Abstract
Background
Frailty is a risk factor for cardiovascular disease (CVD). Underlying mechanisms to explain the connection between frailty and CVD are unclear. We sought to examine the association between frailty and arterial stiffness, a precursor of hypertension and CVD.
Methods
We conducted a cross-sectional analysis of community-dwelling Framingham Heart Study Offspring and Omni participants ≥60 years of age examined in 2005–2008. Frailty was defined primarily according to the Fried physical phenotype definition, which identifies nonfrail, prefrail, and frail individuals. Arterial stiffness was assessed using carotid–femoral pulse wave velocity (CFPWV). Generalized linear regression was used to examine the association between frailty level and CFPWV (modeled as −1000/CFPWV in msec/m, then transformed back to the original scale, m/s), adjusted for age, sex, cohort, mean arterial pressure, heart rate, height, and smoking.
Results
Of 2,171 participants (55% women, 91% white), 45% were prefrail and 7% were frail. Mean ages were 67, 70, and 73 years, and adjusted CFPWV least squares means were 10.0 (95% CI, 9.9–10.1), 10.3 (10.2–10.5), and 10.5 m/s (10.1–11.0); p = .0002 for nonfrail, prefrail, and frail groups, respectively. Results were similar using the Rockwood cumulative deficit model of frailty, and in a sensitivity analysis adjusting for prevalent coronary heart disease and diabetes.
Conclusions
Prefrailty and frailty were associated with higher arterial stiffness in a cohort of community-dwelling older adults. Arterial stiffness may help explain the relationship between frailty and CVD.
Keywords: Arterial Stiffness, Cardiovascular Disease, Epidemiology, Frailty
The incidence of cardiovascular disease (CVD) is strongly age-dependent, but mechanisms by which aging-related processes increase CVD risk have not been fully elucidated (1). Rapid aging of the world population makes understanding relations between aging and CVD ever more relevant (2). Biologic aging occurs at varying rates and is a heterogeneous process that is associated with frailty (3). Frailty is a common age-related syndrome that is associated with poor health outcomes (4) and both prevalent and incident CVD (5). Emerging evidence suggests that frailty is a risk factor for CVD, even after accounting for subclinical atherosclerosis (6). Conversely, CVD risk factors and risk scores may also predict frailty (7,8).
Essential hypertension is closely associated with aging and frailty (9). However, hypertension is no longer considered a natural consequence of aging, as is evidenced by small groups of hunter-gatherers who do not develop hypertension (10). Furthermore, hypertension may be postponed or prevented through healthy lifestyle choices (11). Arterial stiffness shares a bidirectional relationship with hypertension (12) and is an established risk factor for CVD that increases with age (13–15) but does not develop uniformly in all older adults (11,16). That some older adults “escape” age-associated deficits whereas others become frail reflects the heterogeneity of biological aging. However, evidence on relations between levels of frailty and arterial stiffness is limited and conflicting (9,17–19).
Therefore, we sought to examine the cross-sectional association between levels of frailty and arterial stiffness in a sample of community-dwelling older adults using two established theories of frailty. We hypothesized that higher levels of frailty would be associated with higher arterial stiffness.
Methods
Study Sample
The Framingham Heart Study is a multigenerational study that began in 1948 to investigate CVD in the community. The current study investigates the Offspring cohort, the children of the Framingham Heart Study Original cohort, and the Offspring spouses who were enrolled in 1971 and have been examined every 4 to 8 years (20,21). In 1994, a racially and ethnically diverse Omni group 1 cohort (n = 506) was recruited to the Framingham Heart Study, and these participants have been examined with Offspring cohort participants. Offspring and Omni Cohort participants seen at Exam 8/Exam 3 (2005–8) were eligible to participate (n = 3,021 Offspring, n = 298 Omni). These cohort protocols have been published previously (21,22).
Our study sample exclusions are outlined in Supplementary Appendix A. Individuals <60 years (n = 834) were excluded as frailty before 60 years is uncommon, and when it occurs is generally a result of a specific underlying cause of accelerated aging, such as HIV (23). A total of n = 130 individuals missing either >4 items necessary for the Rockwood frailty index (FI) or >1 item for the Fried physical phenotype or both were excluded. In order to focus on community-dwellers, participants examined off-site in nursing homes or personal residences were excluded (n = 9). We further excluded those missing arterial tonometry data (n = 151) or covariates (n = 24) used in the multivariable models. The Offspring and Omni examination included detailed medical history, measures of physical function and performance, cognitive function, and vascular function by trained study personnel. All study participants provided informed consent to participate in this study. All protocols were approved by the Institutional Review Board at Boston University Medical Center.
Frailty Definitions
There are currently two leading theories on how to define frailty. Fried and colleagues operationalized the five-item physical phenotype that consists of five interrelated measures of physical function (3). This has been used previously in the Framingham Offspring cohort (24). Individuals are frail if they have at least three of the following: unintentional weight loss of ≥10 lbs in the past year, self-reported exhaustion, weakness as measured by grip strength, slow walking speed, and decreased physical activity. Physical activity was assessed using the Framingham Physical Activity Index, which creates a weighted score from self-reported time spent in activities during a routine day (24). Those with 1–2 deficits are considered prefrail, and 0 deficits are nonfrail. This definition was used for the primary analysis.
Rockwood and colleagues conceived of frailty as the accumulation of health-related deficits over time (25). The Rockwood cumulative deficit FI can be created using a minimum of 30 variables related to cognition, physical function, mood, and morbidity that are readily available in many existing datasets (26,27).
We used 37 variables available in the Framingham Heart Study database to build a Rockwood FI. Adjudicated outcomes were used for prevalent CVD (including myocardial infarction, stroke, and heart failure), cancer, fractures, and dementia reports. Self-reports of functional and emotional status were taken from validated questionnaires (eg, Katz Activities of Daily Living and Short Form-12). Binary variables were coded as 0 and 1 to indicate absence or presence of the variable, with a higher number representing a deficit. Variables with three responses were graded as 0, 0.5, and 1, and those with five responses were graded as 0, 0.25, 0.5, 0.75, and 1, with higher values indicating poorer rating of health. Frailty indices for each individual were calculated by dividing the numbers of accumulated deficits by the total number of possible deficits (28). Categories were created according to established cutoffs in community-dwelling cohorts to match the Fried physical phenotype: nonfrail (0–0.1), prefrail (>0.1–0.21) and frail (>0.21) (27,29–32). (See Supplementary Appendix B for further FI details.) The Rockwood FI was used both as a categorical and continuous variable as a sensitivity analysis.
Tonometry
To evaluate arterial stiffness, we measured carotid–femoral pulse wave velocity (CFPWV). Arterial tonometry measurements in Framingham have been described in detail previously (14,33). Briefly, recordings were obtained on the right side of the body after participants rested supine for at least 5 minutes. The following were measured in order: resting blood pressure and tonometry with simultaneous ECG at the brachial, radial, femoral, and carotid arteries. For each artery, transit distances from the suprasternal notch were measured. Waveforms of carotid and femoral pressure were used to calculate CFPWV. To correct for parallel transmission, the distance from suprasternal notch to carotid artery was subtracted from the distance from suprasternal notch to femoral artery.
Covariates
Demographics, including age, sex, cohort (Offspring or Omni), self-report of education, employment, retirement status, and marital status were examined. Smoking was assessed as never, former, or current. CVD events were adjudicated. Diabetes was defined as fasting blood glucose greater than 125 mg/dL or use of oral hypoglycemic medications or insulin. Hypertension was defined as the mean of two physician-obtained blood pressures ≥140 or ≥90 mmHg at exam visit or use of antihypertensive medication. Mean arterial pressure was calculated by integration of the calibrated brachial pressure waveform.
Statistical Analysis
We compared the distribution of frailty according to the Fried physical phenotype and Rockwood FI definitions using a weighted Kappa statistic. We stratified the population into nonfrail, prefrail, and frail categories by each frailty definition and performed survival analysis to predict mortality within each frailty group using Kaplan–Meier curves.
CFPWV values were highly skewed. We transformed the values to −1000/CFPWV to reduce skewness and retain the interpretation that higher values indicate greater stiffness. Generalized linear regression was used to examine the association between frailty level and transformed CFPWV. Models were adjusted for age, sex, cohort, mean arterial pressure, height, heart rate, and smoking status based on a priori knowledge of variables that are associated with arterial stiffness (14). Generalized estimating equations were used to account for familial correlation, using the R package geepack (34). We performed a two degree of freedom test of whether the covariate-adjusted means in the three frailty categories differed, where a significant test would indicate at least two categories are significantly different. This single omnibus test was chosen to avoid issues of multiple testing.
Additional sensitivity analysis was performed to adjust for prevalent diabetes and coronary heart disease (CHD), two components of the 37 items in the Rockwood FI. Additional sensitivity analysis excluded the 44 individuals who were missing only one item for the Fried definition. A two-sided p value of <0.05 was considered statistically significant. Least squares means were estimated using R package doBy (34), and the means and 95% confidence bounds were transformed back to the original scale (CFPWV in m/s). Analyses were done using SAS 9.3 and R 3.3.
Results
There were 2,171 participants (55% women, 93% white) with complete tonometry data and sufficient information to calculate a Fried physical phenotype and Rockwood cumulative deficit FI. Age rose steadily across categories of frailty, as did rates of retirement, widowed marital status, current smoking status, CHD, diabetes, and hypertension, although mean arterial pressure remained flat. (Table 1) The racially diverse Omni cohort represented only 7% of the cohort and generally had similar distribution of covariates (Supplementary Appendix C).
Table 1.
Characteristics of the Framingham Frailty Cohort (n = 2,171) According to Frailty Status Defined by the Fried Physical Phenotype and Rockwood Cumulative Deficit Frailty Index
| Fried Physical Phenotype* | Rockwood Frailty Index† | |||||
|---|---|---|---|---|---|---|
| Nonfrail n = 1046 | Prefrail n = 976 | Frail n = 149 | Nonfrail n = 838 | Prefrail n = 865 | Frail n = 468 | |
| Age,mean(SD) | 68.1 (6.1) | 70.6 (7.0) | 75.5 (7.2) | 67.3 (5.7) | 70.1 (6.8) | 73.1 (7.2) |
| Women(%) | 50.7 | 58.5 | 59.7 | 53.2 | 52.3 | 62.4 |
| Race(%) | ||||||
| White | 93.9 | 88.9 | 89.9 | 91.7 | 91.8 | 89.9 |
| Black | 2.6 | 4.5 | 5.4 | 3.3 | 3.4 | 4.7 |
| Other | 3.6 | 6.6 | 4.7 | 5.0 | 4.8 | 5.4 |
| Body mass index,mean(SD) | 27.2 (4.5) | 28.6 (5.2) | 29.2 (7.0) | 26.4 (4.1) | 28.5 (4.9) | 29.8 (6.1) |
| Systolic BP, mmHg,mean(SD)‡ | 142 (20) | 145 (19) | 147 (22) | 141 (19) | 145 (19) | 147 (22) |
| Diastolic BP, mmHg,mean(SD)‡ | 69 (9) | 68 (9) | 66 (10) | 69 (8) | 69 (9) | 67 (10) |
| Mean arterial pressure, mmHg,mean (SD)* | 99 (12) | 99 (11) | 97 (13) | 98 (12) | 99 (12) | 99 (12) |
| Heart rate,mean(SD) | 60 (10) | 62 (10) | 63 (11) | 60 (9) | 61 (10) | 64 (11) |
| Height (inches), mean(SD) | 66.0 (3.8) | 65.0 (3.7) | 64.2 (3.8) | 65.8 (3.9) | 65.5 (3.8) | 64.6 (4.7) |
| Smoking status(%) | ||||||
| Current | 6.8 | 10.5 | 14.1 | 6.9 | 8.6 | 13.3 |
| Former | 55.2 | 48.8 | 53.7 | 52.0 | 52.3 | 52.4 |
| Never | 38.1 | 40.8 | 32.2 | 41.1 | 39.2 | 34.4 |
| Prevalent CHD(%) | 9.3 | 12.5 | 26.2 | 3.2 | 13.4 | 24.6 |
| Prevalent diabetes(%) | 12.0 | 19.3 | 24.1 | 3.6 | 19.7 | 32.0 |
| Prevalent hyperlipidemia(%) | 73.8 | 73.9 | 71.9 | 71.2 | 73.5 | 78.7 |
| Prevalent hypertension(%) | 50.9 | 59.6 | 72.3 | 36.7 | 65.6 | 74.1 |
| Physical activity index score,mean(SD) | 36.9 (4.9) | 34.0 (4.8) | 29.9 (2.7) | 36.3 (5.3) | 35.3 (4.9) | 32.7 (4.3) |
| Gait speed, m/s,mean(SD) | 1.29 (0.21) | 1.08 (0.26) | 0.81 (0.20) | 1.27 (0.23) | 1.17 (0.24) | 0.96 (0.25) |
| Hand grip strength(kg) | 33.9 (10.7) | 27.6 (10.5) | 21.3 (8.7) | 33.1 (11.1) | 30.2 (11.1) | 25.2 (9.6) |
| Physical phenotype criteria(%) | ||||||
| Weight loss | 0 | 12.8 | 42.6 | 3.4 | 7.3 | 20.7 |
| Exhaustion | 0 | 11.3 | 43.5 | 1.0 | 5.1 | 26.4 |
| Low physical activity | 0 | 28.0 | 81.2 | 11.0 | 14.8 | 37.2 |
| Slow gait | 0 | 40.4 | 88.4 | 10.2 | 20.9 | 55.9 |
| Weak grip | 0 | 36.8 | 80.5 | 6.2 | 25.9 | 43.4 |
| Physical phenotype score,mean (SD) | 0 (0) | 1.3 (0.5) | 3.3 (0.6) | 0.3 (0.5) | 0.7 (0.8) | 1.8 (1.2) |
| Frailty index score,mean (SD) | 0.10 (0.07) | 0.18 (0.10) | 0.35 (0.12) | 0.06 (0.03) | 0.15 (0.03) | 0.32 (0.09) |
| Carotid–Femoral pulse wave velocity, m/s,mean(SD) | 10.5 (3.4) | 11.5 (4.1) | 13.1 (4.9) | 10.3 (3.3) | 11.2 (3.7) | 12.6 (4.7) |
| Negative inverse CFPWV | −103.0 (26.6) | −95.1 (26.1) | −85.9 (28.8) | −104.8 (26.0) | −97.4 (26.2) | −88.2 (27.1) |
BP = blood pressure; CFPWV = carotid–femoral pulse wave velocity; CHD = coronary heart disease; SD = standard deviation.
*Physical phenotype: nonfrail = 0 variables, prefrail = 1–2 variables, frail ≥3 variables.
†Frailty index: nonfrail = FI <0.1; prefrail FI = 0.1–0.2; frail FI ≥0.2.
‡Blood pressure and mean arterial pressure values are taken from the time of tonometry assessment.
Using the Fried FI, 45% were prefrail and 7% were frail, whereas using the Rockwood FI, 40% were prefrail and 22% were frail. These observations are consistent with prior reports of community-dwelling older adults (3,27,29). Distributions of the Rockwood FI and Fried FI are shown in Supplementary Appendix B. Agreement between the two definitions of frailty was modest (weighted Kappa 0.36). Both men and women identified as prefrail or frail using either frailty definition were at increased risk for mortality (p < .0001, Supplementary Appendix D: KM survival plots).
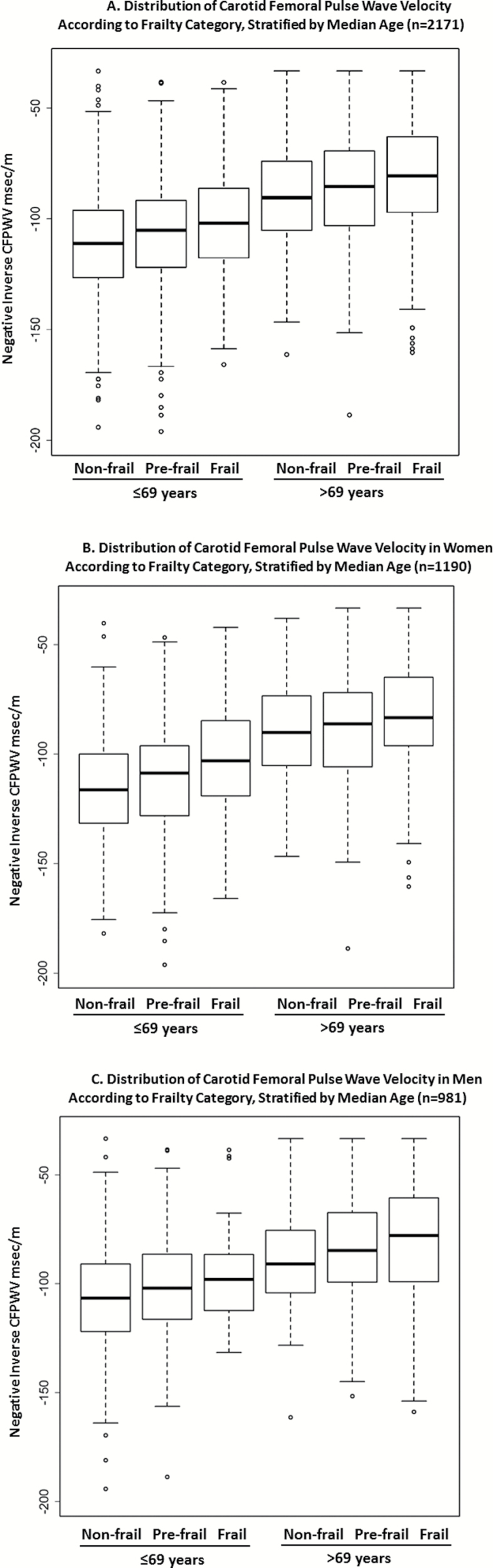
Mean CFPWV was higher according to frailty category using both frailty definitions (Table 1). Adjusted CFPWV least squares means also were higher for higher frailty categories using either frailty definition (Table 2). We further stratified the cohort at the median age (69 years) and found higher CFPWV with higher level of frailty in both age strata, shown in Figure 1A. A similar pattern was seen when dichotomized by sex (Figure 1B and C). There was no evidence for interactions between age or sex and frailty level in the multivariable-adjusted model (p > .1). Shown in Supplementary Appendix E is a series of nested models demonstrating that beyond age and sex, the covariates in the main model had a modest effect on the estimates and p values for frailty defined by Fried phenotype or the Rockwood FI. Results remained similar in sensitivity analyses adjusting for prevalent diabetes, CHD, and both diabetes and CHD (Table 3), although using the Rockwood definition, significance was lost after adjusting for both CHD and diabetes. Results excluding the 44 individuals missing one item for the Fried FI (2% of the cohort) had no substantive effect on the results.
Table 2.
Adjusted Least Squares Means Carotid–Femoral Pulse Wave Velocity (m/s) for 2,171 Framingham Heart Study Participants According to Frailty Group
| Frailty Category | Fried Frailty Phenotype | Rockwood Frailty Index | ||||||
|---|---|---|---|---|---|---|---|---|
| N | LS Means* (m/s) | 95% CI | p Value† | N | LS Means* (m/s) | 95% CI | p Value† | |
| Nonfrail | 1046 | 10.0 | 9.9–10.1 | 0.0002 | 838 | 10.0 | 9.8–10.1 | <.0001 |
| Prefrail | 976 | 10.3 | 10.2–10.5 | 865 | 10.2 | 10.0–10.3 | ||
| Frail | 149 | 10.5 | 10.1–11.0 | 468 | 10.6 | 10.3–10.8 | ||
Note: LS = least squares.
*Adjusted for: age, sex, cohort, smoking, mean arterial pressure, heart rate, and height.
†Two degrees of freedom test of whether the covariate-adjusted means in the three frailty categories differed, where a significant test indicates at least two categories are significantly different.
Figure 1.
(A–C) Distribution of carotid–femoral pulse wave velocity by age and sex. Y-axis: −50 ms/m represents CFPWV of 20 m/s, −100 ms/m is 10 m/s, −150 ms/m is 6.7 m/s, and −200 ms/m is 5 m/s.
Table 3.
Sensitivity Analysis: Least Squares Means Carotid–Femoral Pulse Wave Velocity (m/s) for 2,171 Framingham Heart Study Participants According to Frailty Group Adjusted for Diabetes and CHD
| Model | Group | Fried Frailty Phenotype | Rockwood Frailty Index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | LS Means* (m/s) | 95% CI | p Value† | N | LS Means* (m/s) | 95% CI | p Value† | ||
| Diabetes adjusted | Nonfrail | 1,040 | 10.0 | 9.9–10.1 | 0.001 | 834 | 10.1 | 9.9–10.2 | .01 |
| Prefrail | 971 | 10.3 | 10.2–10.5 | 860 | 10.2 | 10.0–10.3 | |||
| Frail | 145 | 10.5 | 10.1–10.9 | 462 | 10.5 | 10.2–10.7 | |||
| CHD adjusted | Nonfrail | 1,040 | 10.0 | 9.9–10.1 | 0.0004 | 834 | 10.0 | 9.9–10.1 | .006 |
| Prefrail | 971 | 10.2 | 10.2–10.5 | 860 | 10.2 | 10.0–10.3 | |||
| Frail | 145 | 10.5 | 10.1–10.9 | 462 | 10.5 | 10.3–10.8 | |||
| Diabetes and CHD adjusted | Nonfrail | 1,040 | 10.0 | 9.9–10.1 | 0.002 | 834 | 10.0 | 9.9–10.1 | .06 |
| Prefrail | 971 | 10.3 | 10.2–10.5 | 860 | 10.3 | 10.2–10.5 | |||
| Frail | 145 | 10.4 | 10.0–10.9 | 462 | 10.5 | 10.1–10.9 | |||
Notes: CHD = coronary heart disease; CI = confidence interval; LS = least squares.
*Adjusted for: age, sex, cohort, smoking, mean arterial pressure, heart rate, and height.
†Two degrees of freedom test of whether the covariate-adjusted means in the three frailty categories differed, where a significant test indicates at least two categories are significantly different.
Discussion
In our moderate-sized cross-sectional study of community-dwelling older adult participants in the Framingham Heart Study, we found that higher mean levels of frailty were associated with higher levels of arterial stiffness. The association was observed using both of the leading definitions of frailty, even though each definition identified different risk groups, and persisted after stratifying by age and sex.
Frailty and CVD share a common underlying pathobiology that includes changes in endocrine, hematologic, and immunologic systems (2,4). In particular, elevated levels of C-reactive protein, interleukin-6, D-dimer, factor VIII, and insulin resistance have been identified to explain the bidirectional relationship between frailty and clinical CVD (2,35). However, the association between inflammatory markers and frailty in longitudinal studies remains inconclusive (36). These pathophysiologic changes of aging, so-called inflamm-aging, lead to the clinical syndromes of frailty and CVD (37,38) and underlie the pathophysiology of arterial stiffness as well (39). Furthermore, sarcopenia, or age-related muscle loss, and an element of frailty that is also associated with CVD have been shown to be associated with increasing arterial stiffness in cohorts of Japanese older adults (17,40).
We specifically chose to examine the association between frailty and arterial stiffness using the two leading theories of frailty because each is derived from a different conceptual framework of frailty (4). The Fried phenotype focuses primarily on function and is therefore considered a more specific measure of frailty (3). The Rockwood FI, on the other hand, incorporates age-related deficits across the spectrum of health, including cognition, function, mood, and morbidity (25), addressing the concept of immunologic changes across body systems. The Rockwood method has been criticized for being too inclusive and not differentiating between frailty, disability, and morbidity (41). Furthermore, in studies using the Rockwood FI, there is a theoretical risk of over adjustment due to endogeneity when variables included in the FI are also adjusted for in the model. This may be evident in the sensitivity analysis that adjusted for diabetes and CHD.
Although it can be challenging to directly compare prevalence of frailty across cohorts as different definitions of frailty are used and the characteristics of the cohort (eg, age, sex) are often different, the prevalence of frailty in our study was consistent with other community-dwelling cohorts, using each definition of frailty. In the original Cardiovascular Health Study cohort used to develop the Fried phenotype, the prevalence of prefrailty was 46% and frailty was 7% (3). The Rockwood FI is often used as a continuous model; however when stratified, the prevalence of frailty in cohorts of similar age (≥60 years) is near 20% (27,29). In cohorts that have included individuals aged 50 and older, such as NHANES data and the SPRINT trial, the prevalence of frailty was higher than we found in Framingham using the Rockwood criteria, ranging from 27% to 34% (29,32).
Moreover, because the Rockwood FI is more inclusive than the Fried Phenotype, due to the large number of variables included (27,32,42), prior studies have demonstrated that the Rockwood method is more robust than the Fried in identifying risk of mortality (43,44). In the main analysis examining the relationship of stiffness and frailty in our study, we found that using the Rockwood definition, the nonfrail and frail 95% CIs do not overlap, whereas for the Fried definition, these 95% CIs do overlap. This is likely due to the small number of participants defined as frail using the Fried criteria. The trend for both indices is that higher levels of frailty are associated with higher arterial stiffness. However, that we found a similar overall relationship between frailty and arterial stiffness regardless of definition used speaks to the value of using any of the leading definitions of frailty in population studies in order to elucidate relationships between frailty and CVD. By using these two leading theories to define frailty, we were able to shed light on important relationships between frailty levels and arterial stiffness.
Additionally, our results demonstrated that even in those aged 60–69 years, an important age range to consider preventive strategies for CVD, participants who were identified as prefrail or frail had higher CFPWV than the nonfrail group. Whether the effect sizes that we identified between the levels of frailty and CFPWV are clinically relevant are unknown at this time as CFPWV remains a research tool. However, our results provide intriguing preliminary findings to begin to inform underlying potential mechanisms to explain the bidirectional relationship between frailty and clinical CVD.
Our results are consistent with those found in the Atherosclerosis Risk in Communities Study, which examined cardiovascular function and frailty in 3,991 community-dwelling adults (19). Using the Fried frailty definition, 5.3% of participants were identified as frail. Vascular dysfunction was defined with a composite of CFPWV of >13 m/s or ankle–brachial index <0.9. Compared with those who were not frail, the odds ratio for frailty in those with vascular dysfunction was 1.44 (95% CI, 1.06–1.95, p < .05). However, only one frailty definition was examined, prefrail and nonfrail individuals were grouped together, and the composite measure of vascular function represented a mixture of atherosclerosis and stiffness measures. In the Cardiovascular Health Study, 6% of 4,735 participants were identified as frail using the Fried criteria and those with an ankle–brachial index <0.8 or 0.8–0.9 had twofold higher odds of being frail (9). As in the Atherosclerosis Risk in Communities study, the prefrail group was combined with the nonfrail category, whereas CFPWV was not available. In our study, tonometry data were used to derive the gold standard for arterial stiffness measurement (CFPWV), and the cohort was separated into nonfrail, prefrail, and frail categories to better examine the relationship between levels of frailty and arterial stiffness.
Smaller studies in select samples have provided conflicting results. In contrast to our findings, in a cohort of 117 participants (median age 79) in Rotterdam, no association was found between frailty, defined according to Fried, and aortic stiffness, with a reported PWV of 12.1 m/s in each category of frailty (18). The Rotterdam investigation was limited by including only a small sample of older adults and lack of adjustment for important covariates. Consistent with our observations, a Japanese cohort of 496 middle-aged men demonstrated a correlation between sarcopenia (age-related muscle loss and a marker of frailty) and arterial stiffness measured according to brachial–ankle PWV (17).
In a sensitivity analysis, we demonstrated similar findings after adjusting for prevalent diabetes or CHD. Whereas these are components of the Rockwood FI, theoretically, this is not considered overadjustment as diabetes and CHD are individual components of a 37-item equally weighted index (42). However, in the analysis adjusting for both diabetes and CHD, the relationship between frailty level and CFPWV was no longer significant according to the Rockwood definition. This lack of significance may represent a bias toward the null through overadjustment. In the Fried definition which only includes the physical phenotype components, the relationship between frailty levels and CFPWV remains significant and only slightly attenuated relative to the main model.
Our study has several strengths. The Framingham Heart Study is a valuable cohort to study aging phenomena because of the availability of several decades of detailed information on lifestyle, function, mood, cognition, and morbidities, as well as adjudicated outcomes for CHD and mortality. The breadth of phenotype data allowed us to classify frailty according to two leading definitions. While some studies have combined vascular stiffness with ankle–brachial index (measure of generalized atherosclerosis), we had access to precise measures of arterial stiffness using CFPWV, the gold standard measure of arterial stiffness in research. Limitations of our study include the observational and cross-sectional design, limiting our ability to establish causal relations, temporality, or exclude residual confounding. There may be a bidirectional relationship between arterial stiffness and frailty. Future investigation of the longitudinal relationships between frailty and CVD and examination of whether frailty is an intermediate on the pathway between arterial stiffness and CVD is needed. Second, our study focused on community-dwelling older adults and may not be generalizable to those who are institutionalized; however, such older adults are expected to have a higher burden of frailty. Third, although we included the diverse Omni cohort in our study, 91% of the overall cohort was white and that may limit the generalizability of our findings to a more diverse aging population. Fourth, both frailty definitions include self-reported items, which could be inaccurate in participants with cognitive impairment or dementia.
In conclusion, we observed that increasing levels of frailty, defined using either of the two leading models of frailty, were associated with evidence of higher arterial stiffness. Further work is needed to understand the relation between frailty and clinical CVD in order to develop tailored prevention strategies for the aging population.
Funding
This work was supported by NIA R56 AG029451, the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract No. N01-HC-25195 and HHSN268201500001I) and grants HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, and HL126136. Dr. A.R.O is supported in part by the Boston Claude D. Pepper Older Americans Independence Center, NIA grant P30-AG0313679.
Supplementary Material
Acknowledgments
We thank the participants of the Framingham Heart Study for their invaluable contributions to this work.
Conflict of interest statement
G.F.M is the owner of Cardiovascular Engineering, Inc.—a company that develops and manufactures devices to measure vascular stiffness and serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375 [DOI] [PubMed] [Google Scholar]
- 6. Veronese N, Sigeirsdottir K, Eiriksdottir G, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-reykjavik study. Rejuvenation Res. 2017;20:517–524. doi: 10.1089/rej.2016.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gale CR, Cooper C, Sayer AA. Framingham cardiovascular disease risk scores and incident frailty: the English longitudinal study of ageing. Age (Dordr). 2014;36:9692. doi: 10.1007/s11357-014-9692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart. 2013;99:737–742. doi: 10.1136/heartjnl-2012-302922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman AB, Gottdiener JS, Mcburnie MA, et al. ; Cardiovascular Health Study Research Group Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56: M158–M166. [DOI] [PubMed] [Google Scholar]
- 10. Lemogoum D, Ngatchou W, Janssen C, et al. Effects of hunter-gatherer subsistence mode on arterial distensibility in Cameroonian pygmies. Hypertension. 2012;60:123–128. doi: 10.1161/HYPERTENSIONAHA.111.187757 [DOI] [PubMed] [Google Scholar]
- 11. Niiranen TJ, Lyass A, Larson MG, et al. Prevalence, correlates, and prognosis of healthy vascular aging in a western community-dwelling cohort: the Framingham Heart Study. Hypertension. 2017;70:267–274. doi: 10.1161/HYPERTENSIONAHA.117.09026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franklin SS. Arterial stiffness and hypertension: a two-way street?Hypertension. 2005;45:349–351. doi: 10.1161/01.HYP.0000157819.31611.87 [DOI] [PubMed] [Google Scholar]
- 13. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 15. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scuteri A, Morrell CH, Orrù M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64:1219–1227. doi: 10.1161/HYPERTENSIONAHA.114.04127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochi M, Kohara K, Tabara Y, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–332. doi: 10.1016/j.atherosclerosis.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 18. Kannegieter LM, Tap L, Oudshoorn C, Van Bruchem-Visser RL, Mattace-Raso FUS. Mobility and handgrip strength but not aortic stiffness are associated with frailty in the elderly. J Gerontol and Geriatr. 2016;64:2–8. [Google Scholar]
- 19. Nadruz W Jr., Kitzman D, Windham BG, et al. Cardiovascular dysfunction and frailty among older adults in the community: the ARIC study. J Gerontol A Biol Sci Med Sci. 2017;72:958–964. doi: 10.1093/gerona/glw199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 21. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 22. Quan SF, Howard BV, Iber C, et al. The sleep heart health study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 23. Guaraldi G, Palella FJ Jr. Clinical implications of aging with HIV infection: perspectives and the future medical care agenda. AIDS. 2017;31(Suppl 2):S129–S135. doi: 10.1097/qad.0000000000001478 [DOI] [PubMed] [Google Scholar]
- 24. Liu CK, Lyass A, Larson MG, et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr). 2016;38:1. doi: 10.1007/s11357-015-9864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 26. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orkaby AR, Hshieh TT, Gaziano JM, Djousse L, Driver JA. Comparison of two frailty indices in the physicians’ health study. Arch Gerontol Geriatr. 2017;71:21–27. doi: 10.1016/j.archger.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 29. Pajewski NM, Williamson JD, Applegate WB, et al. ; SPRINT Study Research Group Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–E494. doi: 10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24:10–17. [PubMed] [Google Scholar]
- 32. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 33. Mitchell GF, Guo CY, Benjamin EJ, et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733 [DOI] [PubMed] [Google Scholar]
- 34. Moppett IK, Greenhaff PL, Ollivere BJ, Joachim T, Lobo DN, Rowlands M. Pre-Operative nutrition In Neck of femur Trial (POINT)—carbohydrate loading in patients with fragility hip fracture: study protocol for a randomised controlled trial. Trials. 2014;15:475. doi: 10.1186/1745-6215-15-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walston J, McBurnie MA, Newman A, et al. ; Cardiovascular Health Study Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. [DOI] [PubMed] [Google Scholar]
- 36. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 37. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 38. Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72:1218–1225. doi: 10.1093/gerona/glw240 [DOI] [PubMed] [Google Scholar]
- 39. Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237:381–390. doi: 10.1016/j.atherosclerosis.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 40. Kohara K, Okada Y, Ochi M, et al. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J Cachexia Sarcopenia Muscle. 2017;8:557–566. doi: 10.1002/jcsm.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 42. Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K. Aging, frailty and complex networks. Biogerontology. 2017;18:433–446. doi: 10.1007/s10522-017-9684-x [DOI] [PubMed] [Google Scholar]
- 43. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.