To the Editor:
With interest we read Wang et al.’s letter to the editor in which they published results of their studies in rats1 in response to our study in mice.2 Reproducing and supporting our study conclusions, Wang showed that the dietary administration of sucralose (the sweetening ingredient of Splenda) for 6 weeks in rats resulted in increased biomarkers of intestinal inflammation, ie., colitis. From their report, we gather 2 main points; they highlight that (i) sucralose also induces proinflammatory effects in other species (rats) and (ii) such proinflammatory response could be further evidence to support the “unifying hypothesis” that the epidemic of inflammatory bowel diseases (IBDs) could be due to the emergence of such additives in the human diet.1
Although several hypotheses exist on the potential etiologies of IBD, it is important to highlight that a sole cause is unlikely to be the culprit. Many clinical entities are under the IBD “umbrella term,” making drawing conclusions from association studies difficult. Because animal experiments using inbred lines are highly homogeneous compared with human experiments, findings may at best represent the disease of a limited fraction of IBD patients. Genetic studies suggest that IBD is a polygenic predisposition to excessive inflammatory responses, presumably to various elements in the diet or microorganisms in the digestive tract. Studying IBD ileitis, we have shown using germ-free mice (SAMPYit/Fc) that typical 3D lesions of IBD–Crohn’s disease occur in mice in the absence of gut microbes,3, 4 indicating that diet alone is sufficient to drive IBD.
Because diet alters the gut microbiome and numerous microbial byproducts are proinflammatory, we were pleased to see that Wang reproduced our findings in a distinct framework (their rat acute colitis, vs, our mouse chronic ileitis) and that 2 key biomarkers we tested were also abnormal in their study (myeloperoxidase activity [MPO], Proteobacteria). Although other elements are important in Wang’s report (eg, they appreciated histological inflammation, and we did not), we want to emphasize that the use of such biomarkers to monitor the inflammatory response in the digestive tract of humans could be an objective strategy to help patients identify diets that make them feel unwell to minimize chronic inflammation driven by uncharacterized/complex diets. The congruency of our findings is remarkable. However, it is important to note that only ~10% of IBD patients report that sweetened diets make their symptoms worse.2 Clearly, the sole cause of IBD is not sweeteners, for which experimental disease mechanisms have been documented. Unless we accompany this body of experimental data with the personalized, quantitative, and objective assessment of gut inflammation in response to diets, the best that doctors and patients could do is to learn general concepts and continue tracking their diets (including Splenda) subjectively.
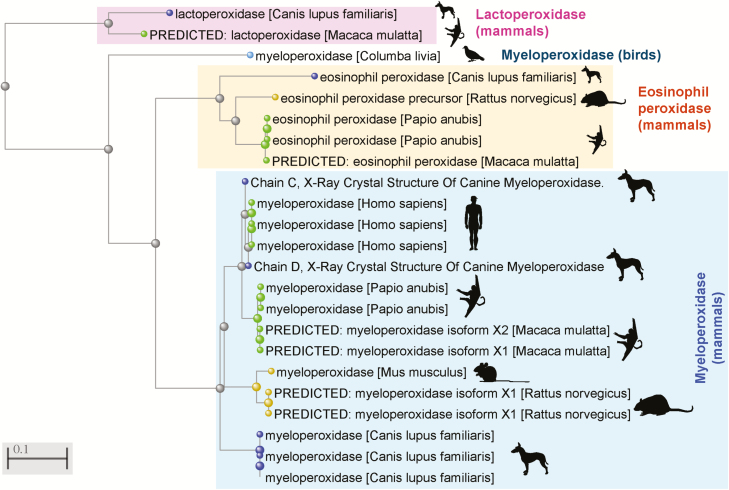
In our study,2 we proposed to use a host-and-bacterial biomarker panel (MPO/Proteobacteria) to assist patients to objectively monitor their “reactivity” to diets. Focused on hosts’ uniqueness and motivated by Wang, herein we expand the concept that MPO is a reliable interspecies biomarker to monitor human–diet interactions. From a biochemical perspective, Figure 1 illustrates the highly conserved amino acid sequence of this enzyme across species1, 2, 5, 6 and contextualizes the clinical/functional reproducibility of measuring its abundance as indicative of digestive inflammation in humans, mice, rats, and more recently in dogs with IBD. In baboons, studies indicate that MPO (an enzyme produced by immune cells, i.e., neutrophils) is also reliable because it is not altered with age-associated remodeling of the intestinal epithelial barrier, which occurs with aging.7 Being exposed to high levels of proinflammatory ingredients, the ultimate goal of a personalized digestive monitoring strategy is to identify diets that each patient can eat or avoid confidently.
FIGURE 1.
Amino acid sequence similarity for the myeloperoxidase gene, predicted or confirmed protein isoforms across various animal species (including humans), in the context of other closely related oxidases of relevance to inflammation. Distance Phylogenetic tree, NCBI Constraint-based Multiple Alignment Tool. Elements clustered together within a given branch are more similar compared to elements in nearby branches; differences increase as branch-branch distances increase. Notice MPO sequence similarity between dogs, humans and experimental mammalian species, mice, rats, and primates.
Disclosure: A patent has been filed for a home-based test to assess the impact of diet on digestive health.
REFERENCES
- 1. Wang X, Guo J, Liu Y, et al. Sucralose increased susceptibility to colitis in rats. Inflamm Bowel Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez-Palacios A, Harding A, Menghini P, et al. The artificial sweetener splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease–like ileitis. Inflamm Bowel Dis. 2018;24:1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Palacios A, Kodani T, Kaydo L, et al. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat Commun. 2015;6:7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez-Palacios A, Aladyshkina N, Ezeji JC, et al. ‘Cyclical bias’ in microbiome research revealed by a portable germ-free housing system using nested isolation. Sci Rep. 2018;8:3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanifeh M, Satu S, Rajamäki MM, et al. S100A12 concentrations and myeloperoxidase activities are increased in the intestinal mucosa of dogs with chronic enteropathies. BMC Vet Res. 2018;14:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J. 1998;45:69–73. [DOI] [PubMed] [Google Scholar]
- 7. Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]