Abstract
Caloric restriction (CR) is the most potent nonpharmacological intervention known to both protect against carcinogenesis and delay aging in laboratory animals. There is a growing number of anticarcinogens and CR mimetics that activate NAD(P)H:quinone oxidoreductase 1 (NQO1). We have previously shown that NQO1, an antioxidant enzyme that acts as an energy sensor through modulation of intracellular redox and metabolic state, is upregulated by CR. Here, we used NQO1-knockout (KO) mice to investigate the role of NQO1 in both the aging process and tumor susceptibility, specifically in the context of CR. We found that NQO1 is not essential for the beneficial effects of CR on glucose homeostasis, physical performance, metabolic flexibility, life-span extension, and (unlike our previously observation with Nrf2) chemical-induced tumorigenesis.
Keywords: Whole-body metabolism, Liver, Aging, Carcinogenesis, Mitochondrial dysfunction, Oxidative stress
Caloric restriction (CR) is among the nutritional strategies that has advanced our understanding of the biology of aging. Although it shares many of the beneficial outcomes as other forms of fasting [ie, intermittent fasting (IF) or fast-mimicking diets (FMD)], CR still constitutes the most powerful mediator at slowing the aging process and improving survival in laboratory animals. CR is associated with a drastic reduction in body weight and central adiposity with concomitant increases in insulin sensitivity and anticancer protection. Anti-inflammatory benefits and improvements in metabolic flexibility, stem cell function, and DNA repair are among the physiological effects stemming from CR intervention (1). At the cellular level, CR triggers an increase in the plasticity and efficiency of mitochondrial bioenergetics (2,3). Mitochondrial biogenesis, stress resistance, regulation of molecular chaperones, and proteostasis represent other outcomes that are positively impacted by CR at the transcriptional level. Overall, CR constitutes a highly attractive model for studying the association of genes with the metabolic regulation of aging (4) in the context of interventions intended to modulate health and longevity.
CR mimetics and other forms of fasting (including CR) activate nuclear factor E2-related factor-2 (Nrf2) (5–8), a transcription factor whose binding to the antioxidant response element (ARE) present in the promoter of genes confers cytoprotection against prooxidant stimuli and inflammatory stressors. Activation of the Nrf2-ARE pathway also regulates energy metabolism and may play a role in regulating species longevity (8–10). However, the CR-mediated increase in maximum life span in mice could not be linked to Nrf2, despite its ability to confer significant anticarcinogenic protection in CR-fed mice (11). Thus, the nature of the Nrf2 target genes involved in longevity, metabolic control and cancer protection remains poorly defined.
NAD(P)H:quinone oxidoreductase 1 (NQO1), is an FAD-dependent flavoprotein, catalyzes a two-electron reduction of various quinones using NADH or NADPH as a cofactor (12). It is presently a target of current therapeutic strategies that aim to delay aging or counteract age-related diseases. Cross-sectional studies have shown a differential change of NQO1 expression and activity with chronological age, and strategies targeting NQO1 activity have been employed to delay aging and age-related metabolic diseases (13–17). Malfunction of NQO1 has been directly linked to age-associated diseases (18–20), and mice lacking NQO1 show increased sensitivity to a skin carcinogenesis model (21,22). Likewise, the C609T (Pro187Ser) null polymorphism of the NQO1 gene is associated with increased risk of complications related to metabolic syndrome (23,24), development of Alzheimer’s disease (25), and overall risk of cancer (26). These findings place NQO1 as an energy sensor that promotes longevity. Although NQO1 is a known transcriptional target of Nrf2, the degree to which NQO1 exerts its physiological and antitumorigenic functions independently of its upstream regulator, Nrf2, needs clarification.
The main focus of the study was to elucidate the role of NQO1 in the aging process, particularly in the context of CR, and to dissociate its effect from Nrf2. In order to achieve this goal, we performed a comprehensive physiological study in NQO1-KO male mice versus littermate (LM) controls, focusing on the main features of CR that included life-span extension, glucose homeostasis, physical performance, and tumor protection.
Methods
Animals
NQO1-knockout (KO) mice were received in a mixed background and were backcrossed (N10) on C57BL/6 background. Littermate male control and NQO1-KO mice on C57BL/6 background were housed at the Gerontology Research Center (Baltimore, MD). Mice had free access to house chow (NIH-31 diet; #T.7917.15, Envigo, Indianapolis, IN) and tap water, and were fed ad libitum (AL). Animal rooms were maintained at 20–22°C with 30–70% relative humidity and a 12-h light/dark cycle. All animal protocols were approved by the Animal Care and Use Committee (352-TGB-2018) of the National Institute on Aging.
Survival Study
Male LM and NQO1-KO mice were housed in cages of two to four animals with AL access to house chow until 30–40 weeks of age. Mice were then randomly assigned to AL or 40% CR groups. At baseline, there was no difference in bodyweight between the two groups. Bodyweight and food intake were monitored biweekly. Survival curves were plotted by using the Kaplan–Meier method using SigmaStat 3.5 (Systat Software Inc., San Jose, CA). Criteria for euthanasia were based on an independent assessment by a veterinarian, according to AAALAC guidelines, and only cases where the condition of the animal was considered incompatible with continued survival are represented in the curves. A total of nine animals were censored in the life-span study (1 ALLM, 4 CRLM, and 4 CRKO). (Total animals at start of the study: ALLM: n = 31, CRLM: n = 33, ALKO: n = 30, and CRKO: n = 30).
Two-Stage Carcinogenesis
An independent cohort of male LM and NQO1-KO mice were maintained on the house chow until 20 weeks of age and were thereafter divided into AL and 40% CR groups before being single-housed. The two-stage carcinogenesis model was performed in this cohort of mice as previously described (11). Briefly, after 5–6 weeks of CR, mice were treated with a single dose of 25 μg of the tumor initiator 7,12-dimethyl-benz(a)anthracene (DMBA) dissolved in 100 μL of acetone. Tumor promotion with 12-O-tetradecanoyl phorbol-13-acetate (TPA) (4 μg dissolved in 100 μL of acetone) treatment began 2 weeks after DMBA initiation and continued twice weekly until at least one papilloma with a radius 1 mm was recorded for tumor incidence data (ALLM: n = 11, CRLM: n = 13, ALKO: n = 9, and CRKO: n = 11). A subset of mice was sacrificed after 36 weeks of DMBA/TPA treatment for immunoblotting.
Body Composition
Lean, fat, and fluid mass were acquired by nuclear magnetic resonance (NMR) using the Minispec LF90 (Bruker Optics, Billerica, MA). (12 months; ALLM: n = 31, CRLM: n = 33, ALKO: n = 30, and CRKO: n = 30; 18 months; ALLM: n = 30, CRLM: n = 26, ALKO: n = 29, and CRKO: n = 24; 24 months; ALLM: n = 21, CRLM: n = 23, ALKO: n = 27, and CRKO: n = 25).
Metabolic Assessment
Activity and metabolism measurements were assessed by indirect calorimetry in open-circuit oxymax chambers using the Comprehensive Animal Metabolic Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) as previously described (27). (12 months; ALLM: n = 12, CRLM: n = 12, ALKO: n = 8, and CRKO: n = 8; 18 months; ALLM: n = 12, CRLM: n = 11, ALKO: n = 12, and CRKO: n = 11; 24 months; ALLM: n = 12, CRLM: n = 12, ALKO: n = 12, and CRKO: n = 12).
Behavioral and Physical Performance Tests
Full methodological details of fear conditioning (5 months: n = 12 per genotype), open field (12 months; ALLM: n = 12, CRLM: n = 11, ALKO: n = 12, and CRKO: n = 11; 18 months; ALLM: n = 12, CRLM: n = 9, ALKO: n = 12, and CRKO: n = 8; 24 months; ALLM: n = 9, CRLM: n = 7, ALKO: n = 10, and CRKO: n = 7), rotarod, and wirehang (12 months; ALLM: n = 12, CRLM: n = 11, ALKO: n = 12, and CRKO: n = 12; 18 months; ALLM: n=12, CRLM: n = 8, ALKO: n = 12, and CRKO: n = 8; 24 months; ALLM: n = 9, CRLM: n = 7, ALKO: n = 10, and CRKO: n = 7) are described in Supplementary Material.
Serum Markers and Glucose Homeostasis
Plasma concentrations of corticosterone were measured using a kit according to the manufacturer’s instructions (MP Biomedicals, Orangeburg, NY) (12-month-old: n = 5 per group). At 12 months of age, plasma concentrations of cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were measured using the COBAS INTEGRA 400 automated analyzer (Roche Diagnostics, Indianapolis, IN) (n = 8 per group). Fasted insulin was measured in serum using an enzyme-linked immunosorbent assay (Crystal Chem., Downers Grove, IL) according to the manufacturer’s instructions (12 months; ALLM: n = 12, CRLM: n = 11, ALKO: n = 12, and CRKO: n = 11; 18 months; ALLM: n = 13, CRLM: n = 10, ALKO: n = 12, and CRKO: n = 8; 24 months; ALLM: n = 8, CRLM: n = 8, ALKO: n = 8, and CRKO: n = 8). Fasted blood glucose was measured using an Ascensia Elite glucose meter (Bayer, Mishawaka, IN). For oral glucose tolerance test (OGTT), after an overnight fast, mice received an oral dose of 2 g/kg of glucose (Sigma-Aldrich, St. Louis, MO) by gavage and blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose administration. (12 months; ALLM: n=12, CRLM: n = 12, ALKO: n = 12, and CRKO: n = 12; 18 months; ALLM: n = 12, CRLM: n = 11, ALKO: n = 12, and CRKO: n = 8; 24 months; ALLM: n = 7, CRLM: n = 8, ALKO: n = 6, and CRKO: n = 7).
Histology
Mice were euthanized and organs fixed for histological analysis in 4% paraformaldehyde. Tissues were embedded in paraffin and stained with hematoxylin and eosin. Pathology was scored by a qualified pathologist blinded to diet and genotype group.
Immunoblotting
Separation of mouse liver extracts for the detection of specific proteins was carried out according to standard procedures as described in the Supplementary Material. The primary antibodies used in this study are presented in Supplementary Table 1 (n = 4–7 per group).
Analysis of Oxidative Markers
Protein carbonyl levels were determined by 2,4-dinitro-phenylhydrazine (DNPH) derivatization. Briefly, two aliquots of 20 μg of protein were prepared; one was used as a negative control, and the other one was treated with 2,4-dinitrophenylhydrazine using an OxyBlot kit (Millipore, Billerica, MA). Proteins were separated by SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and stained with Ponceau S. The derivatized products were then detected by immunoblotting using an antirabbit DNP antibody, as described by the manufacturer. 4-hydroxynonenal (4-HNE)-modified proteins were detected by immunoblotting with an anti-4-HNE antibody (Supplementary Table 1).
Statistics
Data are represented as means ± standard error of the mean (SEM). Student’s t-tests were used for all comparisons, unless otherwise indicated. Mortality during the survival study was assessed through the use of the log-rank test to compare the differences in Kaplan–Meier survival curves. Maximal life span was defined as the 10th percentile of mice still alive. Analyses were performed using Excel 2010 (Microsoft Corp., Redmond, WA), IBM SPSS Statistics (Amonk, NY), or SIGMASTAT 3.0 (Aspire Software International, Ashburn, VA). A p value of < .05 was considered statistically significant.
Results and Discussion
CR Improves Life span Extension and Whole-Body Physiology in NQO1-KO Male Mice
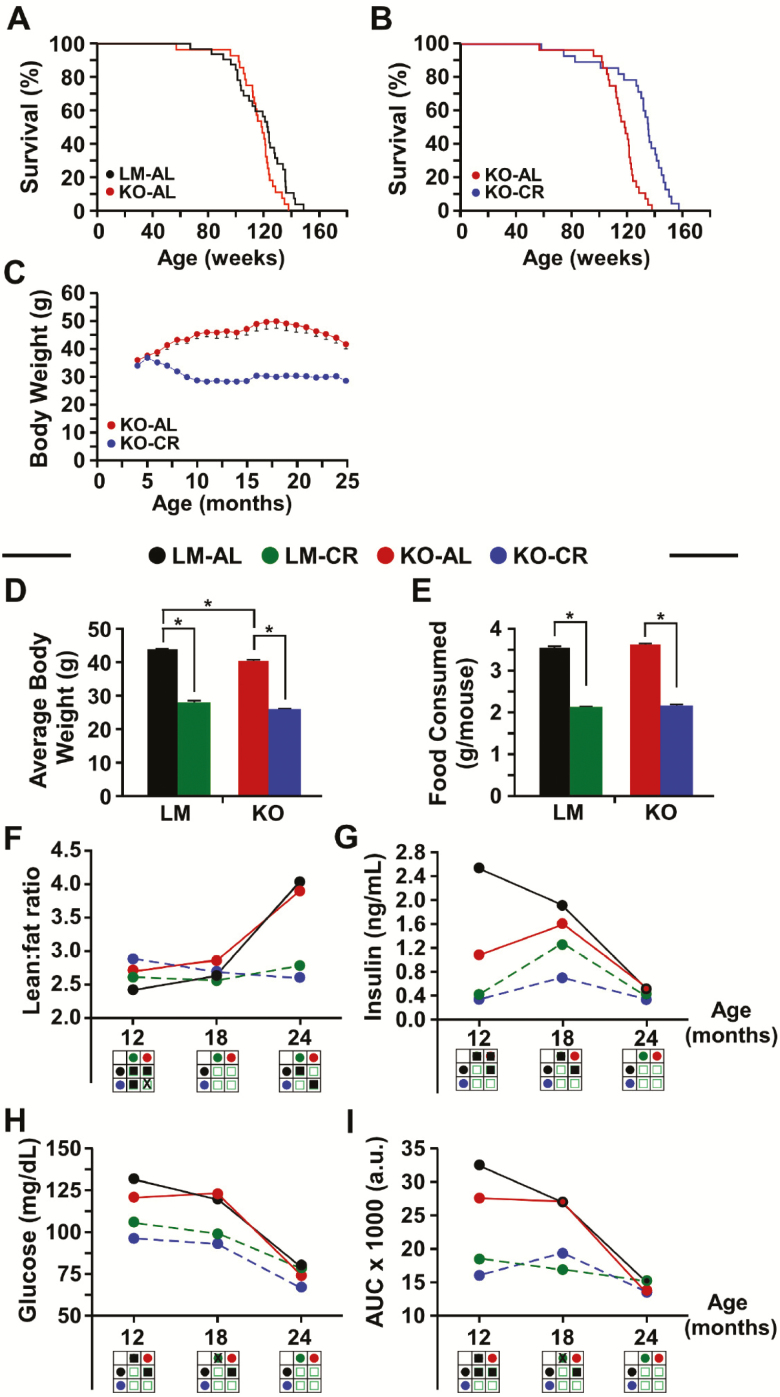
Numerous studies have consistently shown that CR increases life span in rodents (28) through mechanisms that remain poorly understood. In light of the evidence for a pro-longevity function of NQO1, we explored the role of NQO1 in life-span extension and whether the beneficial effects of CR on life span require NQO1. To test both aims, 30–40-week-old male NQO1-KO mice and LMs were placed on an AL or 40% CR diet for the remainder of their life. Although the longevity of AL-fed NQO1-KO mice on a standard AIN-93G diet was unchanged when compared with LM animals (logRank Mantel-Cox analysis, χ2 = 3.185 and p < .74) (Figure 1A), CR feeding resulted in improved survival of NQO1-KO mice, with an increase in median life span of 14% (p < .0001) and in maximum life span of 13.1% (p < .002) when compared with AL-fed NQO1-KO mice [logRank Mantel–Cox analysis; p < .0001] (Figure 1B). While the minimal effects of CR on mean and maximal life span in LM controls (Supplementary Figure 1A) was unexpected, two potential reasons for this result are the type of diet used in this study or the fact that the control animals were LMs from the cross of two heterozygotes, thus not “true” wild-type C57BL/6 mice. The lack of response to CR has been reported earlier and it is now clear that the sex, strain, and diet are important variables that alter survival response to CR (29–32). The percentage of mice presenting major tissue pathologies upon examination at necropsy was lower in CR-fed animals of both genotypes than AL mice; however, CR diet failed to mitigate the higher rate of enlargement of proliferative tissues (ie, spleen) observed in NQO1-KO versus LM mice (Table 1). To determine whether the CR-associated increase in the life span of NQO1-KO mice is stemming from compensation by the paralog NQO2, we conducted immunoblot analysis on whole cell liver extracts from all groups of mice. The results indicate that the hepatic NQO2 levels were not affected by either genotype or diet (CR vs AL) (Supplementary Figure 1B). The fact that the prolongevity effect of CR does not require NQO1 suggests that the life-span extension observed in CR mice entails more than simply an upregulation of the NQO1 pathway.
Figure 1.
Improved life span extension and whole-body physiology by caloric restriction (CR) in NQO1 knockout male mice. Kaplan–Meier survival curves of (A) wild-type [littermate (LM)] and NQO1-KO mice under ad libitum (AL) and (B) AL and 40% CR-fed NQO1-KO mice. (C) Body weight over the life span of NQO1-KO mice on AL and CR diets. (D) Average bodyweight and (E) food consumption of LM and NQO1-KO mice-fed AL and CR diets. Bars represent mean ± SEM. *p < .05. The following parameters were analyzed in LM and NQO1-KO mice at different ages: (F) Lean-to-fat ratio. Circulating (G) insulin and (H) glucose levels were measured after 16 h of fasting; (I) Area under the curve (AUC) for oral glucose tolerance test. Code for filled circles: Black, LM-AL; green, LM-CR; red, KO-AL; blue, KO-CR. Code for statistical analyses (3 × 3 matrices): Filled square, p < .05; open square, not significant; “X”, trend toward significance.
Table 1.
Major Pathologies Identified at Necropsy
| Diet Treatment (n) | |||||
|---|---|---|---|---|---|
| ALLM (25) | ALKO (23) | CRLM (23) | CRKO (19) | ||
| Heart | Enlarged (%) | 4 | 4 | 0 | 0 |
| Liver | Enlarged (%) | 36 | 39 | 15 | 21 |
| Kidney | Enlarged (%) | 8 | 30 | 0 | 5 |
| Spleen | Enlarged (%) | 28 | 52 | 13 | 47 |
As expected, mice on 40% CR weighed less than their AL-fed counterparts (regardless of genetic background), with a gradual reduction in body weight during the first 6 months and stabilization at around 55–60% of the AL-fed controls (Figure 1C; Supplementary Figure 1C). The average body weight of NQO1-KO mice on AL was significantly lower than their LM, AL-fed LMs (Figure 1D), in agreement with a previous study by Gaikwad et al. (18). Body weight trajectories and food consumption were markedly lower, irrespective of genotype, in response to CR (Figure 1D and E). Twelve-month-old NQO1-KO mice on AL had a higher proportion of lean body mass with concomitant loss in fat mass when compared with LM mice (Supplementary Figure 1D and E), which translated into a favorable lean-to-fat ratio (Figure 1F). In contrast, age-matched 18- and 24-month-old mice of both genotypes exhibited no significant differences in lean mass, body fat content (Supplementary Figure 1D and E), and lean-to-fat ratio (Figure 1F) when maintained on AL diet. Importantly, this longitudinal assessment of body composition indicated a sharp increase in lean body content and reduction in fat mass with age, irrespective of NQO1 expression. These changes in body composition, that are normally associated with aging, did not occur in LM or NQO1-KO mice under CR. This observation is in line with our recent report (32) showing no major changes in body composition during the life span of CR-fed LM mice. Body composition of 12-month-old mice on CR revealed a more substantial increase in lean mass and decrease in fat content in NQO1-KO versus LM controls (Supplementary Figure 1D and E), which was accompanied by an increase in lean-to-fat ratio in NQO1-KO animals (Figure 1F). However, the lean mass and fat content differences largely disappeared at 18 and 24 months of age. Thus, combined with the previous observations (32,33), the preservation of fat mass in CR mice at 24 months, most notably in the NQO1-KO background, might play a critical role in maximizing life span.
Next, the relationship between body mass and metabolic rate was explored by evaluating in vivo metabolism and heat production in the four experimental groups of mice using the Comprehensive Lab Animal Monitoring System (CLAMS) over two dark and light cycles. Irrespective of the genotype, CR induced a significant increase in the respiratory exchange ratio (RER) during the light cycle, suggesting a preference for carbohydrate metabolism, but a reduction in RER during the dark cycle, which indicated greater reliance on fatty acid oxidation under CR conditions (Supplementary Figure 1F and G). Both groups of CR animals also exhibited a trend toward higher spontaneous locomotor activity (Supplementary Figure 1H and I) and lower heat production (Supplementary Figure 1J and K). Despite an overall reduction in locomotor activity with age, CR-fed NQO1-KO mice were significantly more active than their LMs when measured at 12, 18, and 24 months (Supplementary Figure 1H and I). There is controversy surrounding the notion that increased locomotor activity is a major factor contributing to extended life span in CR-fed rodents (34).
Because NQO1 plays a role in metabolism (13,16,18), the impact of CR and genotype on whole-body metabolism was investigated. There was a clear age-dependent reduction in fasting insulin and blood glucose levels in all groups of mice, especially between the ages of 18 and 24 months (Figure 1G and H). Mice on CR displayed (regardless of genotype) a significant drop in fasting insulin and blood glucose levels at 12 and 18 months of age when compared with AL-fed animals (Figure 1G and H). Of note, deletion of Nqo1 was accompanied by a significant reduction in fasting insulin levels in 12-month-old AL-fed mice relative to the age-matched LM mice on AL, whereas no differences in fasting glucose levels were observed (Figure 1G and H). Oral glucose tolerance test (OGTT) was performed to measure the mouse’s ability to metabolize and clear glucose from the bloodstream. Area under the curve (AUC) calculations indicate a progressive increase in glucose clearance with age in AL-fed mice of both genotypes, while mice on CR exhibited very active glucose clearance (low AUC values) across their life span (Figure 1I). These data demonstrate an improvement in glucose metabolism in NQO1-KO mice at 12 months of age, and a refractoriness at later time points. A significant increase in circulating levels of corticosterone was found in 12-month-old mice of both genotypes under CR as compared to AL-fed LMs (Supplementary Figure 1L). Moreover, the ratio of corticosterone levels between CR and AL feeding was cross-sectionally increased by 1.5-fold in NQO1-KO versus LM mice. The circulating levels of total, HDL and LDL cholesterol and triglycerides were similar in AL-fed mice of both genotypes, and CR feeding led to a significant reduction in these levels with the exception of LDL cholesterol (Supplementary Figure 1M). Together, the collective data suggest that genetic deletion of Nqo1 does not attenuate the prolongevity effect of CR and its associated net improvement in whole-body physiology.
CR Does Not Require NQO1 for Increased Physical Performance
Various behavioral tests were performed to assess the functional effects of genotype and diet. Cued fear conditioning measures associative memory that predicts aversive events. When fed an AL diet, NQO1-KO mice had a reduced capacity for associative memory formation as compared to LM mice (Figure 2A), which supports the results of an earlier study showing an association between altered NQO1 expression and Alzheimer’s disease (35). In open field testing, there were no differences in overall general activity and exploratory drive performance between AL-fed mice of both genotypes, whereas significant enhancement in performance was observed in 12- and 24-month-old NQO1-KO mice on CR versus AL feeding (Figure 2B). Similarly, age-matched, CR-fed NQO1-KO mice were significantly more active in open field testing than their LM on either diet (Figure 2B), consistent with the spontaneous locomotor activity in metabolic chambers (Supplementary Figure 1H and I). At 12 months of age, NQO1-KO mice on a CR diet showed better motor function in a rotarod test compared to the LM animals on CR (Figure 2C). Furthermore, at 18 months of age, LM mice on CR, as well as NQO1-KO mice, performed better than LM mice on AL (Figure 2C). Due to the increased performance of the LM-CR mice, the difference between NQO1-KO mice on CR seen at 12 months was no longer detected. At 24 months of age, no significant differences between the groups were observed (Figure 2C). Moreover, mice on CR exhibited greater muscle strength, as assessed by the wire hang test, than AL-fed animals, although no differences between genotypes were noted (Figure 2D). Overall, the data indicate that at an early age, Nqo1 deletion is associated with impaired cognitive performance, but an increase in motor function and that these effects become less significant with age.
Figure 2.
CR does not require NQO1 for increased physical performance. (A) Measurement of freezing time for contextual conditioning in littermates (LM) and NQO1-KO mice under ad libitum (AL). Data are mean ± SEM. *p < .05, two-way ANOVA. (B) Total distance traveled in open field and average speed; (C) Time to fall from an accelerating rotarod; and (D) Wire-hanging latency in LM and NQO1-KO mice under AL or 40% caloric restriction (CR). Code for filled circles: Black, LM-AL; green, LM-CR; red, KO-AL; blue, KO-CR. Code for statistical analyses (3 × 3 matrices): Filled square, p < .05; open square, not significant.
The Effects of CR on Carcinogenesis in LM and NQO1-KO Mice
NQO1-KO mice are more susceptible to 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin cancer than LMs, and CR reduces the incidence of carcinogenesis in DMBA-treated mice (11,36). Furthermore, humans carrying a genetic polymorphism of NQO1, which results in the loss of its oxidoreductase activity, are at increased risk for tumor development (37–40). We assessed the role of NQO1 in the antitumorigenesis properties of CR by measuring the rate of formation and growth of epidermal tumors in response to the administration of DMBA/12-O-tetradecanoyl phorbol-13-acetate (TPA) to the skin of mice. This study was performed in an independent cohort of mice from the longevity study. Although not statistically significant (p = .07), we observed a trend that NQO1-KO mice (independent of diet) developed DMBA/TPA-induced tumors at a greater frequency than their LMs (Figure 3, Supplementary Table 2), seemingly consistent with the prior work of Long et al. (36). In addition, we found that CR provided a protective effect against tumor induction, regardless of genotype, suggesting that NQO1 is not essential for the antitumor consequences of CR (Figure 3, Supplementary Table 1). Notably, this latter observation is in contrast to our previous work with Nrf2-KO animals, where it was observed that Nrf2, which regulates NQO1 expression, is necessary for the antitumor effects of CR (11).
Figure 3.
The effects of caloric restriction (CR) on carcinogenesis in littermates (LM) and NQO1-KO mice. Percentage of LM and NQO1-KO mice with at least one papilloma when fed ad libitum (AL) and 40% CR.
NQO1 Partly Mediates the Beneficial Effects of CR on Mitochondria
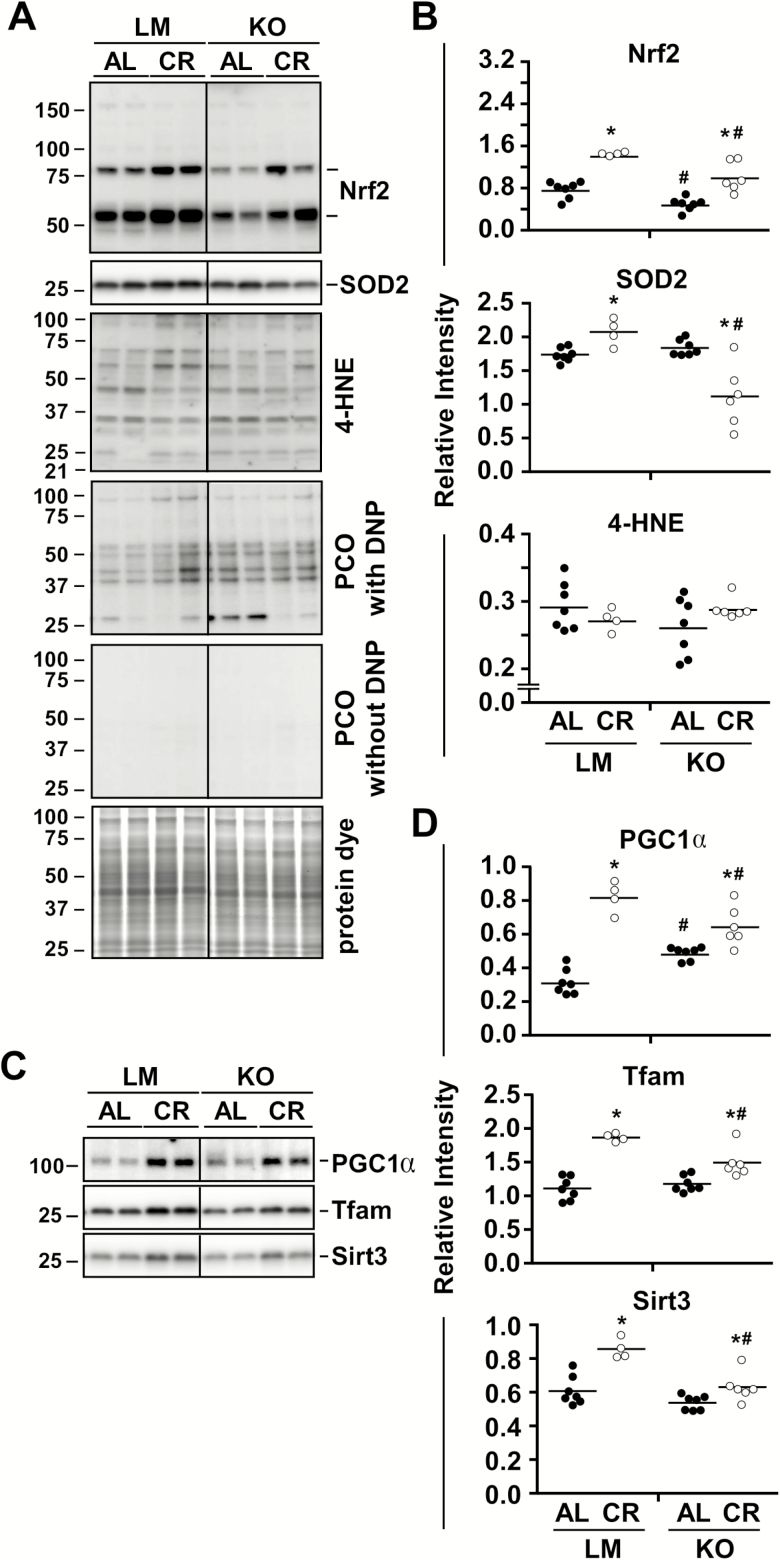
Evidence indicates an important role for NQO1 in the cellular defense against oxidative stress (18,41). Nrf2 is an upstream regulator of NQO1, and the Nrf2-NQO1 cascade elicits mammalian longevity by promoting mitochondrial integrity and conferring protection against dysfunction of liver mitochondria (42,43). We next examined whether deficiency of NQO1 results in oxidative stress and mitochondrial abnormalities in the liver of animals subjected to 36 weeks of DMBA/TPA treatment on their skin. While genetic deletion of Nqo1 had a negative impact on the hepatic expression of its upstream regulator Nrf2, a significant upregulation in Nrf2 protein levels was observed in the liver of CR-fed mice irrespective of genotype (Figure 4A and B, Supplementary Figure 2A). Moreover, the abundance of superoxide dismutase 2 (SOD2), a mitochondrial antioxidant protein, was upregulated in LM but reduced in NQO1-KO livers in response to CR (Figure 4A and B, Supplementary Figure 2A). Nevertheless, we could not detect any significant differences in oxidative stress markers, as assessed by 4-HNE staining and protein carbonyl adducts formation, between diet or genotypes (Figure 4A and B, Supplementary Figure 2A and B).
Figure 4.
NQO1 partly mediates the beneficial effects of caloric restriction (CR) on mitochondria. Immunoblot and densitometric quantification of (A, B) oxidative stress markers [Nrf2, SOD2, 4-HNE, and protein carbonylation (PCO)] and (C, D) mitochondrial players (PGC1α, Tfam, Sirt3), in liver of littermates (LM) and NQO1-KO mice under ad libitum (AL) or 40% CR. AL feeding (LM and KO) are represented by filled symbols and CR feeding (LM and KO) by open symbols. Data are mean ± SEM. *p < .05 compared to AL counterpart; #p < .05 compared to LM counterpart.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) is a master regulator of mitochondrial biogenesis (44), while transcription factor A (Tfam) is a key transcription factor implicated in mitochondrial biogenesis and mitochondrial DNA replication/repair (45). Another key player in mitochondrial biogenesis and function is the mitochondrial NAD+-dependent protein deacetylase sirtuin-3 (Sirt3) (46). The contribution of NQO1 in the expression of these three mitochondrial biomarkers was then assessed by immunoblot analysis. Compared to AL-fed LM controls, NQO1-KO livers exhibited a significant increase in PGC1α, which was also higher in CR-fed LM mice (Figure 4C and D, Supplementary Figure 3), as previously reported (47). A significant increase in abundance of Tfam and Sirt3 was observed in the liver of LM or NQO1-KO mice on CR versus their respective AL-fed counterparts, although to a lesser extent in NQO1-KO versus LM (Figure 4C and D, Supplementary Figure 3).
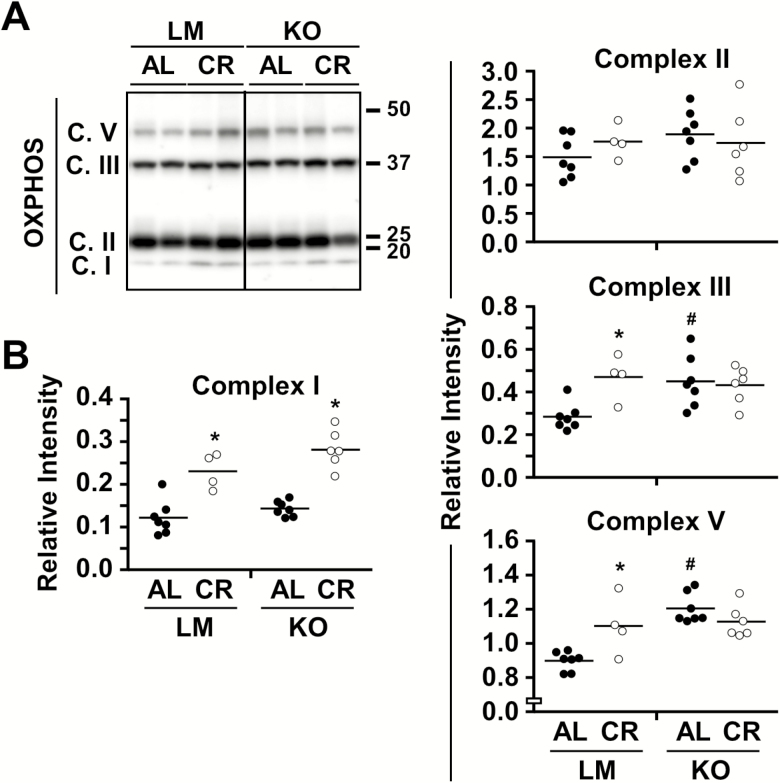
The ability of CR to promote an accumulation of PGC1α and Tfam may also affect cellular bioenergetics through stabilization of mitochondrial oxidative phosphorylation (OXPHOS) complex enzymes. The expression of known markers of several mitochondrial electron transport chain complexes was assessed in total liver lysates of the four experimental groups (Figure 5A and B, Supplementary Figure 3B). With the exception of complex II, CR elicited a significant increase in expression of OXPHOS complexes in LM mice. In the liver of NQO1-KO mice on AL, a significant increase in abundance of complexes III and V subunits was noted compared to AL-fed LM mice, whereas higher complex I expression was found in NQO1-KO mice on CR versus AL (Figure 5A and B, Supplementary Figure 3) in a pattern reminiscent of PGC1α and Tfam protein levels. The data suggest that NQO1 deficiency results in CR refractoriness regarding expression of several mitochondrial genes, but that these effects appear to be insufficient to result in major consequences on longevity and whole-body metabolism.
Figure 5.
Altered OXPHOS protein content as a function of NQO1 status and diet. (A, B) Immunblot and densitometric quantification of components of the OXPHOS complexes in liver of littermates (LM) and NQO1-KO mice under ad libitum (AL) or 40% caloric restriction (CR). AL feeding (LM and KO) are represented by filled symbols and CR feeding (LM and KO) by open symbols. Data are mean ± SEM. *p < .05 compared to AL counterpart; #p < .05 compared to LM counterpart.
Conclusion
The results of the current study show that the longevity of NQO1-KO mice fed AL was only slightly reduced compared to LM. The youngest group of NQO1-KO mice had improved glucose metabolism coincident with an increase in motor function and cognitive impairment when compared with AL-fed LM controls. As mice aged, these differences between genotype disappeared. CR elicited life span extension and conferred beneficial effects on whole-body metabolism that were independent of NQO1 expression. Strikingly, NQO1-KO mice under CR exhibited blunted expression of some key mitochondrial genes in liver without adverse consequences on longevity and whole-body metabolism. In addition, the antitumorigenic function of CR in chemically induced skin cancer was found to be independent of NQO1 expression. These results indicate, in contrast to its transcription factor Nrf2, no separation of function for NQO1 between tumorigenesis and tissue homeostasis/organismal aging. To conclude, the mechanisms involved in CR signaling are complex and ill-defined, and it would appear that pathways unrelated to NQO1 are involved in the regulation of life span and in the reduction of protection against induced tumorigenesis.
Funding
This work was supported in part by the Intramural Research Program of the National Institute on Aging/National Institutes of Health, and by a postdoctoral grant of the Research Foundation—Flanders (FWO/12R2415N) to E.M.M.
Supplementary Material
Acknowledgments
We are grateful to Dawn Nines, Dawn Phillips, and Justine Lucas for their excellent animal care. All experiments were designed by K.J.P., N.L.P., R.d.C., and E.M.M. Lifespan, whole-body and DMBA/TPA experiments were carried out by B.A.C and E.M.M. Behavioral tests were performed by T.M.W. WB analyses were carried out by A.D.R., A.D.F and S.R.L. A.D.R., B.A.C., M.B., R.d.C., and E.M.M. interpreted the data. A.D.R., M.B., R.d.C., and E.M.M wrote the manuscript.
Conflict of Interest
None declared.
References
- 1. Finkel T. The metabolic regulation of aging. Nat Med. 2015;21:1416–1423. doi:10.1038/nm.3998 [DOI] [PubMed] [Google Scholar]
- 2. Martin-Montalvo A, de Cabo R. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal. 2013;19:310–320. doi:10.1089/ars.2012.4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopez-Lluch G, Hunt N, Jones B et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi:10.1073/pnas.0510452103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimokawa I, Chiba T, Yamaza H, Komatsu T. Longevity genes: insights from calorie restriction and genetic longevity models. Mol Cells. 2008;26:427–435. [PubMed] [Google Scholar]
- 5. Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem. 2006;2:275–285. doi:10.2174/157340606776930709 [DOI] [PubMed] [Google Scholar]
- 6. Kulkarni SR, Donepudi AC, Xu J et al. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and sirtuin-1 in mouse and human. Antioxid Redox Signal. 2014;20:15–30. doi:10.1089/ars.2012.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi:10.1073/pnas.0608008103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruns DR, Drake JC, Biela LM, Peelor FF 3rd, Miller BF, Hamilton KL. Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxid Med Cell Longev. 2015;2015:732596. doi:10.1155/2015/732596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14:41–48. doi:10.1097/MCO.0b013e32834136f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vomhof-Dekrey EE, Picklo MJ Sr. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 2012;23:1201–1206. doi:10.1016/j.jnutbio.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 11. Pearson KJ, Lewis KN, Price NL et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA. 2008;105:2325–2330. doi:10.1073/pnas.0712162105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lind C, Cadenas E, Hochstein P, Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi:10.1016/0076-6879(90)86122-C [DOI] [PubMed] [Google Scholar]
- 13. Lee JS, Park AH, Lee SH et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One. 2012;7:e47122. doi:10.1371/journal.pone.0047122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SY, Jeoung NH, Oh CJ et al. Activation of NAD(P)H:quinone oxidoreductase 1 prevents arterial restenosis by suppressing vascular smooth muscle cell proliferation. Circ Res. 2009;104:842–850. doi:10.1161/CIRCRESAHA.108.189837 [DOI] [PubMed] [Google Scholar]
- 15. Kim YH, Hwang JH, Kim KS et al. Enhanced activation of NAD(P)H: quinone oxidoreductase 1 attenuates spontaneous hypertension by improvement of endothelial nitric oxide synthase coupling via tumor suppressor kinase liver kinase B1/adenosine 5’-monophosphate-activated protein kinase-mediated guanosine 5’-triphosphate cyclohydrolase 1 preservation. J Hypertens. 2014;32:306–317. doi:10.1097/HJH.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 16. Hwang JH, Kim DW, Jo EJ et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi:10.2337/db08-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen A, Kim HJ, Oh GS et al. NAD+ augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep. 2017;7:3006. doi:10.1038/s41598-017-03418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaikwad A, Long DJ 2nd, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem. 2001;276:22559–22564. doi:10.1074/jbc.M101053200 [DOI] [PubMed] [Google Scholar]
- 19. Yeo SH, Noh JR, Kim YH et al. Increased vulnerability to β-cell destruction and diabetes in mice lacking NAD(P)H:quinone oxidoreductase 1. Toxicol Lett. 2013;219:35–41. doi:10.1016/j.toxlet.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 20. Nam ST, Hwang JH, Kim DH et al. Role of NADH: quinone oxidoreductase-1 in the tight junctions of colonic epithelial cells. BMB Rep. 2014;47:494–499. doi:10.5483/BMBRep.2014.47.9.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patrick BA, Gong X, Jaiswal AK. Disruption of NAD(P)H:quinone oxidoreductase 1 gene in mice leads to 20S proteasomal degradation of p63 resulting in thinning of epithelium and chemical-induced skin cancer. Oncogene. 2017. doi:10.1038/onc.2016.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iskander K, Gaikwad A, Paquet M et al. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054–2058. doi:10.1158/0008-5472.CAN-04-3157 [DOI] [PubMed] [Google Scholar]
- 23. Martínez-Hernández A, Córdova EJ, Rosillo-Salazar O et al. Association of HMOX1 and NQO1 polymorphisms with metabolic syndrome components. PLoS One. 2015;10:e0123313. doi:10.1371/journal.pone.0123313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramprasath T, Murugan PS, Kalaiarasan E, Gomathi P, Rathinavel A, Selvam GS. Genetic association of Glutathione peroxidase-1 (GPx-1) and NAD(P)H:quinone Oxidoreductase 1(NQO1) variants and their association of CAD in patients with type-2 diabetes. Mol Cell Biochem. 2012;361:143–150. doi:10.1007/s11010-011-1098-5 [DOI] [PubMed] [Google Scholar]
- 25. Luo J, Li S, Qin X et al. Association of the NQO1 C609T polymorphism with Alzheimer’s disease in Chinese populations: a meta-analysis. Int J Neurosci. 2016;126:199–204. doi:10.3109/00207454.2015.1004573 [DOI] [PubMed] [Google Scholar]
- 26. Lajin B, Alachkar A. The NQO1 polymorphism C609T (Pro187Ser) and cancer susceptibility: a comprehensive meta-analysis. Br J Cancer. 2013;109:1325–1337. doi:10.1038/bjc.2013.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minor RK, Baur JA, Gomes AP et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi:10.1038/srep00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi:10.1093/jn/116.4.641 [DOI] [PubMed] [Google Scholar]
- 29. Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr. 1987;117:376–382. doi:10.1093/jn/117.2.376 [DOI] [PubMed] [Google Scholar]
- 30. Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi:10.1111/j.1474-9726.2009.00533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. 2010;45:691–701. doi:10.1016/j.exger.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi:10.1016/j.cmet.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi:10.1111/j.1474-9726.2011.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCarter RJ, Shimokawa I, Ikeno Y et al. Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging (Milano). 1997;9:73–79. [DOI] [PubMed] [Google Scholar]
- 35. Chhetri J, King AE, Gueven N. Alzheimer’s disease and NQO1: is there a link?Curr. Alzheimer Res. 2018;15:56–66. doi:10.2174/1567205014666170203095802 [DOI] [PubMed] [Google Scholar]
- 36. Long DJ 2nd, Waikel RL, Wang XJ, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz[a]-anthracene-induced carcinogenesis in mouse skin. J Natl Cancer Inst. 2001;93:1166–1170. doi:10.1093/carcin/21.10.1813 [DOI] [PubMed] [Google Scholar]
- 37. Wiemels JL, Pagnamenta A, Taylor GM, Eden OB, Alexander FE, Greaves MF. A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. United Kingdom Childhood Cancer Study Investigators. Cancer Res. 1999;59:4095–4099. doi:10.1182/blood.V97.5.1422 [PubMed] [Google Scholar]
- 38. Lafuente MJ, Casterad X, Trias M et al. NAD(P)H:quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis. 2000;21:1813–1819.doi:10.1016/S0169-5002(01)00243-4 [DOI] [PubMed] [Google Scholar]
- 39. Smith MT, Wang Y, Kane E et al. Low NAD(P)H:quinone oxidoreductase 1 activity is associated with increased risk of acute leukemia in adults. Blood. 2001;97:1422–1426. doi:10.1182/ blood.V97.5.1422 [DOI] [PubMed] [Google Scholar]
- 40. Lewis SJ, Cherry NM, Niven RM, Barber PV, Povey AC. Polymorphisms in the NAD(P)H: quinone oxidoreductase gene and small cell lung cancer risk in a UK population. Lung Cancer. 2001;34:177–183. doi:10.1016/S0169-5002(01)00243-4 [DOI] [PubMed] [Google Scholar]
- 41. Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact. 2000;129:77–97. doi:10.1182/blood.V97.5.1422 [DOI] [PubMed] [Google Scholar]
- 42. Kwon J, Han E, Bui CB et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012;13:150–156. doi:10.1038/embor.2011.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi:10.1016/j.freeradbiomed.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi:10.1016/j.bbamcr.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor a regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–929. doi:10.1016/j.bbagrm.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 46. Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol. 2012;4:a013102. doi:10.1101/cshperspect.a013102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adamovich Y, Shlomai A, Tsvetkov P et al. The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1. Mol Cell Biol. 2013;33:2603–2613. doi:10.1128/MCB.01672-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.