Abstract
d-Glucose-3-14C has been prepared by means of three, successive Kiliani syntheses. The process consists of (a) the addition of 14C-labeled-cyanide to d-glyceraldehyde; (b) hydrolysis of the cyanohydrins and conversion of the resulting epimeric d-tetronic-1-14C acids into d-tetroses-1-14C by way of an improved Rosenmund reduction of the acetylated tetronyl chlorides: (c) addition of nonlabeled cyanide to the mixture of the aldehydo acetates of d-threose-1-14C and d-erythrose-1-14C: (d) hydrolysis of the cyanohydrins, and lactonization of the resulting mixture of four d-pentonic-1-14C acids; (e) chromato-graphic separation of these into three fractions; (f) reduction of the fraction containing d-arabinono-2-14C- and d-xylono-2-14C-lactone to the corresponding sugars, and chromatographic separation of these; and (g) conversion of d-arabinose-2-14C into d-glucose-3-14C and d-mannose-3-14C by a third Kiliani synthesis. Approximately 3 percent of the radioactivity was obtained in the form of d-glucose-3-14C The process results in the production of a large number of intermediate labeled compounds.
Keywords: Aldonic acids, carbon- 14-labeled carbohydrates, chromatography, paper, glucose-3-carbon-14: hexoses-3-carbon-14, pentoses-2-carbon-14, radioactive carbohydrates, Rosenmund reductions, synthesis of radioactive sugars, tetroses-l-carbon-14
1. Introduction
Prior publications from this laboratory have reported methods for synthesizing pentoses labeled with carbon 14 at C—1 and C—5 [1, 2, 3],3 and hexoses labeled at C—1, C—2, and C-6 [4, 5, 6, 7], but, heretofore, no methods have been reported for synthesizing tetroses-1-14C, pentoses-2-14C, and hexoses-3-14C In response to the urgent need for some of these sugars in biological research, the preparation of d-glucose-3-14C and certain related compounds was undertaken.
The procedure consisted of a series of three cyano-hydrin syntheses, beginning with 2,3-O-isopropyli-dene-d-glycerose and sodium cyanide-14C. In order to avoid the excessive dilution of radioactivity resulting from the addition of carriers in the separation of the epimers in each step, the use of carriers was limited to the separation of the epimeric hexonic acids and the purification of d-glucose-3-14C. Separations and purifications at earlier stages were accom plished by means of large-scale paper chromatography.
In the first preparation, the tetronic acids were separated by lactonization and chromatography. d-Erythrose-1-14C, prepared from d-erythronic-1-14C acid by an improved Rosenmund synthesis, was converted, by a second cyanohydrin reaction, into d-arabinonic-2-14C and d-ribonic-2-l4C acids. These were lactonized, and the lactones were chromato-graphically separated; the resulting d-arabinono-1,4-lactone-2-14C, on reduction with sodium amalgam, yielded d-arabinose-2-14C Starting with this sugar, a third cyanohydrin synthesis yielded d-glucose-3-14C and d-mannose-3-14C.
In a second, somewhat more efficient, preparation (see fig. 1), it was found convenient to omit separation at the four-carbon level. The tetronic acid mixture was converted into the two d-tetroses-1-14C, and thence into a mixture of the four d-pentonic-2-14C acids. After lactonization and paper chromatography, this gave, as one fraction, a mixture of 2-14C-labeled d-arabinonic and d-xylonic lactones. This mixture of lactones was reduced to a mixture of the corresponding sugars, and d-arabinose-2-14C was separated chromatographically; this sugar was then used for the third cyanohydrin synthesis.4
Figure 1.
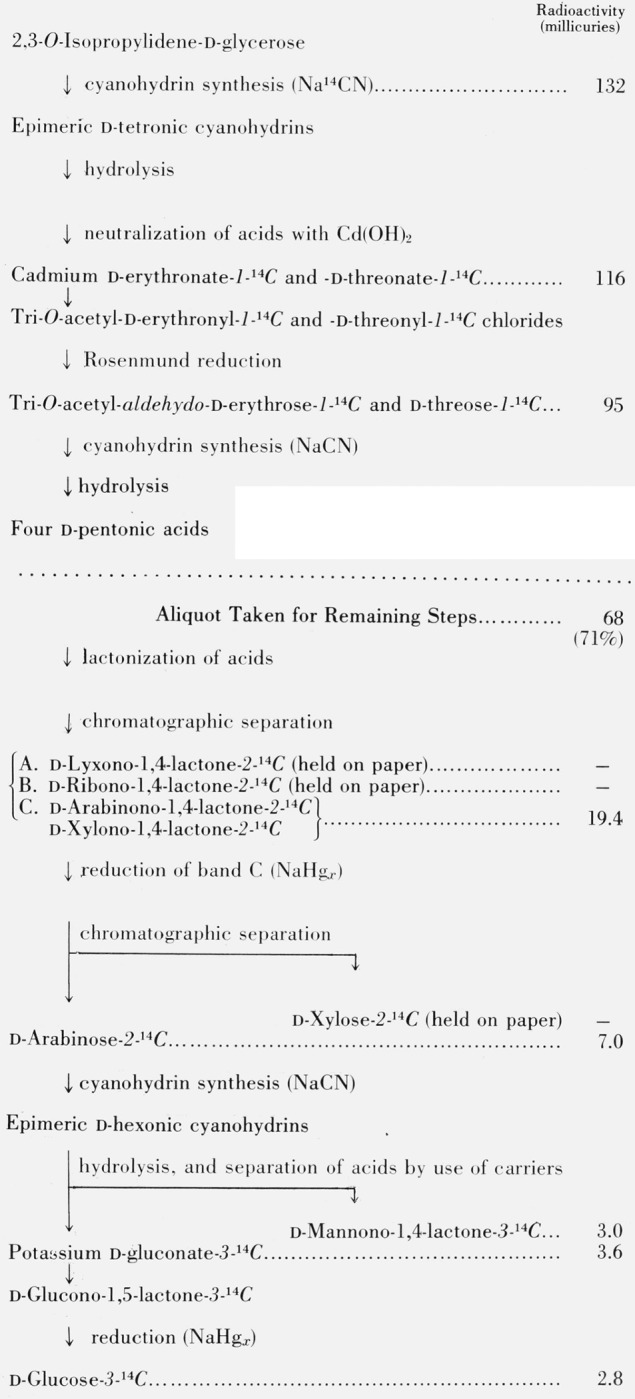
Synthesis of d-glucose-3-14C
2. Development of Experimental Methods
2.1. Determination of Completeness of Addition of Cyanide to 2,3-O-Isopropylidene-d-glycerose
In order to ascertain the optimal conditions for this reaction, the rate and completeness of addition of cyanide to 2, 3–0-isopropylidene-d-glycerose in various buffers were determined. The best results (see table 1) were obtained in a sodium bicarbonate or sodium carbonate buffer; in these, 98 and 94 percent, respectively, of the theoretical uptake of cyanide occurred within ten days. In a sodium acetate —acetic acid buffer, and in a sodium bisulfite buffer, the reaction was less complete.
Table 1.
Completeness of cyanide addition to 2, 3-O-isopropylidene-d-glycerose.
| Buffer | Time (days) | ||||
|---|---|---|---|---|---|
| 3 | 5 | 7 | 10 | 12 | |
| % | % | % | % | % | |
| NaHSO3 | 67.3 | 69.6 | 70.7 | 74.1 | 75.0 |
| NaHCO3 | 78.6 | 88.7 | 94.6 | 97.7 | 98.9 |
| Na2co3 | 72.7 | 82.7 | 88.5 | 93.7 | 95.6 |
| NaOAc | 67.3 | 73.2 | 75.5 | 77.9 | 79.1 |
| HOAc | |||||
2.2. Conversion of d-Erythronic-1-14C Acid Into d-Erythrose-1-14C
Because of the absence of the stabilizing pyranoid ring in the resulting sugar, tetronic lactones cannot be reduced to tetroses by the methods successfully employed for reducing pentonic and higher lactones. Both sodium amalgam reduction and catalytic hydro-genation of an erythronic lactone lead to the fully reduced product, erythritol [8, 9, 10]. However, a modified Rosenmund reduction [11], as first used by Cook and Major on fully acetylated aldonyl chlorides [12], appeared to provide a possible semimicro method for preparing d-erythrose-1-14C The method had already been applied by Glattfeld and Kribben [10] to the macro reduction of DL-erythronic acid to give an overall yield of DL-erythrose of less than 4 percent. Much of the loss occurred during the purification of the aldehydo acetate by distillation. Subsequently, Ladenburg et al. [13] improved the yields of acetylated aldonic acids by use of the cadmium salt of the acid in the acetylation. For the semimicro preparation of tri-O-acetyl-d-erythronyl chloride, it seemed probable that phosphorus pentachloride, used by Cook and Major, would be more convenient as a reagent than thionyl chloride, employed by Glattfeld and Kribben. In pilot experiments on nonlabeled material, one-millimole quantities of cadmium d-erythronate were freeze-dried, acetylated with a mixture of acetic anhydride and hydrogen chloride, converted into tri-O-acetyl-d-erythronyl chloride with phosphorus pentachloride, and reduced by the Rosenmund procedure to tri-0-acetyl-aldehydo-d-erythrose. The overall yield, as determined by titration, was 90 percent.
2.3. Chromatographic Separations
A variety of chromatographic techniques was developed for separating the isomeric lactones and the sugars obtained in the syntheses. Large-scale separations were carried out on heavy paper [14, 15], and bands of material, located by radioautography, were removed from the paper by elution. Of several solvents evaluated, water-saturated 1-butanol was chosen for separating d-erythronic and d-threonic lactones (Rf 0.34 and 0.52, respectively). The subsequent separation of these lactones was found to be complicated by formation, during attempts to achieve complete lactonization, of a complex mixture containing esterlike compounds. The complication was largely overcome by converting the mixed acids into their butyl esters and then converting these into a mixture of the lactones. However, the separation of the tetronic lactones was further complicated by the formation of a fairly stable, slow-moving derivative of d-threonic acid,5 having nearly the same Rf value as that of d-erythronic lactone; consequently, the chroma-tographic separation of d-erythronic lactone was not sharp.
In a second preparation, the d-tetronic acids, without separation, were carried through the Rosenmund reduction (see sees. 2.2 and 3.4). The resulting mixture of acetylated d-tetroses was converted into a mixture of the four d-pentonic acids. These were lactonized, and the lactones were chromatographically separated into three fractions by use of tert-pentyl alcohol, three-quarters saturated with water: A, d-lyxono-l,4-lactone-2-141; B, d-ribono-l,4-lactone-2-14C; and C, a mixture of d-arabinono-l,4-lactone-2-14C and d-xylono-l,4-lactone-2-14C Because the lactones in band C are difficult to separate chromatographically, they were reduced, without separation, to a mixture of the corresponding sugars. d-Arabinose and d-xylose, although separable by use of various developing solvents, were found to have widely different mobilities in 9:1:1 2-butanone — acetic acid —saturated aqueous boric acid [16], and so this solvent mixture was used for their separation.
3. Experimental Details
3.1. Materials and General Methods
Sodium cyanide-14C was prepared from barium carbonate-14C, and was analyzed for specific and total radioactivity by methods previously developed [17]. Nonlabeled 1,2:5,6-di-O-isopropylidene-d-mannitol was prepared in 82-percent yield by condensing d-mannitol with acetone in the presence of zinc chloride [18].
Most of the radioactivity assays for carbon 14 were made with a liquid scintillation counter by use of a scintillation solution having p-dioxane as the solvent [19]. In assaying the salts of aldonic acids (which are relatively insoluble in dioxane), an equal volume of a thixotropic agent6 was added to the scintillation solution before addition of the radioactive sample [20]. The counting efficiency was determined by means of salts of aldonic acids having known radio-” activity. Some of the materials were assayed in formamide solution by means of a 2-π, windowless, gas-flow proportional counter [21].
Reductions of lactones with sodium amalgam (in the form of pellets) [22] were conducted on a semi-micro scale, in the presence of sodium hydrogen oxal-ate as buffer [4, 23].
3.2. Preparation of 2,3-O-Isopropylidene-d-glycerose
A solution of 10 mmoles (2.62 g) of l,2:5,6-di-O>-isopropylidene-d-mannitol in 40 ml of water was mixed with 11 mmoles (2.35 g) of sodium metaperiodate dissolved in 50 ml of water. The resulting solution was kept in the dark at room temperature for 2 hr, cooled in ice, and passed through a column of ice-cold, mixed, cation- and anion-exchange resins to remove sodium iodate and excess sodium metaperiodate. Measurement of conductivity8 showed that the effluent was free of ionic materials. The aqueous solution was immediately concentrated under reduced pressure, and the aldehyde content was determined by oxidation of an aliquot with iodine in alkaline solution [24]. The yield of 2,3-O-isopropylidene-d-glycerose was, by analysis, 16.4 mmoles (82 percent); the material was used without further purification.
3.3. Reaction of 2,3-O-Isopropylidene-d-glycerose With Sodium Cyanide
In order to determine optimal conditions for the cyanohydrin reaction [25], solutions were prepared from equimolar quantities of 2,3-O-isopropylidene-d-glycerose, sodium cyanide, and one of the buffers listed in table 1; the concentration of each compound in the solution was 0.09 mmole per ml. The solutions were kept at room temperature, and aliquots were removed periodically for the determination of residual cyanide [26]. The results, expressed as percent of the cyanide that had reacted with the sugar, are given in table 1.
For the synthesis outlined in figure 1, the following two solutions were combined:
A. Solution of radioactive sodium cyanide (160 ml) containing:
| sodium cyanide | 30.4 mmoles |
| total radioactivity | 132.5 millicuries (mCi) |
| specific radioactivity | 4.36 mCi/mmole |
| sodium hydroxide | 21.2 mmoles |
B. Solution of 2,3-O-isopropylidene-d-glycerose (68 ml) containing:
| 2,3-O-isopropylidene-d-glycerose | 33 mmoles |
| sodium bicarbonate | 52 mmoles.9 |
Solution B was added to solution A, previously frozen onto the sides of a 750-ml, round-bottomed flask. The flask was allowed to stand at room temperature, and was occasionally shaken until the ice had melted.
After 10 days, a solution of 1 g (25 mmoles) of sodium hydroxide in 20 ml of water was added. The flask, equipped with a gas inlet and a reflux condenser, was heated at 90 °C for 1 hr while a gentle stream of nitrogen was passed through the solution.10 The solution was then filtered with the aid of 100 mg of a decolorizing carbon; analysis showed 120.4 mCi of radioactivity (90.9 percent). Cations were removed with a cation-exchange resin.11
3.4. Conversion of d-Tetronic-1-14C Acids Into d-Tetroses-1-14C
After concentration under reduced pressure12 to about 200 ml, the effluent was treated with an excess of cadmium hydroxide, and the suspension was magnetically stirred for two days and filtered. The residue was suspended in water, and the suspension was treated for 15 min with a stream of carbon dioxide gas, heated to boiling, and filtered.13 The combined filtrates and washings (containing 116.1 mCi) were concentrated under reduced pressure, and the concentrate was divided in two parts; each of these was freeze-dried and separately converted into the mixed d-tetroses-1-14C
To 14.6 millequivalents (meq) of mixed cadmium d-erythronate-1-14C and cadmium d-threonate-1-14C (containing 63.5 mCi of radioactivity) was added 75 ml of acetic anhydride. The flask was kept in an ice bath for 20 min, while dry hydrogen chloride was bubbled through the suspension. The flask, containing a magnetic stirring bar, was now closed with a drying tube, and the contents were kept at 50 °C for 1 hr, while being stirred. The suspension was immediately filtered on a 15-ml, coarse, glass-fritted funnel, and the residue was thoroughly washed with acetic anhydride.14 The combined filtrate and washings were concentrated under reduced pressure; traces of acetylating agents were removed by successively adding and evaporating absolute ethanol (twice) and toluene (once). Finally, 50 ml of dry ether, a magnetic stirring bar, and 6.2 g of phosphorus pentachloride were added, the flask was closed with a drying tube, and the contents were stirred overnight at room temperature.
The suspension was filtered through a 15-ml, medium, glass-fritted funnel into a 250-ml, long-necked, round-bottomed flask. Ether and phosphoryl chloride were removed by concentration under reduced pressure. Xylene (50 ml) was twice successively added and distilled.15
The acetylated tetronyl chlorides were then cata-lytically reduced by the Rosenmund procedure [12]. To the sirupy acid chlorides were added 35 ml of dry xylene, a magnetic stirring bar, and 2.75 g of palladium—barium sulfate catalyst [27] (previously heated at 60 °C for 30 min). While the reaction flask was heated under reflux in an oil bath at 130 °C, dry hydrogen was vigorously bubbled through the stirred suspension, and the escaping gases were passed through 15 ml of 0.5-N sodium hydroxide. The receiver and contents were replaced every 30 min, and the hydrogen chloride evolved was determined by neutralizing the excess alkali with nitric acid and titrating the chloride ion with silver nitrate (Mohr method). Reduction was rapid during the first 30 min, and slower thereafter; the reaction, which was continued for 4 hr, evolved 13.1 mmoles of hydrogen chloride (90% of the theoretical).
The hot suspension was filtered on a 60-deg funnel bearing a perforated plate (4-cm diam) and filter paper heavily coated with both paper pulp and diatomaceous earth. The residue was well washed with hot xylene, and the filtrate and washings were combined and evaporated under reduced pressure. The residue was dissolved in 50-percent aqueous ethanol, and the solution was passed through a column containing 35 ml of an anion-exchange resm topped by 5 ml of action-exchange resin. The neutral effluent contained 54.6 mCi of radioactivity. Elution of the resin with 10-percent aqueous acetic acid yielded 1.45 mCi of radioactivity, largely in the form of unreduced tetronic acids.
A repetition of the acetylation and reduction processes, starting with the second portion of mixed cadmium salts (12.0 meq, 52.5 mCi) yielded neutral products containing 40.2 mCi of radioactivity, and unreduced acids containing 3.6 mCi. The total radio-chemical yield of neutral products was 81.7 percent of the tetronic acids.
3.5. Preparation of d-Pentonic-2-14C Acids
The neutral products from the two reductions were combined (21.7 mmoles, 94.8 mCi) and concentrated to about 50 ml under reduced pressure. Sodium bicarbonate (2.18 g, 26 mmoles) was added, and, after the addition of two or three small lumps of solid carbon dioxide, the mixture was frozen onto the sides of the flask. To this solid was added a solution of 1.27 g (26 mmoles) of sodium cyanide in 25 ml of water. The mixture was allowed to thaw in ice water, and was kept at room temperature for one week.
Sodium carbonate (2.1 g) was then added, and the solution was heated under reflux (boiling-water bath) for 2 hr, while a gentle stream of nitrogen was bubbled through it. The hydrolyzate was cooled and passed through a column of 125 ml of action-exchange resin,16 which was then thoroughly washed.
3.6. Lactonization and Separation of d-Pentonic-2-14C Acids
A portion of the mixed acids (15.4 mmoles,67.5 mCi) was lactonized, by way of the butyl esters, by the following rapid method: The solution was concentrated under reduced pressure to a sirup, which was heated at 70 °C for 30 min with a 1-percent solution of hydrochloric acid in l-butanol. Butanol was removed by two successive additions and evaporations, under reduced pressure (50 °C), of a 1:1 mixture of toluene and acetic acid. The sirup was heated at 80 °C for 1 hr with this mixture, and the solvent was removed by evaporation under reduced pressure. Addition and evaporation of the toluene —acetic acid mixture were repeated twice more. Finally, the sirup was dissolved in acetic acid and frceze-dried.
The residue, dissolved in methanol, was applied at once, as a 0.5-cm band, on four 18 in. × 22 in. sheets of prepared seed-test paper [14, 15]. After being dried for 12 hr in the air, the papers were developed for 94 hr17 with tert-pentyl alcohol. ¾ saturated with water.
Radioautograms of the chromatograms (made with industrial x-ray film by exposure for 15 to 20 min) showed three distinct bands. The fastest band (containing both d-arabinono-l,4-lactone-2-14C and d-xylono-l,4-lactone-2-14C) was located on each sheet of paper, and eluted with 50-percent aqueous methanol until the last of the eluate had negligible radioactivity; the combined eluate from this band of the four chromatograms contained 19.4 mCi of radioactivity.18
3.7. Preparation and Separation of d-Arabinose-2-14C and d-Xylose-2-14C
The solution of eluted lactones was treated with a small amount of cation-exchange resin, relactonized, and reduced with sodium amalgam to d-arabinose-2-14C and d-xylose-2-14C by the method previously described [1] for the preparation of d-arabinose-1-14C The first crop had 13.6 mCi of radioactivity.
Unreduced aldonic acids (containing 5.0 mCi of radioactivity, and contaminated with oxalic acid) were eluted from the ion-exchange resins with 100 ml of 10-percent acetic acid. The acetic acid was evaporated under reduced pressure, an aqueous solution of the residue was treated with excess calcium carbonate, and sufficient solution of calcium hydroxide was added to neutralize it (permanent pink color to phenolphthalein). After filtration of the suspension and passage of the filtrate through a column of action-exchange resin, the reclaimed aldonic acids were lactonized by the method described above and reduced with -sodium amalgam. The second crop of sugars, containing 2.4 mCi of radioactivity, brought the combined radiochemical yield of d-arabinose-2-14C and d-xylose-2-14C to 16.0 mCi.
The unreduced acids remaining (2.18 mCi) were again removed from the resins, and shown, by a trace analysis with potassium d-arabinonate. to contain 0.7 mCi of radioactivity in the form of d-arabinonic acid.
The mixture of d-arabinose-2-14C and d-xylose-2-14C (3.6 mmoles, 16.0 mCi) was chromatographed on two 18 in. × 22 in. sheets of Whatman paper No. 17, equipped with stirrups and wicks of paper No. 3 [15]. The solvent, 9:1:1 2-butanonc —acetic acid —saturated boric acid [16], when used in two developments (17 hr and 12 hr), yielded sharp, well defined bands of d-arabinose-2-14C and d-xylose-2-14C as shown by radio-autographs. The d-arabinose-2-14C, eluted with 50-percent methanol, contained 7.0 mCi of radioactivity (1.6 mmoles). Nonlabeled d-arabinosc (210 mg) was dissolved in the eluate, and the solution was filtered through a small amount of decolorizing carbon. Boric acid (from the developing solvent) was removed as methyl borate by several successive additions and evaporations of methanol.
3.8. Preparation of d-Glucose-3-14C
The d-arabinose-2-14C was carried through the third cyanohydrin synthesis essentially as described previously for the synthesis of d-glucose-1-14C [4]. However, the d-gluconic acid was separated by means of carrier crops of potassium d-gluconate, instead of barium d-gluconate.19 Potassium d-gluconate-3-14C, having 3600 μCi of radioactivity, after conversion into d-glucono-l,5-lactone-3-14C and reduction of this, yielded 2380 μCi in the form of chromatographically pure d-glucose-3-14C. Unreduced d-gluconic acid-3-14C was eluted from the ion-exchange resin used in purifying the sugar, and again lactonized and reduced; the total yield of d-glucose-3-14C was 2800 μCi.
Potassium ions were removed from the mother liquor of the potassium d-gluconate-3-14C by action-exchange resin, and the d-mannonic-3-14C acid was separated by use of carrier crops of d-mannono-1,4-lactone and held for other work. Part of the lactone was reduced to d-mannose-3-14C by the procedure reported in reference 6.
Acknowledgments
This work was supported in part by the Psychopharmacology Service Center, National Institute for Mental Health. National Institutes of Health. Public Health Service, Department of Health. Education, and Welfare, Bethesda. Md. 20014.
Footnotes
Preliminary work on this synthesis was reported by one of us (L.T.S.) in a Ph. D. dissertation presented to the university of Maryland (1960).
Figures in brackets indicate the literature references at the end of this paper.
The second prodecure results in the formulation and separation of d-xylose-2-14C and d-lyxono-l,4-lactone-2-14C, in addition to the d-ribono-l,4-lactone-2-14C formed in the first procedure.
On treatment with acid, the compound gave d-threonic acid.
“Cab-O-Sil.” Codfrey L Cabot. Inc.. Boston. Mass.7
Certain commrrcial materials and equipment are identified in this paper in order to specify adequately the-experimental procedure. In no case does such identification imply recommendation or endorsement by the National Bureau of Standards, nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
All effluents from mixed ion-exchange resins were tested for the presence of ionic impurities by use of a commerical conductivity-meter.
In sodium ion, this amount of sodium bicarbonate is equivalent to the reagents in solution A.
The hydrolysis was conducted in an efficient hood, and no attempt was made to determine the ammonia evolved. Preliminary test-hydrolyses, followed by paper chromatography, had shown that the above conditions are satisfactory.
Because of the evolution of gas in the presence of cation-exchange resin, 50 ml of the resin was first stirred with the solution, which was then passed through a column of the resin (150 ml). The column was back-washed several times during the process, and all of the resin was finally washed until the last portions of the effluent had negligible radioactivity.
With one exception, noted later, if a distillate contained appreciable radioactivity, the distillate was redistilled, and the residue was combined with the main product.
The cadmium carbonate remaining still contained 4.3 mCi of radioactivity. This was not reclaimed.
Cadmium salts were removed at this point, in order to obviate “bumping” during evaporation.
The distillate, containing 250 μCi of radioactivity, was discarded.
See footnote 11.
The lactonization of test samples had previously been studied by paper chromatog-raphy. The butyl esters, produced by heating acidified 1-butanol were almost completely converted into the lactones by the toluene-acetic acid treatment described.
The bands of d-lyxono-l,4-laetone-2-14C and d-ribono-l,4-lactone-2-14C were held on paper and not assayed. The remaining paper, when eluted, yielded 18.1 mCi of radioactivity, of which 2.1 mCi was shown, by an isotope dilution technique, to be in the form of d-arabinonic acid.
The potassium salt crystallizes more freely from impure solutions than the barium salt.
4. References
- [1].Frush H. L. and Isbell H. S., J. Res. NBS 51,307 (1953) RP2458. [Google Scholar]
- [2].Isbell H. S., Frush H. L., and Holt N. B., J. Res. NBS 53, 325 (1954) RP2550. [Google Scholar]
- [3].Isbell H. S., Holt N. B., and Frush H. L., J. Res. NBS 57, 95 (1956) RP2697. [Google Scholar]
- [4].Isbell H. S., Karabinos J. V., Frush H. L., Holt N. B., Schwebel A., and Galkowski T. T., J. Res. NBS 48,163 (1952) RP2301. [Google Scholar]
- [5].Isbell H. S., Frush H. L., and Holt N. B., J. Res. NBS 53, 217 (1954) RP2536. [Google Scholar]
- [6].Isbell H. S., Frush H. L., and Schaffer R., J. Res. NBS 54, 201 (1955) RP2581. [Google Scholar]
- [7].Schaffer R. and Isbell H. S., J. Res. NBS 56,191 (1956) RP2667. [Google Scholar]
- [8].Hutton D. Ph. D. dissertation, Univ. of Chicago. 1930. [Google Scholar]
- [9].Glattfeld J. W. E. and Stack A. M., J. Am. Chem. Soc. 59, 753 (1937). [Google Scholar]
- [10].Glattfeld J. W. E. and Kribben B. D., J. Am. Chem. Soc. 61, 1720 (1939). [Google Scholar]
- [11].Rosenmund K. W., Ber. 51, 585 (1918). [Google Scholar]
- [12].Cook E. W. and Major R. T., J. Am. Chem. Soc. 58,2410(1936). [Google Scholar]
- [13].Ladenburg K., Tishler M., Wellman J. W., and Babson R. D., J. Am. Chem. Soc. 66, 1217 (1944). [Google Scholar]
- [14].Brownell H. H., Hamilton J. G., and Casselman A. A., Anal. Chem. 29, 550 (1957). [Google Scholar]
- [15J.Frush H. L. and Sniegoski L. T., unpublished work. [Google Scholar]
- [16].Rees W. R. and Reynolds T. M., Nature 181, 767 (1958). [DOI] [PubMed] [Google Scholar]
- [17].Moyer J. D. and Isbell H. S., Anal. Chem. 29,393(1957). [Google Scholar]
- [18].Baer E., J. Am. Chem. Soc. 67, 338 (1945). [Google Scholar]
- [19].Langham W. H., Eversole W. J., Hayes F. N., and Trujillo T. T., J. Lab. Clin. Med. 47, 819 (1956); Packard Tech. Bull. No. 1 (Packard Instrument Co., LaGrange, 111.). [PubMed] [Google Scholar]
- [20].Blanchard A. and Takahashi I. T., Anal. Chem. 33, 975 (1961). [Google Scholar]
- [21].SchwebeL A. Isbell S., and Moyer J. D., J. Res. NBS 53, 221 (1954) RP2537. [Google Scholar]
- [22].Isbell H. S., Frush H. L., and Holt N. B., J. Res. NBS 64A (Phys. and Chem) No. 1, 135–136 (Jan-Feb 1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Isbell H. S., U.S. Pat. 2,632,005 (Mar. 17, 1953). [Google Scholar]
- [24].Kline G. M. and Acree S. F., BS J. Res. 5, 1063 (1930); Ind. Eng. Chem., Anal. Ed. 2, 413 (1930). [Google Scholar]
- [25].Isbell H. S., U.S. Pat. 2,606,918 (Aug. 12, 1952). [Google Scholar]
- [26].Kolthoff I. M. and Sandell E. B., Textbook of Quantitative Inorganic Analysis, p. 546 (The Macmillan Company, New York, N.Y. 1952). [Google Scholar]
- [27].Mozingo R., Org. Syntheses, Coll. Vol. Ill, 685 (1955). [Google Scholar]


