Abstract
The discovery of miRNAs in the mid-90s has changed the dogma of gene expression regulation. Currently, miRNAs are the main theme of thousands of publications each year and their involvement in human diseases is everyday more deeply understood. With that being known, what are the actual clinical applications of miRNAs and how far are they truly from the patients? To address this question, we reviewed the miRNA diagnostic and therapeutic market. With many companies developing miRNA panels, the activity is high in the diagnostic area. Some products, notably for thyroid cancer (Interpace Diagnostic), are already available to clinician and covered by major insurance companies. In comparison, the therapeutic market, mainly driven by miRNA mimics and antagomiR products, is less advanced. Miravirsen (produced by Roche/Santaris) and RG-101 (produced by Regulus Therapeutics), designed to treat hepatitis C, are considered the flagship products of this class of future drugs. All of the miRNA-based drugs are currently in clinical trials and none have yet reached the pharmaceutical breakthrough. However, acquisition of miRNA-based companies by major pharmas is sending a positive feedback on their potentials. With multiple initiatives on their way, the next years will definitely be determinant for the miRNA market that is still in his infancy.
Key words: microRNA, diagnosis, therapeutics, cancer, drug development
INTRODUCTION
For the last thirty years, the fundamental research into RNA biology has grown at an exponential rate. We are now better positioned than ever to understand the involvement of RNA in almost all critical cellular processes. Indeed, for many years, the number of non-coding RNA discovered has steadily increased. Hence, it is not surprising that several Nobel prizes were awarded for corner stone RNA discoveries, such as those won by Cech and Altman in 1989 (RNA catalytic activities; (1)), Ramakrishnan, Steitz and Yonath in 2009 (ribosome structure; (2)), and of most interest for this review, to Fire and Mello in 2006 (RNA interference; (3)).
Considering the increase in RNA-focused research, one can expect that the advancement of general and specific knowledge about RNA could result in direct clinical applications. For example, more than 45,000 studies were published in 2017 on RNA (Figure 1A). From these, a large proportion of the studies either considered that their work could contribute to the diagnosis or the treatment of disease (about 13,000 and 10,000, respectively; Figure 1B).
Figure 1.
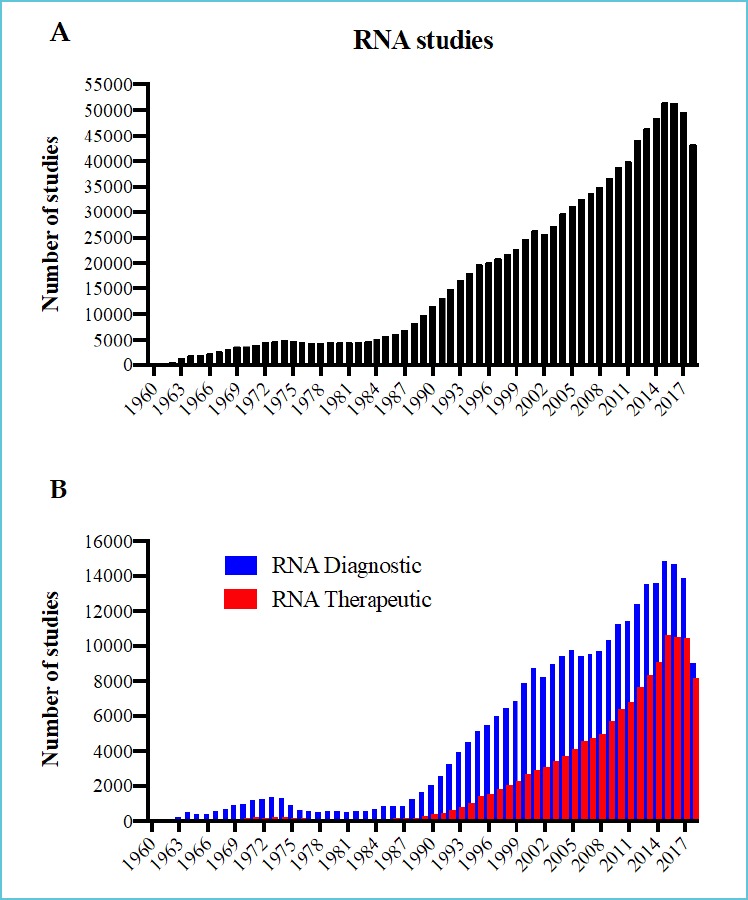
Number of studies published in PubMed per year for: (A) RNA studies; (B) RNA diagnostic and therapeutic studies
From the multitude of RNA discoveries, one of the most important was the discovery of RNA interference by Fire and Mello and miRNAs by Ambros and colleagues (4, 5). Thousands of these small RNAs of approximately 20 nucleotides in length have been identified in humans so far and are conserved across all species (6). Detectable in biopsies and body fluids, miRNAs are considered as very sensitive and specific circulating biomarker (7). The enthusiasm for miRNA in the diagnostics field is reflected by the number of related publications, reaching around 11,000 papers in 2018 (Figure 2A).
Figure 2.
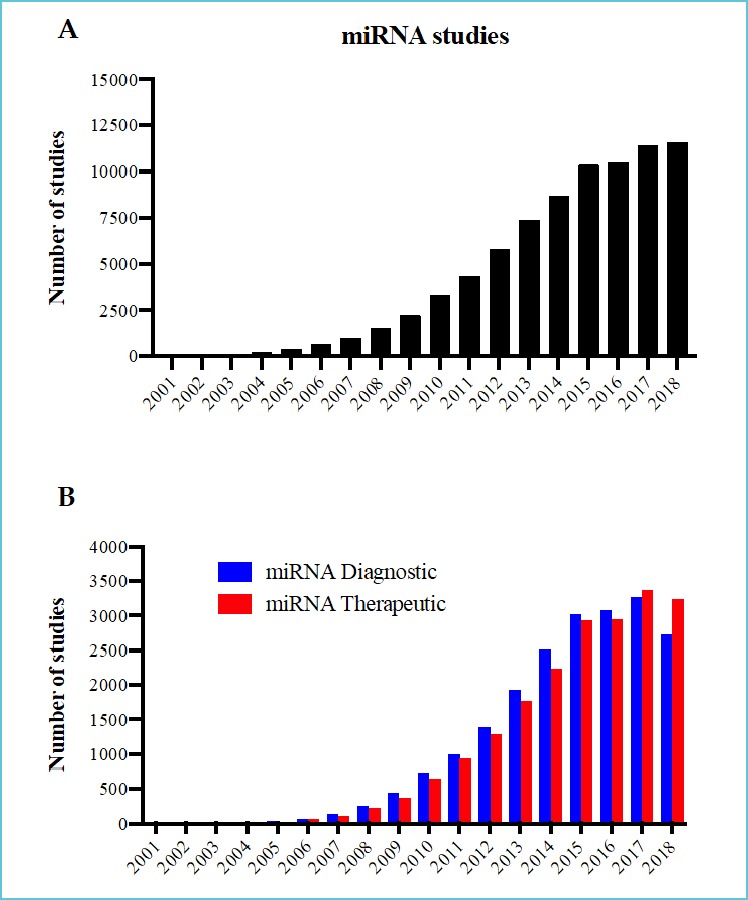
Number of studies published in PubMed per year for: (A) miRNA studies and (B) miRNA diagnostic and therapeutic studies
On the therapeutic side, polypharmacology is gaining a lot of interest in the pharmaceutical era (8). It is now clear that human diseases are complex and that deregulation of multiple genes is often needed to transform a normal cell into a pathological one (9). Furthermore, redundant cellular pathways can limit efficiency of monogenic targeting compounds (10). Conversely, the miRNA’s function is by definition based on multitargeting (11). In fact, it is well established that these small RNAs recognize their mRNA targets mainly by the 2nd to the 8th nucleotides of their 5’ end. Mismatches in the 3’ sequence allow one miRNA to specifically bind to hundreds of different mRNAs simultaneously regulating their expression (11, 12). It is not surprising that these endogenous multitargeting molecules gained a lot of interest in the therapeutic field. In fact, nearly 3,500 studies were published in 2018 on miRNA-based therapeutics (Figure 2B).
Similarly, a multitude of clinical trials were conducted or are currently underway to test new miRNA based treatments. This effervescence is therefore expressing the evolution of this still young and relatively immature field of utilizing the miRNA as a therapeutic tool.
With thousands of academic publications each year, how far from patients are miRNAs? To answer this question and bring a different angle, we reviewed the market of the diagnostic and therapeutic applications of miRNAs. With a lot of companies offering miRNA-profiling services, our focus was to highlight the ones providing specific expression panels for a given clinical application. On the therapeutic side, multiple clinical trials are currently ongoing and we focused on the products that are the most advanced in the field.
miRNA-BASED DIAGNOSTICS
Applications in the cancer field
Since their initial discovery in 1993, miRNA have showed a great diagnostic potential by being associated with various diseases (5, 7). Since then, the number of publications on the diagnostic potential of miRNA grew almost exponentially (Figure 2B), which attracted numerous companies to develop new miRNA-based diagnostic tools. To our knowledge, the first company focusing on miRNA-based diagnosis assays was Rosetta Genomics (NASDAQ: ROSG), an Israeli company incorporated in early 2000. In partnership with Precision Therapeutics, a personalized cancer therapy company, they launched in 2012 miRview™ mets a miRNA panel allowing the identification of cancers of unknown or uncertain primary origin (CUP) (Table 1).
Table 1.
Currently active companies working on miRNA-based diagnostics
| Companies | Product | Targeted miR | Disease type | Development phase | Reference |
|---|---|---|---|---|---|
| Interpace Diagnostics/Asuragen | ThyraMIR/ThyGENX | miR-29b-1-5p, miR-31-5p, miR-138-1-3p, miR-139-5p miR-146b-5p, miR-155, miR-204-5p, miR-222-3p, miR-375 and miR-551b-3p |
Thyroid and pancreatic cancer | Available | thygenext-thyramir.com |
| Rosetta Genomics/Precision Therapeutics Genoptix |
miRview mets Reveal |
miRNA library miRNA library |
Identify tumor origin of cancer Thyroid |
Available Available |
rosettagenomic.comoncotest.co.il genoptix.com |
| TAmiRNA TAmiRNA |
OsteomiR ThrombomiR |
Panel of 19 miRNAs Panel of 11 miRNAs |
Osteoporosis Cardiovasc. Disease |
Available Available |
tamirna.com - |
| Hummingbird Diagnostics | - | Panels (unknown) | Multiple (cancers, brain, heart) | Pre-Clinical & Phase 1 | hummingbird-diagnostics.com |
| DiamiR | CogniMIR Others |
Panel (unknown) Panel (unknown) |
Alzheimer Brain diseases |
Phase 1 | diamirbio.com |
| Mirnext | Panel with miR 423-5p | Heart failure | Development | hmirnext.com | |
| Quanterix/DestiNA Genomics | Simoa | miR-122 | Liver toxicity | Pre-Clinical | quanterix.com |
These cancers account for up to 15% of newly diagnosed cancer in the U.S. every year (13, 14). This CUP classifier was able to identify 42 different tumor types using microarray that measures the expression levels of 64 miRNAs. The miRviewTM mets panel was able to identify accurately 90% of the 509 validation sample set. The assay also showed 88% correspondence with the patient’s clinicopathological evaluation (14). Based on this success, Rosetta Genomic introduced a new product called RossettaGX Reveal™ (Reveal) in 2016.
This new miRNA classifier relied on qRT-PCR to differentiate between benign or indeterminate thyroid nodules using FNA cytology smears. Reveal’s performance was validated using a multicenter retrospective cohort of 189 FNA smears and achieved a negative predictive value of 91%, a sensitivity of 85% and a specificity of 72% (15). Unfortunately, the company declared bankruptcy in May 2018 after a $10 million acquisition deal by Genoptix failed. Interestingly, Interpace Diagnostics acquired most of the equipment through a bankruptcy auction and hired some of Rosetta Genomics employers.
Interpace Diagnostics (NASDAQ: IDXG), is based in New Jersey and is a molecular diagnostic testing company that is offering personalized medicine strategies for the diagnosis of thyroid and pancreatic cancer. Interpace acquired a solution developed by Asuragen combining ThyraMIR®, a miRNA classifier, and ThyGeNEXT®, an oncogene panel for thyroid cancer stratification (Table 1). Initial validation was completed by Asuragen in 2015, over 12 endocrinology centers across the U.S. and 638 surgical and fine needles aspirations (FNA) biopsies were analyzed (16). The combination of ThyraMIR® and ThyGeNEXT® offers an interesting alternative as 15-30% of standard cytological evaluations fail to discriminate benign from the malignant lesions (17, 18). The ThyraMIR® classifier includes the quantification of 10 miRNAs: miR-29b-1-5p, miR-31-5p, miR-138-1-3p, miR-139-5p, miR-146b-5p, miR-155, miR-204-5p, miR-222-3p, miR-375 and miR-551b-3p (Table 1).
This panel was trained using 240 well-characterized, surgically resected, benign or malignant thyroid lesions. A validation set of 54 independent resected thyroid tissues and 235 preoperative thyroid FNAs was then used for threshold optimization (16). Based on this study, Interpace Diagnostic claims a Negative Predictive Value of 94%, a Positive Predictive value of 74% and a reduction of 85% of unnecessary surgeries. Interpace Diagnostic is CLIA certified and CAP accredited, but both tests are not FDA approved. Availability of ThyraMIR® through Labcorp, was announced on January 12, 2016. Interpace also received Medicare coverage in 2016 covering over 50 million patients across the United States. Most recently, in November 2018, the Blue Cross Blue Shield and the U.S. Federal Employee Health Benefit Program have agreed to include the ThyGeNEXT®/ThyraMIR® combined tests for their 5.3 million beneficiaries.
Hummingbird Diagnostics (formerly known as Comprehensive Biomarker Center) was founded in 1998 in Heindelberg, Germany, and has now extended to Boston, Massachusetts, in the United States. Operating as a subsidiary of Febit Holding, this company is hands-on in the development of novel miRNA signatures in liquid biopsies for early detection of various diseases, ranging from cancer (Non-small-cell lung carcinoma, melanoma, breast cancer), to neurodegenerative (multiple sclerosis, Alzheimer, Parkinson), cardiovascular (acute myocardial infarction and heart failure) and inflammatory bowel disease (19-21). With its DIN EN ISO/IEC 17025:2005 accreditations for RNA (including miRNA) extraction and microarray services (Agilent Certified Service Provider), the company profiled more than 7,000 disease-related body fluid samples so far. The bioinformatics and statistical processing of those large expression data led to the identification of multiple disease-related miRNA panels (Table 1). Although none of these are currently commercially available, Hummingbird Diagnostics has 17 granted patents in the field of whole blood expression profiling. The clinical validation of their miRNA signatures for early diagnostic use are ongoing with the funding received through their participation in three European FP7-funded consortia (BestAgeing, RiskyCAD and EURenOmics).
Applications in age-related diseases
DiamiR is located in Monmouth Junction, New Jersey and published their first article describing the use of miRNA biomarkers in mild cognitive impairment in 2012 (22). DiamiR have since published several articles with a similar scope, which is the use of miRNA as markers of neurodegenerative and neurodevelopmental disorders (23-25). This work was funded in part by the Michael J. Fox Foundation for Parkinson’s Research and through a Small Business Innovation Research (SBIR) phase II grant of $1.5M from the National Institute on Aging (NIA) of the National Institutes of Health (NIH) in 2014 and 2015. More recently, in March 2017, the NIA of the NIH awarded DiamiR another SBIR Phase IIB grant of $2.75M over three years to further support the development of their branded lead product, CogniMIR™ (Table 1). CogniMIR™ is currently in clinical trial testing for early detection of Alzheimer’s disease at the presymptomatic, mild cognitive impairment and dementia stages. Using different brain-derived miRNA signatures, DiamiR also expects to test for Parkinson’s disease, frontotemporal degeneration, and amyotrophic lateral sclerosis. However, those products are still in the validation process.
TAmiRNA is another European leader in miRNA diagnostics that was founded in 2013 as a spinoff of two Austrian companies, BOKU and Evercyte. This R&D company develops and offers validated microRNAs panels as additional tools for the diagnostic of age-related disorders. Funded by AWS Seedfinancing and EU Horizon2020 programs, TAmiRNA demonstrated the clinical utility of their licensed miRNAs as biomarkers in osteoporosis (26). OsteomiR™ is their lead product intended to provide the risk of a first fracture in female patients with postmenopausal osteoporosis and type-2 diabetes (27-29). The integration of the expression level of 19 blood-circulating miRNAs gives a calculated fracture-risk index that could be used for preventive therapy and treatment follow-up. Similarly, TAmiRNA also proposes the ThrombomiR™ panel (11 miRNAs) to assess platelet function and the ToxomiR™ panel (19 miRNAs) to evaluate the toxicity occurring in various tissues (Table 1). Kits based on primer-coated qPCR plates can be either purchased (except ToxomiR™) or samples can be directly processed by TAmiRNA, from extraction to data analysis. By starting a partnership agreement with SimplicityBio in February 2017, development of additional miRNA panels are expected. The Swiss-based bioinformatics company has a robust in silico biomarker identification pipeline that could accelerate TAmiRNA’s main goal in offering advanced miRNA markers for the diagnosis and prognosis of age-related disorders.
Applications in cardiac function and liver toxicity
Mirnext is a Dutch biomedical company based in Amsterdam that is interested in the diagnostic potential of miRNAs. It was established in 2014 as a new entity of ACS Biomarker, which was built on the Galectin-3 biomarker of heart failure (30, 31), now out-licensed to BG Medicine and available in clinics. Mirnext is currently financed through Life Sciences Fund Amsterdam and Limburg Ventures, two venture capital investors of The Netherlands. Similar to ACS Biomarker, Mirnext’s main goal is to identify and commercialize biomarkers in the cardiovascular field but with full dedication towards miRNAs. Their high-throughput, disease-based miRNA profiling identified, among others, miR423-5p as a useful marker of heart failure (32). Together with other clinically relevant miRNAs, Mirnext pursued their validation in large patient cohorts with different cardiovascular diseases including heart failure, coronary artery disease and myocardial infarction. Thus, their miRNA panel integrates many different disease mechanisms useful for the identification and stratification of those pathologies. In addition to the diagnosis of heart diseases, Mirnext is aiming to evaluate cardiovascular risk profiles (mortality, hospitalization) of the individuals tested as part of their multi-marker heart failure test. A single test is expected to provide the clinician extensive information on the patient’s cardiovascular health to initiate targeted treatments. At the time of this writing, we were unable to access the company’s website.
Quanterix (NASDAQ QTRX) is a biotech company founded in 2007 in Lexington, Massachusett. Through the development of their ultra-sensitive digital biomarker detection technology Simoa®, Quanterix provides healthcare researchers the ability to investigate the continuum of disease progression. In March 2018, they announced a collaborative effort with DestiNA Genomics to enhance RNA biomarker detection. DestiNA was founded in 2010 in Edinburgh, United Kingdom, where they developed and patented a unique PCR-free, chemical-based technology for the detection and quantification of nucleic acids such as miRNAs, without prior isolation from serum or plasma (33, 34). This highly-specific nucleic acid detection combined to the ultra-sensitive Simoa® system provides a solid support for disease-related miRNA biomarker testing. Accordingly, the collaboration’s first initiative was focused on miR-122 as a liver toxicity marker (35, 36). They demonstrated that their assay detects miR-122 earlier and outperforms the current protein-based biomarkers in specifically detecting and quantifying liver toxicity.
miRNA-BASED THERAPEUTICS
Several pharmaceutical and biotech companies have launched miRNA projects in their development pipeline (Table 2). Companies are mainly working on two types of products; miRNA mimics and antagomiRs. The miRNA mimics are used to re-establish the concentration of a specific miRNA suppressed by the evolution of a given pathology (37, 38). Inversely, antagomiRs are used to suppress the function of specific miRNAs overexpressed and mechanistically involved in a disease (37, 38). In order to allow the development of miRNA therapeutics, scientists must address two main challenges: the stability and delivery. First, RNA molecules are quite unstable because of their 2’-OH chemical group (39). Therefore, several companies, such as Dharmacon, BioSyn and GenScript, can produce natural and chemically modified RNA (2’-O-methyl; 2’-OMe, locked nucleic acid; LNA, of 2’-fluor; 2’-F, phosphorothioate; PS) to stabilize and reduce the high reactivity of RNA molecules. The other major challenge is the delivery of these RNAs to the desired site of action (39). Therapeutic application requires the correct delivery of the RNAs to the targeted organs in order to maintain adequate treatment specificity. When a treatment requires a systemic delivery through intra-venous injection, the delivery strategies are either passive or active (40, 41). The passive strategy utilizes the tendency of several organs, like the liver, the spleen and the lymph nodes to internalize accumulated particles. Using this non-specific approach, designed nanoparticles or liposome-like particles incorporating RNAs can be targeted to these organs (40, 41). Inversely, the active strategies combine the RNA or the particle with a specific molecule that will bind to the cells of interest and will be endocytosed (40, 41). These structural and delivery challenges, albeit being constantly addressed by new design strategies, still complicate the development of miRNA therapeutics.
Table 2.
Currently active companies working on miRNA-based therapeutics
| Companies | Product | Targeted miR | Disease type | Development phase | Reference |
|---|---|---|---|---|---|
| Roche/Santaris | Miravirsen | miR-122 | HCV | Phase 2 | roche.com |
| Regulus Therapeutics | RG-101 RG-012 RG-125 |
miR-122 miR-21 miR-103/107 |
HCV Alport syndrome NASH |
Phase 2 (hold) Phase 1 Phase 1 |
regulusrx.com |
| MiRagen Therapeutics | MRG-201 MRG-106 MRG-107 MRG-110 |
miR-29b miR-155 miR-155 miR-92 |
Fibrosis Lymphoma and leukemia ALS Ischemia |
Phase 2 Phase 1 and Phase 2 Pre-Clinical Phase 1 |
miragentherapeutics.com |
| ENGeneIC | Mesomir | miR-16 | Mesothelioma | Phase 2 | engeneic.com |
| Abivax | ABX464 | miR-124 | IBD | Phase 2 | abivax.com |
| Synlogic | Screening | synlogictx.com | |||
| Opko | Screening | opko.com | |||
| Alnylam Pharmaceuticals | Screening | alnylam.com | |||
| Interna Technologies | Screening | interna-technologies.com | |||
| Mello Biotech | Screening | mellobiotech.com | |||
Applications in liver disease
Among the most advanced products, there is Miravirsen (or SPC3649), an antagomiR targeting miR-122. Santaris Pharma initially developed this drug candidate before Roche acquired the company in 2014 to expand its RNA therapeutic research and development department (Table 2). Miravirsen is a locked nucleic acid (LNA) containing phosphorothioate modifications. MiR-122, is known to be essential in the life cycle of hepatitis C virus (HCV) expressed in the liver (42, 43). Reducing the activity of miR-122 in the context of HCV infection is important. In fact, miR-122 is a host factor that binds to the 5’-UTR region of the HCV genome and enhances its transcription (43, 44). In phase 1 clinical trials, some patients who received high doses of Miravirsen in monotherapy resulted in undetectable HCV RNA levels (43, 44). Because Miravirsen is a modified RNA (LNA and phosphorothioate), it naturally accumulates in the liver and does not require special delivery strategy. Miravirsen is currently undergoing multiple phase 2 clinical trials.
Another product was developed to target miR-122, RG-101, and is produced by Regulus Therapeutics (NASDAQ: RGLS) in collaboration with Ionis Pharmaceuticals and GSK (Table 2). RG-101 is an N-acetyl-D-galactosamine- conjugated RNA antagomiR that also targets miR-122 in HCV infected hepatocytes (45). RG-101, like Miravirsen, shows considerable efficacy with patients displaying undetectable HCV RNA levels (45). However, some serious adverse events of severe jaundice were recently declared in a clinical trial and the FDA put the studies on hold until the situation is clarified. It is worth mentioning that Regulus was also working on RG-125 (also described as AZD4076), an antagomiR targeting miR-103/107, in phase 1 clinical trial for treatment of nonalcoholic steatohepatitis (NASH; Identifier NCT02612662 and NCT02826525) as well as RGLS5040, an anti-miR-27 aiming to reduce cholestatic diseases. However, development of these latter two were recently suspended.
Applications in fibrotic disease
Regulus has also worked with Genzyme (Sanofi) to test the efficacy of RG-012, an antagomiR against miR-21, which reduces the fibrogenesis of organs associated with Alport syndrome (46). This is an X-linked disease and is characterized by kidney disease, hearing loss and visual impairment caused by mutations of the genes encoding type-IV collagen (47). The use of a modified single-stranded antagomiR with phosphorothioate, 2’-O-methoxyethoxy and constrained ethyl modifications showed an important improvement in the survival of a Alport mouse model with a reduction of kidney disease progression (46). Despite interesting results, the phase 1 clinical trial of RG-012 has recently been discontinued mid-2018 because of the reorganization between Regulus and Sanofi (Clinical trial identifier NCT03373786).
Another promising company is MiRagen Therapeutics (NASDAQ: MGEN), based in Boulder, Colorado. First, the company developed MRG-201, also known as Remlarsen, a miRNA mimic that aims to restore the levels of miR-29b, which is a negative regulator of the extracellular matrix deposition processes. The miR-29 family (miR-29a/b/c) is constantly downregulated in fibrotic diseases. MRG-201 is an LNA RNA mimic that is administered by intradermal injection and the phase 2 clinical trial is currently underway (Identifier: NCT03601052). Remlarsen could virtually be used for the treatment or prevention of pathological cutaneous fibrosis, as well as of other fibrotic diseases, including idiopathic pulmonary fibrosis (48).
Applications in cancer
While some companies are having great successes, others struggle to positively impact patient outcomes. This was the case of MiRNA Therapeutics (NASDAQ: MIRN) and a miRNA mimic, MRX34. MiR-34, is a well characterized tumor suppressor downregulated in a broad range of cancers (49-51). MRX34 was delivered as a double stranded RNA encapsulated into a liposome-formulated nanoparticle. Preclinical studies were promising when used in several cancer types such as renal cell carcinoma, acral melanoma and hepatocellular carcinoma (52). However, the FDA halted their phase 1 clinical trial when many immune-related serious adverse events leading to death were registered. It reached a point where MiRNA Therapeutics reduced its staff before Synlogic Inc (NASDAQ: SYBX) finally acquired it in 2017.
MiRagen Therapeutics is actively developing MRG-106, also known as Cobomarsen, an LNA antagomiR that targets miR-155. This miRNA is involved in the differentiation and proliferation of blood and lymphoid cells. Cobormarsen is actually involved in phase 1 trials (Identifier NCT02580552) and phase 2 clinical trials (Identifier NCT03713320), with the goal of treating certain types of lymphoma and leukemia (53). Similarly, MRG-107 also targets miR-155 to alleviate symptoms associated with amyotrophic lateral sclerosis (ALS) but has not yet entered clinical trials. In an ever-growing pipeline, they also work on MRG-110 in collaboration with Servier. This LNA antagomiR targets miR-92 in order to treat ischemic conditions such as heart failure (48). Its phase 1 clinical trial is currently recruiting (Identifier NCT03603431).
Pharmaceutical and biotech companies are heavily engaged in developing successful products and RNA biologics are closer than ever to entering the market. Another indicator of this effervescence is the acquisition of RNA-based companies by pharmaceuticals giants. Santaris Pharma was acquired by Roche in 2014, SiRNA Therapeutics by Merck in 2007, followed by the acquisition of this division by Alnylam Pharmaceuticals in 2014, and more recently MiRNA Therapeutics by Synlogic Inc. However, even more companies are currently testing new miRNA therapeutics. For example, ENGeneIC is currently designing and producing Mesomir, a miRNA mimic that aims to replace miR-16, a tumor suppressor that is reduced in cases of cancer, such as malignant pleural mesothelioma (54). It successfully completed phase 1 clinical trial and will soon start phase 2 (55). On another hand, Abivax produces ABX464, a small molecular compound that triggers the increase of miR-124 to reduce the symptoms of inflammatory bowel disease for patients refractory to anti-TNF biologics and corticosteroids. It is currently in preparation for a phase 2b clinical trial for ulcerative colitis and phase 2a for Crohn’s disease.
Finally, a multitude of companies work in preclinical and large screening studies to identify potential biologic miRNA such as Opko with their CURNA program, Alnylam Pharmaceuticals, Interna Technologies and Mello Biotech. These companies could therefore increase, in the next several years, the number of miRNA therapeutics being tested or entering the market.
CONCLUSION
Enthusiasm, promise and hope are evident in the miRNA industry. As described, multiple companies are dedicating significant efforts and resources to develop miRNA-based products. The diagnostic field is definitely the most advanced with some miRNA panels already offered to clinicians and covered by major insurance companies. However, considering the thousands of publications in this area, miRNAs as diagnostic products can still be considered in their infancy. On the therapeutic side, despite the potentials, the miRNA-based therapeutic breakthroughs have not arrived yet. Recently, an analytical model based on technological growth metrics showed that miRNAs still require time to reach the maturity point needed to yield a significant number of products that could enter the market (56). For this reason, most of the technologies discussed are currently in clinical trials.
The development and commercialization of new diagnostic and therapeutic tools is definitely a long process. Considering the first evidence of the involvement of miRNA in human disease in 2002 and the first detection of miRNAs in blood in 2008, only a decade later, tremendous progress and effort has been made to bring these small RNAs from the bench to the bedside (57, 58).
REFERENCES
- 1.North G. Nobel prizes: chemistry. RNA’s catalytic role. Nature. 1989;341(6243):556. [DOI] [PubMed] [Google Scholar]
- 2.Service RF. Chemistry Nobel. Honors to researchers who probed atomic structure of ribosomes. Science (New York, NY). 2009;326(5951):346-347. [DOI] [PubMed] [Google Scholar]
- 3.Bernards R. [The Nobel Prize in Physiology or Medicine for 2006 for the discovery ofRNA interference]. Nederlands tijdschrift voor geneeskunde. 2006;150(52):2849-2853. [PubMed] [Google Scholar]
- 4.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806-811. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843-854. [DOI] [PubMed] [Google Scholar]
- 6.Tetreault N, De Guire V. miRNAs: their discovery, biogenesis and mechanism of action. Clinical biochemistry. 2013;46(10-11):842-845. [DOI] [PubMed] [Google Scholar]
- 7.De Guire V, Robitaille R, Tetreault N, Guerin R, Menard C, Bambace N, et al. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: promises and challenges. Clin Biochem. 2013;46(10-11):846-860. [DOI] [PubMed] [Google Scholar]
- 8.Proschak E, Stark H, Merk D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J Med Chem. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Boran AD, Iyengar R. Systems approaches to polypharmacology and drug discovery. Curr Opin Drug Discov Devel. 2010;13(3):297-309. [PMC free article] [PubMed] [Google Scholar]
- 10.Nussinov R, Tsai CJ, Jang H. A New View of Pathway-Driven Drug Resistance in Tumor Proliferation. Trends Pharmacol Sci. 2017;38(5):427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769-773. [DOI] [PubMed] [Google Scholar]
- 12.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15-20. [DOI] [PubMed] [Google Scholar]
- 13.Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Semin Oncol. 2009;36(1):6-7. [DOI] [PubMed] [Google Scholar]
- 14.Meiri E, Mueller WC, Rosenwald S, Zepeniuk M, Klinke E, Edmonston TB, et al. A second-generation microRNA-based assay for diagnosing tumor tissue origin. Oncologist. 2012;17(6):801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lithwick-Yanai G, Dromi N, Shtabsky A, Morgenstern S, Strenov Y, Feinmesser M, et al. Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. Journal of clinical pathology. 2017;70(6):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labourier E, Shifrin A, Busseniers AE, Lupo MA, Manganelli ML, Andruss B, et al. Molecular Testing for miRNA, mRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules With Indeterminate Cytology. J Clin Endocrinol Metab. 2015;100(7):2743-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato MA, Buitrago D, Moo TA, Keutgen XM, Hoda RS, Ricci JA, et al. Predictive value of cytologic atypia in indeterminate thyroid fine-needle aspirate biopsies. Ann Surg Oncol. 2011;18(10):2893-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333-339. [DOI] [PubMed] [Google Scholar]
- 19.Fehlmann T, Backes C, Alles J, Fischer U, Hart M, Kern F, et al. A high-resolution map of the human small non-coding transcriptome. Bioinformatics (Oxford, England). 2018;34(10):1621-1628. [DOI] [PubMed] [Google Scholar]
- 20.Keller A, Fehlmann T, Ludwig N, Kahraman M, Laufer T, Backes C, et al. Genome-wide MicroRNA Expression Profiles in COPD: Early Predictors for Cancer Development. Genomics, proteomics & bioinformatics. 2018;16(3):162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahraman M, Roske A, Laufer T, Fehlmann T, Backes C, Kern F, et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Scientific reports. 2018;8(1):11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging. 2012;4(9):590-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheinerman KS, Tsivinsky VG, Abdullah L, Crawford F, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment: biomarker validation study. Aging. 2013;5(12):925-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheinerman KS, Toledo JB, Tsivinsky VG, Irwin D, Grossman M, Weintraub D, et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer’s research & therapy. 2017;9(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheinerman K, Tsivinsky V, Mathur A, Kessler D, Shaz B, Umansky S. Age- and sex-dependent changes in levels of circulating brain-enriched microRNAs during normal aging. Aging. 2018;10(10):3017-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter E, Dellago H, Grillari J, Dimai HP, Hackl M. Costutility analysis of fracture risk assessment using microRNAs compared with standard tools and no monitoring in the Austrian female population. Bone. 2018;108:44-54. [DOI] [PubMed] [Google Scholar]
- 27.Weilner S, Skalicky S, Salzer B, Keider V, Wagner M, Hildner F, et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;79:43-51. [DOI] [PubMed] [Google Scholar]
- 28.Heilmeier U, Hackl M, Skalicky S, Weilner S, Schroeder F, Vierlinger K, et al. Serum miRNA Signatures Are Indicative of Skeletal Fractures in Postmenopausal Women With and Without Type 2 Diabetes and Influence Osteogenic and Adipogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(12):2173-2192. [DOI] [PubMed] [Google Scholar]
- 29.Kocijan R, Muschitz C, Geiger E, Skalicky S, Baierl A, Dormann R, et al. Circulating microRNA Signatures in Patients With Idiopathic and Postmenopausal Osteoporosis and Fragility Fractures. J Clin Endocrinol Metab. 2016;101(11):4125-4134. [DOI] [PubMed] [Google Scholar]
- 30.Amin HZ, Amin LZ, Wijaya IP. Galectin-3: a novel biomarker for the prognosis of heart failure. Clujul medical. 2017;90(2):129-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart failure clinics. 2018;14(1):75-92. [DOI] [PubMed] [Google Scholar]
- 32.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106(6):1035-1039. [DOI] [PubMed] [Google Scholar]
- 33.Bowler FR, Diaz-Mochon JJ, Swift MD, Bradley M. DNA analysis by dynamic chemistry. Angewandte Chemie. 2010;49(10):1809-1812. [DOI] [PubMed] [Google Scholar]
- 34.Marin-Romero A, Robles-Remacho A, Tabraue-Chavez M, Lopez-Longarela B, Sanchez-Martin RM, Guardia-Monteagudo JJ, et al. A PCR-free technology to detect and quantify microRNAs directly from human plasma. The Analyst. 2018;143(23):5676-5682. [DOI] [PubMed] [Google Scholar]
- 35.Venkateswaran S, Luque-Gonzalez MA, Tabraue-Chavez M, Fara MA, Lopez-Longarela B, Cano-Cortes V, et al. Novel bead-based platform for direct detection of unlabelled nucleic acids through Single Nucleobase Labelling. Talanta. 2016;161:489-496. [DOI] [PubMed] [Google Scholar]
- 36.Rissin DM, Lopez-Longarela B, Pernagallo S, Ilyine H, Vliegenthart ADB, Dear JW, et al. Polymerase-free measurement of microRNA-122 with single base specificity using single molecule arrays: Detection of drug-induced liver injury. PloS one. 2017;12(7):e0179669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metias SM, Lianidou E, Yousef GM. MicroRNAs in clinical oncology: at the crossroads between promises and problems. Journal of clinical pathology. 2009;62(9):771-776. [DOI] [PubMed] [Google Scholar]
- 38.Farooqi AA, Fayyaz S, Shatynska-Mytsyk I, Javed Z, Jabeen S, Yaylim I, et al. Is miR-34a a Well-equipped Swordsman to Conquer Temple of Molecular Oncology? Chemical biology & drug design. 2016;87(3):321-334. [DOI] [PubMed] [Google Scholar]
- 39.Haussecker D. Current issues of RNAi therapeutics delivery and development. Journal of controlled release : official journal of the Controlled Release Society. 2014;195:49-54. [DOI] [PubMed] [Google Scholar]
- 40.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chemistry & biology. 2012;19(1):60-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peer D, Lieberman J. Special delivery: targeted therapy with small RNAs. Gene therapy. 2011;18(12):1127-1133. [DOI] [PubMed] [Google Scholar]
- 42.Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic acids research. 2014;42(1):609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindow M, Kauppinen S. Discovering the first microRNA-targeted drug. The Journal of cell biology. 2012;199(3):407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen HL, Reesink HW, Zeuzem S, Lawitz E, Rodriguez-Torres M, Chen A, et al., editors. A randomized, double-blind, placebo (plb) controlled safety and anti-viral proof of concept study of miravirsen (MIR), an oligonucleotide targeting miR-122, in treatment na√VØve patients with genotype 1 (gt1) chronic HCV infection. Hepatology; 2011: WILEY-BLACKWELL COMMERCE PLACE, 350 MAIN ST, MALDEN 02148, MA USA. [Google Scholar]
- 45.Baek J, Kang S, Min H. MicroRNA-targeting therapeutics for hepatitis C. Archives of pharmacal research. 2014;37(3):299-305. [DOI] [PubMed] [Google Scholar]
- 46.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125(1):141-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson S, Bush JS. Alport Syndrome. StatPearls. Treasure Island FL: StatPearls Publishing LLC.; 2018. [Google Scholar]
- 48.Gallant-Behm CL, Piper J, Lynch JM, Seto AG, Hong SJ, Mustoe TA, et al. A microRNA-29 mimic (remlarsen) represses extracellular matrix expression and fibroplasia in the skin. The Journal of investigative dermatology. 2018. [DOI] [PubMed] [Google Scholar]
- 49.Bouchie A. First microRNA mimic enters clinic. Nature biotechnology. 2013;31(7):577. [DOI] [PubMed] [Google Scholar]
- 50.Adams BD, Parsons C, Slack FJ. The tumor-suppressive and potential therapeutic functions of miR-34a in epithelial carcinomas. Expert opinion on therapeutic targets. 2016;20(6):737-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, et al. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ling H, Girnita L, Buda O, Calin GA. Non-coding RNAs: the cancer genome dark matter that matters! Clinical chemistry and laboratory medicine. 2017;55(5):705-714. [DOI] [PubMed] [Google Scholar]
- 53.Seto AG, Beatty X, Lynch JM, Hermreck M, Tetzlaff M, Duvic M, et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. British journal of haematology. 2018;183(3):428-444. [DOI] [PubMed] [Google Scholar]
- 54.Reid G, Pel ME, Kirschner MB, Cheng YY, Mugridge N, Weiss J, et al. Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24(12):3128-3135. [DOI] [PubMed] [Google Scholar]
- 55.van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. The Lancet Oncology. 2017;18(10):1386-1396. [DOI] [PubMed] [Google Scholar]
- 56.Beierlein JM, McNamee LM, Ledley FD. As Technologies for Nucleotide Therapeutics Mature, Products Emerge. Molecular therapy Nucleic acids. 2017;9:379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524-15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141(5):672-675. [DOI] [PubMed] [Google Scholar]


