta-analysis. British Journal of Radiology 2018. 91 20170520 ( 10.1259/bjr.20170520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. Journal of Nuclear Medicine 2006. 47 . [PubMed] [Google Scholar]
- 27.Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, Boland GW. Adrenal lesions:characterizations with fused PET/CT image in patients with proved or suspected malignancy-initial experience. Radiology 2006. 238 . ( 10.1148/radiol.2383042164) [DOI] [PubMed] [Google Scholar]
- 28.Guerin C, Pattou F, Brunaud L, Lifante JC, Mirallie E, Haissaguerre M, Huglo D, Olivier P, Houzard C, Ansquar C, et al Performance of 18F-FDG PET/CT in the characterization of adrenal masses in noncancer patients: a prospective study. Journal of Clinical Endocrinology and Metabolism 2017. 102 . ( 10.1210/jc.2017-00254) [DOI] [PubMed] [Google Scholar]
- 29.Delivanis DA, Bancos I, Atwell TD, Schmidt GD, Eiken PW, Natt N, Erickson D, Maraka S, Young WF, Nathan MA. Diagnostic performance of unenhanced computed tomography and 18F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clinical Endocrinology 2018. 88 . ( 10.1111/cen.13448) [DOI] [PubMed] [Google Scholar]
- 30.Groussin L, Bonardel G, Silvera S, Tissier F, Coste J, Abiven G, Libé R, Bienvenu M, Alberini JL, Salenave S, et al 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. Journal of Clinical Endocrinology and Metabolism 2009. 94 . ( 10.1210/jc.2008-2302) [DOI] [PubMed] [Google Scholar]
- 31.Patel D, Gara SK, Ellis RJ, Boufraqech M, Nilubol N, Millo C, Stratakis CA, Kebebew E. FDG PET/CT scan and functional adrenal tumors: a pilot study for lateralization. World Journal of Surgery 2016. 40 . ( 10.1007/s00268-015-3242-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledo R, Jimenez C. Recent advances in the management of malignant pheochromocytoma and paraganglioma: focus on tyrosine kinase and hypoxia-inducible factor inhibitors. F1000 Research 2018. 30 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassiliadi DA, Ntali G, Vicha E, Tsagarakis S. High prevalence of subclinical hypercortisolism in patients with bilateral adrenal incidentalomas: a challenge to management. Clinical Endocrinology 2011. 74 . ( 10.1111/j.1365-2265.2010.03963.x) [DOI] [PubMed] [Google Scholar]
- 34.Shimzu Y, Matsui S, Harada J. Evaluation of micro-organic pollution in lake sediments: application to Akanoi Bay Lake Biwa, Japan. Environmental Technology 2003. 24 . ( 10.1080/09593330309385625) [DOI] [PubMed] [Google Scholar]
- 35.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nature Reviews: Cancer 2014. 14 . ( 10.1038/nrc3648) [DOI] [PubMed] [Google Scholar]
- 36.Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: imaging with 2-[fluorine-18] fluoro-2-deoxy-D-glucose PET. Radiology 1999. 212 . ( 10.1148/radiology.212.1.r99jl3035) [DOI] [PubMed] [Google Scholar]
- 37.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, et al Stating and Functional characterization of pheochromocytoma and paraganglioma by 18F-flourodeoxyglucose (18F-FDG) positron emission tomography. Journal of the National Cancer Institute 2012. 104 . ( 10.1093/jnci/djs188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari A, Shah N, Sarathi V, Malhotra G, Bakshi G, Prakash G, Khadilkar K, Pandit R, Lila A, Bandgar T. Genetic status determines 18 F-FDG uptake in pheochromocytoma/paraganglioma. Journal of Medical Imaging and Radiation Oncology 2017. 61 . ( 10.1111/1754-9485.12620) [DOI] [PubMed] [Google Scholar]
- 39.Kundu S, Kand P, Basu S. Comparative evaluation of iodine-131 metaiodobenzylguanidine and 18-fluorodeoxyglucose positron emission tomography in assessing neural crest tumors: will they play a complementary role? South Asian Journal of Cancer 2017. 6 . ( 10.4103/2278-330X.202556) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a