A nanoparticle adjuvant enhances protection against heterologous influenza strains
Keywords: adjuvant, Fc Receptor, heterologous challenge, influenza vaccine, CpG ODN
Abstract
The development of a universal influenza vaccine that can provide a robust and long-lasting protection against a broader range of influenza virus strains is a global public health priority. One approach to improve vaccine efficacy is to use an adjuvant to boost immune responses to the target antigens; nevertheless, the role of adjuvants in the context of influenza vaccines is not fully understood. We have previously developed the K3-schizophyllan (SPG) adjuvant, which is composed of nanoparticulated oligodeoxynucleotides K3, a TLR9 agonist, with SPG, a non-agonistic β-glucan ligand of Dectin-1. In this study, K3-SPG given with conventional influenza hemagglutinin (HA) split vaccine (K3-SPG HA) conferred protection against antigenically mismatched heterologous virus challenge. While K3-SPG HA elicited robust cross-reactive HA-specific IgG2c and CD8 T-cell responses, CD8 T-cell depletion had no impact on this cross-protection. In contrast, K3-SPG HA was not able to confer protection against heterologous virus challenge in FcRγ-deficient mice. Our results indicated that FcγR-mediated antibody responses induced by the HA antigen and K3-SPG adjuvant were important for potent protection against antigenically mismatched influenza virus infection. Thus, we demonstrated that the K3-SPG-adjuvanted vaccine strategy broadens protective immunity against influenza and provides a basis for the development of next-generation influenza vaccines.
Introduction
Influenza A viruses (IAVs) can be divided into different subtypes based on the combination of their surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). There are currently 18 known HA subtypes in two groups (group-1 and -2) and 11 NA subtypes. Owing to the high rate of point mutations within the IAV genome, antigenic drift can produce numerous strains within each subtype. IAVs cause seasonal epidemics and occasional pandemics in the human population, resulting in hundreds of thousands of deaths worldwide annually. Currently, annual influenza vaccinations are the most effective strategy to blunt the impact of seasonal influenza infections and for protection from severe influenza disease. However, the efficacy of influenza vaccines varies yearly and between individuals, and is largely dependent on the strains selected and used for the vaccine. The strain selection is typically made by predicting circulating IAV strains in humans on the basis of surveillance data. Thus, a discrepancy between predicted and epidemic strains may not be fully avoided, and the mismatch results in a low vaccine efficacy (1). To overcome this limitation of the current influenza vaccines, there is an urgent need to develop universal vaccines that can confer protection against both seasonal and potential pandemic IAVs (2).
Previous reports have shown that the protective immune response against IAVs induced by the current influenza vaccines is mainly dependent on vaccine-induced neutralizing antibodies targeting HA (3–5). IAV HA protein is composed of two major domains: the globular head and stem domains; the former is immuno-dominant and highly variable between strains, and the latter is relatively conserved among strains and even between subtypes (6). Most HA-specific antibodies elicited by the current vaccines bind to the head domain of HA, and the subsequent neutralizing activities are induced by preventing the binding of viruses to receptors on the host cell surface or preventing the fusion of viruses to the host cell membrane. Nevertheless, the HA head domain is the most mutable region among IAV proteins, limiting the cross-reactivity of vaccine-induced antibodies with other IAV subtypes or antigen-drifted strains. Thus, the current influenza vaccines are only effective against a narrow range of IAV strains that possess antigenically similar HA epitopes compared to those of the vaccine strains, rendering them potentially ineffective against other IAV subtypes, including future pandemic IAVs.
Recent reports indicated that antibodies directed toward the HA stem efficiently protect against IAV infection. Although these antibodies could neutralize various IAV strains in vitro, there are two distinct FcγR-dependent signaling pathways involved in the protection against IAV infection in vivo (7, 8). One mechanism mediating this in vivo protection is through cytotoxic NK cell activation by anti-stem antibody–antigen complexes via antibody-dependent cellular cytotoxicity (ADCC), induction of antibody-dependent cellular phagocytosis (ADCP) and/or complement activation leading to complement-dependent cytotoxicity (CDC). The Fc region of IgG is critical for the efficient induction of ADCC and/or CDC, because the affinity between IgG and FcγR is primarily dependent on the Fc region. In particular, the IgG1 and IgG3 subtypes in human and IgG2 in mice are known to have high affinity for FcγRs (9, 10).
In a previous human clinical study, HA split vaccine given with a novel adjuvant MF59 displayed an increase of antibody titers to not only the HA head but also stem regions (11); thus, adjuvant selection may be critical for the induction of antibodies directed toward the HA stem. Recently, we developed a second-generation CpG oligodeoxynucleotides (ODN) adjuvant, K3-SPG, a nanoparticulate TLR9 agonist derived from B/K-type CpG ODN (K3) conjugated with schizophyllan (SPG), a non-agonistic β-glucan ligand of Dectin-1. We previously demonstrated that vaccination with a model antigen supplemented with the K3-SPG adjuvant strongly induced antigen-specific IgG2 and cytotoxic T lymphocyte responses in mice (12, 13). Furthermore, K3-SPG-adjuvanted seasonal influenza split vaccine provided protection against homologous influenza virus challenge and increased serum antibody titers in both mice and non-human primates (12, 14).
In this study, we further evaluated the potency of the K3-SPG adjuvant given with a seasonal influenza HA split vaccine, by assessing its protective efficacy against heterologous IAV challenge and compared it with protection induced by other common adjuvants, alum and AddaVax, a squalene-based oil-in-water nano-emulsion with a formulation similar to that of MF59. We found that the K3-SPG-adjuvanted HA vaccine protected mice against an antigenically mismatched IAV strain, PR8, which drifted from the 1918 pandemic strain. Further, we demonstrated that the protection was associated with antigen-specific IgG2 antibodies induced by the K3-SPG-adjuvanted HA vaccine, which mediated an FcγR-dependent antibody functionality while independent of the neutralization activity against the heterologous IAV used for challenge. Taken together, our results indicated that wider coverage and stronger protection against IAVs can be achieved for seasonal influenza vaccines through the use of appropriate vaccine adjuvants that are capable of inducing cross-reactive antibodies possessing an Fc-mediated functionality, such as the K3-SPG.
Methods
Reagents
K3 (5′-ATC GAC TCT CGA GCG TTC TC-3′) was synthesized by Gene Design (Osaka, Japan). SPG, aluminum hydroxide gel (alum), and AddaVax were purchased from Invivogen (San Diego, CA, USA). The method for K3-SPG conjugation was described in a previous report (15).
Animals and infection
Six-week-old female C57BL/6J were purchased from CLEA Japan (Tokyo, Japan). FcRγ-deficient mice were described previously (16). All animal experiments were conducted in accordance with the institutional guidelines for the National Institutes of Biomedical Innovation, Health and Nutrition animal facility.
Mice were intra-muscularly vaccinated with influenza A/NC/20/99 HA split vaccine (1 or 10 µg) alone or with alum (50% final volume), AddaVax (50% final volume) or K3-SPG (10 µg) at day 0 and 28. At day 35, the mice were intra-nasally challenged with 2800 pfu (20× LD50) of A/Puerto Rico/8/34 (H1N1) (PR8) or 1 × 108 pfu of A/New Caledonia/20/99 (H1N1) (NC). Anti-CD8a (clone 53–6.7) or isotype control antibody (clone MOPC-173) was intra-peritoneally injected at day 32, 33, 34, 38, 42 and 46 (200 µg per injection per day), and the complete depletion of CD8-positive cells in the blood was confirmed by flow cytometry prior to infection (Supplementary Figure 1). Mice were monitored for survival and body weight until 15 and 17 days after infection, respectively.
Antibodies
The following directly conjugated monoclonal antibodies were used: CD3-Brilliant Violet (BV)570 (17A2), CD3-APC/Fire750 (17A2), CD4-BV510 (GK1.5), CD8a-BV570 (53–6.7), CXCR5-PE/Dazzle594 (L138D7), GL7-BV421 (GL7), IgD-APC/Cy7 (11-26c.2a), IFN-γ-Alexa Fluor 488 (XMG1.2) and PD-1-BV785 (29F.1A12) (all from BioLegend, San Diego, CA, USA); B220-PE/Cy5 (RA3-6B2), CD38-BV786 (90/CD38), CD44-PE/Cy5 (IM7), CD95-PE/CF594 (Jo2), IgG1/BV605 (A85-1), IgM-PerCP/Cy5.5 (R6-60.2), NK1.1-BV570 (PK138) and TNF-α-PerCP/Cy5.5 (MP6-XT22) (all from BD Biosciences, Franklin Lakes, NJ, USA). IgG2c-FITC polyclonal antibody was from Bio-Rad (Hercules, CA, USA). LIVE/DEAD Fixable Blue Dead Cell Stain Kit, streptavidin (SA)-PE and SA-PE/Cy7 were from Life Technologies (Carlsbad, CA, USA).
HA probe preparation
HA constructs had the extracellular domain of HA modified to ablate sialic acid binding and C-terminally fused to a T4 fibritin trimerization motif, biotinylatable AviTag sequence, and a hexahistidine affinity tag were synthesized by Genscript (Piscataway, NJ, USA) and cloned into a cytomegalovirus (CMV) promoter expression plasmid as previously described (17, 18). Briefly, plasmid DNA was prepared using the Qiagen Maxiprep or Megaprep kit (Hilden, Germany). Expi293 cells (Life Technologies) were diluted to 1.5 × 106 cells ml−1 and transfected with 1000 µg l−1 of HA expression plasmids using the 293fectin transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA). At day 5, the cell medium was clarified by centrifugation and filtered, concentrated, diafiltered against four volumes of phosphate-buffered saline (PBS) with 20 mM imidazole (pH 8.0) and loaded onto Ni Sepharose Fast Flow resin (GE Healthcare, Chicago, IL, USA) by gravity flow. The resin was washed with six column volumes of PBS containing 60 mM imidazole, and protein was eluted in five column volumes of PBS containing 500 mM imidazole. The eluted protein was stored at 4°C overnight, concentrated with a centrifugal concentrator and loaded onto a Superdex 200 16/60 column (GE Healthcare), and fractions corresponding to trimeric HA were pooled and concentrated. Subsequently, 800 µl of the protein in 10 mM Tris (pH 8.0) was biotinylated using a biotin-protein ligase kit (Avidity, Aurora, CO, USA) by adding 100 µl of Biomix-A, 100 µl of Biomix-B and 2.5 µl of biotin ligase BirA and incubating the mixture at 37°C for 1 h. The biotinylated HA proteins were buffer-exchanged into PBS with a centrifugal concentrator to remove excess biotin. Biotinylation was confirmed by flow cytometry analysis using SA-coated beads and anti-HA antibody CR9114 for detection.
Polychromatic flow cytometry
Mouse splenocytes were analyzed using a modified LSRII flow cytometer (BD Biosciences) as described previously (19). Briefly, for the analysis of B cells, fresh splenocytes were surface-stained with anti-GL7, anti-CD3, anti-NK1.1, anti-CD38, anti-IgG1, anti-IgD, anti-B220, anti-CD95, anti-IgG2c and anti-IgM antibodies. Following surface staining, the cells were incubated with NC HA biotinylated probe-SA-PE/Cy7 and PR8 HA biotinylated probe-SA-PE on ice for 30 min. UV blue amine-reactive dye was used to exclude dead cells from the analysis.
For the analysis of T cells, fresh splenocytes were cultured in the absence or presence of overlapping peptide against NC [Peptide Array, Influenza Virus A/New Caledonia/20/1999 (H1N1) HA Protein] or PR8 HA protein [Peptide Array, Influenza Virus A/Puerto Rico/8/1934 (H1N1) Hemagglutinin Protein] (BEI Resources, Manassas, VA, USA) in a final concentration of 400 µg ml−1 for each peptide. After 1 h of incubation, monensin (0.7 µg ml−1; BD Biosciences) and brefeldin A (1 µg ml−1; BD Biosciences) were added onto the cells and incubated for 5 h. After washing, the cells were surface-stained with anti-CD8, anti-CD4, anti-PD-1, anti-Ly6C, anti-CD44, anti-CXCR5 and anti-CXCR3 antibodies. The cells were then permeabilized by BD Cytofix/Cytoperm (BD Biosciences) and stained with anti-CD3, anti-TNF and anti-IFN-γ antibodies. Between 3 × 105 and 1 × 106 events were acquired for each sample, and the data were analyzed using the FlowJo software version 9.8.2 (TreeStar, Ashland, OR, USA).
Measurement of antibody titers
The levels of total IgG, IgG1 and IgG2c in the serum were determined by enzyme-linked immunosorbent assay (ELISA) using horseradish peroxidase (HRP)-conjugated anti-mouse total IgG, IgG1 and IgG2c as described previously (12). To measure HA-specific antibody titers, 96-well plates were coated with PR8 or NC protein solution overnight at 4°C. Synthesized peptide encompassing the long alpha helix of HA2 (aa 76–130) was used as the coating antigen for detecting HA2 stem-binding antibodies (20). Coated plates were then washed and incubated for 1 h with a blocking buffer. After blocking, the plates were washed and incubated with diluted serum samples for 2 h at room temperature. To detect bound antibodies, the plates were washed and incubated for 1 h with HRP-conjugated anti-mouse total IgG, IgG1 or IgG2c antibody (Southern Biotech, Birmingham, AL, USA). Subsequently, the plates were washed and incubated with the TMB Microwell Peroxidase Substrate System reagent (KPL, Gaithersburg, MD, USA) to initiate color reaction according to the manufacturer’s protocol. The reaction was stopped by adding 2 N H2SO4, and the optical density was measured at a wavelength of 450 nm (OD450). The antibody titer was defined as the highest serum dilution that yielded an OD450 greater than the OD450 of the negative control serum. HA2 stem-binding IgG titers were expressed as relative concentrations to the standard monoclonal antibody (V15-5) (21).
Production of HA-pseudotyped lentiviral vectors and neutralization assay
Influenza HA-pseudotyped lentiviral vectors expressing a luciferase reporter gene were prepared as previously described (22). Briefly, 293T cells were co-transfected with the following plasmids: 17.5 µg of pCMV-R8.2, 17.5 µg of pHR’CMV-Luc and 1 µg of CMV/R H1 A/Puerto Rico/8/1934 or H1 A/New Caledonia/20/1999. For the production of the H1N1 pseudoviruses, a human type-II transmembrane serine protease TMPRSS2 gene was included in the transfection for the proteolytic activation of HA (23). The cells were transfected overnight, and the medium was replenished with fresh medium. The cells were harvested 48 h later, filtered through a 0.45-µm syringe filter, aliquoted and frozen at −80°C until used.
Prior to performing neutralization assays, serum from vaccinated mice was pretreated with receptor-destroying enzyme II (Denka Seiken, Tokyo, Japan) to eliminate serum non-specific inhibitors according to the manufacturer’s protocol. Subsequently, various dilutions of the serum were mixed with the pseudoviruses for 45 min and then added to 293A cells in 96-well dishes (10000 cells per well). Fresh medium was added to the cells 2 h later. Three days after infection, the cells were lysed in 30 µl of cell culture lysis buffer (Promega, Madison, WI, USA). Luciferase assay reagent (Promega) was added to the cell lysate for the measurement of luciferase activity.
Hemagglutination inhibition assay
Viruses were diluted to 8 HA units per 50 µl, and 25 µl of diluted virus was added into duplicate wells along with an equal volume of serially diluted mouse serum treated with receptor-destroying enzyme II (Denka Seiken) in PBS. Subsequently, 50 µl of 2% chicken red blood cells (Kohjin Bio, Saitama, Japan) was added into the wells and incubated for 30 min at room temperature. The minimum effective concentration was determined according to the final dilution at which hemagglutination was fully inhibited.
Statistical analysis
Experimental variables were analyzed using unpaired t-test with Welch’s correction, log-rank test or Mann–Whitney test. P values <0.05 were considered significant. GraphPad Prism statistical analysis software (version 6; GraphPad Software, San Diego, CA, USA) was used throughout.
Results
K3-SPG-adjuvanted HA split vaccine protects mice from both homologous and heterologous IAV challenge
To investigate the impact of adjuvants in the influenza HA split vaccine on protective immunity, we compared the protective efficacy of the HA split vaccine supplemented with different adjuvants against IAV in a murine challenge model.
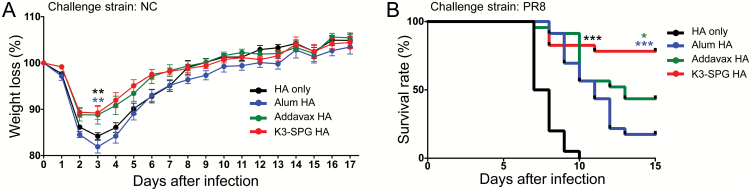
First, we evaluated the efficacy of the HA split vaccine given with or without adjuvant against a homologous challenge. Mice were immunized with 1 µg of HA NC split vaccine along with alum (alum HA), AddaVax (Addavax HA) or K3-SPG (K3-SPG HA). The mice were subsequently challenged using the homologous IAV NC strain. In general, mice immunized with the HA split vaccine alone displayed more profound body weight loss compared to either Addavax HA- or K3-SPG HA-vaccinated mice, and the differences between the vaccine-only or alum HA-vaccinated group and Addavax HA- and K3-SPG HA-vaccinated groups were statistically significant at 3 days post-challenge (Fig. 1A).
Fig. 1.
Protection against homologous and heterologous H1 influenza virus challenges by K3-SPG-adjuvanted vaccination. Mice were vaccinated with 1 µg of HA split vaccine against NC IAV either alone (HA only) or with alum (alum HA), Addavax (Addavax HA) or K3-SPG (K3-SPG HA) as adjuvant at day 0 and 28. Seven days after the last vaccination, the mice were challenged with either NC or PR8 influenza virus (IAV). (A) Weight loss following NC IAV challenge. The body weight of the mice was assessed until day 17 post-infection. The percentage weight change compared to day 0 of each vaccination group was plotted. Differences between the K3-SPG HA and other groups were analyzed using an unpaired t-test with Welch’s correction (n = 10–12, **P < 0.01). (B) Survival curves of mice lethally challenged with PR8 IAV. Survival was assessed until day 15 post-infection. The survival rate (%) was plotted, and differences between the K3-SPG HA and other vaccination groups were analyzed using log-rank test (n = 20–23, *P < 0.05, ***P < 0.001).
We next assessed the protective efficacy of the vaccine given with or without adjuvant in mice challenged with a heterologous IAV strain, PR8. Mice immunized with the HA split vaccine alone died within 10 days after challenge (Fig. 1B). Importantly, mice vaccinated with K3-SPG HA had a significantly higher survival rate than either alum HA- or Addavax HA-vaccinated mice. These results indicate that K3-SPG HA provides a broader protective coverage against IAV strains, including against the antigenically mismatched IAV compared to other vaccines.
K3-SPG HA increases the frequency of cross-reactive HA-specific CD8+ T cells, but they are not involved in the protection against heterologous IAV challenge
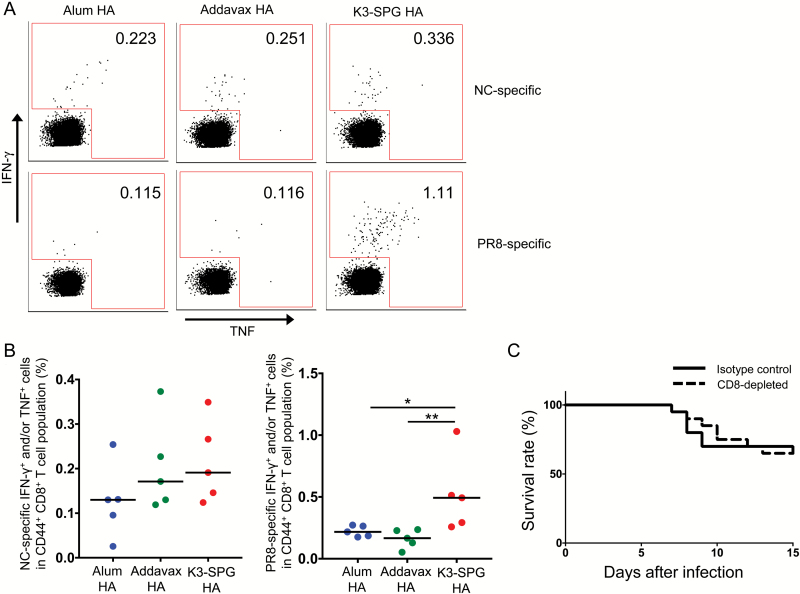
To characterize the immune response in K3-SPG HA-immunized mice, we first evaluated the frequency of NC HA- and PR8 HA-specific CD8+ T cells in splenocytes of vaccinated mice because we previously reported that K3-SPG is a strong adjuvant for eliciting antigen-specific CD8 T cells (15). Seven days after the second vaccination, splenocytes were collected and stimulated with overlapping peptides corresponding to NC or PR8 HA for 6 h. We evaluated the frequency of all functional NC- and PR8-specific CD8+ T cells, on the basis of cell positivity for IFN-γ and/or TNF-α by flow cytometry (Fig. 2A). The frequency of NC-specific CD8+ T cells was not significantly different between the adjuvant groups (Fig. 2B). In contrast, the frequency of PR8-specific CD8+ T cells was significantly higher in the K3-SPG HA group than in the alum HA or Addavax HA groups. Therefore, we further evaluated the role of antigen-specific CD8+ T cells in vivo.
Fig. 2.
Role of HA-specific CD8+ T-cell responses for protection against heterosubtypic IAV challenge in vivo. Splenocytes were harvested from vaccinated mice at day 7 from the last immunization and stimulated with overlapping peptides corresponding to NC or PR8 HA. (A) Representative plots for IFN-γ- and/or TNF-α-positive cells among total CD44+ CD8+ T cells. (B) Frequency of NC HA- or PR8 HA-specific IFN-γ- and/or TNF-α-positive cells in the CD44+ CD8+ T-cell population of the different adjuvant groups. Differences between groups were analyzed using Mann–Whitney U-test (n = 5, *P < 0.05, **P < 0.01). (C) Mice were immunized with 1 µg of the HA split vaccine adjuvanted with K3-SPG at day 0 and 28. At day 35, the mice were challenged with PR8 IAV. Anti-CD8 antibody was injected at day 32, 33, 34, 38, 42 and 46. Survival of the mice was recorded until day 15 post-infection (n = 20).
K3-SPG HA-vaccinated mice were injected with anti-CD8 or isotype control antibody and challenged with IAV PR8 at 7 days after the second vaccination. Surprisingly, there was no significant difference in survival between the two groups (Fig. 2C), suggesting that the functional antigen-specific CD8+ T cells induced by K3-SPG were not involved in the protection against heterologous IAV infection.
K3-SPG HA vaccination changes the quality of serum IgG antibodies
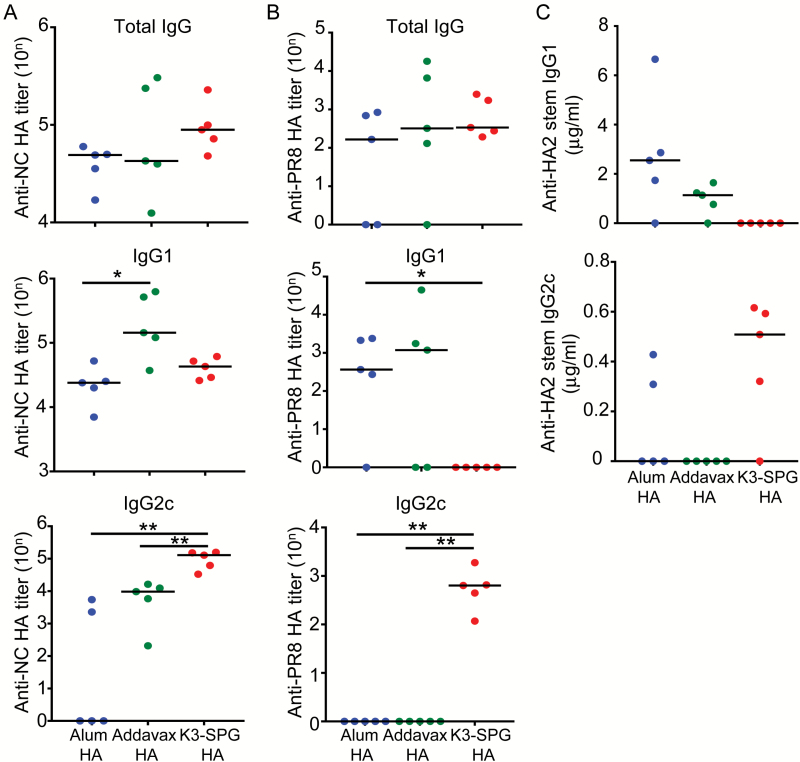
Next, we measured the titer of each IgG isotype in the serum. The total anti-NC HA IgG titers were not significantly different between the different adjuvant groups. However, the titer of anti-NC HA IgG1 in Addavax HA-vaccinated mice was significantly higher than that of alum HA-vaccinated mice (Fig. 3A). Although it was not statistically significant, the titer of anti-NC HA IgG1 in the Addavax HA group was higher than that of the K3-SPG HA group. In contrast, the titer of anti-NC HA IgG2c in K3-SPG HA-vaccinated mice was significantly higher than that in alum HA- or Addavax HA-vaccinated mice (Fig. 3A).
Fig. 3.
Adjuvant selection influenced HA-specific IgG isotypes in immunized mice. NC HA (A), PR8 HA (B) or HA2 stem-specific (C) total IgG, IgG1 and IgG2c titers in the serum of mice vaccinated with 1 µg of HA split vaccine with or without the different adjuvants were measured. Differences between groups were analyzed using Mann–Whitney U-test (n = 5, *P < 0.05, **P < 0.01).
Concerning the heterologous anti-PR8-specific response, total anti-PR8 HA IgG titers were also comparable between the different adjuvant groups (Fig. 3B). However, the anti-PR8 HA IgG1 titer in K3-SPG-vaccinated mice was significantly lower than that in alum HA- or Addavax HA-vaccinated mice (Fig. 3B). Remarkably, the titer of anti-PR8 HA IgG2c of K3-SPG HA-vaccinated mice was significantly higher than that of alum HA- or Addavax HA-vaccinated mice (Fig. 3B). To further characterize the response, we evaluated the production of anti-HA2 stem IgG1 and IgG2c in the different adjuvant groups. K3-SPG HA appeared to induce the production of mainly anti-HA2 stem IgG2c rather than anti-HA2 stem IgG1 (Fig. 3C), although we only measured antibodies directed against linear epitopes of the stem region in this assay, and the differences in titers did not reach statistical significance between the groups.
K3-SPG immunization changes the quality of germinal center B cells
The germinal center (GC) reaction is critical for the induction of immunoglobulin class switching and somatic hypermutations to achieve high-affinity antibodies (24, 25). Because we were not able to detect a sufficient number of events by flow cytometry with 1 µg of the vaccines, we immunized mice with 10 µg of the HA split vaccine without or with adjuvant to investigate differences in the GC reaction induced by the different adjuvants. Using this vaccination dose, clear differences in protective efficacies were observed between alum HA, Addavax HA and K3-SPG HA (Supplementary Figure 2). Therefore, further analyses of the GC reaction were performed using this vaccination dose. We first assessed the frequency of total GC B cells in the different adjuvant groups. At day 7 post-vaccination, the percentage of total GC B cells in the total B-cell population was significantly higher in Addavax HA- than in alum HA-immunized mice, and the percentage appeared to be higher in Addavax HA- than in K3-SPG HA-immunized mice (Supplementary Figure 3A–C). We subsequently assessed differences in the IgG isotype of GC B cells. The frequency of total IgG1-positive GC B cells was significantly higher in the Addavax HA group than in the alum HA or K3-SPG HA groups (Supplementary Figure 3D and E). In contrast, the frequency of total IgG2c-positive GC B cells was significantly higher in the K3-SPG HA group than in the alum HA or Addavax HA groups.
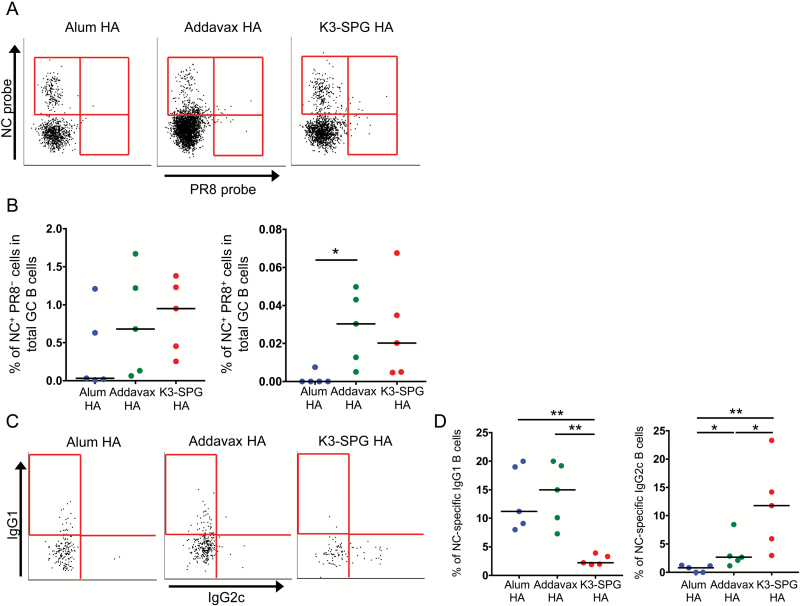
We further evaluated the frequency of antigen-specific GC B cells by staining with NC and PR8 HA probes (Fig. 4A) (17, 18). The frequency of NC HA-specific GC B cells (NC+ PR8− GC B cells) appeared to be higher in the Addavax HA and K3-SPG HA groups than in the alum HA group (Fig. 4A and B). Importantly, the frequency of cross-reactive HA-specific GC B cells (NC+ PR8+ GC B cells) was significantly higher in the Addavax HA than in alum HA groups, and appeared to be higher in the K3-SPG HA than in the alum HA groups, albeit not significantly (Fig. 4B).
Fig. 4.
Development and maintenance of HA-binding isotype-switched GC B cells elicited by different adjuvanted vaccines. Mice were immunized with 10 µg of the HA split vaccine without or with different adjuvants at day 0 and 28. Splenocytes were harvested from vaccinated mice at day 7 from the last immunization and subjected to flow cytometric analysis for the enumeration of total GC (IgM−IgD−CD95+GL7+CD38dull) B cells in the alive-Dump−(CD3−CD14−NK1.1−UV blue−) B220+ cell population. (A) Representative flow cytometry plots of NC and/or PR8 HA probe staining of GC B cells for the different vaccination groups. (B) Frequency of NC HA and/or PR8 HA-positive GC B cells of the different vaccination groups (n = 5). (C) Representative flow cytometry plots of isotype-switched GC IgG1+ and/or IgG2+ NC HA probe-positive B cells in the total NC HA-specific GC B-cell population. (D) Frequency of GC IgG1+ and/or IgG2+ NC HA probe-positive B cells among total NC HA-specific GC B cells of the different vaccination groups (n = 5). Differences between groups were analyzed using Mann–Whitney U-test (*P < 0.05, **P < 0.01).
We also attempted to investigate isotype differences in cross-reactive HA-specific GC B cells for the different adjuvant groups. Unfortunately, we were not able to obtain sufficient number of cells to analyze the phenotype of NC+ PR8+ GC B cells. Nevertheless, we evaluated the phenotype of NC HA-specific GC B cells and observed the same trend of a dominant isotype of NC HA-specific GC B cells as that in the total serum (Fig. 4C). The frequency of NC HA-specific GC IgG2c B cells was significantly higher in K3-SPG HA- than in alum HA- or Addavax HA-vaccinated mice. In contrast, the frequency of NC-HA-specific GC IgG1 B cells was the lowest in the K3-SPG HA group (Fig. 4D).
Taken together, these results suggested that the selection of adjuvant influenced isotype switching of antigen-specific IgG through the GC reaction and were consistent with the serological data. Further, K3-SPG HA elicited a more prominent anti-HA IgG2c response, not only for the homologous NC strain but also for the heterologous IAV PR8, and potentially other antigenically distant IAV strains.
Protection against heterologous challenge is dependent on FcγR-mediated immune responses but not on neutralization activity
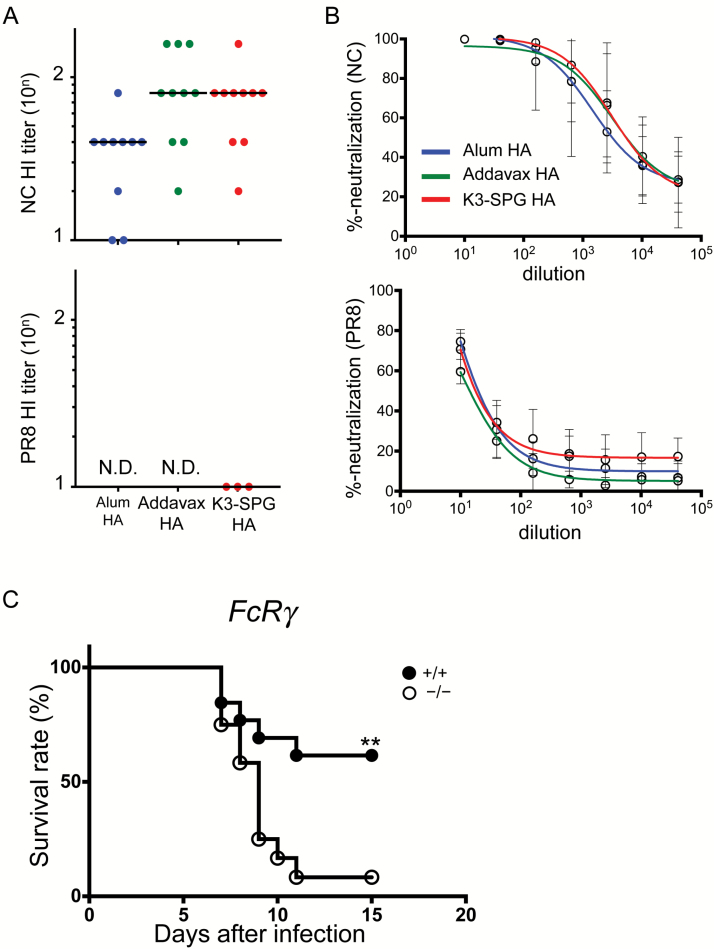
To understand the mechanisms of the protection against heterologous IAV infection in mice vaccinated with the different adjuvants, we first performed an influenza hemagglutination inhibition (HI) assay using serum from each vaccinated mouse. The HI titers against IAV NC in Addavax HA- or K3-SPG HA-vaccinated mice were higher than those in alum HA-vaccinated mice (Fig. 5A). However, we were not able to detect HI against the heterologous IAV PR8 in all groups. Thus, we subsequently performed neutralization assays using HA-pseudotyped lentiviral vectors (26). Using this assay, we were able to measure neutralization activity against IAV NC in all vaccination groups; nevertheless, there was no significant difference in neutralization activity between the groups (Fig. 5B). In contrast, we did not observe any neutralization activity against IAV PR8. These results suggested that the protection against heterologous IAV challenge was not due to a strong neutralization activity.
Fig. 5.
FcγR-mediated immune responses were required for protection against heterosubtypic IAV challenge by K3-SPG adjuvanted vaccination. (A) Serum HI titers against NC and PR8 IAVs of mice given different adjuvanted vaccines. (B) Neutralization activity against H1N1 pseudotyped viruses of mouse serum from the different adjuvant groups. (C) WT (n = 13) and FcRγ−/− mice (n = 12) were immunized with 1 µg of the NC HA split vaccine with different adjuvants at day 0 and 28. At day 7 from the last immunization, the mice were challenged with PR8 IAV. The survival rate (%) was recorded and graphed. Differences between groups were analyzed by log-rank test (**P < 0.01).
Several reports have indicated that FcγR-mediated protection resulted in a broader coverage of IAV strains using broadly neutralizing antibody (bNAb) injection (7, 27), and IgG2c binds to FcγR more efficiently than IgG1 does in mice. Thus, we assessed the role of FcγR-mediated antibody responses in K3-SPG HA-vaccinated mice to elucidate the mechanism of protection against PR8 challenge. We immunized either wild-type (WT) or FcRγ-deficient (FcRγ KO) mice with K3-SPG HA and challenged them with PR8 IAV. The results showed that FcRγ KO mice had significantly lower survival rate when compared to WT mice (Fig. 5C).
Together, these results clearly indicated that the protection against heterologous PR8 IAV in K3-SPG HA-vaccinated mice was dependent on FcγR-mediated immune responses.
Discussion
The generation of cross-reactive antibodies by vaccination is one of the main aims of new influenza vaccine development to protect against a future pandemic (2, 26, 28, 29). Many studies have investigated potential adjuvanted vaccines that can elicit cross-reactive antibodies and provide protection not only against vaccine-matched strains, but also against mismatched heterologous strains. MF59 was previously reported as a promising adjuvant to induce these broad responses, and our data in Fig. 4(B) supported these findings. In this study, we further characterized the impact of adjuvants on eliciting cross-reactive responses and found that K3-SPG is a better adjuvant than MF59-like AddaVax adjuvant in terms of protective efficacy against heterologous challenges.
MF59 induces a strong and robust immune response at the injection site, and the magnitude of the response imparts its superior activity when compared to alum or conventional CpG adjuvants (30). The strong response induced by MF59 may be associated with its potential to induce a wide spectrum of pro-inflammatory chemokines and cytokines at the injection site and stronger recruitment of MHC class II and CD11b+ cells to the injection site despite a weak type-1 interferon induction (30). These cytokines and chemokines promote a more efficient antigen uptake by monocytes, macrophages and granulocytes, and differentiation of monocytes into immature dendritic cells (DCs) (31). MF59 also primes for enhanced antigen processing and presentation for broader epitope recognition (11, 32). Therefore, with MF59-adjuvanted vaccine, memory B cells that may cross react with clade-mismatched H5 viruses (28) and group-mismatched H7N9 viruses are stimulated and elicit a more potent cross-reactive antibody-mediated response than that with the current trivalent inactivated influenza vaccine (TIV) alone (33).
Another report indicated that cationic adjuvant formulation (CAF)01-adjuvanted TIV protected not only against homologous but also against heterologous IAV infection despite the absence of neutralization based on HI (34). CAF01 is a newly developed liposomal adjuvant which is based on liposomes formed by N,N′-dimethyl-N,N′-dioctadecylammonium with the synthetic mycobacterial immunomodulator α,α′-trehalose 6,6′-dibehenate inserted into the lipid bilayers. In the study, Christensen et al. (34) concluded that CAF01-adjuvanted TIV vaccine can reduce virus shedding and systemic disease symptoms, but does not reduce local inflammation in the nasal cavity. Although the subclass of HA-specific IgGs was not assessed in the study as experiments were primarily conducted in ferrets, we speculated that there were indeed differences in isotype switching for HA-specific IgGs when compared to vaccination with the conventional TIV alone, because CAF01 is known to induce Th1/Th17-type T-cell responses. Thus, further investigation is required to clarify the mechanism of protection against heterologous challenge by the CAF01-adjuvanted influenza vaccine.
In our studies, we evaluated the potential of K3-SPG, a next-generation CpG adjuvant, for its use with influenza vaccines. The potent adjuvant activity of K3-SPG may be attributed to its nanoparticulate nature rather than targeting Dectin-1 by SPG, which results in the accumulation and activation of antigen-bearing macrophages and DCs in the draining lymph node. In the present study, we compared the efficacy of K3-SPG adjuvant with that of another promising adjuvant candidate, MF59, described above. We demonstrated that K3-SPG HA vaccine induced better protection against heterologous IAV challenge than MF59 HA, and the difference in protective efficacy was associated with cross-reactive yet non-neutralizing HA-specific antibodies and achieved by FcγR-mediated effector mechanisms.
It is also well established that antigen-specific T cells play an important role in the protection against IAV infection, especially when HA-specific neutralizing antibodies are not effective (35). Several studies have shown that pre-existing T-cell immunity is associated with lower virus shedding and less severe illness by producing anti-viral cytokines and chemokines against IAV infection (35, 36). However, results obtained from our in vivo depletion experiments suggested the lack of CD8 T-cell contribution with our vaccination strategy, although we observed that K3-SPG HA induced a stronger cross-reactive CD8 T-cell response than Addavax HA. This finding also suggests that the mechanism of protection by vaccination adjuvanted with K3-SPG is different than that of vaccination adjuvanted with CAF01, likely due to structural differences between the two adjuvants.
In the case of HA-specific antibodies, several recent reports indicated that stem-specific antibodies are better at mediating ADCC than head-specific antibodies (37–39). While we measured the production of anti-HA2 stem IgG1 and IgG2c in all adjuvant groups in this study, we did not observe any differences between the groups. It suggests that although HA stem-specific antibodies may also provide protection by ADCC via FcγR (7), the amount of HA stem-specific antibodies was not a critical factor for protection against heterologous IAV challenge by K3-SPG HA. Regardless, it is important to note that HA-specific IgG, which can trigger FcγR-mediated antibody responses, is needed for protection against heterologous IAV challenge in our studies. A complex series of events involving various cell types may contribute for the protection through FcγR-mediated immune functions that include ADCC, ADCP, CDC and phagocytosis activities (8, 40–43). Additionally, the wider range of FcγR isoforms, including FcγRI, FcγRIII and FcγRIV, and/or the interaction between FcRγ and c-type lectins may also account for the difference in the induction of ADCC versus phagocytosis to impart robust protection against IAVs (8). Further investigations are still needed to explore additional details of the mechanisms behind FcγR-mediated antibody responses.
Nevertheless, we could not rule out the role of neutralizing antibodies for protection against IAVs. Cross-reactive neutralizing antibodies can provide protection against heterologous IAV challenge, as long as sufficient antibody titers are achieved. However, in a clinical setting, it is challenging to induce such responses by vaccination, even with the use of promising adjuvants. Even if vaccination can elicit stem-specific bNAbs, it is also important to consider the potential short life of stem-specific cross-reactive B cells (44) and poly-reactivity of stem-specific bNAbs (45). In such cases, HA-specific cross-reactive non-neutralizing antibody induced by vaccination may play a role for heterologous protection.
Our findings may have implications for future development of influenza vaccines, and we anticipate that the incorporation of potent adjuvants would enable broader protection against IAV infections by inducing high levels of HA-specific antibodies that provide cross-protection through FcγR-mediated mechanisms.
Funding
This study was supported by National Institutes of Biomedical Innovation, Health and Nutrition, and in part by Japan Agency for Science and Technology (JST) CREST (grant number JPMJCR18H1), Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (A) (grant number JP17H05087) and by Agency for Medical Research and Development (grant number JP17ak0101068, JP17fk0108124 and JP17fk0108207).
Supplementary Material
Acknowledgements
We thank Drs Cevayir Coban and Etsushi Kuroda, and other members of the laboratories of Ken J. Ishii for their valuable comments and assistance. We also thank Akiko Okabe and Mariko Nakamura for their excellent technical assistance. T.Y. and K.J.I. conceived and designed the experiments; T.Y., Y.M., M.M., T.K., S.T., E.M. and Y.T. performed the experiments and analyzed the data; M.K., Y.Y., T.S. and B.S.G. contributed reagents, materials or analysis tools; and T.Y., Y.T., and K.J.I. wrote the manuscript.
Conflicts of interest statement: K.J.I. has a patent pending for K3-SPG. Y.M. is an employee of Nippon Shinyaku Co., Ltd. The remaining authors declare no commercial or financial conflict of interest.
References
- 1. Pavia A. T. 2016. Influenza vaccine effectiveness: mysteries, enigmas, and a few clues. J. Infect. Dis. 213:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wheatley A. K. and Kent S. J. 2015. Prospects for antibody-based universal influenza vaccines in the context of widespread pre-existing immunity. Expert Rev. Vaccines 14:1227. [DOI] [PubMed] [Google Scholar]
- 3. Knossow M., Gaudier M., Douglas A., et al. 2002. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology 302:294. [DOI] [PubMed] [Google Scholar]
- 4. Wiley D. C. Wilson I. A. and Skehel J. J. 1981. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289:373. [DOI] [PubMed] [Google Scholar]
- 5. Dreyfus C., Laursen N. S., Kwaks T., et al. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang T. T. and Palese P. 2011. Biochemistry. Catching a moving target. Science 333:834. [DOI] [PubMed] [Google Scholar]
- 7. DiLillo D. J. Tan G. S. Palese P. and Ravetch J. V. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med. 20:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry Dunand C. J., Leon P. E., Huang M., et al. 2016. Both neutralizing and non-neutralizing human H7N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe 19:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jegaskanda S. Reading P. C. and Kent S. J. 2014. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J. Immunol. 193:469. [DOI] [PubMed] [Google Scholar]
- 10. Seidel U. J. Schlegel P. and Lang P. 2013. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front. Immunol. 4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khurana S., Verma N., Yewdell J. W., et al. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobiyama K., Aoshi T., Narita H., et al. 2014. Nonagonistic dectin-1 ligand transforms CpG into a multitask nanoparticulate TLR9 agonist. Proc. Natl Acad. Sci. USA 111:3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobiyama K., Temizoz B., Kanuma T., et al. 2016. Species-dependent role of type I IFNs and IL-12 in the CTL response induced by humanized CpG complexed with β-glucan. Eur. J. Immunol. 46:1142. [DOI] [PubMed] [Google Scholar]
- 14. Koyama S., Aoshi T., Tanimoto T., et al. 2010. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci. Transl. Med. 2:25ra24. [DOI] [PubMed] [Google Scholar]
- 15. Masuta Y., Yamamoto T., Natsume-Kitatani Y., et al. 2018. An antigen-free, plasmacytoid dendritic cell-targeting immunotherapy to bolster memory CD8+ T cells in nonhuman primates. J. Immunol. 200:2067. [DOI] [PubMed] [Google Scholar]
- 16. Park S. Y., Ueda S., Ohno H., et al. 1998. Resistance of Fc receptor-deficient mice to fatal glomerulonephritis. J. Clin. Invest. 102:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whittle J. R., Wheatley A. K., Wu L., et al. 2014. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol. 88:4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joyce M. G., Wheatley A. K., Thomas P. V., et al. ; NISC Comparative Sequencing Program 2016. Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto T., Lynch R. M., Gautam R., et al. 2015. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci. Transl. Med. 7:298ra120. [DOI] [PubMed] [Google Scholar]
- 20. Wang T. T., Tan G. S., Hai R., et al. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl Acad. Sci. USA 107:18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wada Y., Nithichanon A., Nobusawa E., et al. 2017. A humanized mouse model identifies key amino acids for low immunogenicity of H7N9 vaccines. Sci. Rep. 7:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Z. Y., Wei C. J., Kong W. P., et al. 2007. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Böttcher E. Matrosovich T. Beyerle M. Klenk H. D. Garten W. and Matrosovich M. 2006. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 80:9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mesin L. Ersching J. and Victora G. D. 2016. Germinal center B cell dynamics. Immunity 45:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Victora G. D. and Wilson P. C. 2015. Germinal center selection and the antibody response to influenza. Cell 163:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanekiyo M., Wei C. J., Yassine H. M., et al. 2013. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiLillo D. J. Palese P. Wilson P. C. and Ravetch J. V. 2016. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 126:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galli G., Hancock K., Hoschler K., et al. 2009. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl Acad. Sci. USA 106:7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei C. J., Boyington J. C., McTamney P. M., et al. 2010. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060. [DOI] [PubMed] [Google Scholar]
- 30. Mosca F., Tritto E., Muzzi A., et al. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl Acad. Sci. USA 105:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Hagan D. T. 2007. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 6:699. [DOI] [PubMed] [Google Scholar]
- 32. Bihari I. Pánczél G. Kovacs J. Beygo J. and Fragapane E. 2012. Assessment of antigen-specific and cross-reactive antibody responses to an MF59-adjuvanted A/H5N1 prepandemic influenza vaccine in adult and elderly subjects. Clin. Vaccine Immunol. 19:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrews S. F., Joyce M. G., Chambers M. J. et al. 2017. Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2:eaan2676. [DOI] [PubMed] [Google Scholar]
- 34. Christensen D., Christensen J. P., Korsholm K. S., et al. 2017. Seasonal influenza split vaccines confer partial cross-protection against heterologous influenza virus in ferrets when combined with the CAF01 adjuvant. Front. Immunol. 8:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinfurter J. T., Brunner K., Capuano S. V., 3rd, et al. 2011. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 7:e1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilkinson T. M., Li C. K., Chui C. S., et al. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18:274. [DOI] [PubMed] [Google Scholar]
- 37. Tete S. M., Krammer F., Lartey S. et al. 2016. Dissecting the hemagglutinin head and stalk-specific IgG antibody response in healthcare workers following pandemic H1N1 vaccination. NPJ Vaccines 1:16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jegaskanda S., Vandenberg K., Laurie K. L., et al. 2014. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J. Infect. Dis. 210:1811. [DOI] [PubMed] [Google Scholar]
- 39. de Vries R. D., Nieuwkoop N. J., Pronk M., et al. 2017. Influenza virus-specific antibody dependent cellular cytotoxicity induced by vaccination or natural infection. Vaccine 35:238. [DOI] [PubMed] [Google Scholar]
- 40. Bournazos S. and Ravetch J. V. 2017. Fcγ receptor function and the design of vaccination strategies. Immunity 47:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bournazos S. and Ravetch J. V. 2017. Anti-retroviral antibody FcγR-mediated effector functions. Immunol. Rev. 275:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He W., Chen C. J., Mullarkey C. E., et al. 2017. Alveolar macrophages are critical for broadly-reactive antibody-mediated protection against influenza A virus in mice. Nat. Commun. 8:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mullarkey C. E., Bailey M. J., Golubeva D. A. et al. 2016. Broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an Fc-dependent manner. Mbio 7:e01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wheatley A. K., Whittle J. R., Lingwood D., et al. 2015. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J. Immunol. 195:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andrews S. F., Huang Y., Kaur K., et al. 2015. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 7:316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.