Abstract
MicroRNAs (miRNAs) are small, protein noncoding RNAs that regulate gene expression post-transcriptionally. Their role is considered to set the gene expression to the optimal level, or in other words to provide “fine tuning” of gene expression. They regulate essential physiological processes such as differentiation, cell growth, apoptosis and their role is known in tumor development too.
At tissue level differential miRNA expression in endocrine disorders including endocrine malignancies has also been reported. A new era of miRNAs-related research started when miRNAs were successfully detected outside of cells, in biofluids, in cell-free environments. Their significant role has been demonstrated in cell-cell communication in tumor biology.
Due to their stability circulating miRNAs can serve as potential biomarkers. In common diseases circulating miRNAs can be potentially proposed as screening biomarkers and they are also useful to detect tumor recurrence hence they can be applied in post-surgery follow-up too. MiRNAs as diagnostic markers can also be helpful at tissue level when certain histology diagnosis is challenging. Beside diagnosis, tissue miRNAs have the potential to predict prognosis.
Intensive research is carried out regarding endocrine tumors as well in terms of miRNAs. However, until now miRNAs as biomarkers do not applied in routine diagnostics, probably due to the challenging preanalytics. In this review we summarized tissue and circulating miRNAs found in thyroid, adrenal, pituitary and neuroendocrine tumors. We aimed to highlight the most important, selected miRNAs with potential diagnostic and prognostic value both in tissue and circulation. Common miRNAs across different endocrine neoplasms are summarized and miRNAs enriched at 14q31 locus are also highlighted suggesting their general role in tumorigenesis of endocrine glands.
Key words: miRNA, endocrine tumors, adrenal, pituitary, thyroid, circulating miRNA, biomarker
INTRODUCTION
MicroRNAs (miRNAs) are small, protein noncoding RNAs that regulate gene expression post-transcriptionally. Through RNA interference miRNAs target mRNAs mainly at 3’ untranslated regions but even the coding sequence or 5’UTR were described to be miRNA target regions [1]. After biogenesis the mature miRNA incorporates into miRNA-induced silencing complex (miRISC) [1]. In the miRISC complex, based on sequence complementarity miRNAs cause translational repression, mRNA destabilization or mRNA cleavage. However in some particular cases miRNAs can enhance gene expression as well [1]. It is thought that approximately 3050% of all protein-coding genes might be controlled by miRNAs [2]. One miRNA potentially targets several transcripts, and one gene’s transcription is influenced by numerous miRNAs. Therefore, the net physiological outcome is the result of a miRNA target network. Their role is considered to set the gene expression to the optimal level, or in other words provide “fine tuning” and adaptive setting of gene expression [3]. Their roles have been described in the regulation of several physiological and pathophysiological cellular processes such as proliferation, differentiation, metabolism and apoptosis.
A new era of miRNA-related research started when miRNAs’ presence was proved outside of cells, in biofluids. Henceforth extracellular miRNAs have been considered as a novel type of biomarkers that are secreted and can be taken up by various cells. Furthermore, as signal mediators they can function similarly to hormones or cytokines. Several studies showed correlations between circulating miRNA dysregulation and pathophysiological conditions. Regarding neoplastic diseases extracellular miRNAs recently have been investigated and linked to diagnosis, prognosis and recurrence [4]. Differential miRNA expression in endocrine disorders including malignancies has also been reported [5, 6].
Extracellular (EC) miRNAs are secreted by nearly all kinds of cells and therefore they are detectable practically in all body fluids [7]. As unprotected miRNAs are sensitive to degradation mainly through RNAses present in large amount in these fluids, miRNAs in circulation are bound to proteins (mainly Argonaute (AGO)) or they are packaged into vesicles that protect miRNAs against degradation or cleavage. Based on size, EC vesicles are mainly categorized as exosomes, microvesicles or apoptotic bodies. Exosomes are small (approximately 30-100 nm) membrane-limited secreted vesicles [8]. They are formed in the endosomal compartments of cell (multivesicular endosomes or MVEs) and can be released to extracellular space. Microvesicles are more variable in size, typically between 501000 nm. They are generated by directly budding or shedding off the plasma membrane. Both exosomes and microvesicles contain various molecules including mRNA, miRNA, proteins, cytokines and different surface receptors specific for their cell origin. Interestingly, several miRNAs were found in high density lipoprotein (HDL) particles as well [8].
Since their discovery there is exponentially increasing information regarding EC vesicle function. Studies showed their significant role in cell-cell communication in immunology and tumor biology. For instance, exosomes secreted by dendritic cells carry antigens and are able to induce immune response [9]. They can mediate paracrine signals of cancer cells influencing tumor microenvironment by exosome secretion in promoting growth by inhibiting antitumor immune response and by facilitating angiogenesis, cell migration and metastasis [10]. Also, tumor cells were found to exhibit self-promoting effect by secreting microvesicles [11]. However, gastric cancer cells were detected to eliminate tumor-suppressor miRNAs by exosome secretion [12].
On the whole, in a malignant tumor overexpressed (oncogenic) and downregulated (tumor suppressor) miRNAs can be useful as potential biomarkers. MiRNAs are tissue specific, they may be unique identifiers of certain tumor types both in tissue and in circulation. The purpose of miRNA biomarkers can be various and they have a great potential in many ways. In frequent diseases, e.g., thyroid nodule (where prevalence is 2-6%, 19-35% and 8-65% by palpation, ultrasound and autopsy, respectively) circulating miRNAs can be potentially recommended as screening biomarkers [13]. miRNAs as diagnostic markers can also be helpful when cytology following fine-needle aspiration biopsy (FNAB) has to be performed, as from 3-6% to 10-25% of FNAB are interpreted as indeterminate without definitive diagnosis regarding thyroid tumors [13]. Additionally, in any tumor where histological diagnosis can be challenging (e.g. adrenal carcinoma or pheochromocytoma) miRNA biomarkers can be used to help diagnosis. Beside diagnosis, tissue miRNAs have the potential to predict prognosis and therapy response as well. Circulating miRNAs are also useful to detect tumor recurrence hence they can be applied in post-surgery follow-up.
In this review we focus on tissue and circulating miRNAs in thyroid, adrenal, pituitary and neuroendocrine tumors. Although the frame of this review cannot allow assessing all miRNAs in all the above mentioned neoplasms, for that many excellent reviews are available targeting a single tumor type, rather we would like to highlight the most important, selected miRNAs as potential diagnostic and prognostic tissue and circulating biomarkers.
THYROID TUMORS
Thyroid cancer is the most frequent endocrine malignancy. The majority of thyroid tumors (~95%) arise from follicular cells and they are categorized as papillary (PTC, 75-80%) and follicular (FTC, 10-15%) or anaplastic thyroid cancer (ATC, 0.2-2%). Tumors developing from parafollicular C cells are called medullary thyroid cancer (MTC) representing ~5-10% of all thyroid cancers. Although commonly they occur sporadically, some of them (25-30%) are hereditary and part of multiple endocrine neoplasia type 2 (MEN2), caused by germline mutations of the RET proto-oncogene [14]. Most of the well differentiated thyroid cancers (DTC, including PTC, FTC) have excellent prognosis, however patients with ATC have 6-12 months’ median survival [13]. Thyroglobulin is widely applied as tumor marker for tumors arising from follicular cells. It is used for evaluation of tumor residuum or recurrence in patients treated by total thyroidectomy and/or radioidine ablation. On the other side calcitonin, a product of parafollicular C cells, is used for the diagnosis and follow-up of MTC. Both tumor markers have limitations therefore miRNAs can be practical in these diseases too [15].
Diagnostic miRNAs
Interestingly, global miRNA downregulation was detected in malignant thyroid cancer compared to normal tissue together with decreased DICER gene expression that was associated with aggressive features [13, 15].
Since the first publication on miRNAs in thyroid cancer in 2005, numerous studies have been published and extensively reviewed e.g., by Malek et al. and Celano et al. in 2017. Among several miRNA alterations described, recent meta-analyses emphasize the role of couple of commonly upregulated miRNAs: miR-146b, miR-221, miR-222, miR-181b in PTC compared to normal tissue [13, 15]. Regarding the role of some of these miRNAs, downregulation of KIT was identified as the common potential regulating mechanism [16]. In FTC, miR-637, miR-181c-3p, miR-206, and miR-7-5p were discovered as de novo potential FTC markers based on another meta-analysis comprising 3 independent datasets [17]. In anaplastic thyroid cancer, similarly to DTC cases, overexpression of miR-146b, miR-221, and miR-222 was described together with downregulation of miR-200 and miR-30 families leading to enhanced epithelial-to-mesenchymal transition (EMT) [18]. miRNAs have been dysregulated in MTC too, and this dysregulation was described as a probable early event in C-cell carcinogenesis [19]. Similar to ATC, the underexpressed miR-200 family through regulating E-cadherin by directly targeting ZEB1 (zinc finger E-box binding homeobox 1) and (ZEB2 zinc finger E-box binding homeobox 2) leads to enhanced expression of transforming growth factor β (TGFβ)-2 and TGFβ-1 [13, 15].
Apart from tissue samples fine needle aspiration biopsy (FNAB) specimens were also subjected to miRNA analysis and yielded highly comparable results [15]. Meta-analyses showed that multiple miRNA assays showed a higher diagnostic accuracy than single miRNA and the test results indicated 77% sensitivity, 75% specificity with 0.83 AUC in a receiver operating characteristic analysis [20]. However, several other studies reported promising sets of miRNAs in discriminating benign vs. malignant thyroid lesions from FNAB samples (Table 1).
Table 1.
miRNAs discriminating benign vs. malignant thyroid lesions from FNAB samples
| Authors | Panel of markers | Groups | n (sample number) | Sensitivity (%) | Specificity (%) | Diagnostic accuracy (%) |
|---|---|---|---|---|---|---|
| Stokowy et al. 2016 | miR-484, miR-184b-3p |
mutation-negative follicular thyroid carcinomas and follicular thyroid adenomas | 44 | 89 | 87 | na |
| Paskas et al. 2015 | BRAF V600E, miR-221, miR-222, galectin-3 |
benign vs. malignant thyroid nodule | 120 | 73.5 | 89.8 | 75.7 |
| Panebianco et al. 2015 | miR-146b, miR-222, KIT, TC1 |
benign vs. malignant thyroid nodule | 118 | 92.3 | 94.7 | 96 |
| Shen et al. 2012 | miR-146b, miR-221, miR-187, miR-30d |
benign vs. malignant thyroid nodule | 68 | 88.9 | 78.3 | 85.3 |
| Keutgen et al. 2012 | miR-328, miR-222, miR-21, miR-197 |
benign vs. malignant thyroid lesions | 72 | 100 | 86 | 90 |
| Mazeh et al. 2012 | miR-21, miR-31, miR-146b, miR-187, miR-221, miR-222 |
benign vs. malignant thyroid nodule | 11 | 88 | 100 | 90 |
Circulating miRNAs in both serum and plasma were tested and excellently summarized by Celano et al. Although the summary reported a highly variable miRNA expression [15], some miRNAs were identified by multiple groups. In PTC patients, the levels of miR-146b, miR-221 and miR-222 were detected to be higher compared to controls [13, 15, 21]. Post-surgically, significantly reduced miR-151 and miR-222 expression was reported compared to pre-operative samples in more than one study [21]. Also, miR-146b reportedly discriminated benign and malignant tumors with 61.4% sensitivity and 74.3% specificity, while miR-155 had 57.9% sensitivity and 63.2% specificity in serum/plasma [13, 15]. The levels of these two miRNAs were also correlated with lymph node metastases and tumor size [13, 15].
Plasma exosomal miRNAs were also assessed and miR-31 was found to be over-represented in the samples of patients with PTC compared to benign tumors, while miR-21 helped to distinguish between FTC and benign tumors. Using both miR-21 and miR-181a-5p helped to distinguish between PTC and FTC with 100 % sensitivity and 77 % specificity [23].
Prognostic miRNAs
Several studies investigated the potential prognostic value of miRNAs that was summarized in Celano et al. Higher expression of miR-146b, miR-221 and miR-222 showed association with prognostic parameters on tissue level [15]. The expression of these miRNAs showed association with tumor size, capsular and vascular invasion, extra-thyroidal extension, lymph node metastases and TNM stage [15]. Besides, the overexpression of miR-146b correlated with multifocality and miR-221 with distant metastases as well in PTC. Overall survival was significantly decreased in patients with higher miR-146b expression in tumor tissue [24]. In a recent study 7 miRNAs (miR-146b, miR-184, miR-767, miR-6730, miR-6860, miR-196a-2 and miR-509-3) were associated with the overall survival and miR-184, miR-146b, miR-509-3 and lysophosphatidic acid receptor 5 (LPAR5) were identified as independent risk factors for prognosis by multivariate analysis [25]. In FTC, the level of miR-221-3p, miR-222-3p, miR-222-5p, miR-10b and miR-92a was higher in metastatic cases vs. non-metastatic. Regarding MTC, Abraham et al. published that miR-183 and miR-375 were associated with lymph node metastases, distant metastases and mortality [26]. Also, upregulation of miR-224 was described as potential prognostic biomarker associated with a better outcome in MTC patients [19].
Among circulating miRNAs the level of miR-222, miR-221, miR-146b and miR-151-5p was described to be decreased after tumor removal [15]. Serum level of miR-146a-5p and miR-221-3p was found to be consistent with response to therapy, including patients with structural evidence of disease whose thyreoglobulin (Tg) test remained negative [13, 15]. Therefore it is suggested that these two miRNAs could be applied as post-treatment monitoring biomarker of PTC patients, especially when Tg assay results are uninformative [13, 15].
ADRENAL TUMORS
Adrenal tumors can develop from the adrenal cortex or the medulla. Adrenocortical tumors (ACT) are common and their prevalence reaches 6% after the age of 60 years [27]. Most of them are benign (adrenocortical adenomas, ACA) in nature. Although the most common tumors, so called incidentalomas are non-functioning, some are associated with hormone overproduction syndromes (such as Cushing’s, Conn’s syndrome, hyperandrogenism). Adrenocortical carcinomas (ACC) however, are rare and aggressive tumors (0.5–2/million per year) [27] with a dismal prognosis where surgical resection is the only curative treatment. The differential diagnosis between ACA and localized ACC can be challenging as radiological and pathological features can be similar but the distinction between them is essential due to the completely different therapy.
HORMONE PRODUCING ADRENAL ADENOMAS
Regarding functional adenomas, peripheral blood hormone testing can help in diagnosis. In aldosterone producing adenomas (APAs) high expression of miR-23 and miR-34a was described [27]. He et al. reported 31 miRNAs which were significantly differentially expressed in APAs when compared to normal adrenal cortex. Of these, 23 were downregulated with miR-375 being the most underexpressed [28]. Between expression of miR-375 and miR-7 a strong positive correlation was found indicating a potential synergistic function [28]. MiR-375 substitution in NCI-H295R ACC cell line resulted in decreased cell growth and it inhibited its target gene metadherin (MTDH). Also, miR-375 expression was negatively correlated with APA tumor size reflecting its potential role as a prognostic marker. In cortisol producing adenoma and carcinoma, circulating, extracellular, vesicle-associated miR-22-3p, miR-27a-3p and miR-320b were significantly overrepresented compared to nonfunctional adenomas [29].
ADRENOCORTICAL CARCINOMAS (ACC)
Diagnostic miRNAs in ACC
Similarly to thyroid tumors, numerous publications reported differentially expressed miRNA profile in ACA and ACC which are exceedingly summarized by Igaz et al., Cherradi et al., and Hassan et al. [30–32]. The most frequently reported overexpressed miRNAs were miR-483-5p and -3p, miR-503, miR-210 and miR-184 in ACC vs ACA. Certainly, miR-483-5p and miR-483-3p have a significant role in adrenal tumorigenesis, its gene is located in the intron of the insulin like growth factor 2 (IGF2) gene, a well-known overexpressed gene in ACC. Expectedly, a positive correlation was described between miR-483-5p and IGF2 expression levels [33]. Experiments, however, demonstrated that miR-483-5p has an independent role in ACC’s pathogenesis as IGF2 transgenic animals did not develop tumors [31]. Indeed, downregulation of miR-483-5p (and miR-483-3p) led to decreased proliferation of ACC cell line [34], and it protected cells from apoptosis by targeting proapoptotic PUMA (p53-upregulated modulator of apoptosis or BBC3: BCL2 binding component 3) [30]. Wang et al, suggested the use of the combination of SMAD4 (SMAD family member 4) negative/low expression with elevated miR-483-3p expression that provided a diagnostic specificity of 92.8% for distinguishing ACA vs. ACC (while SMAD4 expression itself demonstrated high sensitivity of 92%) [35]. The overexpression of miR-210 has been also documented by several publications [31, 32]. This miRNA is widely overexpressed in different tumors and it is also called as a “hypoxamir” due to its upregulation by both hypoxia inducible factor 1 subunit alpha and beta (HIF1α, HIF1β). It has a role in tumorigenesis through regulating arrest of cell proliferation, repression of mitochondrial respiration, arrest of DNA repair, vascular biology, and angiogenesis [36]. Among downregulated miRNAs in ACC miR-195 has been frequently reported [31, 32]. As a member of miR-15/16/195/424/497/6838 family it promotes apoptosis together with inhibiting cell proliferation in ACC and other cells targeting cell cycle regulators such as cyclin D1 (CCND1) or cyclin dependent kinase 6 (CDK6) [31].
Beside adrenocortical tumor tissues, miR-483-5p was found to be increased in serum, plasma and circulating exosomes derived from patients with ACC compared to patients with ACA [31, 32]. Circulating miR-100, miR-181b, miR-184, miR-210 and miR-34a were found to be upregulated and miR-195, miR-335, miR-376a downregulated in ACC samples compared to ACA [31, 32]. After tumor removal, the initially highly expressed miR-483-5p decreased and low level of miR-195-5p increased in blood stream of patients with ACC. Therefore, it is suggested that these miRNAs directly derived from ACC tissues [32]. Interestingly, opposite expression of miRNAs in tissue vs. serum was also described in independent publications too. While miR-34a was detected increased in serum and decreased in ACC tissues, miR-376a showed the opposite pattern suggesting a potential selecting mechanism of extracellular secretion [31, 32]. Assessing diagnostic potential of miRNAs Chabre et al. also investigated miR-195, miR-335 and miR-376a and found that miR-195 showed the highest sensitivity (90,9%) and specificity (100%) in discriminating ACC patients [37].
Prognostic miRNAs in ACC
The decreased expression of miR-195 and miR-497 in ACC was reported to directly regulate TARBP2 (TARBP2 subunit of RISC loading complex) and DICER1 (dicer 1, ribonuclease III) expression in ACC cells therefore contributing to a global downregulation of miRNA expression [32, 34]. Also, a significant overexpression of TARBP2, DICER, and DROSHA (drosha ribonuclease III) in ACC compared with ACA or normal adrenal cortices were found and inhibition of TARBP2 in human ACC cell line resulted in a decreased cell proliferation and induction of apoptosis [38]. Interestingly, low DICER1 expression was also associated with poor clinical outcome in adrenocortical carcinoma [30–32].
Lower expression of miR-483-5p in combination with increased miR-195 level was reported as predictor of poor prognosis in ACC [30–32]. High expression of miR-503 was associated with shorter survival [34], and increased expression of miR-210 with ACC clinicopathologic parameters of aggressiveness and a poor prognosis [39, 40]. In a recent study, three miRNA clusters were identified related to prognosis [40]. “Mi1” and “Mi2” miRNA clusters including 11 upregulated miRNAs located at Xq27.3 and 38 downregulated miRNAs derived from 14q32 locus were associated with better prognosis (C1B molecular group) while “Mi3” miRNA cluster associated with poor prognosis (C1A group).
Using next-generation sequencing expression levels of 6 microRNAs (miR-503-5p, miR-483-3p, miR-450a-5p, miR-210, miR-483-5p and miR-421) predicted malignant/non-malignant status with over 95% accuracy [41]. In this study the best single miRNA for malignancy was miR-483-3p [41]. MiR-139-5p and miR-376a levels significantly increased in aggressive ACC compared with non-aggressive ACC patients in tumor samples but not in circulation [37]. Additionally, high circulating levels of miR-483-5p or low circulating levels of miR-195 were associated with both shorter recurrence-free survival and shorter overall survival in the study of Chabre et al. 2013 [37].
PHEOCHROMOCYTOMA-PARAGANGLIOMA
Although the localization is different pheochromocytomas (PCC) and paragangliomas (PGL) arise from the same type of neural crest tissue of the sympathetic and parasympathetic paraganglia [42]. Tumors of the adrenal medulla are called PCCs and neoplasms developing from thoracic, abdominal or pelvic region paraganglia are named as PGLs. It represents a rare disease as its estimated incidence is 1-8 cases per million worldwide annually [42]. PCCs and PGLs are usually benign (10-year overall survival is around ~96%), however 10% of PCC and even 40% of PGL become metastatic resulting in a poorer prognosis (5-year survival below 50%) [42]. Unfortunately, there are neither clear histopathological signs of malignant behavior nor efficient therapy for malignant PCC/PGL. Therefore, investigating miRNAs as potential biomarkers can be useful in this regard. Nonetheless, compared to thyroid and adrenocortical neoplasms miRNAs are not so extensively investigated in PCC/PGL.
More than 30% of the cases are attributed to germline mutation leading to autosomal dominant genetic syndromes such as multiple endocrine neoplasia type 2A and 2B caused by RET mutations, von Hippel Lindau syndrome due to VHL mutations, neurofibromatosis type 1 with NF1 mutations or hereditary PG syndromes caused by mutations of succinate dehydrogenase (SDH) genes. Lately, as a result of next-generation sequencing, novel genes, including KIF1b, PHD2, TMEM127, MAX, FH, MDH2, GOT2 and SLC25A11 were identified in the pathogenesis of PCC/PGL [43].
Diagnostic miRNAs
miRNA profile in different genetic subtypes is also distinct. Hypoxia induced miR-210 was found overexpressed commonly in pseudohypoxia-associated PCC/PGLs harboring SDHB and VHL mutations. Upregulation of miR-139-3p, miR-541, miR-765 and miR-133b was described in VHL associated tumors, while miR-96 and miR-183 were found to be overexpressed in neoplasms with SDHB mutations [30, 44, 45]. NGF (nerve growth factor) treatment in vitro in PCC cell line significantly decreased the level of miR-139-3p and miR-210 and led to differentiation raising the role of these two miRNAs in tumor development [46]. Similarly, through targeting ezrin, miR-96 and miR-183 also suppressed cell adhesion and differentiation [30]. In MEN2B associated PCs miR-885-5p was repeatedly found to be overexpressed [30]. miR-885-5p regulates molecules involved in apoptosis (Casp3) and cell cycle (CDK2) [30], and by targeting IGF-binding proteins its role was supposed in RET mutation associated PCC/PGL pathogenesis [30]. Interestingly, miR-137 and miR-382 can be considered as general PCC/PGL markers as they were found overexpressed in most cases except for MAX mutation associated tumors [30]. Interestingly, similarly to ACCs, miRNAs coded at 14q32 genomic region (DLK-MEG3 region) was described to be downregulated in MAX mutated and a subset of sporadic PC samples as well [42]. The DLK-MEG3 locus was reported to be hypermethylated in approximately 10% of PC samples and the role of downregulated miRNAs located here was also proposed [30].
Prognostic miRNAs
Similarly to ACC, miR-483-5p was found to be upregulated in malignant PCC/PGL in more than one study [30]. Also, the well-known tumor suppressor miR-15a, miR-16 were underexpressed in malignant PCC vs. benign tumors [47]. These miRNAs were downregulated in several neoplasms and they are considered tumor suppressors by targeting BCL2 and CCND1 [30, 47]. Igaz et al. described miR-1225-3p overexpressed compared to sporadic non-recurring, MEN2-, VHL-, and NF1-associated PCs [30]. By targeting Notch signaling, the role of miR-1225-3p is considered in PC tumorigenesis as in in vitro experiments Notch-1 inhibited proliferation of PC cell [30].
Compared with benign PCCs, miR-101 level was higher in patients with malignant PCCs and the level of miR-101 was higher in SDHD mutation associated tumors [48]. In discriminating malignant from benign PCs AUCs for miR-101 in all investigated PCCs samples were 0.79 and 0.77 for non-SDHD mutant samples [48]. Another study by Patterson et al. also proposed that miR-483-5p, miR-101, and miR-183 could serve as useful diagnostic markers (AUC: 0.7; 0.78 and 0.82, respectively) for distinguishing malignant from benign PCCs [44].
PITUITARY TUMORS
Pituitary adenomas are among the most frequent intracranial tumors with a high incidence rate, approx. 10–15 % [49]. Although the great majority of pituitary tumors are benign they can lead to significant morbidity through compressing adjacent structures (visual impairment, headache) or by hormonal disturbance (either hypo- or hyperfunction). Of these 95% are sporadic, only the remaining 5% are associated with genetic syndromes such as MEN1, MEN4, Carney complex or McCune-Albright syndrome. Interestingly, miRNAs are an extensively investigated field in pituitary tumors. Since the first study published in 2005 by Bottoni et al.[50], more than a hundred hits have appeared for a search with keywords “microrna” AND “pituitary” AND “adenoma” in Pubmed in February, 2019. These studies broadly evaluated not only miRNA signature characteristics of different types of pituitary adenomas but also target genes and miRNA function as well. Accordingly, several reviews summarizing the expression profile and role of tissue miRNAs widely investigated in pituitary adenoma have been published, one of the most recent by Feng et al. in 2018 [51] who extensively summarized miRNAs reported in pituitary adenomas dissected by adenoma subtypes. Here, we aim to highlight the roles of miRNAs by their targets’ function. Although the expression profile and role of tissue miRNAs are widely investigated in pituitary adenoma, we lack information about the diagnostic potential of miRNAs present in circulation. Pituitary adenomas are hormone secreting which give an excellent opportunity to monitor tumor growth and function by hormone tests, therefore the role of miRNAs might be less important. However, regarding non-functional pituitary adenomas a blood-based miRNA biomarker could help the diagnosis and patient follow-up after surgery.
Diagnostic miRNAs
Pituitary subtype-specific miRNA profile was described by several studies [51].
In GH producing adenomas several miRNAs target insulin growth factor binding proteins (IGFBP-3, -6, -7) that regulates organ development and growth [51]. The overexpressed miR-26a has a key role in the pathogenesis of GH-secreting adenoma by directly regulating PI3K/Akt signaling pathway and LEF-1 that is involved in anterior pituitary development [51]. The overexpression of Pituitary Tumor-Transforming Gene 1 (PTTG1) was demonstrated in ~90% of pituitary adenoma independently of adenoma type. A set of downregulated miRNAs (miR-126, miR-381, miR-665, miR-300, miR-381, miR-329) is suggested to contribute to PTTG1 overexpression as restoring their level in vitro led to suppressed viability and proliferation of pituitary cells [52]. High Mobility Group AT-Hook Protein 2 encodes a non-histone chromosomal high mobility group (HMG) protein, as a transcriptional regulator it controls cell cycle and pituitary cell proliferation. Numerous miRNAs were identified targeting HMGA1 and 2 in GH, PRL and nonfunctioning pituitary adenomas [51]. In sporadic GH-producing adenomas miR-34a and miR-107 were found to regulate AIP a well-known tumor suppressor frequently mutated in familial pituitary adenomas.
In corticotroph adenomas miR-26a was massively overexpressed compared to normal pituitary. MiR-26a downregulated Cyclin A and Cyclin E and it targeted PRKCD (protein kinase C delta) that was reported to suppress ACTH secreting pituitary cells [51].
In prolactinomas HMGA1 and 2 as a target of several miRNAs were also validated. Interestingly the expression of miR-432 were found to be positively correlated with serum prolactin [51]. The role of downregulated miR-410 was also proved in pathogenesis of prolactinoma by targeting Cyclin B1 [51].
In non-functioning pituitary adenomas (NFPAs) the role of HMGA1 and 2 proteins also has to be mentioned regulated by several miRNAs [51]. Cell cycle in NFPAs is regulated in G2M transition by miRNAs through targeting Wee1 and CDC25A [53]. The TGFβ signaling is also controlled by miRNAs through SMAD3 [54]. Additionally, miR-106b influenced migration and invasion of pituitary adenoma cells via regulating PTEN and further activity of the PI3K/AKT signaling pathway and MMP-9 expression [51].
In pituitary carcinoma metastasis expression of miR-20a, miR-106b and miR-17-5p were increased compared to the primary neoplasm and these miRNAs were proved to be involved in pituitary carcinoma metastasis by attenuating PTEN and TIMP2 (TIMP metallopeptidase inhibitor 2) [55]. In ACTH producing carcinomas miR-493 was significantly upregulated compared to ACTH adenomas while miR-122 overexpressed in both corticotroph adenomas and carcinomas compared to normal pituitary [56]. LGALS3 and RUNX2 are both predicted targets of miR-493 and these genes have been shown to have roles in pituitary tumor cell growth [56].
Prognostic miRNAs
Level of several miRNAs (miR-24, miR-34a, miR-93, miR-148-3p, miR-152, miR-132, miR-15a, and miR-16) are significantly lower in invasive pituitary adenomas compared with non-invasive ones [57]. In prolactinomas, miR-183 was found downregulated in aggressive tumors and it inhibited tumor cell proliferation by inhibiting KIAA0101 that is involved in cell cycle activation and inhibition of p53-p21 mediated cell cycle arrest [58]. In corticotroph adenomas miR-93-3p, miR-93-5p, miR-25-3p and miR-106b-5p were detected to be overexpressed in invasive tumors compared to non-invasive ones through targeting MCM7 the overexpression of these miRNAs led to increased invasiveness and unfavorable outcomes after resection [51, 59]. Patients with corticotroph adenomas with decreased level of miR-141 had higher chance of remission [60].
In NFPAs several miRNAs showed correlation with tumor size some of the (miR-450b-5p, miR-424, miR-503, miR-542-3p, miR-629, and miR-214) were underexpressed and target SMAD3 [54]. In invasive adenomas expression levels of miR-181b-5p, miR-181d, miR-191-3p, and miR-598 were upregulated, and the expression levels of miR-3676-5p and miR-383 were down-regulated [61]. In GH3 cells Caveolin-1 (CAV1) was reported to promote invasion while silencing CAV1 indirectly induced miR-145, miR-124, and miR-183 that suppressed the migration and invasion of pituitary adenoma cells through targeting FSCN1, PTTG1IP and EZR, respectively [57].
NEUROENDOCRINE TUMORS (NETs)
Neuroendocrine tumors (NETs) consist of heterogeneous neoplasms of different origin arising from neuroendocrine cells throughout the body (most commonly from the lungs, pancreas, small intestine, and rectum). Gastroentero-pancreatic NETs (GEP-NETs) represent less than 1% of digestive cancers and 7-21% of all neuroendocrine neoplasms [62]. Lung NETs originate from pulmonary neuroendocrine cells accounting for approximately 25% of primary lung neoplasms. Lung NETs classified into the following subtypes: typical carcinoids (TCs, well differentiated, low-grade); atypical carcinoids (ACs, well-differentiated, intermediate-grade); large cell neuroendocrine carcinomas (LCNECs, poorly differentiated, high-grade); and small cell lung cancer (SCLCs, poorly differentiated, high-grade) [63]. NET as a heterogeneous group have different behavior and prognosis but they express neuroendocrine markers such as chromogranin A (CgA) and synaptophysin. Based on the World Health Organization (WHO) classification the prognosis is characterized by the grade of neuroendocrine differentiation and the proliferative index (Ki-67) from G1-G3. However, guidelines indicate that prognosis is also influenced by several other factors, such as patient age, tumor site, metastatic spread and hormonal production [64]. Unfortunately as more than 80% of patients usually present with metastatic disease, NET prognosis is poor, due to the relative lack of effective therapy [63, 64]. Among circulating biomarkers, CgA has been measured in several NETs, but its value as a prognostic biomarker in NETs is limited [64], therefore identification of prognostic factors to predict outcome would be fundamental.
Diagnostic miRNAs
Interestingly, little information is available regarding miRNA profile in gastric NETs (gNETs), however it was described that gastrin-induced miR-222 overexpression resulted in reduced p27, which in turn caused actin remodeling and increased migration in human stably CCK2 receptor expressing gastric adenocarcinoma cell line [65].
In circulation, serum miR-222 expression was increased in hypergastrinemic patients with autoimmune atrophic gastritis and type 1 gastric NET. Because its level decreased in patients after CCKR2 agonist treatment miR-222 was proposed to be a promising biomarker for gastrin induced premalignant changes in the stomach [65].
However, different miRNA sets with altered expression were described regarding pancreatic NET (pNET), comparing functional, nonfunctional NET, normal pancreas, normal pancreatic islets in any combinations that is reviewed in detail by Malczewska et al. and Zatelli et al. [64, 65]. The overexpressed miR-103, miR-107 and the underexpressed miR-155 discriminated sporadic pancreatic NET (insulinoma and non-functioning) from acinar cell carcinomas. The role of downregulated miR-155 was suggested in pathogenesis by targeting proapoptotic tumor protein p53 inducible nuclear protein 1 (TP53INP). Also, miR-144/451 cluster and miR-21 was found overexpressed compared to normal pancreatic islets [65]. MiR-204 was found primarily expressed in insulinomas and correlated with immunohistochemical expression of insulin [66]. Interestingly, several miRNAs located at 14q32 region showed dysregulated expression, including miR-144/451 cluster [65]. Interestingly, these miRNAs showed overexpression in insulinomas compared to other endocrine tumors where miRNAs of this region are frequently downregulated. In in vitro experiments miR-144 induced cell proliferation in murine pancreatic β cells and regulated Akt signaling by targeting PTEN [65]. Additionally, miR-451 also promoted cell proliferation by regulating cell cycle through targeting p19 [67]. Overexpression of miR-21 were reported over-expressed in pNET [65, 66]. Expression of miR-642 correlated with Ki67 (MiB1) score and miR-210 correlated with metastatic disease [68].
13 miRNAs were identified by comparing serum from pNET patients and healthy volunteers and miR-193b was up-regulated in both pNET tissue and serum when compared to controls described by Thorns et al. [68]. In addition, miR-1290 showed overexpression in pNETs and the latter could accurately distinguish patients with low-stage pancreatic cancer from healthy controls and subjects with chronic pancreatitis [69].
MiRNA profile of small bowel NET (sbNET) was also investigated, however fewer experimental information are available regarding miRNA’s function studies and target validation. MiR-7-5p, miR-182, miR-183 and miR-96-5p were found to be upregulated in sbNET compared to normal small bowel consequently in different studies [65]. Furthermore, miR-182, miR-183 and miR-96 overexpressed in NET metastases compared to primary tumors [65]. Similarly, upregulation of miR-196a was described in numerous studies however its role in cell proliferation could not be confirmed [65, 69]. In addition, the downregulation of miR-129-5p and miR-133a was also established in sbNET metastases vs. primary tumors [65].
Similarly to tissue, overexpression of miR-182, miR-196a and miR-200a and downregulation of miR-31, miR-129-5p and miR-133a were detected in blood of sbNET patients and the level of some of them changed upon somatostatin treatment [65].
Similarly to sbNET, in appendiceal carcinoids without metastases low levels of miR-96 and high levels of miR-133a were detected [65]. In colorectal NET (cNET) patients underexpression of miR-186 was found in tumor tissue, blood and stool samples compared to controls. In parallel, PTTG1 upregulation was detected in the same samples together with decreased miR-186 expression therefore the authors suggested that upregulation of PTTG1 was induced by the loss of miR-186 [70].
Interestingly, based on miRNA expression profile analysis common origin for pulmonary carcinoids and GI-NETs was suggested by Yoshimoto et al. [71].
Lung NETs. The expression profiles of pulmonary carcinoids and SCLCs were quite different, indicating the distinct genesis of these neuroendocrine neoplasms [71].
Prognostic miRNAs
A study by Sadanandam et al. identified molecular subtypes of pNETs (islet-like, intermediate and metastasis-like primary types) [72]. As these subtypes exhibit distinct metabolic profiles marked by differential pyruvate metabolism, substantiating the significance of their separate identities they may have role to predict different behavior. Expectedly, the three subtypes have distinct mRNA and miRNA signatures as well [72]. Roldo et al. reported the overexpression of miR-21 as positively correlated with Ki-67 proliferation index and presence of liver metastases [66]. Besides, expression of miR-642 was also described to correlate with Ki67 index score while miR-210 correlated with metastatic disease [68]. Additionally miR-196a was identified as prognostic factor in pNET as its expression significantly associated with stage, and mitotic count [64]. Also, high miR-196a level was associated with decreased overall survival and disease-free survival. The hazard ratio for recurrence of patients with high miR-196a expression was 16.267 [73].
In sbNET increased plasma miR-21 and decreased miR-150-5p were characteristic to metastatic tumors [74]. In line with this, low plasma miR-21 and high miR-150-5p levels were associated with significantly prolonged overall survival [74]
In rectal carcinoids miR-885-5p was identified as upregulated in tumors with lymphovascular invasion. Also, high miR-885-5p expression was independently associated with lymphovascular invasion. Therefore miR-885-5p is suggested as a potential biomarker for predicting malignancy [75].
Regarding NET in the lung Zatelli et al. thoroughly summarized miRNAs with prognostic role [64]. miR-150 and miR-886-3p were found downregulated while miR-92a2* and miR-7 upregulated in SCLC that showed correlation with overall or disease-free survival. The latter miRNAs were found to be associated with chemoresistance too [64]. In other publications including typical, atypical carcinoids and large cell neuroendocrine carcinomas the upregulated miR-21 and the downregulated miR-409-3p, miR-409-5p, and miR-431-5p correlated with the presence of lymph node metastases and set of other 5 upregulated miRNAs with overall survival [64].
COMMON miRNAs DETECTED IN VARIOUS ENDOCRINE TUMORS
Although miRNAs’ expression and their functions are tissue specific, discussing common miRNAs among different endocrine tumors can still be an interesting aspect reflecting potential common pathomechanism. For instance, miR-210 was overexpressed in several endocrine tumors, such as ACC and PCC/PGL [30, 31]. Also, its increased expression seems to be an unfavorable prognostic factor correlating clinicopathologic parameters of aggressiveness and a poor prognosis in ACC and in pNET [39, 40, 68]. Usually, the increased expression of miR-210 might be a consequence of hypoxic environment within tumor tissues or activation of pseudohypoxia signaling pathway in PPC/PGL. As a general phenomenon increase of miR-210 was also described in other, non-endocrine solid tumors (i.e. breast, lung, head and neck, pancreatic and glioblastoma).
Overexpression of miR-483-5p and miR-483-3p in tissues and in circulation (serum, plasma and exosomes) is almost a unique characteristic of ACC and is considered as a prognostic marker [30, 31, 32, 33]. It predicted malignant/non-malignant status and it associated with both shorter recurrence-free survival and shorter overall survival. Interestingly, not only for tumors of adrenal cortex but for tumors originated from adrenal medulla (PCC/PGL) miR-483-5p is also proposed as useful diagnostic markers for malignancy [30].
Two well-known tumor suppressor miRNAs: miR-15a and miR-16 are downregulated in pituitary adenomas and in PCC where they were described to be able to discriminate benign and malignant forms of the disease [47, 51]. In addition, the lost expression of general tumor suppressor miRNAs targeting BCL2 and CCND1 have been described in other, non-endocrine tumors as well [30, 47].
Additionally, Pituitary Tumor-Transforming Gene 1 (PTTG1) is another target gene that is regulated by miRNAs in endocrine tumors. Several miRNAs (miR-126, miR-381, miR-665, miR-300, miR-381, miR-329) are suggested to contribute to PTTG1 overexpression in pituitary adenomas [51], that is also upregulated in colorectal NET by the loss of miR-186 [70]. However, PTTG1 overexpression is not unique for endocrine tumors as it was described in several other malignancies with prognostic potential.
MiR-21, a widely investigated oncomiR, has been found up-regulated in many types of human tumors. Indeed, in lung NET and pNET it was described overexpressed and in pNET its expression positively correlated with proliferation index and with metastases [65, 66]. Additionally, its level was reported elevated in circulation in sbNET where it was described to be characteristic to metastatic tumors and associated with significantly prolonged overall survival [74]. In follicular thyroid cancer plasma level of exosomal miR-21 helped to distinguish between FTC and benign tumors [23].
Similarly to miR-21, miR-222 is differentially expressed in several endocrine tumors, such as in gastric NET (where it is induced by gastrin) and in thyroid cancer (both papillary and anaplastic) [13, 15, 18, 65], whereas in follicular thyroid cancer the tissue level of miR-222 was prognostic (higher in metastatic cases vs. non-metastatic). In the blood stream of PTC patients, miR-222 expression significantly decreased after surgery [21].
CONCLUSIONS AND FUTURE PERSPECTIVES
It is obvious that miRNAs are potentially useful and promising tissue and circulating biomarkers. They are extensively investigated in different neoplasms including tumors of the neuroendocrine system. Interestingly, miRNAs coded at 14q32 region seems to be dysregulated in almost all endocrine neoplasms (Figure 1) and several other tumors. This region is frequently found hypermethylated in cancer compared to normal tissues. The downregulation of miRNAs located at 14q32 region shows important correlations with poor prognosis and aggressiveness in different cancer types. Studies also suggest chromatin remodeling by lncRNA-mediated mechanisms in this region beside DNA methylation that may also influence miRNA expression. Additionally, as 14q32 is an imprinted region and imprinting imbalance could also result in alteration of paternally and maternally expressed genes.
Figure 1.
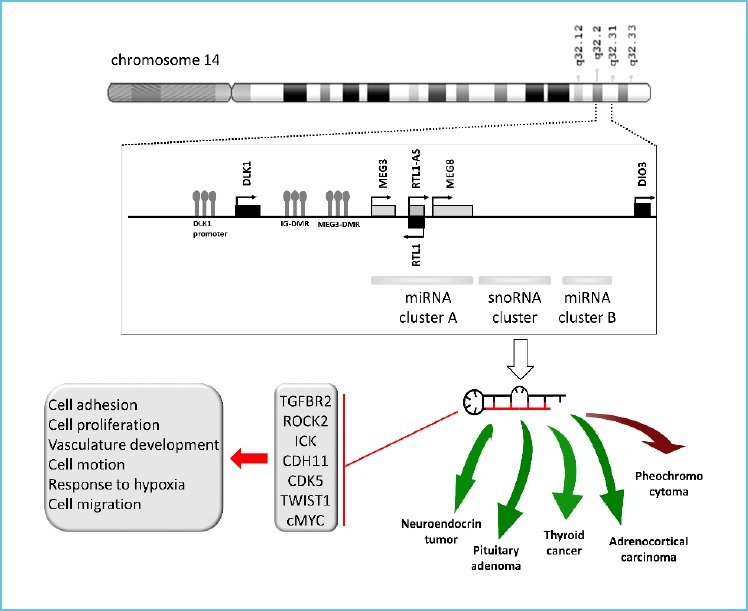
miRNAs coded at 14q32 is commonly dysregulated in endocrine neoplasms: downregulated in neuroendocrine tumors, pituitary adenomas, thyroid cancer and adrenocortical carcinoma (indicated by green arrows) and overexpressed in pheochromocytomas (showed by red arrow)*
Regarding their application to routine diagnostics thyroid tumors and FNAB samples seem to be investigated the most intensively. Although high sensitivity and diagnostic accuracy of miRNAs have been reported in thyroid cancer which may be suitable for differentiation of benign vs malignant thyroid nodules, routine use of miRNAs as biomarkers has not been widespread yet either in thyroid or other endocrine and non-endocrine tumors. In literature it is suggested that this may be due to challenging preanalytics, especially in case of bio-fluids. Additionally, there are no IVD-CE (IVD: applicable for In Vitro Diagnostic, CE: complies with the essential requirements of the relevant European health, safety and environmental protection legislations) marked assays to measure miRNAs in clinical care. That also may delay miRNAs’ application as potential diagnostic biomarkers.
However, regarding thyroid nodules there are common miRNAs among different studies discriminating benign and malignant nodules (e.g. miR-221, miR-222, and miR-146b). These miRNAs should be further investigated as potential candidates for clinical use.
In pituitary neoplasms, however there is the most information gathered regarding the pathogenesis and miRNA-regulated networks, there is basically no information on miRNAs in biofluids. It can be a consequence of negative results or the difficulty of sample group homogenization due to the different adenoma groups especially in the light of the 2017 WHO classification. In adrenocortical carcinoma the role of one miRNA (miR-483-5p) has been repeatedly proved by several studies in both tissue level and in circulation. And finally, the prognostic role of miRNAs is also intensively investigated in NETs however, again, due to the heterogeneous tumor group there is still a need for data regarding the application of any miRNA biomarker in clinical use. Behind the discrepant results found in literature not only the tumor heterogeneity but also the differences in study design and technical implementation could be suspected. Regarding circulating biomarkers, currently there are many biological (age, gender, BMI, smoking status, disease stage, other accompanying illnesses, medications, etc.) and technical (serum, plasma, extracellular vesicle as source, different nucleic acid extraction methods, miRNA detection methods, etc.) factors known and unknown which need standardization and harmonization in order to establish evidences before application of miRNAs as biomarkers.
Acknowledgement
This work was supported partly by grants from Hungarian Scientific Research Grants (OTKA, K125231 to Attila Patocs). Henriett Butz is a recipient of Bolyai Research Fellowship and supported by ÚNKP-18-4-SE-8 New National Excellence Program of The Ministry of Human Capacities.
REFERENCES
- 1.Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M. (2014) Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int J Genomics 2014:970607 10.1155/2014/970607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis BP, Burge CB, Bartel DP. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20. 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 3.Mattick JS, Makunin IV. (2005) Small regulatory RNAs in mammals. Hum Mol Genet 14 Spec No 1:R121-R132. 10.1093/hmg/ddi101 [DOI] [PubMed] [Google Scholar]
- 4.Silva J, García V, Zaballos Á, et al. (2011) Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J 37:617–623. 10.1183/09031936.00029610 [DOI] [PubMed] [Google Scholar]
- 5.Szabo PM, Butz H, Igaz P, et al. (2013) Minireview: mi-Romics in endocrinology: a novel approach for modeling endocrine diseases. Molecular endocrinology 27:573–585. 10.1210/me.2012-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farazi TA, Hoell JI, Morozov P, Tuschl T. (2013) MicroRNAs in human cancer. Adv Exp Med Biol 774:1–20. 10.1007/978-94-007-5590-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JA, Baxter DH, Zhang S, et al. (2010) The microRNA spectrum in 12 body fluids. Clin Chem 56:1733–1741. 10.1373/clinchem.2010.147405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turchinovich A, Weiz L, Burwinkel B. (2012) Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 37:460–465. 10.1016/i.tibs.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Regnault A, Lozier A, et al. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594-600 [DOI] [PubMed] [Google Scholar]
- 10.Barros FM, Carneiro F, Machado JC, Melo SA. (2018) Exosomes and Immune Response in Cancer: Friends or Foes? Front Immunol 9:730 10.3389/fimmu.2018.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri M, Paone A, Calore F, et al. (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 109:E21102116 10.1073/pnas.1209414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohshima K, Inoue K, Fujiwara A, et al. (2010) Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 5:e13247 10.1371/journal.pone.0013247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek A, Sleptsov I, Cheburkin Y, Samsonov R, Kolesnikov N. (2017) miRNA as Potential Tool for Thyroid Cancer Diagnostics and Follow up: Practical Considerations. JSM Thyroid Disord Manag 2(1): 1007. [Google Scholar]
- 14.Raue F, Frank-Raue K. (2015) Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. Recent Results Cancer Res 204:61–90. 10.1007/978-3-319-22542-53 [DOI] [PubMed] [Google Scholar]
- 15.Celano M, Rosignolo F, Maggisano V, et al. (2017) MicroRNAs as Biomarkers in Thyroid Carcinoma. In: International Journal of Genomics. https://www.hindawi.com/journals/ijg/2017/6496570/. Accessed 27 Feb 2019 [DOI] [PMC free article] [PubMed]
- 16.He H, Jazdzewski K, Li W, et al. (2005) The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 102:19075–19080. 10.1073/pnas.0509603102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokowy T, Wojtas B, Fujarewicz K, et al. (2014) miRNAs with the potential to distinguish follicular thyroid carcinomas from benign follicular thyroid tumors: results of a meta-analysis. Horm Metab Res 46:171–180. 10.1055/s-0033-1363264 [DOI] [PubMed] [Google Scholar]
- 18.Fuziwara CS, Kimura ET. (2014) MicroRNA Deregulation in Anaplastic Thyroid Cancer Biology. Int J Endocrinol 2014:743450 10.1155/2014/743450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mian C, Pennelli G, Fassan M, et al. (2012) MicroRNA profiles in familial and sporadic medullary thyroid carcinoma: preliminary relationships with RET status and outcome. Thyroid 22:890–896. 10.1089/thy.2012.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhong Q, Chen X, et al. (2014) Diagnostic value of microRNAs in discriminating malignant thyroid nodules from benign ones on fine-needle aspiration samples. Tumour Biol 35:9343–9353. 10.1007/s13277-014-2209-1 [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Zhao JT, Clifton-Bligh RJ, et al. (2013) MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer 119:4358–4365. 10.1002/cncr.28254 [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Liu Y, Wang J, et al. (2012) Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab 97:2084–2092. 10.1210/jc.2011-3059 [DOI] [PubMed] [Google Scholar]
- 23.Samsonov R, Burdakov V, Shtam T, et al. (2016) Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumour Biol 37:12011–12021. 10.1007/s13277-016-5065-3 [DOI] [PubMed] [Google Scholar]
- 24.Chou C-K, Yang KD, Chou F-F, et al. (2013) Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J Clin Endocrinol Metab 98:E196-E205. 10.1210/jc.2012-2666 [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Kong D, Cui Q, et al. (2018) Bioinformatic analysis and identification of potential prognostic microRNAs and mRNAs in thyroid cancer. PeerJ 6:e4674 10.7717/peerj.4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham D, Jackson N, Gundara JS, et al. (2011) MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin Cancer Res 17:4772–4781. 10.1158/1078-0432.CCR-11-0242 [DOI] [PubMed] [Google Scholar]
- 27.Lerario AM, Moraitis A, Hammer GD. (2014) GENETICS AND EPIGENETICS OF ADRENOCORTICAL TUMORS. Mol Cell Endocrinol 386:67–84. 10.1016/j.mce.2013.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Cao Y, Su T, et al. (2015) Downregulation of miR-375 in aldosterone-producing adenomas promotes tumour cell growth via MTDH. Clin Endocrinol (Oxf) 83:581–589. 10.1111/cen.12814 [DOI] [PubMed] [Google Scholar]
- 29.Perge P, Decmann Á, Pezzani R, et al. (2018) Analysis of circulating extracellular vesicle-associated microRNAs in cortisol-producing adrenocortical tumors. Endocrine 59:280–287. 10.1007/s12020-017-1506-z [DOI] [PubMed] [Google Scholar]
- 30.Igaz P, Igaz I, Nagy Z, et al. (2015) MicroRNAs in adrenal tumors: relevance for pathogenesis, diagnosis, and therapy. Cell Mol Life Sci 72:417–428. 10.1007/s00018-014-1752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherradi N. (2015) microRNAs as Potential Biomarkers in Adrenocortical Cancer: Progress and Challenges. Front Endocrinol (Lausanne) 6:195 10.3389/fendo.2015.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan N, Zhao JT, Sidhu SB. (2017) The role of microRNAs in the pathophysiology of adrenal tumors. Mol Cell Endocrinol 456:36–43. 10.1016/j.mce.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 33.Patterson EE, Holloway AK, Weng J, et al. (2011) MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 117:1630–1639. 10.1002/cncr.25724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Özata DM, Caramuta S, Velázquez-Fernández D, et al. (2011) The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr Relat Cancer 18:643–655. 10.1530/ERC-11-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Sun Y, Wu H, et al. (2014) Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology 64:567–576. 10.1111/his.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan YC, Banerjee J, Choi SY, Sen CK. (2012) miR-210: the master hypoxamir. Microcirculation 19:215–223. 10.1111/j.1549-8719.2011.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabre O, Libé R, Assie G, et al. (2013) Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr Relat Cancer 20:579–594. 10.1530/ERC-13-0051 [DOI] [PubMed] [Google Scholar]
- 38.Caramuta S, Lee L, Ozata DM, et al. (2013) Clinical and functional impact of TARBP2 over-expression in adrenocortical carcinoma. Endocr Relat Cancer 20:551–564. 10.1530/ERC-13-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duregon E, Rapa I, Votta A, et al. (2014) MicroRNA expression patterns in adrenocortical carcinoma variants and clinical pathologic correlations. Hum Pathol 45:1555–1562. 10.1016/j.humpath.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Assié G, Letouzé E, Fassnacht M, et al. (2014) Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46:607–612. 10.1038/ng.2953 [DOI] [PubMed] [Google Scholar]
- 41.Koperski Ł, Kotlarek M, Swierniak M, et al. (2017) Next-generation sequencing reveals microRNA markers of adrenocortical tumors malignancy. Oncotarget 8:49191–49200. 10.18632/oncotarget.16788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro-Vega LJ, Letouzé E, Burnichon N, et al. (2015) Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun 6:6044 10.1038/ncomms7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkadi B, Grolmusz VK, Butz H, et al. (2018) [Evolution of molecular genetic methods in the clinical diagnosis of hereditary endocrine tumour syndromes]. Orv Hetil 159:285–292. 10.1556/650.2018.31036 [DOI] [PubMed] [Google Scholar]
- 44.Patterson E, Webb R, Weisbrod A, et al. (2012) The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endocr Relat Cancer 19:157–166. 10.1530/ERC-11-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsang VHM, Dwight T, Benn DE, et al. (2014) Over-expression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr Relat Cancer 21:415–426. 10.1530/ERC-13-0519 [DOI] [PubMed] [Google Scholar]
- 46.Hamada N, Fujita Y, Kojima T, et al. (2012) MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int 60:743–750. 10.1016/j.neuint.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 47.Meyer-Rochow GY, Jackson NE, Conaglen JV, et al. (2010) MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer 17:835–846. 10.1677/ERC-10-0142 [DOI] [PubMed] [Google Scholar]
- 48.Zong L, Meng L, Shi R. (2015) Role of miR-101 in pheochromocytoma patients with SDHD mutation. Int J Clin Exp Pathol 8:1545–1554 [PMC free article] [PubMed] [Google Scholar]
- 49.Aflorei ED, Korbonits M. (2014) Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol 117:379–394. 10.1007/s11060-013-1354-5 [DOI] [PubMed] [Google Scholar]
- 50.Bottoni A, Piccin D, Tagliati F, et al. (2005) miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol 204:280–285. 10.1002/jcp.20282 [DOI] [PubMed] [Google Scholar]
- 51.Feng Y, Mao Z-G, Wang X, et al. (2018) MicroRNAs and Target Genes in Pituitary Adenomas. Horm Metab Res 50:179–192. 10.1055/s-0043-123763 [DOI] [PubMed] [Google Scholar]
- 52.Liang H, Wang R, Diao C, et al. (2015) The PTTG1-targeting miRNAs miR-329, miR-300, miR-381, and miR-655 inhibit pituitary tumor cell tumorigenesis and are involved in a p53/PTTG1 regulation feedback loop. Oncotarget 6:29413–29427. 10.18632/oncotarget.5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butz H, Likó I, Czirják S, et al. (2010) Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab 95:E181-E191. 10.1210/jc.2010-0581 [DOI] [PubMed] [Google Scholar]
- 54.Butz H, Rácz K, Hunyady L, Patócs A. (2012) Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol Sci 33:382–393. 10.1016/j.tips.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 55.Wei Z, Zhou C, Liu M, et al. (2015) MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary 18:710–721. 10.1007/s11102-015-0648-3 [DOI] [PubMed] [Google Scholar]
- 56.Stilling G, Sun Z, Zhang S, et al. (2010) MicroRNA expression in ACTH-producing pituitary tumors: up-regulation of microRNA-122 and -493 in pituitary carcinomas. Endocrine 38:67–75. 10.1007/s12020-010-9346-0 [DOI] [PubMed] [Google Scholar]
- 57.Yang Q, Li X. (2019) Molecular Network Basis of Invasive Pituitary Adenoma: A Review. Front Endocrinol (Lausanne) 10:. 10.3389/fendo.2019.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roche M, Wierinckx A, Croze S, et al. (2015) Deregulation of miR-183 and KIAA0101 in Aggressive and Malignant Pituitary Tumors. Front Med (Lausanne) 2:54 10.3389/fmed.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garbicz F, Mehlich D, Rak B, et al. (2017) Increased expression of the microRNA 106b~25 cluster and its host gene MCM7 in corticotroph pituitary adenomas is associated with tumor invasion and Crooke’s cell morphology. Pituitary 20:450–463. 10.1007/s11102-017-0805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amaral FC, Torres N, Saggioro F, et al. (2009) MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J Clin Endocrinol Metab 94:320–323. 10.1210/jc.2008-1451 [DOI] [PubMed] [Google Scholar]
- 61.Wu S, Gu Y, Huang Y, et al. (2017) Novel Biomarkers for Non-functioning Invasive Pituitary Adenomas were Identified by Using Analysis of microRNAs Expression Profile. Biochem Genet 55:253-267. 10.1007/s10528-017-9794-9 [DOI] [PubMed] [Google Scholar]
- 62.Yao JC, Hassan M, Phan A, et al. (2008) One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26:3063–3072. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 63.Hendifar AE, Marchevsky AM, Tuli R. (2017) Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 12:425–436. 10.1016/j.jtho.2016.11.2222 [DOI] [PubMed] [Google Scholar]
- 64.Zatelli MC, Grossrubatscher EM, Guadagno E, et al. (2017) Circulating tumor cells and miRNAs as prognostic markers in neuroendocrine neoplasms. Endocr Relat Cancer 24:R223–R237. 10.1530/ERC-17-0091 [DOI] [PubMed] [Google Scholar]
- 65.Malczewska A, Kidd M, Matar S, et al. (2018) A Comprehensive Assessment of the Role of miRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 107:73–90. 10.1159/000487326 [DOI] [PubMed] [Google Scholar]
- 66.Roldo C, Missiaglia E, Hagan JP, et al. (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24:4677–4684. 10.1200/JCO.2005.05.5194 [DOI] [PubMed] [Google Scholar]
- 67.Jiang X, Shan A, Su Y, et al. (2015) miR-144/451 Promote Cell Proliferation via Targeting PTEN/AKT Pathway in Insulinomas. Endocrinology 156:2429–2439. 10.1210/en.2014-1966 [DOI] [PubMed] [Google Scholar]
- 68.Thorns C, Schurmann C, Gebauer N, et al. (2014) Global microRNA profiling of pancreatic neuroendocrine neoplasias. Anticancer Res 34:2249–2254 [PubMed] [Google Scholar]
- 69.Li A, Yu J, Kim H, et al. (2013) MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res 19:3600–3610. 10.1158/1078-0432.CCR-12-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang M, Xia X, Chu W, et al. (2015) Roles of miR-186 and PTTG1 in colorectal neuroendocrine tumors. Int J Clin Exp Med 8:22149–22157 [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimoto T, Motoi N, Yamamoto N, et al. (2018) Pulmonary Carcinoids and Low-Grade Gastrointestinal Neuroendocrine Tumors Show Common MicroRNA Expression Profiles, Different from Adenocarcinomas and Small Cell Carcinomas. Neuroendocrinology 106:47–57. 10.1159/000461582 [DOI] [PubMed] [Google Scholar]
- 72.Sadanandam A, Wullschleger S, Lyssiotis CA, et al. (2015) A Cross-Species Analysis in Pancreatic Neuroendocrine Tumors Reveals Molecular Subtypes with Distinctive Clinical, Metastatic, Developmental, and Metabolic Characteristics. Cancer Discov 5:1296–1313. 10.1158/2159-8290.CD-15-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee YS, Kim H, Kim HW, et al. (2015) High Expression of MicroRNA-196a Indicates Poor Prognosis in Resected Pancreatic Neuroendocrine Tumor. Medicine (Baltimore) 94:e2224 10.1097/MD.0000000000002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowden M, Zhou CW, Zhang S, et al. (2017) Profiling of metastatic small intestine neuroendocrine tumors reveals characteristic miRNAs detectable in plasma. Oncotarget 8:54331–54344. 10.18632/oncotarget.16908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitsuhashi K, Yamamoto I, Kurihara H, et al. (2015) Analysis of the molecular features of rectal carcinoid tumors to identify new biomarkers that predict biological malignancy. Oncotarget 6:22114–22125 [DOI] [PMC free article] [PubMed] [Google Scholar]


