Abstract
Background
This study compared long-term outcomes of biological and mechanical mitral valve replacement (MVR) in patients requiring replacement of the mitral valve where repair was not feasible.
Methods
A single-centre registry of patients receiving MVR between 2005 and 2015 was established. Thirty-day mortality and long-term outcomes were analysed and compared.
Results
Three hundred twenty four patients underwent MVR (265 biological; 59 mechanical valves). Patients receiving biological valves were older (p < 0.001), had a higher log EuroSCORE (p < 0.001) and received less minimally invasive surgery (p < 0.001).
Immediate procedural mortality was 1.9%, which only occurred in the biological valve group. At 30 days, 9.0% of patients had died, 4.0% experienced stroke, 8.0% received a pacemaker and 10.5% suffered an acute renal failure. The rate of re-thoracotomy (14.2%) was lower in the biological (12.5%) than in the mechanical valve group (22.0%; adjOR 0.45 [0.20–1.00]; p = 0.050). Frequent long-term complications were stroke (9.2%) and bleeding (4.8%), with bleeding complications being higher in the mechanical valve group (p = 0.009). During the follow-up period biological valves showed a numerically higher survival rate during the first years, which shifted after 3 years in favour of mechanical valves. At 10 years, survival rates were 62.4% vs. 77.1% in the biological and mechanical valve groups (p = 0.769). Hazard ratio after adjustment was 0.833 (95% CI 0.430–1.615).
Conclusion
These data confirm that mechanical valve implantation is associated with an increased risk of bleeding. While there was a potential survival benefit during the first years after surgery for patients receiving a biological valves the difference became insignificant after a follow-up of 10 years.
Electronic supplementary material
The online version of this article (10.1186/s13019-019-0943-6) contains supplementary material, which is available to authorized users.
Keywords: Mitral valve replacement, Biological valve, Mechanical valve
Background
In accordance with guidelines from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), mitral valve replacement (MVR) is a surgical procedure that is used when the patient’s heart valve is so severely compromised that mitral valve (MV) repair is no longer a viable option [1, 2]. The American Heart Association (AHA) guidelines recommend MV surgery for asymptomatic patients with chronic severe primary mitral regurgitation (MR) and left ventricular (LV) dysfunction [3, 4]. Again, MV repair is preferred over MVR when possible [3, 4].
Data on the choice of biological versus mechanical MV is controversial. Biological MVs are generally considered to be associated with lower bleeding complications due to the need for less or no anticoagulation, but they lack of durability. Mechanical valves are durable, but are associated with thromboembolism and bleeding complications mostly because of inevitable lifelong anticoagulation therapy [2, 5, 6].
In a study of 279 patients undergoing MVR (154 biological and 125 mechanical valves), clinically satisfactory results were obtained in both groups. After 15 years, fewer patients in the mechanical valve group were free of bleeding events (92.5% vs. 100%) and patients in the biological valve group had a lower freedom from thromboembolism (72.2% vs. 93.5%), valve failure (22.0% vs. 87.0%) and cardiac events (16.5% vs. 47.2%). The study concluded that the use of mechanical valves was preferential and biological valves should be reserved for patients older than 65 years and only be used with concomitant anticoagulant therapy [5]. Conversely, the results of a 10-year follow-up study published in 2001 showed no significant clinical differences between patient groups receiving biological and mechanical valves [7]. These results confirm the findings of a study from the 1980s [8].
This paper aims to compare the outcomes after biological versus mechanical MVR in patients who were not suitable or ineligible for MV repair.
Methods
This study is a single-centre registry analysis of MVR procedures performed at the Kerckhoff-Heart Center, in Bad Nauheim, Germany, between January 2005 and December 2015. It was approved by the site’s Ethical Committee and complied with the Declaration of Helsinki and its amendments. Given the use of anonymised data already collected as part of routine diagnosis and treatment, written informed consent was neither feasible nor required.
Patient population
All patients undergoing MVR at our site within the specified time period (January 2005–December 2015) were included. The study also included patients receiving MVR combined with tricuspid valve repair and ablation therapy, patent foramen ovale (PFO) or atrial septal defect (ASD) closure.
Age, comorbidity and lifestyle were considered when recommending biological valves to patients. Patients with a contraindication for vitamin K antagonists (VKA), women with a desire to become pregnant, certain professions (e.g., pilots) and those with a likely lack of compliance were also considered for biological valves even if they were less than 65 years of age.
Patients with endocarditis had their infection carefully resected. Patients with severe calcification and some of those with endocarditis received neochordae implanted at the mitral annulus. Patients with mitral insufficiency without calcification and without endocarditis who received MVR after two failed attempts had their anterior mitral leaflet (AML) resected and the posterior leaflet (PML) preserved to protect the subvalvular apparatus.
Exclusion criteria were a simultaneous coronary artery bypass grafting (CABG) or an aortic valve replacement. Of note, all patients with biological valves routinely receive acetylsalicylic acid (ASA) 100 mg daily and patients with mechanical valves and/or atrial fibrillation received VKA.
Data and outcomes
In patients who had undergone an MVR, we checked electronic medical records (inpatient and outpatient notes and the results of any diagnostic testing). Clinical variables of interest were patient age, sex, comorbidity, prior cardiological interventions, echocardiography (pre-operative transthoracic echocardiogram [TTE], perioperative transoesophageal echocardiogram, one-week post-operative and upon follow-up) and relevant medical/surgical history. Follow-up data collected at the patient’s last follow-up hospital or outpatient visit were valve-related complications and echocardiography parameters.
Statistics
Data were analysed using descriptive statistics, with categorical variables presented as absolute values and frequencies (%) and normally distributed continuous variables as means with standard deviations (SDs). Non-normally distributed continuous variables are presented as the median and interquartile range (IQR with the borders for the first and third quartile). Comparisons between biological and mechanical valve groups were carried out using a Student’s T-test with Levine’s homogeneity of variance or the Mann-Whitney U-test for continuous variables, as appropriate, and a Fisher’s exact or Chi-square test for categorical variables. For outcome analyses odds ratios (OR) were calculated by logistic regression. Survival analysis was presented as Kaplan-Meier (KM) curve. Hazard ratios (HR) were calculated by Cox-regression and adjusted for major baseline variables. Freedom from major complications (embolism/stroke and bleeding) were displayed as KM curves. For patients without a documented time-point for the documented event (embolism/stroke or bleeding), the time to death or the time of the last follow-up visit was used and divided by two for the KM curves. In all cases, a two-tailed P-value of < 0.05 was considered statistically significant. All statistical tests were performed using IBM SPSS Statistics software version 24.0 (Armonk, NY. IBM Corporation).
Results
Between 2005 and 2015, 1357 patients received MV surgery at the Kerckhoff-Heart Center, including 324 patients receiving MVR. Of these patients, 265 patients received a biological valve and 59 a mechanical valve (Fig. 1).
Fig. 1.
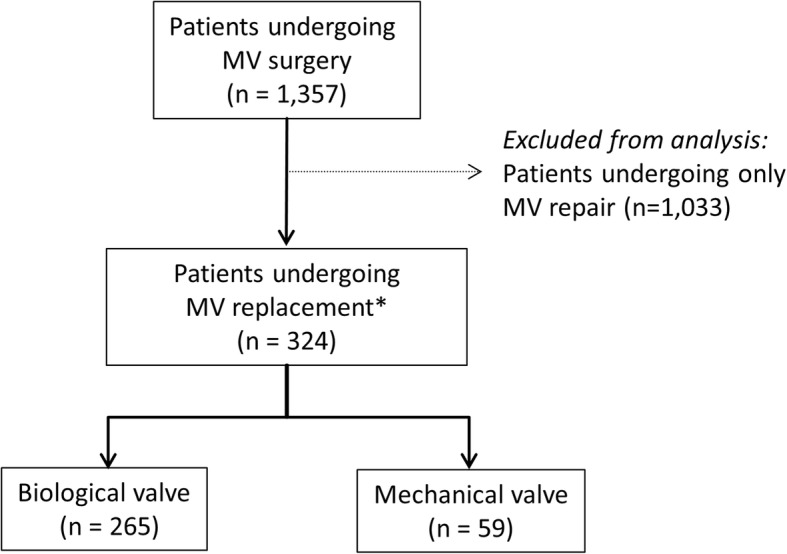
Flow Chart. Legend: MV, mitral valve. *123 patients underwent MV replacement after a MV repair was attempted
Patient characteristics
Patient characteristics, MV pathologies and echocardiographic parameters are presented in Tables 1 and 2. Overall, 49.7% of patients were female and 38.9% had atrial fibrillation. The majority of patients had degenerative MV disease (96.0%) with a dilated annulus (71.3%) and MV insufficiency grade ≥ II (96.6%). 28.7% of the patients presented with MV stenosis and 14.5% suffered from acute endocarditis. Patients receiving biological valves were older (71.0 vs. 56.0 years; p < 0.001) and had a significantly higher median log EuroSCORE I (8.4% vs. 4.5%; p < 0.001).
Table 1.
Patient characteristics
| Total N = 324 |
Biological MVR N = 265 |
Mechanical MVR N = 59 |
p-value | |
|---|---|---|---|---|
| Age in years | 69.0 [58.0–76.0] [324] | 71.0 [62.0–77.0] [265] | 56.0 [50.0–62.0] [59] | < 0.001 |
| ≤ 65 years, % | 133/324 (41.0) | 86/265 (32.5) | 47/59 (79.7) | < 0.001 |
| Female gender, % | 161/324 (49.7) | 137/265 (51.7) | 24/59 (40.7) | 0.126 |
| BMI (kg/m2) | 26.2 [23.2–29.0] [256] | 26.2 [23.0–28.9] [214] | 26.9 [23.2–31.7] [42] | 0.337 |
| CV risk factors | ||||
| Hypertension, % | 173/324 (53.4) | 147/265 (55.5) | 26/59 (44.1) | 0.112 |
| Dyslipidemia, % | 52/324 (16.0) | 46/265 (17.4) | 6/59 (10.2) | 0.174 |
| Comorbidity general | ||||
| Diabetes mellitus, % | 35/324 (10.8) | 31/265 (11.7) | 4/59 (6.8) | 0.271 |
| Kidney failure (Crea. > 2.26 mg/dL) | 8/324 (2.5) | 7/265 (2.6) | 1/59 (1.7) | 1.000 |
| Stroke, % | 30/324 (9.3) | 27/265 (10.2) | 3/59 (5.1) | 0.221 |
| COPD, % | 44/324 (13.6) | 39/265 (14.7) | 5/59 (8.5) | 0.206 |
| PAD, % | 17/324 (5.2) | 16/265 (6.0) | 1/59 (1.7) | 0.328 |
| Comorbidity cardiac | ||||
| Atrial fibrillation, % | 126/324 (38.9) | 102/265 (38.5) | 24/59 (40.7) | 0.755 |
| Coronary artery disease, % | 45/324 (13.9) | 41/265 (15.5) | 4/59 (6.8) | 0.081 |
| Prior MI (≤90 days), % | 4/324 (1.2) | 3/265 (1.1) | 1/59 (1.7) | 0.554 |
| Prior aortic valve replacement, % | 15/324 (4.6) | 11/265 (4.2) | 4/59 (6.8) | 0.489 |
| Prior CABG, % | 25/324 (7.7) | 23/265 (8.7) | 2/59 (3.4) | 0.277 |
| Prior pacemaker, % | 13/324 (4.0) | 11/265 (4.2) | 2/59 (3.4) | 1.000 |
| NYHA class III/IV, % | 273/324 (84.3) | 225/265 (84.9) | 48/59 (81.4) | 0.498 |
| CCS class III %a | 16/324 (4.9) | 12/265 (4.5) | 4/59 (6.8) | 0.505 |
| Pulmonary hypertension, % | 56/324 (17.3) | 47/265 (17.7) | 9/59 (15.3) | 0.648 |
| Emergency indication for surgery, % | 31/324 (9.6) | 23/265 (8.7) | 8/59 (13.6) | 0.249 |
| Cardiac decompensation, % | 112/324 (34.6) | 95/265 (35.8) | 17/59 (28.8) | 0.304 |
| Log EuroSCORE I (%) | 7.2 [3.5–15.6] [322] | 8.4 [3.6–17.5] [263] | 4.5 [2.9–8.0] [59] | < 0.001 |
Legend: Values are patients applicable/patients with available information with percentage in brackets OR medians with IQR in brackets and available patient number in brackets; aNo patient presented with CCS class IV in this collective
BMI body mass index, CV cardiovascular, CABG coronary artery bypass graft, CCS Canadian Cardiovascular Society, COPD chronic obstructive pulmonary disease, MI myocardial infarction, NYHA,New York Heart Association, PAD peripheral artery disease, SD standard deviation
Table 2.
MV pathologies and echocardiographic parameters
| Total N = 324 |
Biological MVR N = 265 |
Mechanical MVR N = 59 |
p-value | |
|---|---|---|---|---|
| MV pathologies | 0.712 | |||
| Functional, % | 13/324 (4.0) | 10/265 (3.8) | 3/59 (5.1) | |
| Degenerative, % | 311/324 (96.0) | 255/265 (96.2) | 56/59 (94.9) | |
| Acute endocarditis, % | 47/324 (14.5) | 37/265 (14.0) | 10/59 (16.9) | 0.556 |
| MV stenosis, % | 93/324 (28.7) | 71/265 (26.8) | 22/59 (37.3) | 0.107 |
| Annulus dilatation, % | 231/324 (71.3) | 189/265 (71.3) | 42/59 (71.2) | 0.984 |
| Annulus calcification, % | 91/324 (28.1) | 72/265 (27.2) | 19/59 (32.2) | 0.437 |
| AML prolapse, % | 78/324 (24.1) | 69/265 (23.0) | 17/59 (28.8) | 0.346 |
| AML flail, % | 35/324 (10.8) | 27/265 (10.2) | 8/59 (13.6) | 0.395 |
| PML prolapse, % | 119/324 (36.7) | 99/265 (37.4) | 20/59 (33.9) | 0.618 |
| PML flail, % | 83/324 (25.6) | 72/265 (27.2) | 11/59 (18.6) | 0.175 |
| Chordae elongation, % | 54/324 (16.7) | 42/265 (15.8) | 12/59 (20.3) | 0.403 |
| Restrictive leaflet, % | 157/324 (48.5) | 126/265 (47.5) | 31/59 (52.5) | 0.487 |
| MV insuff. Grade ≥ IIa, % | 313/324 (96.6) | 260/265 (98.1) | 53/59 (89.8) | 0.006 |
| Echocardiographic parameters | ||||
| LVEF, % | 56.0 [50.0–60.0] [324] | 58.0 [50.0–60.0] [265] | 55.0 [48.3–60.0] [59] | 0.292 |
| LVEDD (mm) | 54.0 [49.0–59.0] [214] | 54.0 [49.0–59.0] [200] | 54.0 [50.0–57.0] [49] | 0.950 |
| LVESD (mm) | 35.0 [31.0–40.5] [237] | 35.0 [31.0–41.0] [191] | 35.0 [32.0–40.0] [46] | 0.444 |
| Left atrium (mm) | 55.0 ± 10.5 [251] | 54.5 ± 10.2 [203] | 57.2 ± 11.9 [48] | 0.107 |
| Right atrium (mm) | 45.0 [38.0–53.0] [251] | 45.0 [38.0–53.0] [203] | 48.5 [39.3–54.5] [48] | 0.318 |
| Mitral opening (mm) | 3.4 ± 1.8 [93] | 3.4 ± 1.6 [79] | 3.3 ± 2.6 [14] | 0.966 |
| PISA radius (mm) | 1.0 [1.0–1.2] [56] | 1.0 [1.0–1.2] [46] | 1.0 [0.9–1.1] [10] | 0.426 |
| Vena contracta (mm) | 6.0 [5.0–7.0] [90] | 6.0 [4.0–7.0] [78] | 6.0 [5.0–7.0] [12] | 0.717 |
Legend: Values are patients applicable/patients with available information with percentage in brackets OR means ± SD with available patient numbers in brackets OR medians with IQR in brackets and available patient number in brackets; a patients with MR grade < II initially underwent mitral valve repair and, in cases where the result was not satisfactory, underwent mitral valve replacement
AML anterior mitral valve leaflet, LVEDD left ventricular end diastolic pressure, LVEF left ventricular ejection fraction, LVESD left ventricular end systolic pressure, MV mitral valve, PISA proximal isovelocity surface area, PML posterior mitral valve leaflet, SD standard deviation
Procedural details and outcomes
Patients in the biological valve group received less minimally invasive MV surgery (MIC; 37.4% vs. 61.0%; p = 0.001) and more conventional sternotomy (CS) (Table 3). While the operating time was comparable between the two groups there was a shorter cardiopulmonary bypass (CPB) time in the biological valve group (p = 0.030). Hospital stay was lower in the biological valve group (Table 3) (p = 0.012). Noteworthy was a higher need for secondary MV repair (11.9 vs. 0.8%; p < 0.001) in the mechanical valve group.
Table 3.
Procedural details
| Total N = 324 |
Biological MVR N = 265 |
Mechanical MVR N = 59 |
p-value | |
|---|---|---|---|---|
| Operative approach | 0.001 | |||
| MIC, % | 135/324 (41.7) | 99/265 (37.4) | 36/59 (61.0) | |
| CS, % | 189/324 (58.3) | 166/265 (62.6) | 23/59 (39.0) | |
| MV size | 0.652 | |||
| 25 mm, % | 2/324 (0.6) | 2/265 (0.8) | – | |
| 26 mm, % | 1/324 (0.3) | 1/265 (0.4) | – | |
| 27 mm, % | 45/324 (13.9) | 40/265 (15.1) | 5/59 (8.5) | |
| 29 mm, % | 99/324 (30.6) | 82/265 (30.9) | 17/59 (28.8) | |
| 31 mm, % | 128/324 (39.5) | 100/265 (37.7) | 28/59 (47.5) | |
| 33 mm, % | 49/324 (15.1) | 40/265 (15.1) | 9/59 (15.3) | |
| Times | ||||
| Procedure time (min) | 196.5 [166.3–240.0] [324] | 197.0 [165.5–239.0] [265] | 192.0 [170.0–258.0] [59] | 0.539 |
| CPB time (min) | 117.0 [166.3–146.8] [324] | 114.0 [94.0–145.0] [265] | 126.0 [105.0–154.0] [59] | 0.030 |
| x-clamp time (min) | 72.0 [59.0–92.8] [324] | 71.0 [58.0–91.0] [265] | 74.0 [62.0–99.0] [59] | 0.235 |
| Length of intubation (h) | 12.0 [9.0–18.0] [324] | 12.0 [9.0–18.0] [265] | 11.0 [8.0–17.0] [59] | 0.230 |
| Length of ICU (h) | 34.5 [22.0–75.8] [324] | 34.0 [22.0–72.0] [265] | 47.0 [23.0–108.0] [59] | 0.095 |
| Length of hospital stay (d) | 12.0 [9.0–17.0] [324] | 11.0 [9.0–17.0] [265] | 14.0 [11.0–19.0] [59] | 0.012 |
| Concomitant procedures | ||||
| Cryo ablation, % | 82/323 (25.4) | 65/265 (24.5) | 17/58 (29.3) | 0.448 |
| LAA closure, % | 88/324 (27.2) | 74/265 (27.9) | 14/59 (23.7) | 0.512 |
| Tricuspid valve repair, % | 63/323 (19.5) | 53/264 (20.1) | 10/59 (16.9) | 0.584 |
| PFO closure, % | 13/324 (4.0) | 10/265 (3.8) | 3/59 (5.1) | 0.712 |
| ASD closure, % | 6/324 (1.9) | 5/265 (1.9) | 1/59 (1.7) | 1.000 |
| Second MV repair, % | 9/324 (2.8) | 2/265 (0.8) | 7/59 (11.9) | < 0.001 |
| Conversion to CSa, % | 9/137 (6.6) | 9/101 (8.9) | 0/36 (0) | 0.112 |
Legend: Values are patients applicable/patients with available information with percentage in brackets OR medians with IQR in brackets and available patient number in brackets; aReasons for conversion were severe forms of trichterbrust, severely elevated diaphragm or severe adhesion of the right pleura, as well as severe intraoperative bleeding
ASD atrial septum defect, CPB cardiopulmonary bypass, CS conventional sternotomy, ICU intensive care unit, LAA left atrial appendage, MIC minimally invasive mitral valve surgery, MV mitral valve, PFO patent foramen ovale, SD standard deviation
Frequent procedure related complications after MVR were atrial fibrillation (21.0%) and pneumonia (9.9%) (Table 4). There were no statistically significant differences between groups. Immediate procedural mortality (1.9%) was only seen in patients undergoing biological valve replacement.
Table 4.
Procedure-related complications
| Biological MVR N = 265 |
Mechanical MVR N = 59 |
Odds Ratio 95% CI |
Adjusted Odds Ratioa 95% CI |
|
|---|---|---|---|---|
| Wound infection, % | 9/265 (3.4) | 1/59 (1.7) | 2.04 (0.25–16.41) | 1.01 (0.11–9.77) |
| Pericardial tamponade, % | 21/265 (7.9) | 5/59 (8.5) | 0.93 (0.34–2.58) | 0.67 (0.22–2.05) |
| AV block grade III, % | 22/265 (8.3) | 5/59 (8.5) | 0.98 (0.35–2.70) | 0.57 (0.18–1.75) |
| Pneumonia, % | 27/265 (10.2) | 5/59 (8.5) | 1.23 (0.45–3.33) | 1.02 (0.35–2.97) |
| Pneumothorax, % | 2/265 (0.8) | 2/59 (3.4) | 0.22 (0.03–1.57) | 0.22 (0.02–1.98) |
| Pleural effusion, % | 12/265 (4.5) | 1/59 (1.7) | 1.59 (0.19–13.44) | 1.62 (0.18–14.45) |
| Atrial fibrillation, % | 59/265 (22.3) | 9/59 (15.3) | 1.60 (0.74–3.44) | 1.09 (0.47–2.51) |
| MVI ≥ II post OP, % | 6/264 (2.3) | 1/59 (1.7) | 1.35 (0.16–11.42) | 1.86 (0.20–17.31) |
| Immediate 72 h procedural mortality, % | 6/265 (2.3) | 0/59 (0) | n.a. | n.a. |
Legend: Values are patients applicable/patients with available information with percentage in brackets
AV atrioventricular, MVI mitral valve insufficiency, n.a. not applicable
aOdds Ratios were calculated by logistic regression and adjusted for age, logistic EuroScore -I, CAD and MV insuff. Grade ≥ II (preOP)
Echocardiography data
The mean post-operative diastolic MV gradient was 5.0 mmHg for all patients and patients receiving a biological valve, but 4.0 mmHg for patients receiving mechanical valves (p = 0.833). After a mean follow-up of 6.4 years, the difference between the groups became significant (p = 0.018). The recorded peak diastolic MV gradient was 13.0 mmHg in the biological valve group and 9.5 mmHg in the mechanical valve group (p = 0.117), after a mean follow-up of 5.7 years.
Follow-up
At 30 days, 9.0% of all patients had died, 4.0% experienced stroke, 10.5% acute renal failure, 8.0% received a pacemaker and 14.2% had to undergo re-thoracotomy (Table 5). OR between groups were, however, not statistically different for either the unadjusted and adjusted analyses. Re-thoracotomies had the highest absolute difference of 9.5% (12.5% vs. 22.0%; adjusted OR 0.45, 95% CI 0.20–1.00; p = 0.050).
Table 5.
30-day Outcomes
| Biological MVR N = 265 |
Mechanical MVR N = 59 |
Odds Ratio 95% CI |
Adjusted Odds Ratioa 95% CI |
|
|---|---|---|---|---|
| Death, % | 23/265 (8.7) | 6/59 (10.2) | 0.84 (0.33–2.16) | 0.50 (0.17–1.52) |
| Cardiac death, % | 15/265 (5.7) | 4/59 (6.8) | 0.88 (0.28–2.75) | 0.66 (0.18–2.42) |
| Non-cardiac death, % | 8/265 (3.0) | 2/59 (3.4) | 0.89 (0.18–4.29) | 0.37 (0.06–2.32) |
| Stroke, % | 11/265 (4.2) | 2/59 (3.4) | 1.23 (0.27–5.72) | 0.47 (0.08–2.66) |
| Acute renal failure, % | 27/265 (10.2) | 7/59 (11.9) | 0.85 (0.35–2.05) | 0.45 (0.16–1.24) |
| Myocardial infarction, % | 0/265 (0) | 2/59 (3.4) | n.a. | n.a. |
| Pacemaker implantation, % | 20/264 (7.6) | 6/59 (10.2) | 0.45 (0.16–1.29) | 0.72 (0.28–1.89) |
| Re-thoracotomy, % | 33/265 (12.5) | 13/59 (22.0) | 0.50 (0.25–1.03) | 0.45 (0.20–1.00) |
Legend: Values are patients applicable/patients with available information with percentage in brackets
n.a. not applicable
aOdds ratios were calculated by logistic regression and adjusted for age, logistic EuroScore-I, CAD and MV insuff. ≥ grade II (preOP)
The most frequent major complication was embolism, which occurred in 9.2% (19/229) of patients (9.2% [17/184] vs. 4.4% [2/45] in the biological and mechanical valve groups, respectively; p = 0.381) and mostly affected the brain. Bleeding complications occurred in 4.8% (11/229) of patients, but were significantly higher in the mechanical valve group (13.3% [6/45] vs. 2.7% [5/184], respectively; p = 0.009) (Additional file 1: Table S1). In Additional file 2: Figure S1 the occurrences of these two complications over time are shown as KM curves.
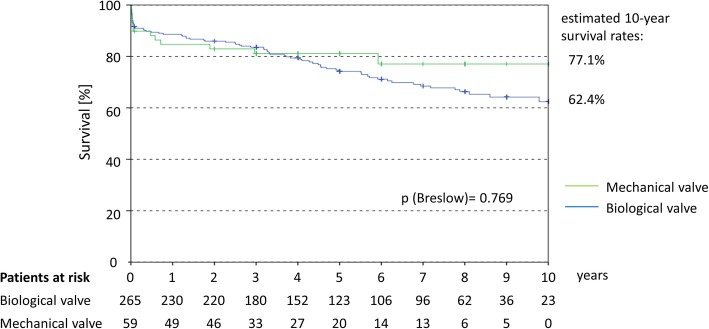
In the overall follow-up period of up to 10 years, we found that during the first post-treatment years patients receiving a biological MV had a nominally better survival rate. 3 years post-treatment, this shifted nominally in favour of patients receiving mechanical valves. Survival rates were 62.4% for biological and 77.1% for mechanical valves at 10 years (p = 0.769). Differences were not statistically significant at any time point (Fig. 2). The HR was 1.264 (95% CI 0.687–2.325; p = 0.451). After adjustment for key baseline variables (age, CAD, MV regurgitation grade ≥ II, logistic EuroScore-I) no significant statistical difference between biological and mechanical valves was found (HR 0.833 (95% CI 0.430–1.615); p = 0.589).
Fig. 2.
Kaplan–Meier curve for the long-term survival after MVR. Legend: HR calculated by COX regression was 1.264 (95% CI 0.687–2.325; p = 0.451) After adjustment for age, logistic EuroScore-I, CAD and MV regurgitation grade ≥ II (preOP) the HR was 0.833 (95% CI 0.430–1.615; p = 0.589) in favour for biological valves
Discussion
This single centre study provides a contemporary picture of the safety and effectiveness of the two valve concepts. Event rates at 30 days may appear slightly high and we consider this the result of our clinic getting referrals of very complex cases that may result in longer procedural times and hazards. Furthermore our clinic is frequently chosen for re-interventions after failed procedures. Our study revealed a success rate of MVR that is quite comparable between valves, but confirmed that biological valves were used in older patients, while mechanical valves were used in younger patients and associated with an increased risk of bleeding.
Both types of valves have advantages and disadvantages, but also specific patient factors need to be taken into consideration when making the decision on which valve type to use [9, 10]. Historically, biological MVs were generally considered to have superior antithrombotic properties but lacked durability, while mechanical valves were thought to be more durable but were associated with thromboembolism and bleeding events [2, 5, 6, 9]. The data from our study confirm the observation that the use of anticoagulation along with mechanical valve implantation is associated with an increased risk of bleeding and that there is a potential survival benefit for patients receiving a biological MV.
Earlier studies report that mechanical valves were associated with increased durability [2, 5, 6]. The KM data confirm that at 10 years patient survival is higher with the mechanical valve than the biological valve (77.1% vs. 62.4%, respectively), despite survival being nominally higher with the biological valve within the first years of follow-up. Our Cox regression analysis revealed that after adjustment for key baseline variables there was no significant difference between the two valve types (HR 0.833; 95% CI 0.430–1.615; p = 0.589). Further information is needed to determine if the durability of the mechanical and biological valve affects patient survival because the biological valve recipients were 15 years older in our dataset. In addition, biological valves have been associated with an increased risk of re-operation and structural valve deterioration, which may start to occur at 3 years but, on the other hand, a durability of 12-plus years has also been reported [11–15].
Patient-specific factors, including age, surgical factors, comorbidities and patient preference, also influence the choice of valve type [9, 16]. With respect to the patient’s age, the general recommendations are that patients younger than 65 years should receive a mechanical valve because of their increased durability, while patients older than 65 years should be considered for a biological valve, as they are less likely to outlive the valve’s life expectancy [9, 17, 18]. Our study mirrors this approach, with the median age of patients in the mechanical valve group being 15 years lower. Comorbidities, such as atrial fibrillation, renal failure and diabetes, and surgical factors, such as the need for concurrent aortic root replacement, also affect valve selection [9, 19], although we did not observe any statistically significant difference in the rate of these comorbidities.
In the future, improvements in mechanical valve structure may lower the risk of thromboembolism thus potentially reducing the intensity of lifelong anticoagulation, which may result in a preference for these valve types [20]. Furthermore, newer oral anticoagulants may also make mechanical valves more attractive from both the patient’s perspective and from a medical standpoint [21].
Limitations
Overall, 324 patients were included in this study. The majority of these study patients received a biological valve (n = 265) and only 59 patients received a mechanical valve. Furthermore, patients receiving mechanical valve were typically younger than those receiving biological valve. Finally, there is an evolution of surgical techniques over time. As such, we adjusted the outcomes for differences in baseline variables to overcome this limitation. Data on major complications and echo data, collected at the patient’s last follow-up visit, were not available for some patients as they only recently received their implant. The data, however, provide a useful insight into post-procedural major complications and echocardiographic data.
Conclusions
Despite a significant passage of time since MVR was first performed, many of the findings remain the same – biological valves tend to be implanted in older patients while mechanical valves are preferred in younger patients but associated with a higher risk of bleeding. The estimated 10-year survival rate tended to be higher in patients receiving mechanical valves; but adjusted Cox regression analysis showed no significant difference between the two valve types. It appears, therefore, as if valve selection may not be as important for patient survival as prior data suggested.
Additional files
Table S1. Major complications (embolism/stroke and bleeding) and their localisation. Legend: Values are patients applicable/patients with available information with the percentage in brackets. MI, myocardial infarction. (DOCX 12 kb)
Figure S1. Kaplan–Meier curves for embolism/stroke (A) and bleeding (B) complications. (TIF 1692 kb)
Acknowledgements
Not applicable.
Abbreviations
- adj
Adjusted
- AHA
American Heart Association
- AML
Anterior mitral valve leaflet
- ASA
Acetylsalicylic acid
- ASD
Atrial septal defect
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CCS
Canadian Cardiovascular Society
- COPD
Chronic obstructive pulmonary disease
- CPB
Cardio-pulmonary bypass
- CS
Conventional sternotomy
- CV
Cardiovascular
- EACTS
European Association for Cardio-Thoracic Surgery
- ESC
European Society of Cardiology
- HR
Hazard ratio
- ICU
intensive care unit
- IQR
Interquartile range
- KM
Kaplan–Meier
- LAA
Left atrial appendage
- LV
Left ventricular
- LVEDD
Left ventricular enddiastolic pressure
- LVEF
Left ventricular ejection fraction
- LVESD
Left ventricular endsystolic pressure
- MI
Myocardial infarction
- MV
Mitral valve
- MVI
Mitral valve insufficiency
- MVR
Mitral valve replacement
- MVRep
Mitral valve repair
- NYHA
New York Heart Association
- OR
Odds ratio
- PAD
Peripheal artery disease
- PFO
Patent foramen ovale
- PISA
Proximal isovelocity surface area
- PML
Posterior mitral valve leaflet
- SD
Standard deviation
- TEE
Transoesophageal echocardiography
Authors’ contributions
AC, SH, MS, MD and MR performed the surgery and collected the data. AC, KB, PB and JP worked on the datset, designed the analyses and developed the concept of the paper. AC and PB drafted the manuscript which was critically revised by SH, KB, MS, MD, JP and MR. All authors approved the final version of the manuscript and can be held accountable for the integrity of the work.
Funding
This work was supported by funding from Edwards Lifesciences.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the site’s Ethical Committee and complied with the Declaration of Helsinki and its amendments. Given the use of anonymised data already collected as part of routine diagnosis and treatment, written informed consent was neither feasible nor required.
Consent for publication
Not applicable.
Competing interests
Peter Bramlage received research funding from Edwards Lifesciences related and unrelated to the present work. The other authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayse Cetinkaya, Email: a.cetinkaya@kerckhoff-klinik.de.
Julia Poggenpohl, Email: Julia.poggenpohl@web.de.
Karin Bramlage, Email: karin.bramlage@ippmed.de.
Stefan Hein, Email: s.hein@kerckhoff-klinik.de.
Mirko Doss, Email: m.doss@kerckhoff-klinik.de.
Peter Bramlage, Phone: +49 3379 3147890, Email: peter.bramlage@ippmed.de.
Markus Schönburg, Email: m.schoenburg@kerckhoff-klinik.de.
Manfred Richter, Email: m.richter@kerckhoff-klinik.de.
References
- 1.Ribeiro AH, Wender OC, de Almeida AS, Soares LE, Picon PD. Comparison of clinical outcomes in patients undergoing mitral valve replacement with mechanical or biological substitutes: a 20 years cohort. BMC Cardiovasc Disord. 2014;14:146. doi: 10.1186/1471-2261-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1e95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 5.Yao H, Miyamoto T, Mukai S, Yamamura M, Tanaka H, Nakagawa T, et al. Long-term results of mitral valve replacement: biological xenograft versus mechanical valves. J Artif Organs. 2003;6:30–36. doi: 10.1007/s100470300005. [DOI] [PubMed] [Google Scholar]
- 6.Iung B, Rodes-Cabau J. The optimal management of anti-thrombotic therapy after valve replacement: certainties and uncertainties. Eur Heart J. 2014;35:2942–2949. doi: 10.1093/eurheartj/ehu365. [DOI] [PubMed] [Google Scholar]
- 7.Demirag M, Kirali K, Omeroglu SN, Mansuroglu D, Akinci E, Ipek G, et al. Mechanical versus biological valve prosthesis in the mitral position: a 10-year follow up of St. Jude Medical and Biocor valves. J Heart Valve Dis. 2001;10:78–83. [PubMed] [Google Scholar]
- 8.Hammond GL, Geha AS, Kopf GS, Hashim SW. Biological versus mechanical valves. Analysis of 1,116 valves inserted in 1,012 adult patients with a 4,818 patient-year and a 5,327 valve-year follow-up. J Thorac Cardiovasc Surg. 1987;93:182–198. [PubMed] [Google Scholar]
- 9.Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence. 2011;5:91–99. doi: 10.2147/PPA.S16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fino C, Iacovoni A, Pibarot P, Pepper JR, Ferrero P, Merlo M, et al. Exercise hemodynamic and functional capacity after mitral valve replacement in patients with ischemic mitral regurgitation: a comparison of mechanical versus biological prostheses. Circ Heart Fail. 2018;11:e004056. doi: 10.1161/CIRCHEARTFAILURE.117.004056. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Cohn LH, Aranki SF. Tissue valve is the preferred option for patients aged 60 and older. Circulation. 2013;128:1365–1371. doi: 10.1161/CIRCULATIONAHA.113.002584. [DOI] [PubMed] [Google Scholar]
- 12.Poirer NC, Pelletier LC, Pellerin M, Carrier M. 15-year experience with the Carpentier-Edwards pericardial bioprosthesis. Ann Thorac Surg. 1998;66:S57–S61. doi: 10.1016/S0003-4975(98)01110-2. [DOI] [PubMed] [Google Scholar]
- 13.Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124:146–154. doi: 10.1067/mtc.2002.121672. [DOI] [PubMed] [Google Scholar]
- 14.Banbury MK, Cosgrove DM, 3rd, White JA, Blackstone EH, Frater RW, Okies JE. Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2001;72:753–757. doi: 10.1016/S0003-4975(01)02992-7. [DOI] [PubMed] [Google Scholar]
- 15.McClure RS, Narayanasamy N, Wiegerinck E, Lipsitz S, Maloney A, Byrne JG, et al. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010;89:1410–1416. doi: 10.1016/j.athoracsur.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Manghelli JL, Carter DI, Khiabani AJ, Gauthier JM, Moon MR, Munfakh NA, et al. A 20-year multicenter analysis of dialysis-dependent patients who had aortic or mitral valve replacement: implications for valve selection. J Thorac Cardiovasc Surg. 2018. 10.1016/j.jtcvs.2018.10.168. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 17.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the veterans affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/S0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 18.Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55:2413–2426. doi: 10.1016/j.jacc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 19.de Vincentiis C, Kunkl AB, Trimarchi S, Gagliardotto P, Frigiola A, Menicanti L, et al. Aortic valve replacement in octogenarians: is biologic valve the unique solution? Ann Thorac Surg. 2008;85:1296–1301. doi: 10.1016/j.athoracsur.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 20.van Geldorp MW, Eric Jamieson WR, Kappetein AP, Ye J, Fradet GJ, Eijkemans MJ, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881–886. doi: 10.1016/j.jtcvs.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Borris LC. Rivaroxaban and dabigatran etexilate: two new oral anticoagulants for extended postoperative prevention of venous thromboembolism after elective total hip arthroplasty. Arch Orthop Trauma Surg. 2010;130:583–589. doi: 10.1007/s00402-009-0930-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Major complications (embolism/stroke and bleeding) and their localisation. Legend: Values are patients applicable/patients with available information with the percentage in brackets. MI, myocardial infarction. (DOCX 12 kb)
Figure S1. Kaplan–Meier curves for embolism/stroke (A) and bleeding (B) complications. (TIF 1692 kb)
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.