Abstract
Objectives:
This study tested the hypothesis that longer duration of any type of respiratory support is associated with an increased rate of death or neurodevelopmental impairment (NDI) at 18–22 months.
Methods:
Retrospective cohort study using the Generic Database of NICHD Neonatal Research Network from 2006 to 2010. Infants were born at <27 weeks gestational age with birth weights of 401–1000g. Respiratory support received during initial hospitalization from birth was characterized as follows: no support, only invasive support, only non-invasive support or mixed invasive and non-invasive support. The primary outcome was death after 24 hours of life or NDI at 18–22 months corrected age.
Results:
In a cohort of 3651 infants, 1494 (40.9%) died or had NDI. Cumulative respiratory support of any type beyond 60 days was associated with the likelihood of death or NDI. Infants who only received invasive support had the highest rate (89.1%), followed by those received mixed support (26.1%). Infants who received only non-invasive support had the lowest rate (7.7%). When compared to the only non-invasive support group, both invasive [OR 62.7 (95% CI 25.7, 152.6)] and mixed [OR 6.1 (95% CI 2.6, 14.4)] support groups were significantly more likely to die or have NDI.
Conclusion:
Prolonged respiratory support, whether invasive or non-invasive, is associated with increased odds of a poor outcome. The proportion of infants with a poor outcome increased in a dose dependent manner by 2 factors: the cumulative duration of respiratory support beyond 60 days, and the extent to which invasive support is provided.
Keywords: Newborn, extremely low birth weight infant, neurodevelopmental impairment, prematurity, respiratory support
Summary
In summary, our findings demonstrate that prolonged respiratory support, regardless of whether invasive or non-invasive support, is associated with an increased risk of mortality or neurodevelopmental disability. In addition, there is a dose-dependent increase in the risk of death or NDI from NI-RS to mixed RS to I-RS. Further studies are needed to clarify whether the different modes of noninvasive support have any differential impact on outcomes; or whether the timing (early versus late) of the mode of respiratory support have any impact on neurodevelopmental outcomes.
INTRODUCTION
Over the past three decades, the survival rate of extremely low birth weight (ELBW, BW<1,000g) infants with immature lung development has improved 1,2. Disappointingly, the outcomes in survivors have not changed significantly 3,4. Such ELBW infants often require prolonged ventilation support. Recognition of the hazards of prolonged mechanical ventilation has resulted in increased use of non-invasive respiratory support (NI-RS) modalities. These non-invasive modalities include Nasal Intermittent Positive Pressure Ventilation (NIPPV), Nasal Continuous Positive Airway Pressure (NCPAP) and High Flow Nasal Cannula (HFNC) 5–11. Each of these may be used as first line respiratory support, or immediately following mechanical ventilation. Application of these non-invasive modalities can prevent application of invasive support, but may be needed for prolonged periods of time 12,13.
In an earlier era (1995–1998), when invasive respiratory support (I-RS) dominated the course of an ELBW infant’s time in the newborn intensive care unit (NICU), Walsh et al. showed a dose-dependent relationship between the duration of invasive ventilation and both in-hospital mortality and 24-month neurodevelopmental disability 14. It is still unclear, however, whether reducing the duration of I-RS, through aggressive use of NI-RS, avoids or reduces similar adverse outcomes. Our objective was to assess the hypothesis that increasing duration of any RS is associated with increased death or adverse neurodevelopmental outcomes in survivors at 18–22 months.
METHOD
This retrospective multi-site study evaluated prospectively collected data from the NICHD Neonatal Research Network (NRN) Generic Database (GDB) and Generic Follow-Up (FU) studies. Neonatal outcome data are collected for the entire hospitalization including any time before transfer to the network centers. The Institutional Review Board at each participating institution approved the GDB and Follow-up studies. The study population included all premature infants born in NRN sites between 2006 and 2010 at <27 weeks gestational age (GA), with birth weight between 401 and 1,000 grams who were enrolled in the generic database. A comprehensive neurodevelopmental follow-up examination was conducted at 18–22 months corrected age among survivors. In addition to GA and birth weight, inclusion criteria also included: 1) complete respiratory support data; 2) no significant congenital cardiac disease (other than a patent ductus arteriosus); 3) no congenital chromosomal abnormalities; 4) survival beyond the first 24 hours of life. The time point of death after 24 hours was chosen because critically ill infants dying soon after birth carry no risk of NDI and may, in many cases, reflect other disease processes. Trained study coordinators abstracted data from medical records using standardized definitions. Data were coordinated and analyzed at a central data center (RTI International, Research Triangle Park, NC).
Respiratory Support Data
Infants were classified into 4 groups by ascending degree of respiratory support: 1) No respiratory support (No-RS), 2) Non-invasive respiratory support only (NI-RS), which included non-intubated respiratory support (NIPPV, NCPAP, or NC); 3) Mixed invasive and non-invasive respiratory support (Mixed RS); and 4) Invasive respiratory support (I-RS) via an endotracheal tube only. Data on respiratory support (RS) were recorded as cumulative number of days on each mode of ventilation. A day of cumulative RS is assigned if the infant spent any time on RS. Furthermore, if an infant received multiple types of support in one day, the highest level of RS was used to count the type of support for that day. So, for an infant receiving both mechanical ventilation and non-invasive support on a given day, that day was analyzed only as a day of invasive support.
Primary Outcome:
The primary outcome was the composite of death after 24 hours, or neurodevelopmental impairment (NDI) at 18–22 months corrected age. Follow-up assessment at 18 to 22 months PMA was performed by standard NRN study protocols, incorporating the Bayley Scales of Infant Development-III (BSID-III). NDI at 18–22 months was determined if impairment was indicated in one or more of the NDI component examinations of cognition, motor function, vision, and hearing. Cognition was measured by certified psychologists using the BSID-III cognitive score and cognitive delay was indicated by a cognitive score < 70 (at least two standard deviations below the mean). Cerebral palsy (CP) was characterized by abnormal muscle tone and impaired range or control of movements using medical history, physical examination and neurological examinations. Moderate-to-severe CP, which indicated impairment for NDI, was defined as level II or higher on the Gross Motor Function Classification Scale (GMFCS). Severe or non-ambulatory CP was defined as GMFCS IV or V 15,16. Severe vision impairment was defined as blindness in both eyes with possibly some functional vision. Severe hearing impairment was defined as permanent hearing loss that requires bilateral amplifications.
NDI was deemed present if one or more of the component outcomes were present, or absent if none of the components indicated any impairment. If one or more NDI components were missing, and if NDI was not indicated by the non-missing components, the value of NDI was uncertain and it was left missing. The composite primary outcome of NDI or death was indicated if either NDI was present or if the infant died.
Secondary Outcomes:
The individual components of the primary outcome (death and NDI) are examined as secondary outcomes. Mild cognitive delay was also analyzed as a secondary outcome, being a BSID-III score < 85 (one standard deviation below the mean). In addition, in-hospital morbidity outcomes were examined. These included: late-onset sepsis (positive blood culture at ≥72 hours of age), grade III or IV intraventricular hemorrhage (IVH), cystic periventricular leukomalacia (PVL), postnatal steroid exposure, and necrotizing enterocolitis (NEC, modified Bells criteria, stage 2 or 3).
Statistical Analysis
The primary outcome assessed was a composite of death or NDI at 18–22 month corrected age. All outcomes were analyzed for differences between respiratory groups in the total cohort. On-going need for I-RS and NI-RS (or duration) was expressed as mean, median and inter-quartile range. Separate descriptive analyses were performed for each interval assessed (1, 7, 14, 28, 60, 90, 120 days). Survivors were compared with non-survivors by unadjusted analyses with Wilcoxon rank-sum tests for continuous variables, and chi-square or Fisher’s exact tests for categorical variables. Adjusted associations between impairment and RS (I-RS, NI-RS, or ‘mixed’) as well as duration in days were explored using a logistic regression model. Duration on any RS was defined as a categorical variable in days, to be consistent with assumption of the linearity of the logits of numeric variables. Results were expressed as odds ratios and 95% confidence intervals. In addition, we adjusted for covariates chosen a-priori, similar to those reported in the Walsh study in 2005. These covariates included: antenatal steroid use, maternal education, birth weight, gestational age, gender, NEC, Apgar score at 1 minute, mother’s age, maternal hypertension, antenatal hemorrhage, prenatal care visit, PDA, and surfactant. Since all infants diagnosed with BPD required a period of respiratory support, we will not be able to cleanly separate out the effects of respiratory support from the effects of BPD. Therefore, BPD was not included in the analysis to avoid the confounding effect. Birth weight and maternal factors (age and education) were also included to minimize selection bias due to lost to follow up. To test if longer duration of support is associated with increased death or NDI, the Mantel-Haenszel Chi-Square Test and Cochran-Armitage Trend Tests were performed.
Additionally, we performed the logistic regression analysis via multiple imputation of the missing data. The method of fully conditional specification was implemented. In order to mitigate the risk of selection biases for the application of different respiratory therapies, we conducted the propensity score modeling analysis. A logistic model of receiving invasive support (vs. mixed or noninvasive supports or providing no support) was estimated. We then stratified the study population into quintile groups based on the propensity model predicted probability of receiving invasive support, and ran the modeling estimation within each group.
RESULTS
Study population
Between January 2006 and December 2010, a total of 5086 live born neonates with birth weight between 401 and 1,000 g and gestational age <27 weeks were enrolled in the GDB. A total of 1109 infants were excluded from further analysis: 3 for missing data, 73 with congenital heart disease or chromosomal anomalies, and 1033 died within the first day of life. Of the remaining 3977 infants, 1,050 (26.4%) died during their initial hospital stay, another 58 (1.5%) infants died after hospital discharge, and 326 (8.2%) infants were lost to follow-up leaving 2543 (63.9%) infants with a neurodevelopmental assessment at 18–22 months (E-figure 1).
Demographics of the study sample are presented in table 1. Infants in whom the primary outcome was assessed were born at median of 25.1(quartile range 24.4 and 26.0) weeks gestational age and at a median birth weight of 710 (quartile range 612 and 820) grams. 51% of the infants were male and 42% were African American. The mothers had a mean age of 27±6 years at delivery, 40% had private insurance, and 66% had a high school or higher degree.
Table 1.
Patient characteristics
| Characteristic | Overall N = 3,651 |
Respiratory Group | P-value1 | |||
|---|---|---|---|---|---|---|
| No RS N = 9 |
NI-RS N = 104 |
Mixed RS N = 2,650 |
I-RS N = 888 |
|||
| Birth weight, grams Median (Q1, Q3) |
710 (612,820) |
880 (608, 970) |
845 (765, 920) |
730 (640, 830) |
630 (550,730) |
<0.001* |
| Gestational age, week Median (Q1, Q3) |
25.1 (24.4, 26.0) |
26.3 (24.0, 26.3) |
26.3 (25.7, 26.6) |
25.4 (24.6, 26.1) |
24.6 (23.9, 25.4) |
<0.001* |
| Mother’s education, % <HS degree HS degree or higher Unknown |
21 66 14 |
33 56 11 |
32 62 7 |
21 71 8 |
19 49 32 |
<0.001* |
| Mother’s insurance, % Private Medicaid Self-pay/Other/Unknown |
40 50 10 |
33 67 0 |
42 48 10 |
41 50 10 |
36 51 13 |
0.028* |
| Age of mother, years Mean (SD) |
27.2 (6.4) |
27.2 (7.8) |
26.3 (6.5) |
27.3 (6.4) |
26.8 (6.4) |
0.078 |
| Prenatal care visit, % | 95 | 100 | 91 | 95 | 95 | 0.304 |
| Maternal hypertension, % | 22 | 11 | 16 | 23 | 21 | 0.217 |
| Antenatal hemorrhage, % | 21 | 0 | 20 | 20 | 24 | 0.073 |
| Antenatal steroids, % | 87 | 89 | 90 | 89 | 81 | <0.001* |
| Male gender, % | 51 | 67 | 40 | 50 | 54 | 0.015* |
| Infant’s race, % Black White Other |
42 52 5 |
56 44 0 |
51 46 3 |
42 53 5 |
43 51 6 |
0.468 |
| Apgar score at 1 minute % <=3 % <=5 |
50 61 |
33 33 |
14 22 |
47 59 |
62 74 |
<.001* |
| Apgar score at 5 minutes % <=3 % <=5 |
14 21 |
22 22 |
1 3 |
12 18 |
23 33 |
<.001* |
The p-value is from a test for any difference across all four groups. Frequencies were tested with a chi-square test or a Fisher’s exact test. Medians were tested with a Kruskal-Wallis test.
We assessed whether the infants lost to follow up differed from those followed. The two groups were similar in maternal insurance status, prenatal care visits, incidence of maternal hypertension, antenatal hemorrhage, and antenatal steroids, or infants’ gender, race, gestational age at birth and one/five minutes Apgar scores. However, there were statistically significant differences in birth weight, maternal age and education between the two groups (Table S-1).
Outcomes by respiratory support groups
In this study cohort, the vast majority of infants received some level of respiratory support (3966 out of 3977 infants or 99.7%). In the 3651 infants with follow up data available, accumulated days of respiratory support was over 28 days in 2402 (65.8%) patients and over 60 days in 1125 (30.8%) patients. Outcome data by respiratory support groups are shown in table 2.
Table 2.
Rates of composite outcome, death or NDI in survivors and adjusted Odds Ratios for risk of composite outcome, death or NDI in survivors by RS Groups
| RS Groups |
Duration of RS Median weeks (Q1,Q3) |
NDI or death N (%) |
Death N (%) |
NDI in survivors N (%) |
|---|---|---|---|---|
| No RS N=9 |
0 | 4 (44.4) | 4 (44.4) | 0/5 (0) |
| NI-RS N=104 |
2.2 (0.1, 8.9) | 8 (7.7) | 3 (2.9) | 5/101 (5) |
| Mixed RS N=2650 |
7.3 (0.3, 30) | 691(26.1) | 335(12.6) | 356/2315 (15.4) |
| I-RS N=888 |
1.6 (0.1, 17.3) | 791(89.1) | 766(86.3) | 25/122 (20.5) |
| Overall* N=3651 |
- | 1494(40.9) | 1108(30.4) | 386/2543 (15.2) |
| RS Groups Comparison | NDI or death OR Estimate (95% CI) |
Death OR Estimate (95%CI) |
NDI in survivors OR Estimate (95%CI) |
|
| I-RS vs. Mixed RS | 10.3 (7.6, 13.8) | 18.3 (13.3, 25.2) | 1.0 (0.5, 1.7) | |
| Mixed RS vs. NI-RS | 6.1 (2.6, 14.4) | 18.6 (4.8, 71.9) | 1.1 (0.4, 3.1) | |
| I-RS vs. NI-RS | 62.7 (25.7, 152.6) | 341.0 (85.7, 1356.3) | 1.0 (0.3, 3.4) | |
No RS: no respiratory support; NI-RS: Non-invasive respiratory support; I-RS: Invasive respiratory support; Mixed RS: invasive +non-invasive respiratory support.
Logistic regression models were used to compare infants who received I-RS and Mixed RS to the NI-RS group. Similarly, infants who received I-RS were compared to those received mixed RS.
Outcomes by total cumulative days of any RS
There is a bimodal distribution of mortality as evaluated by the total cumulative days of any respiratory support in Figure 1. Mortality rate was >30% in those who received any RS for less than 7 days, decreased to <20% for those received any RS for 14–89 days and then steadily increased to 39% for any RS over 120 days. In addition, figure 1 also demonstrates an increasing rate of impairment as duration of any respiratory support increases. In these patients, NDI remained steady at 10–15% until after 60 days of support after which NDI increased. Percentage of infants with CP, blindness, deafness and decreased cognitive scores <85 and <70 all increased after receiving any RS for more than 60 days. Statistical analysis demonstrated significant increasing trends of the outcome rates with duration of respiratory support (p-value<0.001) (Table 3).
Figure 1. Outcome by duration of any respiratory support.
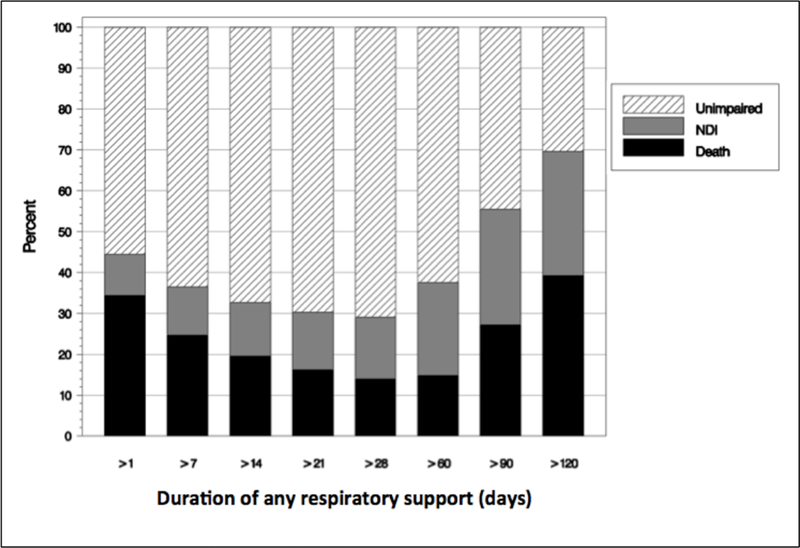
Mortality and neurodevelopmental outcomes of the cohort at each duration of respiratory support were analyzed. Duration of support is presented in 7-day interval. For example, respiratory support of 1–6 days was presented as >1 and 7–13 days as >7. This shows a clear gradient of risk that becomes apparent if an infant requires >60 days of RS.
Table 3.
Neurodevelopmental outcome in survivors by duration of any respiratory support
| Duration of support (number of patients) |
>1d N=92 |
>7d N=107 |
>14d N=122 |
>21d N=154 |
>28d N-1106 |
>60d N=717 |
>90d n=186 |
>120d N=54 |
P-value |
|---|---|---|---|---|---|---|---|---|---|
| No NDI (%) | 98 | 95 | 94 | 93 | 90 | 78 | 65 | 50 | <0.001* |
| CP (%) | 5 | 3 | 7 | 8 | 10 | 18 | 21 | 38 | <0.001* |
| Moderate-severe CP (%) | 1 | 0 | 2 | 3 | 4 | 10 | 15 | 28 | <0.001* |
| Blind (%) | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 4 | <0.001* |
| Deaf (%) | 1 | 2 | 2 | 2 | 2 | 5 | 7 | 15 | <0.001* |
| BSID-III Cognitive score, mean (SD) | 97.4 (12.3) | 95.4 (14.3) | 92.7 (12.3) | 94.1 (13.2) | 91.1 (14.2) | 84.7 (14.9) | 78.4 (16.0) | 74.8 (17.0) | <0.001** |
| Cognitive score <70 (%) <85 (%) |
1 8 |
3 15 |
2 19 |
4 17 |
6 23 |
14 40 |
27 54 |
37 63 |
<0.001*
<0.001* |
Note: This table includes infants that received respiratory support for at least one day and survived to follow-up and had NDI information (n=2538). Each column represents a distinct group of infants. For instance, the column labeled as >1d contains the statistics for infants that had support for at least 1 days, but less than 7 days; and the column labeled as >7d contains the statistics for infants that had support for at least 7 days, but less than 14 days. NDI=neurodevelopmental impairment, CP=cerebral palsy.
Mantel-Haenszel Chi-Square Test. Additionally, Cochran-Armitage Trend Tests indicated significant increasing trends of the outcome rates with duration of respiratory support (Left-sided P-value<0.001). Exact test statistics were calculated due to small sample sizes.
One-way analysis of variance (ANOVA) F test.
Outcomes by RS groups
Table 2 shows the number of infants falling into each of the subgroups of RS, and the total duration of receipt of RS by weeks. The primary outcome and its breakdown by components are shown. The overall rate of the primary outcome was 40.9%. In order to determine the relative impact of the mode of RS, we also derived the odds ratios from the estimated logistic regression models comparing each subgroup of RS against each other. A gradient of risk of NDI or death is seen for IRS as compared to NI-RS (62.7, CI 25.8, 152.6). Comparison of IRS to mixed RS showed lower OR (10.3, CI 7.6, 13.8). However, this risk seems mainly related to death with the risk dramatically higher in infants that never come off mechanical ventilation (I-RS vs. NI-RS: OR 341.0, CI 85.7, 1356.3). In survivors, however, neither I-RS nor Mixed RS increased the odds of NDI compared to the NI-RS group or when compared to each other.
Risk factors for the composite primary outcome
In further modeling, we assessed potential risk modifiers. Several other neonatal or demographic factors were significantly associated with increased risk of the composite primary outcome; but carried lower OR estimates than RS type. These factors include male gender, maternal education less than high school diploma, maternal antenatal hemorrhage, lack of prenatal visit, NEC and PDA. Factors associated with decreased risk of death or neurodevelopmental impairment include increased birth weight, increased gestational age, and maternal hypertension. Overall 87% of infants had received one or more doses of antenatal corticosteroids. Antenatal steroid administration and surfactant use were not associated with decreased risk of the composite primary outcome (Table S-2).
Risk Factors for NDI at 18–22 month
In patients who survived to follow up, the overall rate of NDI was 15.2%. Figure 2 shows that the odds of impairment significantly increased by a factor of 1.22 (CI 1.17–1.26, p<0.05) per additional week of RS. However, when the duration of RS and other factors were adjusted, neither I-RS nor Mixed RS by itself significantly increased the risk of NDI in survivors as compared to Non-invasive RS (I-RS vs. NI-RS: OR 0.8; CI 0.26–2.41 and Mixed RS vs. NI-RS: OR 0.95; CI 0.37–2.45).
Figure 2. Risk factors for NDI in survivors at 18–22 months.
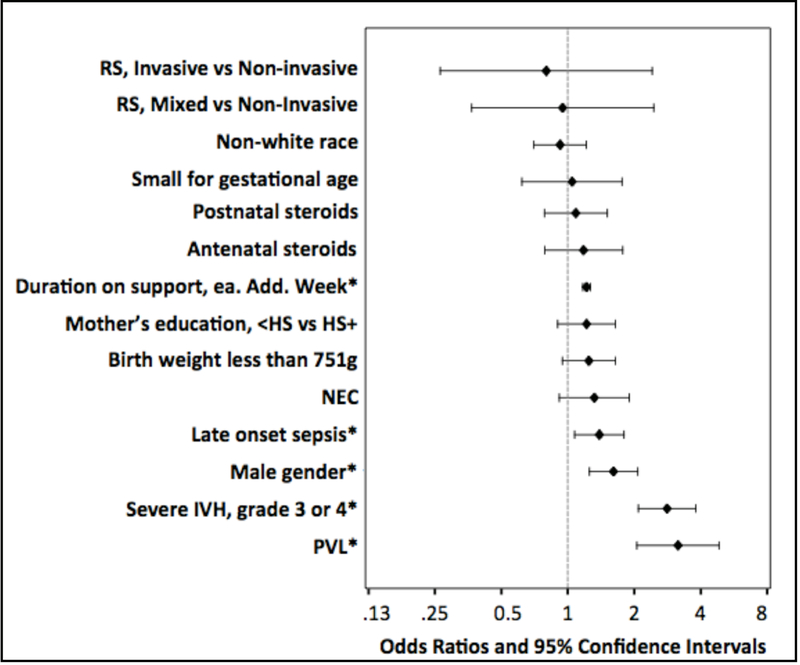
Multivariate logistic regression model was used to explore risk factors for NDI in survivors. Results are expressed as odds ratios and 95% confidence intervals. * Duration on respiratory support (each additional week of RS), late onset sepsis, male gender, severe IVH and PVL were associated with NDI after adjustment, p<0.05. HS= high school, NEC= necrotizing enterocolitis, IVH= intraventricular hemorrhage, and PVL=periventricular leukomalacia
Additional factors significantly associated with increased risk of neurodevelopmental impairment are graphically depicted (Figure 2) and included male gender, severe IVH, PVL, and late onset sepsis. In this cohort of infants, we did not find an independent association between antenatal steroids or postnatal steroids and risk of NDI.
DISCUSSION
This study evaluated a large, recent multi-center cohort of ELBW infants for the composite outcome of either death or NDI by 18–22 months. Our data showed that even in an era of non-invasive ventilation, the primary outcome of mortality and/or NDI remains a burden in this cohort of ELBW infants.
This cohort demonstrated a bimodal distribution of risk with high rates in the first few days of life and after 60 days of support, which is our definition of prolonged respiratory support. The high initial mortality is expected and consistent with the experience of neonatal caregivers around the world. Also, consistent with the experience of most neonatal caregivers is the high mortality seen in infants that never wean from invasive support, 86%. These infants have the most complicated courses and therefore the highest risk of death. However, the increased late mortality after 90 days of any RS is mainly contributed by infants in the Mixed-RS group as the majority of infants in either NI-RS or I-RS groups received short duration of those supports.
Prolonged RS of any type over 60 days, regardless of invasive or non-invasive support, was associated with increased risk of poor neurodevelopmental outcome. The findings for NDI mirrored the findings for mortality that the rates of NDI increased as the need for any RS extended beyond 60 days of support. Highest rates were of course seen in the group of infants that were unable to wean off I-RS. However, our analysis indicates that the duration of respiratory support, not the mode of support (I-RS or mixed RS) is associated with NDI in survivors. These data suggest simply avoiding invasive ventilation does not alone mitigate the risks to neurodevelopment associated with extreme preterm birth and having severe bronchopulmonary dysplasia.
This current cohort demonstrates similar findings to a previous cohort of infants from the NICHD Neonatal Research Network. Walsh et al previously reported that invasive respiratory support beyond 60 days increased the odds of death or NDI. 14 These findings are consistent with the many previous clinical studies and the biological reports of the injurious effects of mechanical ventilation 17,18 that continued use of mechanical ventilation increases the odds of death or poor neurodevelopmental outcomes. However, this study adds on to the previous data to reflect changes in respiratory support modalities in the contemporary cohort. Although clinicians now use non-invasive modalities more frequently, and the length of invasive support has decreased, we continue to see a similar trend of increasing rate of death or NDI as duration of any respiratory support extends beyond 60 days, regardless of invasive or noninvasive support. There is also a dose-response relationship with higher death or NDI rate in the only I-RS group, followed by the Mixed RS group, and lowest in the only NI-RS group. These data may aid clinicians when discussing short and long-term prognosis with families and participate in decision-making. Prior data and predictive models have only drawn a link between duration of RS and the outcome of BPD 22–24. In addition, those models routinely concentrate on the duration of mechanical ventilation and do not extend predictions out as far as 18–22 months. Finally, many of these instruments are complex mathematical models, rendering them difficult to grasp. An external validation study done by Onland et al24 demonstrated that most of these models do not perform well enough to be used in routine clinical care. The data as presented as in Figure 1, may assist clinicians in having these discussions with parents of infants who received prolonged respiratory support.
This study has several strengths and weaknesses. Among the strengths are the large multi-center cohort of ELBW infants, the low lost to follow-up rate (8.2%), and the standardized data collection. Our data represents care at large academic centers in the United States. However, outcomes in individual centers may differ from these aggregated results. In addition, without quantitative disease severity scores, it is difficult to separate sicker patients from the ones with milder illness in each RS groups and compare patients with similar disease severity between different RS groups, although the adjusted analyses take account of some of these variations. Further, due to the limitation of the dataset, and the nature of a retrospective study, we were unable to differentiate data miscoding, nor could we control the variations in clinical practice such as indications or thresholds for initiating respiratory support or the selection of different respiratory support methods. We attempted to reduce the risk of selection bias for the application of different respiratory support through propensity score matching, however, the bias cannot be eliminated given there is no alternative to I-RS in the most critically ill infants and all subjects in this study were recruited from large academic centers that potentially could underrepresent infants with milder disease from smaller local NICUs. Although the estimated odds ratios varied by group, the summary odds ratios were close to our original estimates but had wider 95% confidence intervals (Table S-3). A more detailed analysis of the timing of when the different respiratory support occurred might be beneficial to further differentiate the impact of different respiratory support on death and/or NDI. While the primary composite outcome appears to be robust by several analytical steps, we note that figure 2 for antecedents of NDI alone, suggest a more nuance interpretation. It is possible that while low, the 8% loss to FU may have affected this finding, and reflects attrition bias. To minimize bias of the missing sample, we have adjusted for birth weight and maternal factors including age and education in the primary analysis and performed multiple imputation analysis, which demonstrated that missing data to lost to follow up did not alter the results (Table S-2). We have not performed imputation analysis on the deaths since the potential of those died before the NDI measurements could have NDI is unlikely to affect the result of the composite outcome. However, we think it is reasonable to speculate that the sickest infants – had they survived – would have had higher risk of NDI. Future studies may consider separate outcomes of death and NDI when more NDI data become available.
We noticed that the neurodevelopmental outcome in survivors has improved as compared to that reported by Walsh et al. We believe there are several possible explanations: this could be a true improvement in developmental outcomes with improved neonatal management such as better treatment for ROP. Efficient non-invasive support could also contribute to a true improvement, as some patients in our cohort never received any invasive respiratory support and had a low NDI rate of only 5%. Perhaps a more important reason, is that the Bayley-III was used in this study, as opposed to the Bayley-II used by the prior study. Recent studies report that Bayley-III scales may underestimate developmental delay 19–21. Despite the difference between the two versions of Bayley testing, a simple qualitative (delayed vs. intact survival) comparison of 18 to 22-month outcome data can be performed between the recent era (2006–2010) and the earlier era (1995–1998), and the finding of a similar relationship between prolonged respiratory support and NDI is consistent between the two cohorts and supports the findings.
Supplementary Material
There were 5086 ELBW infants born at <27 weeks GA and between 401–1000g birth weight registered in GDB. 1109 (3 for missing data, 73 with congenital heart disease or chromosomal anomalies, and 1033 died within the first day of life) were excluded for not meeting inclusion criteria, leaving a study cohort of 3977 infants. 3615 were analyzed for the composite primary outcome and 2543 were analyzed for neurodevelopmental outcome.
Acknowledgements
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Mr. Douglas Kendrick (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine. Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbott R. Laptook, MD; Angelita M. Hensman, RN BSN; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Barbara Alksninis, PNP; Theresa M. Leach, MEd CAES; Victoria E. Watson, MS CAS. Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA. Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Jean J. Steichen, MD; Kimberly Yolton, PhD. Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC. Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA. Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; Anna M. Dusick, MD; Leslie Dawn Wilson, BSN CCRC; Faithe Hamer, BS; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP. Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Leif D. Nelin, MD. RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP. Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi, Alexis S. Davis, MD MS Epi; M. Bethany Ball, BS CCRC; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Jean G. Kohn, MD MPH; Hali E.Weiss, MD. Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN BSN; Elisabeth C. McGowan, MD. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA. University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Wade Rich, BSHS RRT. University of Iowa, Children’s Hospital (U10 HD53109, M01 RR59) – Edward F. Bell, MD; Michael J. Acarregui, MD; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA. University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Rebecca Montman, BSN. University of Pennsylvania (U10 HD68244) – Barbara Schmidt, MD MSc. University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, UL1 RR24160, M01 RR44) – Dale L. Phelps, MD; Gary J. Myers, MD; Linda J. Reubens, RN CCRC; Erica Burnell, RN; Diane Hust, MS RN CS; Julie Babish Johnson, MSW; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Kelley Yost, PhD; Lauren Zwetsch, RN MS PNP. University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Nora I. Alaniz, BS; Patricia W. Evans, MD; Charles Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT(ASCP). University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Gaynelle Hensley, RN; Sally S. Adams, MS RN CPNP; Linda A. Madden, RN CPNP; Elizabeth T. Heyne, MS MA PA-C PsyD; Janet S. Morgan, RN; Catherine Twell Boatman, MS CIMI; Lizette E. Torres, RN. University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 RR25764) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN BSN; Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RNC; Michael Steffen, MS CPM. Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD. Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Athina Pappas, MD; Rebecca Bara, RN BSN; Laura A. Goldston, MA. Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, M01 RR125) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN.
Financial Support: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Abbreviations:
- ELBW
extremely low birth weight
- NDI
neurodevelopmental impairment
- GA
gestational age
- PMA
postmenstrual age
- OR
odds ratio
- RS
respiratory support
- NI-RS
non-invasive respiratory support
- I-RS
invasive respiratory support
Footnotes
Conflicts of Interest: The authors do not have any relevant financial interests or conflicts of interest to disclose.
Contributor Information
Huayan Zhang, The Children’s Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine.
Kevin Dysart, Division of Neonatology and Department of Pediatrics; The Children’s Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Douglas E Kendrick, RTI International, Research Triangle Park, NC.
Lei Li, RTI International, Research Triangle Park, NC.
Abhik Das, RTI International, Rockville, MD.
Susan R. Hintz, Departments of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University School of Medicine and Lucile Packard Children’s Hospital, Palo Alto, CA.
Betty R. Vohr, Department of Pediatrics, Division of Neonatology, Women & Infants’ Hospital, Alpert Medical School of Brown University, Providence, RI.
Barbara J Stoll, Department of Pediatrics, McGovern Medical School at UTHealth, Houston, Texas.
Rosemary D. Higgins, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
Leif Nelin, Department of Pediatrics, The Ohio State University and Nationwide Children’s Hospital, Columbus, Ohio.
David P. Carlton, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA.
Michele C Walsh, Department of Pediatrics, Rainbow Babies & Children’s Hospital, Case Western Reserve University, Cleveland, Ohio.
Haresh Kirpalani, Division of Neonatology and Department of Pediatrics; The Children’s Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
REFERENCE:
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: final data for 2011. Natl Vital Stat Rep. 2013;62(1):1–69, 72. [PubMed] [Google Scholar]
- 3.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147 e141–148. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. Jama. 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari V Nasal intermittent positive pressure ventilation in the newborn: review of literature and evidence-based guidelines. J Perinatol. 2010;30(8):505–512. [DOI] [PubMed] [Google Scholar]
- 6.Meneses J, Bhandari V, Alves JG. Nasal intermittent positive-pressure ventilation vs nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(4):372–376. [DOI] [PubMed] [Google Scholar]
- 7.Verder H, Bohlin K, Kamper J, Lindwall R, Jonsson B. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr. 2009;98(9):1400–1408. [DOI] [PubMed] [Google Scholar]
- 8.Bancalari E, Claure N. Non-invasive ventilation of the preterm infant. Early Hum Dev. 2008;84(12):815–819. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Fetus and Newborn. Respiratory support in preterm infants at birth. Pediatrics. 2014;133(1):171–174. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23(2):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandari V, Finer NN, Ehrenkranz RA, Saha S, Das A, Walsh MC, Engle WA, Van Meurs KP. Synchronized nasal intermittent positive-pressure ventilation and neonatal outcomes. Pediatrics. 2009;124(2):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMauro SB, Millar D, Kirpalani H. Noninvasive respiratory support for neonates. Curr Opin Pediatr. 2014;26(2):157–162. [DOI] [PubMed] [Google Scholar]
- 13.Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med. 2013;369(7):611–620. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole K, Tyson JE, Wright LL, Ehrenkranz RA, Stoll BJ, Fanaroff AA. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146(6):798–804. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, Wood E, Bartlett DJ, Galuppi BE. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288(11):1357–1363. [DOI] [PubMed] [Google Scholar]
- 16.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428. [DOI] [PubMed] [Google Scholar]
- 17.Vendettuoli V, Bellu R, Zanini R, Mosca F, Gagliardi L. Changes in ventilator strategies and outcomes in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2014;99(4):F321–324. [DOI] [PubMed] [Google Scholar]
- 18.Fischer HS, Buhrer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics. 2013;132(5):e1351–1360. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Collaborative Victorian Infant G. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–356. [DOI] [PubMed] [Google Scholar]
- 20.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012;160(4):553–558. [DOI] [PubMed] [Google Scholar]
- 21.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, Newman JE, Peralta-Carcelen M, Yolton K, Dusick AM, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–228 e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol. 2012;39(3):585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onland W, Debray TP, Laughon MM, Miedema M, Cools F, Askie LM, Asselin JM, Calvert SA, Courtney SE, Dani C, et al. Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study. BMC Pediatr. 2013;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There were 5086 ELBW infants born at <27 weeks GA and between 401–1000g birth weight registered in GDB. 1109 (3 for missing data, 73 with congenital heart disease or chromosomal anomalies, and 1033 died within the first day of life) were excluded for not meeting inclusion criteria, leaving a study cohort of 3977 infants. 3615 were analyzed for the composite primary outcome and 2543 were analyzed for neurodevelopmental outcome.


