Abstract
A new method is introduced allowing seamless assembly of independent, functionally tested, blunt-end double strand nucleic acid parts (DNA fragments not supplied in vectors such as plasmids) into more complex biological devices (e.g. protein expression vectors) and higher order multi-device systems (e.g. biochemical pathways). Individual parts include bacterial selection markers and origins of replication, promoters useful in a variety of species, transcription terminators, shuttle sequences and a variety of “N” and “C” terminal solubility/affinity protein tags. Parts are not subjected to pre-assembly manipulation with nucleic acid modifying enzymes. Instead, they are simply mixed in appropriate pre-defined combinations and concentrations and then seamlessly linked into devices employing a specialized thermostable enzyme blend. Combinatorial assembly of parts is an inherent time-saving feature of the new method, in contrast to hierarchical binary assembly (“one part at a time”) methods. This feature substantially simplifies and speeds optimization of device and system development. The versatility and functionality of the new method was shown by combinatorial assembly of parts into vector devices, one of which optimally expressed protein from a model gene. Also, a four-enzyme biosynthetic pathway system was re-created by combinatorial construction from parts and devices. Concepts discussed in this paper provide synthetic biologists, chemists and bio-engineers with improved and expanded capability to create novel biological molecules and systems.
Introduction
The discipline of synthetic biology has greatly benefitted from key enabling technologies such as DNA synthesis and sequencing becoming accessible to more researchers due to the reduction in the previously prohibitive financial entry point. To date however, a third enabling technology, molecular cloning, has not kept pace with technological advances made in DNA synthesis and DNA sequencing. One of the most highly recognized collection of techniques and materials developed to improve conventional cloning of biological parts, devices and systems is “BioBricks” [1, 2]. Briefly, the “bricks”, or parts, of this technology represent cloned DNA sequences possessing defined functions, such as antibiotic resistance and ribosome binding sites. Parts are assembled to create larger devices such as protein expression vectors and several devices are joined into a system such as a biosynthetic pathway. BioBrick devices and systems are constructed by “hierarchical binary assembly” of parts, or “one-brick-at-a-time.” More specifically, BioBricks represent functional double strand DNA molecules housed within carrier plasmids flanked by universal and precisely defined upstream and downstream sequences that are technically not part of the BioBrick. These universal sequences contain restriction enzyme recognition sites for one of two closely related enzymes, each having slightly different recognition sequences but upon cleavage generate identical termini (isocaudomers). Linking two BioBricks together requires isolation of the individual parts from their carrier plasmids by specific isocaudomer(s) digestion, end repair in some cases, ligation and finally bacterial transformation. A major drawback to this technique is that BioBrick parts must not contain these restriction enzyme recognition sites within the sequences to be assembled. Also, BioBrick hierarchical binary assembly is time consuming, tedious and not conducive to combinatorial assembly.
Current assembly methods that convert parts into devices also rely on the isolation of parts and devices from dedicated BioBrick-like “destination vectors” (BioBricks [1, 2], SLIC [3], Gibson [4], CPEC [5], SLiCE [6], and In-Fusion [http://www.clontech.com/US/Products/Cloning_and_Competent_Cells/Cloning_Resources/Selec-tion_Guides/In-Fusion_Cloning_Kits]). In other methods, significant parts manipulation with either one or more Type-II restriction enzymes is required (GoldenGate [7], MoClo [8], GoldenBraid [9]). Alternatively, parts manipulation with T5-exonuclease or a combination of Pfu and Taq DNA polymerases are required for Gibson [4] and DATEL [10] assembly methods, respectively, to create overlaps for subsequent annealing and ligation. In summary, assembly methods are complicated when restriction enzyme specificity must be considered at each stage of parts and devices design. Also, creating small parts between 50 and 250 base pairs with one or more enzymes possessing exonuclease activity is difficult due to the propensity of these enzymes to completely degrade the parts. It is apparent that these limitations curtail combinatorial experimental design and significantly slow the process of identifying optimal devices and systems.
Providing functionally validated parts to researchers without the need for retrieval from destination vectors, combined with a seamless protocol conducive to combinatorial assembly of parts into devices and higher order systems would represent a significant improvement in synthetic biology molecular cloning. This paper describes such a system. Parts are provided such that a wide variety of devices can be rapidly assembled. Appropriately chosen parts are combined and assembled in a single-tube reaction creating molecules that transform/transfect and properly function as devices in E. coli, mammalian and yeast (Saccharomyces cerevisiae) cells. Multiple assemblies can be performed in parallel to generate a collection of unique devices that can be used in combination to optimize novel biological systems. The utility of this technology, referred to as “SureVector” (SV), was validated by assembling parts into a collection of plasmid devices that were easily screened to identify clones expressing the largest amount of protein from a model gene of interest (GOI). To further demonstrate the applicability of the SV process, a multi-device system was designed and constructed to reconstitute a four-enzyme biosynthetic pathway in E. coli. This multi-device system enabled identification of several over-expressing clones within one week.
Materials and methods
SureVector (SV) parts
SureVector parts include bacterial origins of replication [OR], bacterial selectable markers [SM], XP-1 and XP-2 referred to as “Expansion parts” containing sequences allowing replication and selection in a variety of organisms (Saccharomyces cerevisiae and mammalian cells; defined in more detail below), promoters [P] (E. coli, Saccharomyces cerevisiae and mammalian), and protein expression tags [T] (both C-terminal and N-terminal tags are available). Each part is flanked by unique 30 base pair (bp) sequences not found in any global DNA databases, such as NCBI’s Basic Local Alignment Search Tool BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and allow specific assembly of parts into devices (example shown in Table 1) and ultimately systems.
Table 1. SureVector overlaps (N-terminal fusions).
| 5' overlap | Fragment | 3' overlap | ||
|---|---|---|---|---|
| OV 6 | GCTCATTTCTACAACGCGGCACTTCTCGAG | Promoter | CCTTGTTTAACTTTAAGAAGGAGATATACAT | G4S |
| G4S | CCTTGTTTAACTTTAAGAAGGAGATATACAT | Expression Tag | GGTGGCGGAGGTTCTGGAGGCGGTGGAAGT | OV 5 |
| OV 5 | GGTGGCGGAGGTTCTGGAGGCGGTGGAAGT | GOI 5’ → 3’ |
CATTTGGTTTAGTGTACAATATCTCCTCGAG | OV 4 |
| OV 4 | CATTTGGTTTAGTGTACAATATCTCCTCGAG | Expansion Slot 2 | CAACAGGAGGTGAAGCTGTAACGTCTCGAG | OV 3 |
| OV 3 | CAACAGGAGGTGAAGCTGTAACGTCTCGAG | Expansion Slot 1 | GATTTTGAGACGTGCTCACAGTTTTCTCGAG | OV 2 |
| OV 2 | GATTTTGAGACGTGCTCACAGTTTTCTCGAG | Origin of Replication | CAGTTCTTCTGGTTGGAGGACTTCCTCGAG | OV 1 |
| OV 1 | CAGTTCTTCTGGTTGGAGGACTTCCTCGAG | Bacterial Selectable Marker | GCTCATTTCTACAACGCGGCACTTCTCGAG | OV 6 |
XP1 and XP2 expansion parts
XP1 parts contain either the yeast autonomous replication sequence (γARS or 2-micron circle) allowing plasmid replication in Saccharomyces cerevisiae or a linker derived from a unique nucleotide sequence not possessing a function other than to tether a chosen bacterial origin of replication to an XP2 fragments during parts assembly.
The XP2 parts include either a non-functional tether sequence, as above, or the lacI repressor found in E. coli, or mammalian selection markers or yeast auxotrophic markers, allowing yeast to grow in the absence of an essential amino acid. XP2 parts designed specifically for use in mammalian cells include blasticidin, puromycin or hygromycin selection markers. For yeast, XP2 parts include the auxotrophic markers URA3 and HIS3 and the selection marker for hygromycin resistance. XP2 parts also contain a transcription terminator; either the bovine growth hormone poly-A signal (bGH pA) specific for mammalian cells or a rho independent terminator for bacterial transcripts provided by the strong hairpin forming sequence (5’-GCCGCCAGCGGAACTGGCGGC-3’). These terminator sequences are positioned and oriented within the XP2 part to terminate transcripts from an upstream GOI.
Large scale production and purification of SV parts
Large scale parts synthesis was performed by PCR in 96-well plates using sequence verified master plasmid templates. Each reaction contained 1 ng of plasmid template, 1x Herculase II reaction buffer (Agilent Technologies, Inc.), 0.25 mM of each dNTP, 0.4 μM of each PCR primer and 2 μl of pre-formulated Herculase II enzyme (Agilent Technologies, Inc.) in a final volume of 100 μL. Thermocycling conditions were: 1 cycle at 95°C for 2 min.; 30 cycles at 95°C for 20 sec., 55°C for 20 sec. and 72°C for 30 sec.; 1 cycle at 72°C for 3 min. Contents of multiple 96-well plates for each SV part were pooled and purified using AMPure XP magnetic beads according to the manufacturer’s instructions (Beckman-Coulter). Correct lengths and purities of SV parts were assessed with an Agilent Technologies, Inc. BioAnalyzer.
General assembly of SV parts into devices
SV parts designed to assemble into a desired device were combined with a SV adapted GOI part and other reaction components were added as follows: 1x SureVector reaction buffer, 0.25 mM of each dNTP, 5.0 nM of each part (e.g. SM + OR + XP1 + XP2 + T + GOI + P) and 1 μL of pre-formulated thermostable enzymes including high fidelity DNA polymerase, dUTPase, “flap-endonuclease” and ligase in a final volume of 20 μL. Thermocycling of these components consisted of 1 cycle at 95°C for 2 min.; 8 cycles at 95°C for 20 sec., 55°C for 20 sec. and 68°C for 30 sec.; 1 cycle at 68°C for 3 min. Following thermocycling, one unit of Dpn I restriction enzyme was added to the reaction and incubated for 5 minutes at 37°C. One μL of the reaction was transformed into XL1-Blue Supercompetent E. coli cells (Agilent Technologies, Inc.) according to instructions and varying amounts (10, 20 and 50 μL) of the transformation mixtures were spread onto LB agar plates containing the appropriate antibiotic and incubated at 37°C until colonies were easily visualized (12–16 hrs.). Device DNA was purified from select colonies and either analyzed by restriction digestion, or sequenced to verify correct parts assembly, or used directly in downstream processes.
SV parts assembly into Nedd5 protein expression devices
To demonstrate an obvious use of the new SV cloning method, the human Nedd5 gene was chosen as a model GOI for performing an expression screening experiment. Nedd5 is a mammalian septin known to associate with actin-based structures such as the contractile ring and stress fibers and is involved in the process of cytokinesis in human brain tumors [11], although the specific nature of Nedd5 is not pertinent to this paper. The Nedd5 gene containing a start and a stop codon was adapted by PCR to be SV compatible by using the primers listed below:
N-terminal forward primer 5’- GGTGGCGGAGGTTCTGGAGGCGGTGGAAGTATGGGATCCATGTCTAAGCAACAACCAACTC-3’ and N-terminal reverse primer 5’- TCGAGGAGATATTGTACACTAAACCAAATGTCACACATGCTGCCCGAGAGCCCCGCTGTCAC-3’.
The Nedd5 gene containing a start codon but lacking a stop codon was adapted by PCR to be SV compatible by using the following primers:
C-terminal forward primer 5’- CCTTGTTTAAACTTTAAGAGGAGGGCCACCATGGGATCCATGTCTAAGCAACAACCAACTC-3’ and C-terminal reverse primer 5’-CCACCGCCTCCAGAACCTCCGCCACCCACATGCTGCCCGAGAGCCCCGCTGTCACTGTCAC-3’.
Primers show the start codon or stop codon in bold-italicized type and all primers include unique 30 bases (underlined) for assembly with adjacent parts. The resulting Nedd5 PCR products were used as the model GOI and assembled into a variety of twelve expression constructs each containing a different C- or N-terminal expression tag. The following parts were used in these assemblies: Amp [SM] + pBR322 [OR] + XP-1 linker + XP-2 lacI + Nedd5 [GOI] with either C-terminal Tags[T] (c-Myc, thioredoxin, streptavidin binding protein, calmodulin binding protein, His6 or hemagglutinin) or N-terminal tags (GST, MBP, His6, SBP, CBP, or HisDbsA, calmodulin binding protein or hemagglutinin) + pTac [P] (Refer to section entitled “General assembly of SV parts into devices”). As a comparison the Nedd5-Fusion expression region from these SureVector assembled plasmids was isolated by PCR and sub-cloned, using restriction enzymes, into a commercially available ptac based expression vector. Both plasmids were then subjected to the identical protein expression protocol.
SV Nedd5 expression devices screening
SureVector derived, and the ptac constructed, Nedd5 clones with different expression tags were cultured overnight at 37°C with shaking at 250 rpm in 1 ml of LB broth containing ampicillin (50 μg/ml). The following day, 10 ml cultures of LB broth containing ampicillin (50 μg/ml) were inoculated with these clones and incubated at 37°C with shaking at 250 rpm until the OD600, a measure of bacterial growth, reached 0.6 (time zero, approximately 1 hour). Protein expression was induced by the addition of IPTG to a final concentration of 0.5 mM followed by incubation for 20 hours at 30°C with shaking at 250 rpm. Volumes of cells equal to OD600 of 3.0 were removed from each culture at time zero (uninduced samples) and after 20 hours of incubation (induced samples). The samples were then centrifuged, and cell pellets resuspended in 120 μl of 8M urea. The mixtures were vortexed well and incubated at 75°C for 5 min. Cell lysates were centrifuged and supernatants analyzed for Nedd5 expression by SDS-gel electrophoresis.
SV assembly of parts and devices into higher order systems expressing DMRL
SV parts and devices were assembled to reconstitute the four enzyme E. coli biosynthetic pathway (system) for 6,7-dimethy-8-ribityllumazine (DMRL), the fluorescent precursor to riboflavin (rib pathway genes). The DMRL system construction was accomplished by first assembling devices with zero, one or two rib genes. PCR primers used to amplify rib open reading frame gene parts A (ribA– 591 bp), B (ribB– 654 bp), D (ribD– 1104 bp) and E (ribE—471 bp) with appended SV overlaps to make them SV compatible were:
E. coli ribA–Gene ID = 945763
1. ribA_Forward Primer-N-Tag (56 bp)
ggtggcggaggttctggaggcggtggaagtATGCAGCTTAAACGTGTGGCAGAAGC
2. ribA_Reverse Primer-RBS (67 bp)
gaaattgttaaattatttctagattcgaaaggagctcgaattcTTATTTGTTCAGCAAATGGCCCAT
E. coli ribB–Gene ID = 947526
1. ribB_Forward Primer-N-Tag (56 bp)
ggtggcggaggttctggaggcggtggaagtATGAATCAGACGCTACTTTCCTCTTT
2. ribB_Reverse Primer-RBS (68 bp)
gaaattgttaaattatttctagattcgaaaggagctcgaattcTCAGCTGGCTTTACGCTCATGTGCC
E. coli ribD–Gene ID = 945620
1. ribD_Forward Primer-RBS (73 bp)
ttcgaatctagaaataatttaacaatttcacataaaggaggtaaataATGCAGGACGAGTATTACATGGCGCG
2. ribD_Reverse Primer (54 bp)
ctcgaggagatattgtacactaaaccaaatgTCATGCACCCACTAAATGCAGGC
E. coli ribE—Gene ID = 946453
1. ribE_Forward Primer-RBS (74 bp)
ttcgaatctagaaataatttaacaatttcacataaaggaggtaaataATGAACATTATTGAAGCTAACGTTGC
2. ribE_Reverse Primer: (55 bp)
ctcgaggagatattgtacactaaaccaaatgTCAGGCCTTGATGGCTTTCAATAC
Two sets of bi-cistronic devices were designed and assembled, one containing the ribA and ribD genes and the other containing the ribB and ribE genes. A ribosome binding site (RBS) was included in the 3’ region of the ribA and ribB genes downstream of their native stop codon and the same sequence was also included in the 5’ region upstream of the ATG start codon of the ribD and ribE genes. This RBS sequence was used as the overlap by which the ribD and ribE genes were positioned downstream of ribA and ribB genes, respectively. The intended outcome was to place two rib genes under control of one promoter and couple expression of the upstream and downstream rib genes via a second RBS between the two rib genes. This was done to attempt to balance expression levels of the individual rib genes. This second RBS overlap region between the rib genes was designed in such a way that the downstream rib gene was not in the same reading frame as the upstream rib gene thus preventing two gene products in the same device from becoming physically linked. An additional stop codon was also added to each upstream rib gene to further guard against translation read-through. Bi-cistronic vectors of this type have been used previously for preparation of platelet activating factor acetylhydrolase (PAF-AH) alpha domain heterodimer for crystal structure studies [12] and for the analysis of BORG/Septin heterodimer filament formation [13]. Devices lacking either the upstream or downstream rib gene parts were correctly assembled into circular molecules using 90 bp N-terminal and C-terminal “Non-Coding” linker parts NC-N and NC-C, respectively, and were made by overlap extension [14]:
N-terminal “NC” replaces ribA or ribB parts
rib—“NC” N-term_Forward ggtggcggaggttctggaggcggtggaagtgaaactgcactcatcgtccctcgaggagct
rib—“NC” N-term_Reverse gaaattgttaaattatttctagattcgaagagctcctcgagggacgatgagtgcagtttc
C-terminal “NC” replaces ribD or ribE parts
rib—“NC” C-term_Forward ttcgaatctagaaataatttaacaatttcacataaaggaggtatagacagcatacgagtc
rib—“NC” C-term_Reverse ctcgaggagatattgtacactaaaccaaatgactcgtatgctgtctatacctcctttatg
Bi-cistronic devices that only contain a single rib gene required either a NC-N or NC-C part in lieu of the corresponding rib gene part. Three standard parts were used in both sets of rib devices; the T7 promoter-HIS6, XP1 linker and XP2 lacI. These 3 standard parts were used in various combinations with either the ampicillin or kanamycin selectable markers and the pBR322 or p15a bacterial origins of replication. Therefore, all SV rib devices were assembled from just seven SV parts. A total of 18 device level plasmids were constructed. These system level devices were designated by letter-number codes. “K” devices consisted of the kanamycin resistance marker (kan), the p15a origin of replication and either zero, one or two rib genes. “A” devices consisted of the ampicillin resistance marker (amp), the pBR322 origin of replication and either zero, one or two rib genes (Table 2). Higher order systems were created using various combinations of these device level plasmids by the co-transformation of two devices. For example, devices K6 and A7 resulted in co-expression of ribA-ribD genes from the K6 device and ribB-ribE genes from the A7 device. Combinations of devices were transformed into Agilent BL21(Gold) DE3 E. coli and spread onto LB-agar plates containing 100 μg/ml each of kanamycin and ampicillin (LB-kan-amp) plus 0.5 mM IPTG. Plates were incubated at 37°C for 12 to 18 hours and examined under UV light to identify DMRL expressing clones (systems) as evidenced by fluorescent colonies surrounded by fluorescent halos.
Table 2. SV devices required to re-create the DMRL biosynthetic pathway.
| Kanamycin/p15a Rib “K” Devices | ||||||
| Device ID | SM | Origin | Promoter | GOI1 | GOI2 | Type |
| K1 | Kan | p15a | T7-His | RibA | NC-C | Control |
| K2 | Kan | p15a | T7-His | RibB | NC-C | Control |
| K3 | Kan | p15a | T7-His | NC-N | RibD | Control |
| K4 | Kan | p15a | T7-His | NC-N | RibE | Control |
| K5 | Kan | p15a | T7-His | NC-N | NC-C | Control |
| K6 | Kan | p15a | T7-His | RibA | RibD | Test Plasmids |
| K7 | Kan | p15a | T7-His | RibB | RibE | Test Plasmids |
| K8 | Kan | p15a | T7-His | RibA | RibE | Test Plasmids |
| K9 | Kan | p15a | T7-His | RibB | RibD | Test Plasmids |
| Ampicillin/pBR322 Rib “A” Devices | ||||||
| Device ID | SM | Origin | Promoter | GOI1 | GOI2 | Type |
| A1 | Amp | pBR322 | T7-His | RibA | NC-C | Control |
| A2 | Amp | pBR322 | T7-His | RibB | NC-C | Control |
| A3 | Amp | pBR322 | T7-His | NC-N | RibD | Control |
| A4 | Amp | pBR322 | T7-His | NC-N | RibE | Control |
| A5 | Amp | pBR322 | T7-His | NC-N | NC-C | Control |
| A6 | Amp | pBR322 | T7-His | RibA | RibD | Test Plasmids |
| A7 | Amp | pBR322 | T7-His | RibB | RibE | Test Plasmids |
| A8 | Amp | pBR322 | T7-His | RibA | RibE | Test Plasmids |
| A9 | Amp | pBR322 | T7-His | RibB | RibD | Test Plasmids |
Each device contains zero, one or two rib genes, and either the kanamycin resistance marker and p15a origin of replication prefabs (labeled “K”) or the ampicillin resistance marker and pBR322 origin prefabs (labeled “A”). Single rib gene control plasmids (K1 –K4; A1-A4) require either N- or C- terminal “non-coding” parts (NC-N and NC-C, respectively) to assemble complete devices. Zero rib gene control devices (K5 and A5) contain both NC-N and NC-C parts in place of both rib genes to assemble into complete devices. Co-transformation of one “K” device (e.g. K6 through K9) with one “A” device (e.g. A6 through A9) results in a higher order system expressing either 0, 1, 2, 3 or 4 rib genes.
Validation of DMRL system synthesis—assay, purification and MS analysis
Monitoring clones for DMRL synthesis on agar plates was straightforward as the DMRL fluoresces within colonies and is secreted into the surrounding media creating fluorescent halos around DMRL positive colonies. DMRL production from clones cultured in liquid media was also straightforward as the compound possesses a characteristic visible light absorption spectrum with λ max OD490 [15] that can be measured in cell free supernatants. Validation of DMRL synthesis required its production, purification and analytical characterization. Clone K6A7 produced pronounced fluorescent colony-halos and was chosen for this purpose. A single colony was inoculated into 3 ml of LB-kan-amp liquid media and incubated overnight at 37°C with shaking at 250 rpm. A 2.0 ml sample of this culture was inoculated into a 500 ml Erlenmeyer flask containing 100 ml of LB-kan-amp and incubation continued as before until the OD600 value reached 0.35. IPTG was added to a final concentration of 0.5 mM and incubation continued for an additional 18 hours. Cells were removed by centrifugation and acetic acid added to the supernatant to a final concentration of 5%. This sample was applied to a 2.5 x 3.5 cm column of Florisil (Sigma-Aldrich) equilibrated with 5% acetic acid. The column was washed with one liter of 5% acetic acid and DMRL eluted with 100 ml of 3% pyridine. Solvent was removed by evaporation and the residue suspended in 5 ml of water. This sample was applied to a 2.5 x 35 cm column of chromatography grade cellulose (Sigma-Aldrich) equilibrated with water and DMRL was eluted with water. Forty milligrams of DMRL were recovered from 0.8g of cells (wet weight) and was analyzed by mass spectrometry. The sample was run on an Agilent 6538 QTOF coupled to an Agilent 1100/1200 lc stack. The column was a Zorbax SB-C18 0.5 x 150 mm. Flowrate = 20μl/min. Injection volume = 5μl. Solvent A was H2O – 0.1% formic acid and solvent B was acetonitrile– 0.1% formic acid. Gradient: T0: 95% A—5% B; T10: 75% A—25% B; T12: 50% A—50% B; T15: 5% A—95% B; T20: off. Five min. re-equilibration time. For the ms run, a range from m/z = 85 to m/z = 1100 was scanned. For the ms/ms run, m/z 327.1 was targeted, with three different collision energies: ce = 10V, ce = 20V, and ce = 40V.
Quantities and rates of DMRL synthesis from devices and systems
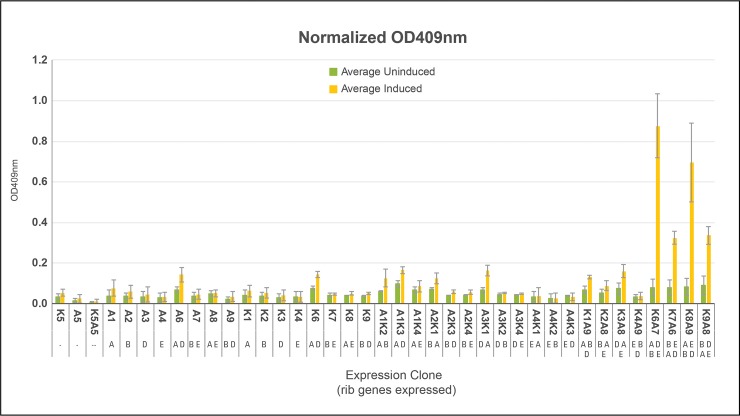
Single colonies from devices and systems listed in Table 2 were purified by re-streaking onto fresh LB-kan-amp plates without IPTG. Three colonies from each plate were cultured overnight at 37°C with shaking at 250 rpm in separate tubes containing 3 ml of LB-kan-amp. The next day, one hundred microliters of each culture were added to 4.9 ml of LB-kan-amp and incubated until OD600 reached between 0.3 and 0.5. IPTG was added to a final concentration 0.5 mM and incubation continued for three hours after which OD600 values were re-measured. Cultures were centrifuged to remove cells and OD409 values of supernatants obtained. OD409 values were normalized relative to OD600 values measured post IPTG addition and the resulting numbers compared. DMRL was synthesized exclusively by clones containing all four rib genes (two devices each expressing two rib genes)–systems K6A7, K7A6, K8A9, K9A8.
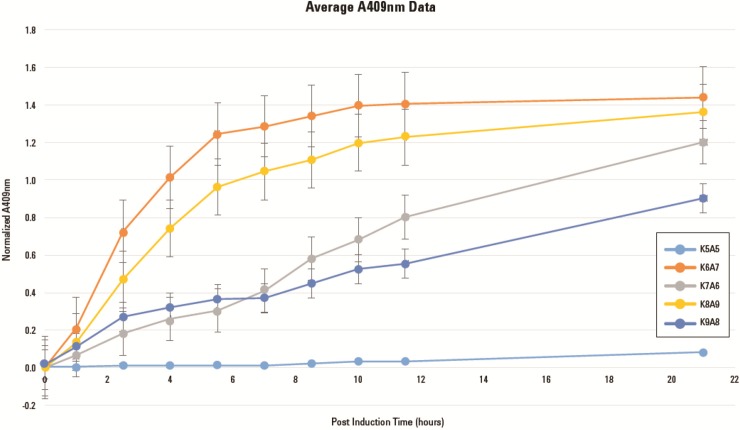
DMRL synthesis rates of systems K6A7, K7A6, K8A9, K9A8 and negative control K5A5 were obtained by inoculating single colonies into 5 ml of LB-kan-amp media followed by overnight incubation at 37°C with shaking at 250 rpm. One ml of these starter cultures was inoculated into separate 250 ml flasks each containing 49 ml of LB-kan-amp. Incubation at 37°C with shaking at 250 rpm continued until OD600 values reached between 0.3 and 0.5. IPTG was added to a final concentration of 0.5 mM and incubation continued. At regular intervals, 1.0 ml samples were retrieved from each culture and OD600 values measured. Samples were then centrifuged to remove cells and OD409 values of supernatants obtained. OD409 values were normalized relative to OD600 values measured post IPTG addition and the resulting numbers compared.
Results and discussion
Design features of SV parts and assembly process
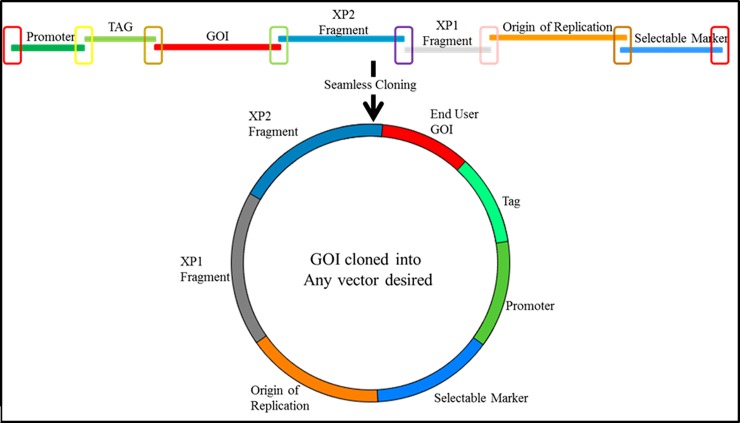
The key design features of SV parts, ensuring precise and ordered joining, are the unique 30 bp sequences incorporated into each PCR primer used to generate a part (see Materials and Methods section). Fig 1 schematically represents how seven SV parts align and overlap due to this design feature.
Fig 1. Schematic of a seven SV part assembly into a GOI expressing device.
Different colored open rectangles highlight unique 30 bp overlaps between functional parts. Sequences represented by the two end rectangles (red) also overlap. The mixture of parts is treated with the SureVector enzyme assembly blend resulting in a device, represented by the closed circle that will transform, replicate and express a GOI in E.coli.
Assembling parts listed in Table 1 in the manner described in the Materials and Methods section and in the Fig 1 legend generated devices expressing GOI’s with an N-terminal fusion protein: -Bacterial Selectable Marker-Bacterial Origin of Replication-XP1-XP2-GOI←Expression Tag←Promoter- (← denotes direction of promoter and gene expression)
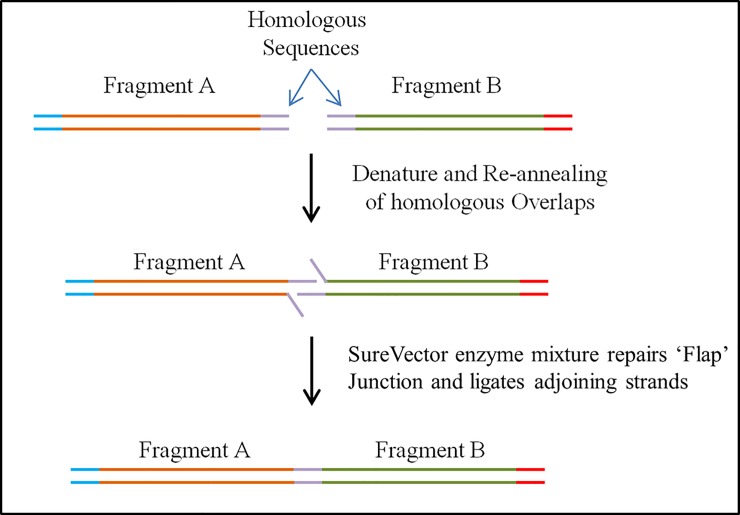
The assembly mechanism of linking parts into higher order devices is represented in Fig 2. Parts are denatured, and adjacent parts anneal due to the 30 bp overlaps. Exposed 3’-OH ends are partially extended by a polymerase resulting flaps that are digested by an endonuclease and covalently joined by a ligase.
Fig 2. Schematic Showing How Adjacent SV Parts are Assembled; Parts A and B Possess Homologous Ends.
Following denaturation and annealing, resulting free 3’ ends are extended, “flaps” digested and the two parts ligated.
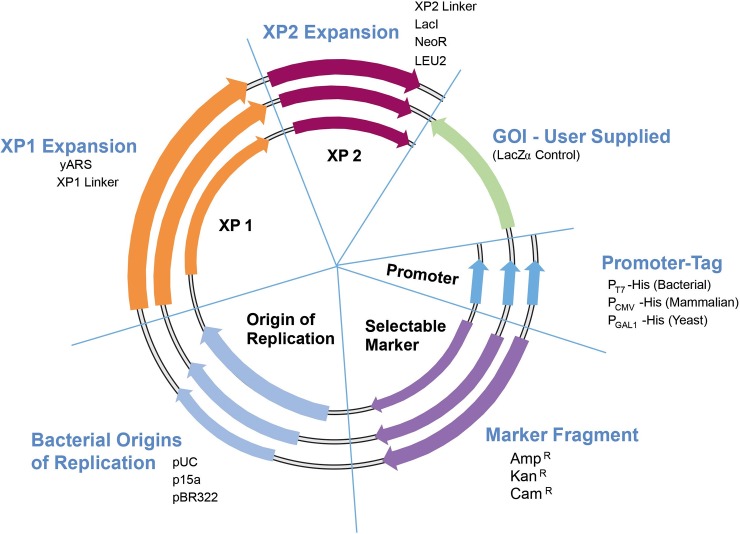
Fig 3 represents the combinatorial assembly power of the SV process. Different functional parts are rapidly assembled into multiple configurations in parallel assembly experiments to determine the best organization for, in this case, expression of a single GOI.
Fig 3. Collection of SV parts and assembly design.
A variety of parts and GOI’s can be assembled into many functional devices.
SV Nedd5 expression devices screening
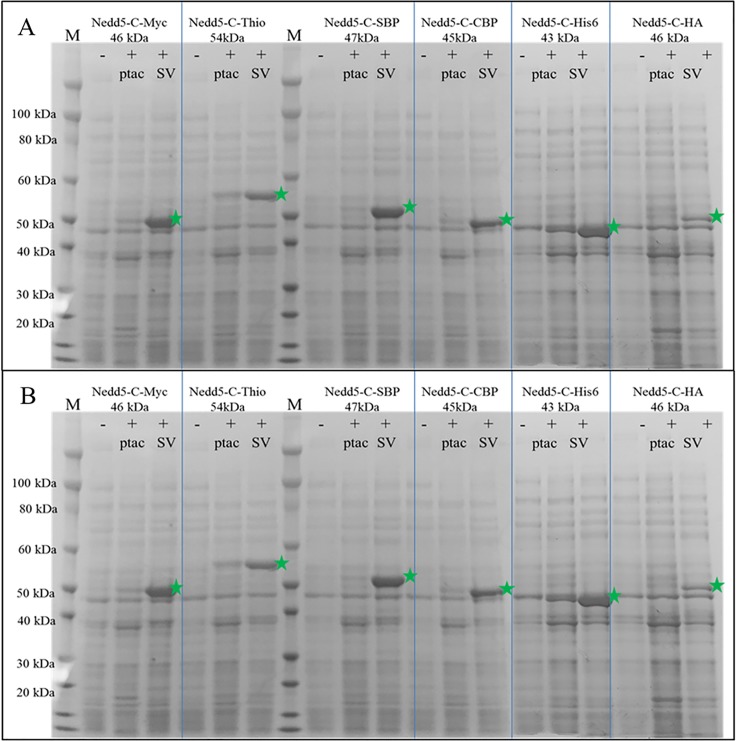
SDS-page analysis of twelve Nedd5 expression devices, fused with N- or C- terminal tags identified the best expression constructs (green arrows shown in Fig 4A and Fig 4B highlight proteins from induced cultures). Quantities of expressed fusion proteins varied with the type of expression tag and were highest with N-terminal tagged MBP/Nedd5, HisDbsA/Nedd5, and CBP/Nedd5 devices and C-terminal tagged Nedd5/SBP, Nedd5/c-myc and Nedd5/His6 devices. Lesser quantities of fused proteins were expressed as N-terminal tagged GST/Nedd5 and His6/Nedd5 devices and C-terminal tagged Nedd5/Thioredoxin and Nedd5/CBP devices. The least quantities of fusion Nedd5 were N-terminal tagged SBP/Nedd5 and C-terminal tagged Nedd5/HA devices. It is worth emphasizing that this protein expression screening experiment, starting from assembly of parts into devices and analyzing protein expression was completed in less than three days.
Fig 4.
a. SDS-PAGE gel of SV Nedd5 expression devices with different N-terminal tags. M = protein molecular weight marker; (-) = Uninduced sample; (+) Induced samples. SV denotes expression from SureVector assembled plasmids, ptac denotes expression from the commercially available ptac based plasmids. Green stars denote expressed N-terminal tagged Nedd5-fusion proteins. Fig 4b. SDS-PAGE Gel of SV Nedd5 Expression Devices with Different C-Terminal Tags. M = protein molecular weight marker; (-) = Uninduced samples; (+) = Induced samples. SV denotes expression from SureVector assembled plasmids, ptac denotes expression from the commercially available ptac based plasmids. Green stars denote expressed N-terminal tagged Nedd5-fusion proteins.
Re-creating a biosynthetic pathway
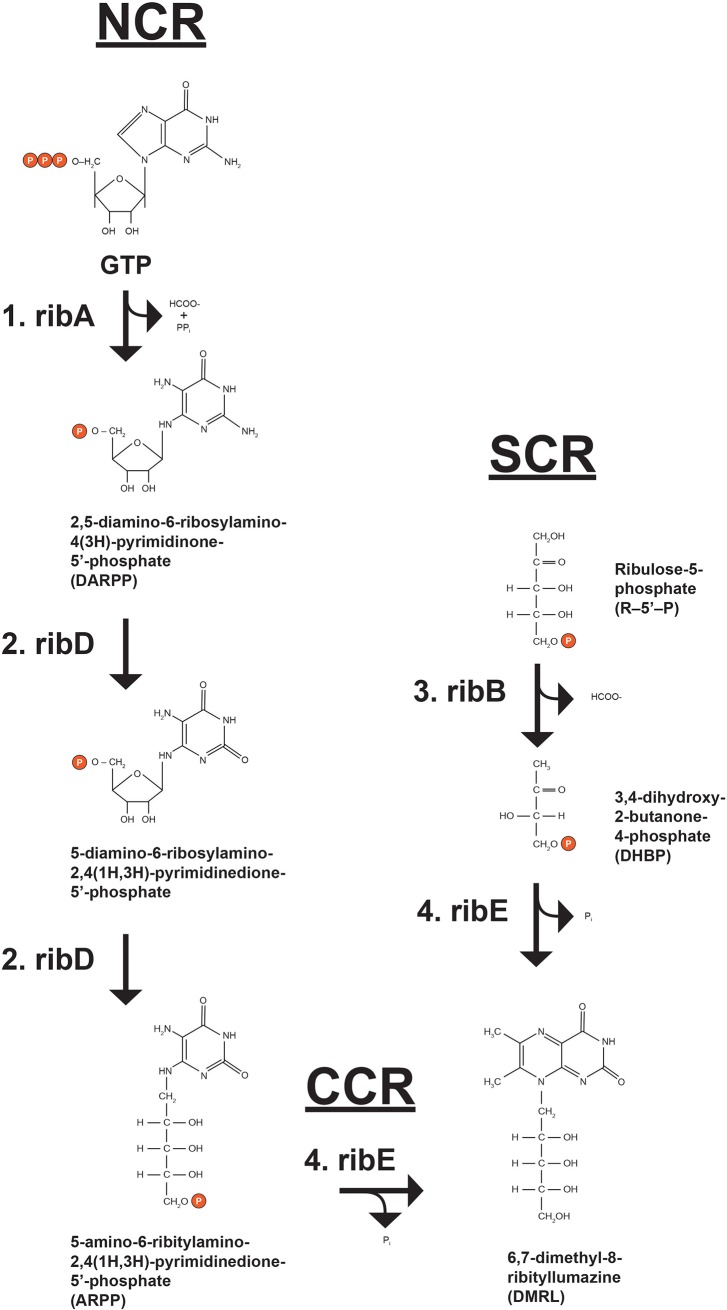
The utility of SV parts assembly into devices and ultimately higher order systems was demonstrated by recreating the E. coli biosynthetic pathway for 6,7-dimethy-8-ribityllumazine (DMRL), the fluorescent precursor to riboflavin (Fig 5; [16]). DMRL is synthesized by four unique enzymes (expressed from ribA, ribB, ridD and ribE genes) and substrates. The critical initial substrates for this pathway are GTP and ribulose-5’-phosphate (R5P). They are funneled through a “nucleotide conversion route” (NCR), a “sugar conversion route” (SCR) and a “converging condensation route” (CCR). The NCR starts by the hydrolytic removal of carbon atom 8 from the imidazole ring of GTP by GTP Cyclohydrolase II (ribA gene product; [17]) yielding 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone-5’-phosphate (DARPP). DARPP is converted to 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione-5’-phosphate (ARPP) by diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione reductase (ribD gene product; [18, 19]), a “fused” enzyme possessing both NCR and SCR-relevant activities. As the enzyme name describes, the nucleotide base of DARPP is deaminated followed by reduction of the ribosyl moiety to ribityl with NADPH serving as reductant. Separately, L-3,4-dihydroxy-2-butanone-4-phosphate synthase, an enzyme exclusively in the SCR (ribB gene product; [20]) converts ribulose-5’-phosphate (R-5-P) to L-3,4-dihydroxy-2-butanone-4-phosphate (DHBP) and formate. Both ARPP and DHBP are dephosphorylated and enter the CCR via DMRL synthase (ribE gene product; [21]) resulting in DMRL.
Fig 5. DMRL biosynthetic pathway.
The imidazole ring of GTP is hydrolytically removed by GTP Cyclohydrolase II (rxn. 1; ribA) yielding 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone-5’-phosphate (DARPP), formate and pyrophosphate. DARPP is converted to 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione-5’-phosphate (ARPP) by fused diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione reductase (rxns. 2; both ribD). Separately, ribulose-5-phosphate (R5P) is converted to L-3,4-dihydroxy-2-butanone-4-phosphate (DHBP) and formate by L-3,4-dihydroxy-2-butanone-4-phosphate synthase (rxn. 3; ribB). ARPP and DHPB are dephosphorylated and then condensed by DMRL synthase (rxn. 4; ribE) producing 6,7-dimethyl-8-ribityllumazine (DMRL).
Pathway construction
DMRL pathway construction was accomplished by first assembling SV parts into devices (Table 2) containing zero, one and two (“bi-cistronic”) rib genes. PCR primers used to amplify rib gene parts A, B, D and E are shown in Materials and Methods. They allowed amplification of rib open reading frames ribA– 591 bp, ribB– 654 bp, ribD– 1104 bp and ribE—471 bp with appended correct overlaps to make them SV compatible. Two sets of bi-cistronic devices, one containing the ribA and ribD genes and the other containing the ribB and ribE genes were also designed and assembled (Table 2). Ribosome binding sites (RBS) were designed and incorporated into the 3’and 5’ flanking regions of the rib genes and subsequently used as unique overlaps between the genes such that ribD and ribE genes were positioned downstream of the ribA and ribB genes, respectively. The intended outcome of placing two rib genes under control of one T7 promoter and coupling expression of the upstream and downstream rib genes was balanced expression levels. The RBS placed in the 5’ regions of both ribD and ribE genes promoted downstream rib gene translation efficiency. The RBS overlap between the upstream and downstream rib genes was designed so that the downstream rib genes were out of frame with the upstream rib genes thus preventing two gene products in the same device from becoming physically linked. An additional stop codon was also added to each upstream rib gene as an added prevention to translation read-through. As mentioned previously, bi-cistronic vectors of this type have been used previously for preparation of platelet activating factor acetylhydrolase (PAF-AH) alpha domain heterodimer for crystal structure studies [12] and for the analysis of BORG/Septin heterodimer filament formation [13]. SV devices lacking one or both rib genes were also assembled using N-terminal and C-terminal “Non-Coding” parts; NC-N and NC-C, respectively (Table 2). Zero or single rib gene control devices required either an NC-N or NC-C part, or both in the case of the double negative control, in lieu of a rib gene part. Common SV parts used in both sets of rib devices were the T7 promoter-HIS6, XP1 linker and XP2 lacI (allowing IPTG induction of rib gene devices expression) in addition to various combinations of ampicillin and kanamycin selectable markers with pBR322 or p15a bacterial origins of replication. Therefore, all 18 SV rib device plasmids were assembled from just 7 seven SV parts, not including the rib gene parts.
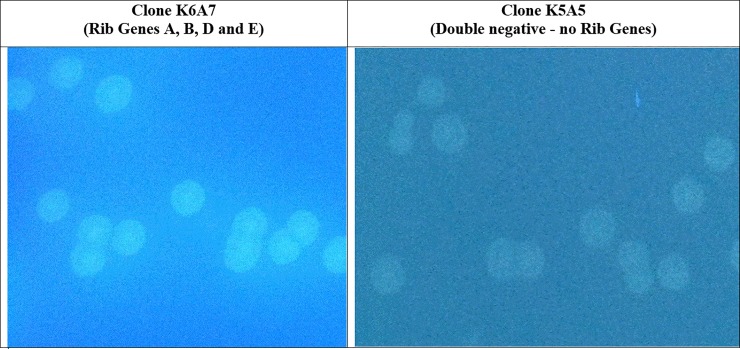
Table 2 devices were transformed either individually or in various co-transformation scenarios (Table 3) into Agilent BL21(Gold) DE3 E. coli and spread onto LB- agar plates containing 100 μg/ml each of kanamycin and ampicillin (LB-kan-amp) plus 0.5 mM IPTG. Plates were incubated at 37°C for twelve to eighteen hours. All colony devices and systems were easily monitored for DMRL synthesis by irradiating plates with UV light and visualizing fluorescent colonies surrounded by fluorescent halos. DMRL producing colony systems contained all four rib genes (Fig 6 - left panel). Colonies containing only 1, 2 or 3 rib genes, as well as the double negative control lacking all 4 rib genes (clone K5A5 assembled with NC-N and NC-C parts), did not produce DMRL (Fig 6 - right panel; no fluorescent colonies and no halos).
Table 3. Combinations of Table 2 functional and control devices used to re-create the DMRL biosynthetic pathway system.
| Transformations into BL21(GOLD) | |||||
|---|---|---|---|---|---|
| System | rib Genes Expressed | Rib genes Missing | Plate on | Assay | |
| Device 1 | Device 2 | ||||
| K1 | - | A | B, D and E | Kan | Control |
| K2 | - | B | A, D and E | Kan | Control |
| K3 | - | D | A, B and E | Kan | Control |
| K4 | - | E | A, B and D | Kan | Control |
| K5 | - | - | A, B, D and E | Kan | Control |
| K6 | - | A and D | B and E | Kan | Control |
| K7 | - | B and E | A and D | Kan | Control |
| K8 | - | A and E | B and D | Kan | Control |
| K9 | - | B and D | A and E | Kan | Control |
| A1 | - | A | B, D and E | Amp | Control |
| A2 | - | B | A, D and E | Amp | Control |
| A3 | - | D | A, B and E | Amp | Control |
| A4 | - | E | A, B and D | Amp | Control |
| A6 | - | A and D | B and E | Amp | Control |
| A7 | - | B and E | A and D | Amp | Control |
| A8 | - | A and E | B and D | Amp | Control |
| A9 | - | B and D | A and E | Amp | Control |
| A5 | - | - | A, B, D and E | Amp | Control |
| K1 | A9 | A, B and D | E | Kan+Amp | Control |
| K2 | A8 | B, A and E | D | Kan+Amp | Control |
| K3 | A8 | D, A and E | B | Kan+Amp | Control |
| K4 | A9 | E, B and D | A | Kan+Amp | Control |
| K6 | A7 | A, D, B and E | - | Kan+Amp | Test |
| K7 | A6 | B, E, A and D | - | Kan+Amp | Test |
| K8 | A9 | A, E, B and D | - | Kan+Amp | Test |
| K9 | A8 | B, D, A and E | - | Kan+Amp | Test |
Fig 6. Co-transformation of two SV compatible devices containing all 4 rib biosynthetic genes results in DMRL synthesis.
Devices K6 and A7 (see Table 2) were co-transformed into Agilent BL21(Gold)DE3 E.coli and spread onto LB-kan-amp-IPTG plates. Resulting colonies were examined under unfiltered UV light. All resulting K6A7 systems contained four rib genes and produced DMRL as evidenced by fluorescent colonies with fluorescent halos. Control (device K5A5) through three rib genes (see Table 2) did not produce DMRL as no fluorescent halos were detected.
Characterization of DMRL synthesized by SV rib system K6A7
DMRL synthesized and purified from system K6A7 (Table 3) was characterized by mass spectrometry (see Materials and Methods). For the ms run, a range from m/z = 85 to m/z = 1100 was scanned. The theoretical m/z value for DMRL is 327.12990 and the observed m/z value was 327.12914. In addition, for the ms/ms run the m/z value of 327.1 was targeted, using three different collision energies at 10V, 20V, and 40V. The expected exact mass of fragmented DMRL was 192.06 and the observed value was 192.07 confirming that SV system K6A7 produced DMRL.
Quantities and rates of DMRL synthesis from DMRL devices and systems
Fig 7 confirms that only SV systems K6A7, K7A6, K8A9, K9A8, each containing two rib gene devices expressing all four rib genes, produced DMRL. Systems K6A7 and K8A9 produced the highest levels of DMRL and most rapidly (Fig 8). Both systems are composed of devices K6 and K8 expressing enzymes from ribA and ribD genes and from ribA and ribE genes, respectively. The ribA gene is positioned immediately downstream of the T7 promoter in both K6 and K8 devices. In devices A7 and A9, the ribB gene is downstream of the T7 promotor. Total DMRL accumulation and slower synthesis rates are characteristic of systems K7A6 and K9A8. These systems are composed of devices K7 and K9 expressing enzymes from ribB and ribE genes and from ribB and ribD genes, respectively. In K7 and K9 devices the ribB gene is positioned immediately downstream of the T7 promotor. The A6 and A8 devices express enzymes from ribA and ribD genes and ribA and ribE genes, respectively. In these devices ribA is downstream of the T7 promoter. How these system configurations resulted in different totals and rates of DMRL synthesis were not examined. However, there may be a connection between over-expression of ribA (GTP Cyclohydrolase II, the first committed step in the NCR) and production of DARPP (Fig 5) in kanamycin resistant hosts that leads to saturating substrate levels for the balance of NCR and CCR enzymes. Also, over-expression of ribB (L-3,4-dihydroxy-2-butanone-4-phosphate synthase, the first committed step in the SCR) and production of DHBP in ampicillin resistant hosts leads to saturating co-substrate levels for the CCR enzyme DMRL synthase (Fig 5). The net result of attaining saturating levels these substrates would be that all DMRL synthesis enzymes could operate at Vmax.
Fig 7. Total quantities of DMRL synthesis from devices and systems.
Single colonies from devices and systems listed in Table 3 were purified by re-streaking onto fresh LB-kan-amp plates without IPTG. Three colonies from each plate were cultured in separate tubes containing 3 ml of LB-kan-amp. One hundred microliters of each culture were added to 4.9 ml of LB-kan-amp and incubated until OD600 reached between 0.3 and 0.5. IPTG was added to a final concentration 0.5 mM and incubation continued for three hours after which OD600 values were re-measured. Cultures were centrifuged to remove cells and OD409 values of supernatants obtained. OD409 values were normalized relative to OD600 values measured post IPTG addition and the resulting numbers compared. DMRL was synthesized exclusively by systems containing two devices each expressing two rib genes–systems K6A7, K7A6, K8A9 and K9A8.
Fig 8. DMRL synthesis rates.
DMRL synthesis rates of systems K6A7, K7A6, K8A9, K9A8 and negative control K5A5 were obtained by inoculating single colonies into 5 ml of LB-kan-amp media followed by overnight incubation at 37°C with shaking at 250 rpm. One ml of these starter cultures was inoculated into separate 250 ml flasks each containing 49 ml of LB-kan-amp. Incubation at 37°C with shaking at 250 rpm continued until OD600 values reached between 0.3 and 0.5. IPTG was added to a final concentration of 0.5 mM and incubation continued. At regular intervals, 1.0 ml samples were retrieved from each culture and OD600 values measured. Samples were then centrifuged to remove cells and OD409 values of supernatants obtained. OD409 values were normalized by dividing by the OD600 values and these numbers plotted as a function of OD409 post-IPTG addition.
Conclusions
A new method (SureVector) for seamless assembly of biological parts into functional devices and higher order systems is presented that takes advantage of principles learned from prefabrication engineering design and assembly. These are: (1) Functional DNA parts are manufactured and quality controlled at a location away from the assembly site (Agilent Technologies, Inc.); (2) Parts, other requisite assembly materials and detailed assembly instructions are delivered to the site of construction (the research laboratory) for assembly; (3) from a synthetic biology standpoint, processes (1) and (2) enable rapid and reliable combinatorial assembly of desired devices destined for introduction into E. coli, mammalian and yeast cells. The salient features of this new method were demonstrated by constructing a set of protein expression devices to identify the best device for the expression of a target GOI (Nedd5). To further illustrate the adaptability of this assembly method, a higher order plasmid system was constructed to recreate a four-gene biosynthetic pathway. The biosynthetic pathway to synthesize DMRL was selected as it served as a good model of a multi-gene system that could be easily evaluated. The combinatorial assembly power of this process was also utilized in developing a synthetic DMRL expression system. A total of 18 protein expression devices were assembled in one day possessing zero, one or two rib gene (bi-cistronic) parts. Expression screening was initiated the following day and positive systems were selected for structural and functional testing in less than one week. While assembled devices were being sequenced for validation of their structural integrity, DMRL was purified and characterized by mass spectrometry. Experiments were also performed to determine optimal DMRL synthesis rates and maximum production. The same type of project out-sourced to a third-party vendor would have taken several months to complete (previous experience). While optimizing DMRL production was not the goal of these experiments, it is worth noting that 40 mg of DMRL was purified from 100 ml of media collected after growth of 0.8 g (wet weight) of E. coli clone K6A7. In stark contrast, Maley and Plaut (15) required 5 kg of the mold A. gossypii to obtain 160 mg of DMRL.
The combinatorial power, simplicity and assembly accuracy of the SureVector process will facilitate building many “multi-device” systems including unique biochemical synthetic pathways and novel regulatory circuits. Production of fine chemical intermediates and end-products represents an obvious high-value application.
Acknowledgments
The authors would like to thank Dr. Bill Webb at “The Scripps Research Institute Center for Metabolomics and Mass Spectrometry” for his expertise in performing and analyzing all mass spectrometry data.
Data Availability
All relevant data are within the paper.
Funding Statement
Agilent internally funded. The authors commercial affiliation did not play a role in the study design, data collection and analysis, decision to publish or preparation of the manuscript and only provided financial support in the form of authors salaries and research materials. More specifically, the funder provided support in the form of salaries and research materials for authors (JCB and PJS) but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section of the online submission form.
References
- 1.Knight, T.F. (2003) Idempotent vector design for standard assembly of BioBricks. Tech. Report., MIT Synthetic Biology Working Group Technical Reports.
- 2.Shetty R.P., Endy D. and Knight T.F. Jr. (2008) Engineering BioBrick vectors from BioBrick parts. J. Biol. Eng. 2, 5 10.1186/1754-1611-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M.Z. and Elledge S.J. (2007) Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nature Methods, 4, 251–256. 10.1038/nmeth1010 [DOI] [PubMed] [Google Scholar]
- 4.Gibson D.G., Young L., Chuang R-Y., Venter J.C., Hutchison C.A. and Smith H.O. (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 6, 343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 5.Quan J. and Tian J. (2009) Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 4(7): e6441 10.1371/journal.pone.0006441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yongwei Z., Werling U. and Edelmann W. (2012) SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 40 (8): e55 10.1093/nar/gkr1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engler C., Gruetzner R., Kandzia R. and Marillonnet S. (2009) Golden Gate Shuffling: A one-pot DNA shuffling method based on Type IIs restriction enzymes. PLoS ONE 4(5): e5553 10.1371/journal.pone.0005553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber E., Engler C., Gruetzner R., Werner S. and Marillonnet S. (2011) A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 6(2): e16765 10.1371/journal.pone.0016765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrion-Perdigones A., Falconi E.E., Zandalinas S.I., Juárez P., Fernández-del-Carmen A., Granell A. et al. (2011) GoldenBraid: an iterative cloning system for standardized assembly of reusable genetic modules. PLoS ONE 6 (7): e21622 10.1371/journal.pone.0021622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin P., Ding W., Du G., Chen J. and Kang Z. (2016) DATEL: A scarless and sequence-independent DNA assembly method using thermostable exonucleases and ligase. ACS Synth. Biol. 5 (9): 1028–32. 10.1021/acssynbio.6b00078 [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M., Kumar S., Mizoguchi A., Ide C., Kinoshita A., Haraguchi T., et al. (1997) Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11 (12): 1535–47. 10.1101/gad.11.12.1535 [DOI] [PubMed] [Google Scholar]
- 12.Sheffield P.J. (2001) Preparation and crystal structure of the recombinant α1/α2 catalytic heterodimer of bovine brain platelet-activating factor acetylhydrolase Ib. Protein Engineering 14: 513–519. [DOI] [PubMed] [Google Scholar]
- 13.Sheffield P. J. Oliver C.J., Kremer B.E., Sheng S., Shao Z. and Macara I. G. (2003) Borg/Septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278: 3483–3488. 10.1074/jbc.M209701200 [DOI] [PubMed] [Google Scholar]
- 14.Bryksin A.V. and Matsumura I (2010) Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques 48 (6): 463–465. 10.2144/000113418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maley G.F. and Plaut G.W.E. (1959) The isolation, synthesis and metabolic properties of 6,7-dimethyl-8-ribityllumazine. J. Biol. Chem. 234: 641–647. [PubMed] [Google Scholar]
- 16.Zhenquan L., Lin Z., Xu Z., Li Y., Wang Z., Chen T. et al. (2014) Metabolic engineering of Escherichia coli for the production of riboflavin. Microbial Cell Factories 13: 104 10.1186/s12934-014-0104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foor F. and Brown G.M. Purification and properties of guanosine triphosphate cyclohydrolase II from Escherichia coli. J. Biol. Chem. 250: 3545–3551 (1975). [PubMed] [Google Scholar]
- 18.Burrows R.B. and Brown G.M. Presence in Escherichia coli of a deaminase and a reductase involved in biosynthesis of riboflavin. J. Biol. Chem. 136: 657–667 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter G., Fischer M., Krieger C., Eberhardt S., Lüttgen H., Gerstenschläger I. et al. (1997) Biosynthesis of riboflavin: characterization of the bifunctional deaminase-reductase of Escherichia coli and Bacillus subtilis. J. Bact. 179: 2022–2028. 10.1128/jb.179.6.2022-2028.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volk R. and Bacher A. (1990) Studies on the 4-carbon precursor in the biosynthesis of riboflavin. J. Biol. Chem. 265: 19479–19486. [PubMed] [Google Scholar]
- 21.Mörtl S., Fischer M., Richter G., Tack J., Weinkauf S. and Bacher A. (1996) Biosynthesis of riboflavin. Lumazine synthase of Escherichia coli. J. Biol. Chem. 271: 33201–33207. 10.1074/jbc.271.52.33201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.