Abstract
Turning is common in daily activity and requires rapid, coordinated reorientation of the head, trunk, and pelvis toward the new direction of travel. Yet, turning gait has not been well explored in populations with mild traumatic brain injury (mTBI) who may alter their turning behavior according to self-perceived symptoms or motor dysfunction. The purpose of this study was to examine turning velocities and coordination in adults with chronic mTBI (>3 months post-injury and still reporting balance complaints) during a task simulating everyday ambulation. We hypothesized that individuals with chronic mTBI would reduce their angular velocity when turning and increase the variability of head-pelvis coordination compared with controls, and that the reduction in velocity and increased variability would be associated with their self-reported symptom score. Forty-two adults (14 chronic mTBI, 28 controls) completed the Neurobehavioral Symptom Inventory before walking 12 laps around a marked course containing two 45-degree turns, four 90-degree turns, and two 135-degree turns. Inertial sensors collected angular velocities of the head and pelvis. After adjusting for covariates, participants with chronic mTBI had significantly slower lap times and peak angular velocities of the pelvis (p < 0.01) compared with the control group. The peak velocity timing (PVT) between peak velocities of the head and pelvis, and the variability of that timing was significantly greater in participants with chronic mTBI (p < 0.01). Within the chronic mTBI group, somatosensory symptoms were associated with slower angular velocities of the head and pelvis (p = 0.03) and increased PVT variability (p < 0.01). The results suggest individuals with chronic mTBI with worse somatic symptoms have impaired head stabilization during turning in situations similar to everyday life. These results encourage future research on turning gait to examine the causal relationship between symptoms and daily locomotor function in adults with chronic mTBI.
Keywords: brain injury, gait, head stabilization, inertial sensors, turning
Introduction
Abnormal gait has been well documented in individuals following sport-related concussion or nonsporting mild traumatic brain injury (mTBI). Slower gait speeds,1 altered gait termination strategies,2 and larger mediolateral sway3,4 have been reported in recently concussed individuals during straight walking tasks. However, daily activities are not confined to straight paths. People perform between 800 and 1000 turns per day,5 and 35–45% of daily steps are not straight.6
Only two small-scale studies have examined turning specifically in individuals with sport-related concussion or nonsporting mTBI to date. Powers and associates7 found concussed individuals had more variability in the onset of turning across body segments during a light-induced turning task and that the individuals continued to demonstrate increased reorientation variability after being medically cleared for athletic competition. A later study by Fino and colleagues8 found turning kinematics were abnormal in athletes after sport-related concussion, including less whole-body roll into the turn and lower path curvatures, and that these abnormal kinematics gradually returned to normal over the course of one year. Both studies provided preliminary evidence that turning may be impaired after sport-related concussions or in nonsport-related mTBIs. However, these studies involved highly specific turns: light-induced turns to 45 degrees or 60 degrees, and 90-degree turns around a pylon, which may not be highly representative of the pre-planned turns and various turning angles used in everyday ambulation.6
Turning and change of direction tasks involve the rapid, coordinated reorientation of the head, trunk, and pelvis.9 During turning, dynamic shifts in the body-sensed gravitoinertial acceleration reference frame10 and asymmetrical loadings11 alter vestibular and proprioceptive sensory information, requiring dynamic reweighting of sensorimotor information and sophisticated oculomotor reflexes to stabilize spatial information.10 Similar rapid movements can exacerbate self-reported symptoms,12 suggesting that individuals with persistent symptomology may limit their reorientation velocity and avoid turning at fast speeds to minimize the dynamic shifts in sensory information.
To date, no study has examined turning in individuals with mTBI and persistent symptomology, referred to here as chronic mTBI, despite the high percentage of adults with mTBI (11–64%) who will experience prolonged self-reported symptoms lasting longer than three months.13 While persistent symptoms have a significant, detrimental influence on the quality of life and community integration,14–16 measures of gait and balance have not been consistently associated with self-reported symptoms,17,18 and it is unclear how symptoms impact mobility. Yet, turning performance may be particularly abnormal in persons with chronic mTBI if self-reported symptoms are associated with turning gait.
In this study of a preliminary subset of participants recruited for a larger study,19 we examined (1) the turning characteristics of individuals with chronic mTBI and controls and (2) the association between turning characteristics and self-reported symptomology in those with chronic mTBI. We hypothesized that persons with chronic mTBI would exhibit slower reorientation velocity and increased variability of segmental coordination compared with healthy controls and that such deficits would be associated with their self-reported symptom score. In addition, we hypothesized that vestibular-related symptoms (including dizziness, coordination, and imbalance) would be most strongly related to turning performance given the complex coordination and shift in gravitoinertial information during turning. Finally, we hypothesized that individuals with chronic mTBI, in an effort to limit the exacerbation of symptoms, would avoid increasing the turning velocity when asked to walk at a faster speed.
Methods
Participants
Participants were recruited through posted flyers in athletic facilities, physical therapy clinics, hospitals, concussion clinics, community notice boards, and cafes around the Portland, Oregon metropolitan area. All participants with chronic mTBI had self-reported complaints of imbalance as determined from a nonzero symptom score for balance problems on the SCAT3 symptom inventory20 and a diagnosed mTBI >3 months before the testing session. For the purposes of this study, mTBI was defined and classified using the criteria from the United States Department of Defense: no CT scan, or a normal CT scan if obtained, loss of consciousness not exceeding 30 min, alteration of consciousness/mental state up to 24 h, and post-traumatic amnesia not exceeding one day.21 The mechanism of injury was not restricted. All diagnoses of mTBI were confirmed by an Oregon Health & Science University (OHSU) physician.
Inclusion and exclusion criteria were defined by Fino and coworkers.19 To be included in the study, participants (1) were between 21 and 50 years old, (2) had minimal cognitive impairment as assessed by the Short Blessed test, and (3) had either a diagnosis of mTBI with persisting symptoms for more than three months post-injury for the chronic mTBI group or no history of mTBI or brain injury within the past year for the control group. Exclusion criteria included: any other neurological illness or major surgical procedure that could explain balance deficits; moderate to severe substance abuse; significant pain during testing; pregnancy; history of balance complaints, peripheral vestibular pathology, or oculomotor deficits before the mTBI; hearing loss no worse than 60 dB HL (PTA 0.5–3 kHz); or inability to abstain from medications that might impair balance. All participants were required to abstain from medications that could impair their balance or gait for at least 24 h before testing, including sedating antihistamines, benzodiazepines, sedatives, narcotic pain medications, and alcohol. Inclusion and exclusion criteria were evaluated by research assistants over the phone or in-person before enrolling participants. Recruitment procedures and experimental protocols were approved by the OHSU and Veterans Affairs Portland Health Care System (VAPORHCS) joint Institutional Review Board.
Procedures
The full protocol for this study is described by Fino and coworkers19; specific components pertaining to the present study are presented here in further detail.
Symptoms were assessed at the beginning of testing using the Neurobehavioral Symptom Inventory (NSI). The NSI total symptom score was divided into four subscores based on the domain of the reported symptom: somatosensory score (headache, nausea, vision problems, sensitivity to light, sensitivity to noise, numbness/tingling, change in taste/smell), affective score (fatigue, sleep, anxiety, depression, irritability, frustration), cognitive score (concentration/distraction, forgetfulness, difficulty making decisions, slowed thinking), and vestibular score (dizziness, imbalance, coordination).22 Symptoms of post-traumatic stress disorder (PTSD) were assessed using the appropriate civilian or military version of the PTSD Checklist, depending of the veteran-status of the participant, to control for the potential mediation between PTSD and physical health.23
Participants then were instrumented with five inertial sensors (Opals, APDM, Inc., Portland, OR), one on each foot, over the lumbar spine, sternum, and on the forehead. Only data from the lumbar spine, sternum, and forehead sensors are presented here. Before the turning protocol, participants completed several balance and gait tests as part of a larger study, including a self-paced walk eight times down and back between two lines spaced 13 meters apart in a quiet hallway. Straight, self-selected gait speed was extracted from the straight sections of this long walk using MobilityLab (Software version 2, Analysis version 3, APDM, Inc. Portland, OR) for comparison purposes. Other protocols and data pertaining to the straight walking protocol are not presented in this initial analysis of turning.
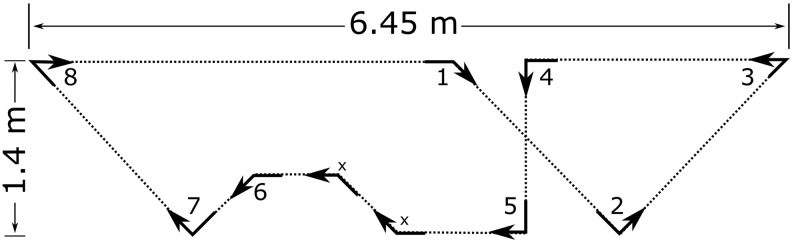
Participants were introduced to a marked course containing four 45-degree turns, four 90-degree turns, and two 135-degree turns specifically designed for this study (Fig. 1). The course was designed to feature a variety of turns at angles common in everyday ambulation. Participants were led through the course by a research assistant before following the course on their own for a minimum of two laps or until they were familiar with the walking pattern. After the familiarization, the participants walked around the course for 12 continuous laps at their self-selected pace.
FIG. 1.
Turning course marked with arrows including four 45-degree turns, four 90-degree turns, and two 135-degree turns. Two 45-degree turns (x) were excluded, leaving eight total turns per lap.
After completing 12 laps at the comfortable pace, participants were instructed to walk “as fast as possible without running or jogging” around the course. The participants completed a minimum of two laps at the fast walking pace to familiarize themselves with the course at the new pace. Participants then walked at their fast pace around the course for four continuous laps. Data were recorded from the inertial sensors at 128 Hz.
As peripheral vestibular12,24 and oculomotor25,26 dysfunction is common after mTBI, each participant also completed clinical oculomotor and vestibular tests of saccadic latency, smooth pursuit, gaze fixation, convergence, calorics, head impulse, cervical vestibular evoked myogenic potentials (cVEMP), and ocular vestibular evoked myogenic potentials (oVEMP) at least one day before mobility testing. These clinical oculomotor and vestibular tests were used to provide further descriptive characteristics of each participant.
Analysis
For each trial, raw data were extracted from the inertial sensors, and angular velocities were filtered using a 1.5 Hz, fourth order phaseless low-pass Butterworth filter. The continuous data were segmented into 12 laps and the peak velocity of each turn. Two 45-degree turns were excluded from the analysis because a clearly defined peak velocity was not present across all participants (Fig. 1). All turns from the first and final lap were excluded from the analysis, leaving 10 laps of eight analyzed turns at normal walking speed. For the fast walking trials, only the final lap was excluded, leaving three laps of eight analyzed turns.
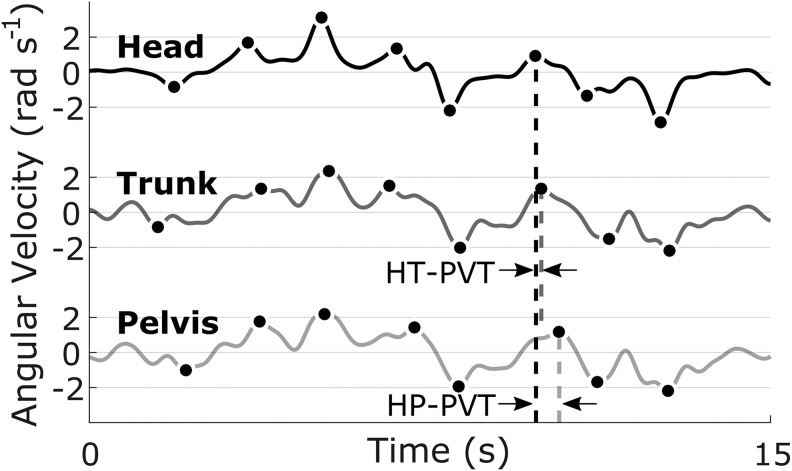
For each turn, the magnitude and time of the peak angular velocity of the head and pelvis, relative to the global reference frame, were extracted. For each participant and at each speed, the peak angular velocities were averaged across turns of the same angle. Peak velocity timing (PVT) was defined as the difference in time between peak velocities of head and trunk (HT-PVT) or head and pelvis (HP-PVT) for each turn (Fig. 2).
FIG. 2.
Example angular velocity traces of the head (top), trunk (middle), and pelvis (bottom). The peak angular velocity for each turn is indicated with a filled marker. The head-to-trunk and head-to-pelvis peak velocity timings (HT-PVT and HP-PVT, respectively) are indicated by the temporal difference between the peak velocities at each turn.
The PVT differs from traditionally defined reorientation timing in that it measures the coordination of angular velocities, rather than angular displacements or time of turn onset. A positive PVT indicates the peak velocity of the head that occurred before the peak velocity of the trunk or pelvis, with more positive values indicating greater separation between the peak head and peak trunk or pelvis angular velocities. Similarly, a negative PVT indicates the peak velocity of the head that occurred after the peak velocity of the trunk or pelvis. Larger PVT magnitudes, regardless of direction, indicate the head reorientations are less coupled with the reorientations of the trunk or pelvis. The HT-PVT and HP-PVT were each averaged across turns of the same angle. The variability of HT- and HP-PVT was determined from the standard deviation across all turns of the same speed. The average time to complete each lap was also calculated and recorded.
Statistical analysis
Two-sample independent t tests compared the symptom scores, age, body mass index (BMI), and average lap time and gait speed between groups. To test whether the peak angular velocity of the head, peak angular velocity of the pelvis, or the HT- or HP-PVT differed between persons with chronic mTBI and controls, we fit a linear mixed model for each outcome. Each linear mixed model was adjusted for group, average lap time, turning angle, age, BMI, and with a random intercept for each subject. To test whether the persons with chronic mTBI had more variable HT- or HP-PVT compared with controls, linear regression models were fit for the variability of HT- and HP-PVT and adjusted for group, average lap time, age, and BMI.
To compare the relationship of peak angular velocity of the head, peak angular velocity of the pelvis, HT- or HP-PVT to symptom severity, we fit four linear mixed models per outcome. Each model was adjusted for one of the NSI symptom subscores: somatosensory score, affective score, cognitive score, and vestibular score. Each model was also adjusted for potential confounders of time since injury, age, BMI, and PTSD symptom score, turning angle, and included a random intercept for each subject. To similarly compare the relationship between HT-PVT, HP-PVT variability, average lap time, or straight gait speed to symptom severity, four linear regression models were fit for each outcome and adjusted for one of the NSI symptom subscores and potential confounders of time since injury, age, BMI, and PTSD symptom score.
To assess whether individuals with chronic mTBI increased reorientation velocities as much as the control group, post hoc linear mixed models were fit for peak angular velocity of the head, trunk, and pelvis, and for average lap time. Each model was adjusted for group, speed, group*speed, and a random intercept for each subject. Turn angle was included as a covariate for peak head, trunk, and pelvis angular velocity.
All analyses were performed in MATLAB R2016a (The MathWorks, Inc., Natick, MA). Statistical analyses were performed with the MATLAB R2016a Statistics and Machine Learning Toolbox using a two-sided significance level of 0.05.
Results
Fifty-seven individuals were screened for inclusion and exclusion criteria, and 42 participants (14 chronic mTBI, 28 healthy controls) subsequently enrolled and provided informed written consent to participate. Participant demographic information is provided in Table 1. The chronic mTBI group was significantly older (t = 2.85 p = 0.007) and had higher BMIs compared with the control group (t = 3.05 p = 0.004). Of the 14 participants with chronic mTBI included in this study, all had evidence for peripheral vestibular or oculomotor dysfunction on at least one clinical test. No controls had any abnormal clinical vestibular or oculomotor results. Clinical vestibular and oculomotor results for each subject with chronic mTBI are presented in Table 2.
Table 1.
Means (Standard Deviations), unless Otherwise Noted, of Demographic Information and Self-Reported Symptom Scores for Each Group
| mTBI | Controls | |
|---|---|---|
| N | 14 | 28 |
| Age (years) | 38.4 (9.9) | 25.6 (5.4) |
| Height (cm) | 174 (9) | 171 (6) |
| Mass (kg) | 86.8 (16.7) | 73.6 (14.3) |
| BMI | 28.9 (4.3) | 25.1 (4.3) |
| Previous concussions / mTBIs* | 2 (1–10) | 0 (0–2) |
| Years since most recent concussion / mTBI* | 0.72 (0.39–13.01) | 15.54 (3.30–27.47) |
| NSI Symptom Scores | ||
| Total | 36.8 (11.3) | 4.8 (5.6) |
| Somatosensory | 9.4 (4.9) | 1.1 (2.0) |
| Affective | 11.8 (5.7) | 2.1 (2.0) |
| Cognitive | 7.7 (3.3) | 0.6 (1.2) |
| Vestibular | 5.8 (2.0) | 0.6 (1.2) |
| PTSD Severity Score | 48.8 (14.2) | 23.4 (10.9) |
mTBI, mild traumatic brain injury; BMI, body mass index; NSI, Neurobehavioral Symptom Inventory; PTSD, post-traumatic stress disorder.
Shown as median (range).
Table 2.
Vestibular and Oculomotor Testing Results for Each Mild Traumatic Brain Injury Participant*
| Subject | vHIT | cVEMP | oVEMP | Gaze | Saccades | Smooth Pursuit | Dix-Hallpike | Convergence | Calorics |
|---|---|---|---|---|---|---|---|---|---|
| mTBI-1 | Normal | Absent right | Absent bilateral | Normal | Normal | Borderline normal | Normal | Normal | Weakness right, WNL |
| mTBI-2 | Normal | Asymmetric -Weakness Left | Normal | Normal | Normal | Borderline normal | Normal | Normal | Symmetric |
| mTBI-3 | Some catch-up overt saccades, WNL | Normal | Normal | Normal | Normal | Normal | Normal | Abnormal | Weakness left |
| mTBI-4 | Normal | Asymmetric - Weakness Left | Normal | Normal | Normal | Normal | Nystagmus (fast phase right) | Normal | Weakness left, WNL |
| mTBI-5 | Some overt saccades, WNL | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Weakness right, WNL |
| mTBI-6 | Normal | Asymmetric - Weakness Right | Absent bilateral | Normal | Normal | Normal | Normal | Normal | No Test |
| mTBI-7 | Overt catch-up saccades (left lateral), WNL | Absent right | Absent bilateral | Normal | Normal | Normal | Normal | Normal | Weakness left, WNL |
| mTBI-8 | Normal | Absent bilateral | Normal | Normal | Normal | Normal | Normal | Normal | Symmetric |
| mTBI-9 | Normal | Normal | No Test | Normal | Normal | Normal | Normal | Abnormal | No Test |
| mTBI-10 | Normal | Absent bilateral | Absent bilateral | Normal | Normal | Normal | Normal | Normal | Weakness right |
| mTBI-11 | Mild catch-up saccades, WNL | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| mTBI-12 | Normal | Asymmetric - Weakness Left | Asymmetric right | Normal | Normal | Normal | Normal | Abnormal | Weakness right |
| mTBI-13 | Normal | Normal | Asymmetric left | Normal | Normal | Normal | Nystagmus (fast phase right) | Normal | No Test |
| mTBI-14 | No Test | Absent left | Absent right | Normal | Normal | Borderline normal | Nystagmus (fast phase left) | Normal | Normal |
vHIT, video head impulse test; cVEMP, cervical vestibular evoked myogenic potentials; oVEMP, ocular vestibular evoked myogenic potentials; mTBI, mild traumatic brain injury; WNL, within normal limits.
No control participant had any abnormal test.
The chronic mTBI group had significantly slower average lap times (chronic mTBI mean, standard deviation [SD] = 20.3, 7.1 sec; control mean, SD = 16.5, 1.9 sec; t = 2.74 p = 0.009) and straight gait speed (chronic mTBI mean, SD = 1.08, 0.11 sec; control mean, SD = 1.20, 0.11 sec; t = −3.44 p = 0.001). Univariate means and SDs for each outcome are provided in Table 3. Results from the statistical models comparing each outcome between groups are provided in Tables 4 and 5.
Table 3.
Univariate Descriptive Means (Standard Deviations) for Peak Head, Peak Trunk, and Peak Pelvis Angular Velocities, and Head-to-Trunk and Head-to-Pelvis Peak Velocity Timings by Turning Angle and Group*
| mTBI | Controls | |||||
|---|---|---|---|---|---|---|
| 45-degree angle | 90-degree angle | 135-degree angle | 45-degree angle | 90-degree angle | 135-degree angle | |
| Peak head angular velocity (rad/sec) | 0.85 (0.22) | 1.46 (0.36) | 2.22 (0.47) | 1.01 (0.23) | 1.74 (0.32) | 2.54 (0.35) |
| Peak trunk angular velocity (rad/sec) | 1.11 (0.26) | 1.55 (0.36) | 2.13 (0.41) | 1.33 (0.19) | 1.88 (0.26) | 2.45 (0.32) |
| Peak pelvis angular velocity (rad/sec) | 1.09 (0.23) | 1.56 (0.35) | 2.09 (0.42) | 1.40 (0.22) | 1.93 (0.28) | 2.50 (0.34) |
| Head-to-trunk segmental coordination timing (HT-PVT) (msec) | 149 (135) | 132 (128) | 67 (113) | 86 (74) | 99 (80) | 83 (82) |
| Head-to-pelvis segmental coordination timing (HP-PVT) (msec) | 203 (199) | 161 (117) | 97 (158) | 128 (110) | 80 (77) | 74 (85) |
| Variability of HT-PVT (ms) | 264 (128) | 187 (83) | ||||
| Variability of HP-PVT (ms) | 358 (81) | 249 (64) | ||||
mTBI, mild traumatic brain injury.
The variability of HT- and HP-PVT are presented over all turning angles.
Table 4.
Beta Coefficients, 95% Confidence Intervals, and p Values from the Linear Mixed Models for Peak Head, Trunk, and Pelvis Angular Velocities, Head-to-Trunk and Head-to-Pelvis Peak Velocity Timings*
| Peak head angular velocity | Peak trunk angular velocity | Peak pelvis angular velocity | Head-to-trunk peak velocity timing (HT-PVT) (msec) | Head-to-pelvis peak velocity timing (HP-PVT) (ms) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | 95% CI of Beta | p value | Beta (SE) | 95% CI of Beta | p value | Beta (SE) | 95% CI of Beta | p value | Beta (SE) | 95% CI of Beta | p value | Beta (SE) | 95% CI of Beta | p value | |
| Intercept | 1.976 (0.324) | [1.333, 2.619] | <0.001 | 2.216 (0.291) | [1.640, 2.792] | <0.001 | 2.384 (0.294) | [1.802, 2.966] | <0.001 | 146 (95) | [−42, 334] | 0.125 | 183 (111) | [−37, 404] | 0.103 |
| Group | –0.173 (0.114) | [−0.398, 0.052] | 0.131 | –0.202 (0.102) | [−0.404, −0.001] | 0.049 | –0.228 (0.103) | [−0.432, −0.024] | 0.029 | 45 (33) | [−21, 111] | 0.176 | 96 (39) | [19, 174] | 0.015 |
| Average Lap Time | –0.017 (0.011) | [−0.039, 0.005] | 0.130 | –0.029 (0.010) | [−0.049, −0.009] | 0.005 | –0.031 (0.010) | [−0.051, −0.011] | 0.003 | 0 (3) | [−6, 7] | 0.978 | 0 (4) | [−7, 8] | 0.966 |
| Age | –0.006 (0.005) | [−0.016, 0.004] | 0.228 | 0.000 (0.004) | [−0.009, 0.009] | 0.998 | –0.002 (0.004) | [−0.011, 0.007] | 0.666 | 1 (1) | [−2, 4] | 0.378 | 0 (2) | [−4, 3] | 0.856 |
| BMI | 0.009 (0.010) | [−0.011, 0.029] | 0.371 | 0.006 (0.009) | [−0.012, 0.024] | 0.500 | 0.005 (0.009) | [−0.013, 0.023] | 0.577 | –4 (3) | [−10, 2] | 0.188 | –4 (3) | [−11, 3] | 0.231 |
| 45-degree angle | –0.692 (0.043) | [−0.777, −0.608] | <0.001 | –0.527 (0.034) | [−0.595, −0.459] | <0.001 | –0.513 (0.031) | [−0.574, −0.453] | <0.001 | 7 (12) | [−17, 30] | 0.575 | 61 (18) | [25, 96] | 0.001 |
| 135-degree angle | 0.778 (0.043) | [0.694, 0.863] | <0.001 | 0.571 (0.034) | [0.503, 0.639] | <0.001 | 0.555 (0.031) | [0.494, 0.616] | <0.001 | –35 (12) | [−58, −11] | 0.004 | –27 (18) | [−62, 9] | 0.145 |
SE, standard error; CI, confidence interval; BMI, body mass index.
Bold values indicate significant group differences between mild traumatic brain injury and controls at a 0.05 significance level.
Table 5.
Beta Coefficients, 95% Confidence Intervals, and p Values from the Linear Mixed Models for the Variability of Head-to-Trunk and Head-to-Pelvis Peak Velocity Timings*
| Variability of HT-PVT | Variability of HP-PVT | |||||
|---|---|---|---|---|---|---|
| Beta (SE) | 95% CI of Beta | p value | Beta (SE) | 95% CI of Beta | p value | |
| Intercept | 236 (104) | [25, 45] | 0.030 | 163 (81) | [−1, 329] | 0.053 |
| Group | 103 (37) | [29, 177] | 0.008 | 95 (29) | [36, 153] | 0.002 |
| Average Lap Time | 6 (3) | [−1, 14] | 0.100 | 6 (3) | [0, 12] | 0.037 |
| Age | 0 (2) | [−3, 4] | 0.759 | 0 (1) | [−2, 3] | 0.921 |
| BMI | −7 (3) | [−14, −1] | 0.031 | −1 (2) | [−6, 4] | 0.666 |
HY-PVT, head-to trunk peak velocity timing; HP-PVT, head to pelvis peak velocity timing; SE, standard error; CI, confidence interval; BMI, body mass index.
Bold values indicate significant group differences between mild traumatic brain injury and controls at a 0.05 significance level. The control group was used as a reference condition.
Slower peak trunk and pelvis angular velocities and longer delays between the peak head and peak pelvis angular velocities were observed in the chronic mTBI group compared with the controls, after adjusting for covariates of average lap time, age, and BMI. No group differences were found for peak head angular velocity or HT-PVT. Across all turns, participants with chronic mTBI had more variable HT-PVT and HP-PVT compared with controls (Fig. 3). No group differences between peak head angular velocities were detected after controlling for covariates.
FIG. 3.
Box and scatter plot of the variability in head-to-pelvis peak velocity timing (HP-PVT) for the chronic mild traumatic brain injury (mTBI) and control groups. A significant difference was found between groups (p = 0.001). HP-PVT variability was defined as the standard deviation, across all turns, of the temporal difference between peak head and pelvis angular velocities.
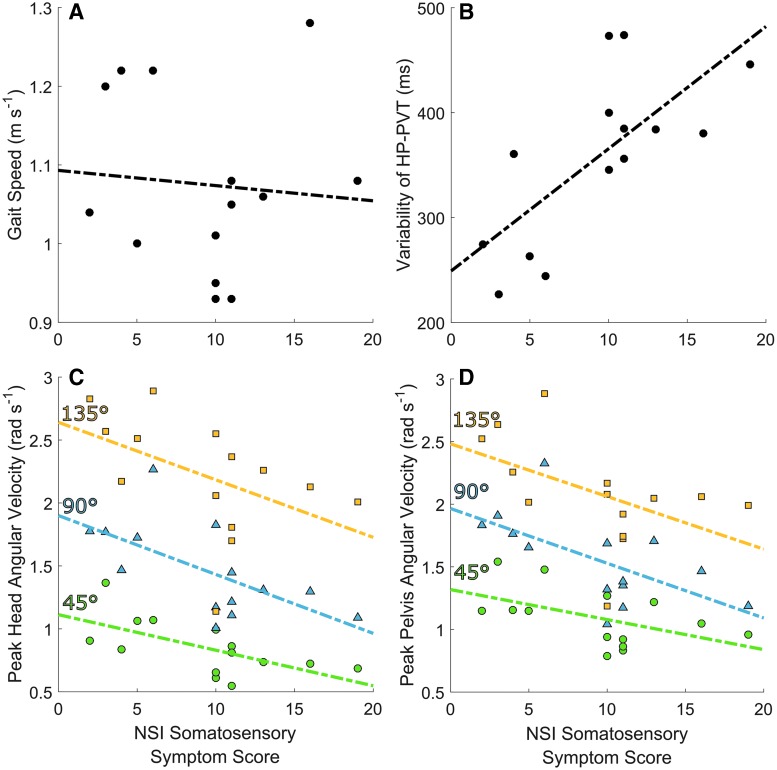
Within the chronic mTBI group, peak head, trunk, and pelvis angular velocities, HT-PVT, HP-PVT, and variability of HP-PVT (Fig. 4) were highly associated with the NSI Somatosensory Symptom Score after adjusting for potential confounders (Table 6). Average lap time, straight gait speed, and HT-PVT variability were not associated with any symptom score. Cognitive (p = 0.40–0.86), affective (p = 0.26–0.96), and vestibular (p = 0.42–0.97), symptom subscores were not associated with any outcome after adjusting for confounders.
FIG. 4.
Scatter plots of (A) self-selected gait speed obtained from a straight walking test, (B) variability of the head-to-pelvis peak velocity timing (HP-PVT), (C) peak head angular velocity, and (D) peak pelvis angular velocity versus the Neurobehavioral Symptom Inventory (NSI) somatosensory symptom score in the chronic mild traumatic brain injury (mTBI) group only. For (C) and (D), green circles, blue triangles, and yellow squares indicate 45-degree, 90-degree, and 135-degree turns, respectively. Self-selected straight gait speed was not associated with NSI somatosensory symptom scores before (R2 = 0.006) or after (p = 0.506) adjusting for covariates. PVT variability was significantly associated with higher NSI somatosensory symptom scores before (R2 = 0.504) and after (p = 0.009) adjusting for covariates. Peak head angular velocity was significantly associated with higher NSI somatosensory symptom scores before (R2 = 0.398 for 45-degree turns, R2 = 0.401 for 90-degree turns, R2 = 0.230 for 135-degree turns) and after (p < 0.001) adjusting for covariates. Peak pelvis angular velocity was significantly associated with higher NSI somatosensory symptom scores before (R2 = 0.258 for 45-degree turns, R2 = 0.381 for 90-degree turns, R2 = 0.245 for 135-degree turns) and after (p = 0.002) adjusting for covariates. Color image is available online at www.liebertpub.com/neu
Table 6.
Beta Coefficients and p Values from the Linear Regression Models between Each Outcome and Neurobehavioral Symptom Inventory Somatosensory Symptoms Score*
| Peak head angular velocity | Peak trunk angular velocity | Peak pelvis angular velocity | Head-to-trunk peak velocity timing (HT-PVT) | Head-to-pelvis peak velocity timing (HP-PVT) | Variability of HT-PVT | Variability of HP-PVT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | |
| Intercept | 0.830 (0.677) | 0.229 | 1.402 (0.829) | 0.101 | 1.318 (0.785) | 0.103 | 521 (284) | 0.077 | 1015 (264) | <0.001 | 728 (389) | 0.103 | 345 (159) | 0.155 |
| NSI Somatosensory Score | −0.052 (0.012) | <0.001 | −0.046 (0.015) | 0.004 | −0.046 (0.014) | 0.002 | 13 (5) | 0.018 | 16 (5) | 0.001 | 12 (7) | 0.115 | 14 (3) | 0.009 |
| Time since 1njury (*10-2) | −0.011 (0.004) | 0.018 | 0.000 (0.000) | 0.070 | −0.009 (0.005) | 0.089 | 0 (0) | 0.369 | 2 (2) | 0.154 | 0 (0) | 0.255 | 1 (1) | 0.221 |
| Age | −0.003 (0.006) | 0.623 | −0.004 (0.007) | 0.543 | −0.004 (0.007) | 0.534 | 1 (2) | 0.717 | −3 (2) | 0.161 | 1 (3) | 0.694 | 1 (1) | 0.532 |
| BMI | 0.042 (0.016) | 0.014 | 0.029 (0.020) | 0.159 | 0.026 (0.019) | 0.179 | −14 (7) | 0.045 | −24 (6) | <0.001 | −19 (9) | 0.079 | −7 (4) | 0.238 |
| PTSD | −0.003 (0.003) | 0.492 | 0.001 (0.005) | 0.857 | 0.004 (0.005) | 0.388 | −3 (2) | 0.099 | −4 (2) | 0.011 | −2 (2) | 0.400 | 1 (1) | 0.855 |
| 45° Angle | −0.616 (0.077) | <0.001 | −0.448 (0.062) | <0.001 | −0.465 (0.057) | <0.001 | 22 (23) | 0.351 | 69 (33) | 0.046 | ||||
| 135° Angle | 0.738 (0.077) | <0.001 | 0.571 (0.062) | <0.001 | 0.544 (0.057) | <0.001 | −67 (23) | 0.006 | −69 (33) | 0.047 | ||||
HT-PVT, head-to trunk peak velocity timing; HP-PVT, head to pelvis peak velocity timing; SE, standard error; BMI, body mass index; PTSD, post-traumatic stress disorder.
Bold values indicate outcomes that were significantly associated with the NSI somatosensory symptom score after adjusting for covariates. The 90° angle was used as the reference condition for peak angular velocities and peak velocity timings.
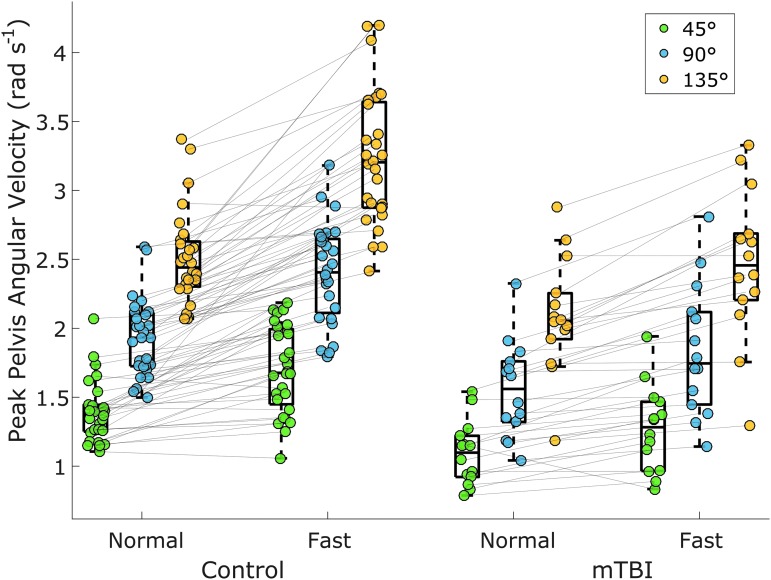
A significant group*speed interaction was found for peak trunk angular velocity (β = −0.121, SE = 0.057, p = 0.034) and pelvis angular velocity (β = −0.228, SE = 0.062, p < 0.001; Fig. 5), where controls increased angular velocities more than the chronic mTBI group. No significant interaction was found for peak head angular velocity (β = −0.061, SE = 0.069, p = 0.379) or for lap completion time (β = −0.348, SE = 0.603, p = 0.566).
FIG. 5.
Peak pelvis angular velocity for each group stratified by turning angle and speed. A significant group*speed interaction was found (p < 0.001) with controls increasing their peak angular velocity across speeds more than the chronic mild traumatic brain injury (mTBI) group. Lines between the normal and fast speed correspond to each participant at each turning angle. Color image is available online at www.liebertpub.com/neu
Discussion
We investigated whether people with chronic mTBI exhibit different turning characteristics compared with healthy controls, and whether abnormal turning characteristics are associated with self-reported symptoms. In support of our hypothesis, we found that individuals with chronic mTBI turned their trunk and pelvis slower, had longer PVT, more variable HP-PVT, and took longer to complete the turning course compared with controls.
These results present the first study of how chronic mTBI affects turning and agree with previous work that investigated turning in acute and resolving sport-related concussion.7,8 Specifically, slower lap times and slower pelvis angular velocities in the chronic mTBI group are consistent with previous reports of slower center-of-mass velocities in recently concussed, asymptomatic athletes during a 90-degree turn.8 Additionally, the increased PVT variability during pre-planned turns reported here extends the results of Powers and associates7 who found increased segmental reorientation variability during a light-induced turning task in concussed athletes. However, it is important to note that chronic mTBI and sport-related concussion may have significantly different characteristics. Our chronic mTBI individuals were not restricted by mechanism of injury, while both Powers and associates7 and Fino and colleagues8 examined athletes with sport-related concussion. Here, all subjects were at least three months post-mTBI and still reported symptoms. The previous studies examined athletes with acute or subacute conditions whose symptoms resolved within three weeks. Further, all subjects with chronic mTBI had at least one abnormal clinical vestibular or oculomotor test. Combined, this suggests that the subjects with chronic mTBI examined here may have had more severe injuries, and potentially different physiological abnormalities, than those studied by Powers and associates7 or Fino and colleagues.8 Despite these potential differences in injury severity, the similar results across studies suggests turning abnormalities may be a consistent characteristic of traumatically induced brain injuries including sport-related concussion and mTBI.
To date, we are unaware of any other study examining the association between turning performance and symptoms. Our results found that several aspects of turning, including coordination variability and peak angular velocity, were strongly associated with self-reported symptoms. While clinical balance tests are often not correlated with self-reported symptoms,18,27 it is unclear whether other measures of mobility are associated with symptomology. Gait speed was strongly correlated with self-reported symptoms in adults four years post-mTBI.28 Another study reported weak correlations between gait speed and symptoms in adolescents 46 days post-concussion,17 and a different study reported gait speed was unrelated to self-reported dizziness in persons with peripheral vestibular disorders.29 Interestingly, we found straight gait speed and average lap time during the turning task were not correlated with symptoms. While it is difficult to say whether turning is better than other motor signs for diagnostic or return-to-activity evaluation, the ecological validity during the present turning task is likely higher than most other motor evaluations, including static stability, because it is modeled off of natural turning actions performed several hundred times per day.5,6 These results indicate that measures of turning coordination and angular velocity, more than straight gait speed or task completion times, may be better indictors of the impact of symptoms on everyday mobility.
Contrary to our hypothesis, somatosensory symptoms (e.g., headache, nausea, vision problems, sensitivity to light/noise) were strongly associated with turning coordination and speed, but other symptom domains, such as vestibular symptoms (e.g., dizziness, imbalance, coordination) and affective symptoms (e.g., fatigue, sleep, anxiety, depression, irritability, frustration) were not. Symptoms such as headache and nausea are more perceptible than other symptoms,30 suggesting that individuals may alter their behavior to minimize somatic symptoms rather than other, less perceptible symptoms. Somatic symptom severity has also been associated with prolonged recovery from concussion,31 suggesting somatosensory symptom severity may be associated with injury severity.
This cross-sectional study cannot determine the order of cause and effect between the symptoms and turning performance. However, two explanations are likely. First, symptomatic individuals may be altering their turning behavior to limit the exacerbation of their somatosensory symptoms. We did not administer a symptom inventory after the turning task to see if the turning task did elicit symptoms, but at least one subject with chronic mTBI specifically commented that turning quickly made them nauseous, and previous reports have noted that rapid movements can elicit symptoms.12 Therefore, it is possible that individuals with chronic mTBI turn slower to limit the rapid reorientation associated with turning and the worsening of symptoms. Our results showing that individuals with chronic mTBI did not increase their peak pelvis velocity as much as controls when asked to walk fast directly support this interpretation. This significant interaction between speed and group also suggests individuals with chronic mTBI show greater impairments during turning tasks in everyday life that demand turns outside normal speeds, such as occupational, military, or athletic environments. An equally likely explanation is that greater somatic symptoms and abnormal turning are covarying characteristics associated with more severe injuries. These two interpretations are not mutually exclusive; somatic symptoms and abnormal turning may be representative of underlying pathophysiology, and individuals may also alter their movements to prevent further worsening of symptoms. Additional studies should further examine the causal relationship between turning performance and symptoms.
In addition to turning slower, our results indicate people with chronic mTBI decoupled their head from their pelvis more, and did so with more variability, than controls during pre-planned turns. During turning, segmental reorientation and postural adjustments preempt turns to stabilize visual information, initiate disequilibrium, and plan the next trajectory,9,32–34 but visual targets can change the reorientation timing. Greater separation between the peak head and peak pelvis angular velocities has been reported in healthy adults when turning toward a visually marked known location compared with an unmarked known location.35 Our protocol used arrows on the floor to guide visually the direction of the next turn and could be interpreted as the former, visually marked condition. Solomon and coworkers35 reported HP-PVTs of 171 msec for visibly marked 90-degree turns and 90 msec during unmarked 90-degree turns. Our chronic mTBI and control groups had average HP-PVTs of 161 msec and 80 msec, respectively, during 90-degree turns. Therefore, differences in PVT could be because of the utilization of visual cues; individuals with chronic mTBI may have been more reliant on the marked arrows, while control subjects may have remembered the direction of the course and ignored the visual references.
Methodological differences between our definition of HT- or HP-PVT, and head-trunk or head-pelvis coordination used in previous studies must be acknowledged. Previous studies examining segmental reorientation have predominantly examined angular displacements from singular turns to determine the relative onset time of the turn.7,33,34,36–40 Conversely, we defined HT- and HP-PVT as the relative difference in time between the peak velocities of each segment35 as the conclusion of one turn and initiation of the next could not always be determined due to the compact nature of the course. While HT- and HP-PVT likely share some association to onset timing,41 caution should be applied when comparing these results with results obtained from other methods. In addition, future research is needed to identify the clinically important differences in HT- and HP-PVT.
We were surprised that persons with chronic mTBI did not have slower head velocity during turning, particularly because they had peripheral vestibular or oculomotor deficits. Reorientation of the head, however, is a highly stereotyped behavior that often anticipates a turn.9,34 It is possible the small sample size of this preliminary study was underpowered for this comparison. Conversely, the speed of head reorientation during turning may be more centrally programmed and less variant than the speed of the pelvis during turning. Peak head angular velocities have been associated with the amplitudes of shifts in gaze,42,43 suggesting that the peak speed of head reorientation during turning may not differ in individuals with chronic mTBI without significant dysfunction of the central nervous or oculomotor systems. As some but not all, participants had oculomotor deficits, there may have been variability within the chronic mTBI group. Nonetheless, slower head velocity was significantly associated with higher somatosensory symptom scores, suggesting that head velocity may not differ in individuals with low symptom burdens, but that individuals with high symptom burdens can have nonnatural reorientation velocities. We plan to examine further the relationship between head, trunk, and pelvis reorientation velocities and symptoms in a larger cohort.
Taken together, the slower peak pelvis and trunk velocities, increased HP-PVT, increased HP-PVT and HT-PVT, and equivocal peak head velocities suggest persons with chronic mTBI have impaired head stabilization during turning compared with controls (i.e., the head is allowed to move more freely). Similar head stabilization deficits have been reported in chronic mTBI during straight walking.44 The precise mechanism underlying this dysfunction is unknown, but impaired integration of vestibular and proprioceptive information is a potential candidate as integration of vestibular and pelvis-to-feet proprioceptive information is necessary to maintain a heading direction.35,45 It is currently unclear how sensory integration deficitis in persons with chronic mTBI during static postural tasks46 translate into nonstatic tasks such as gait and turning. Yet, it is possible that difficulty integrating vestibular and proprioceptive information could be associated with the abnormal turning characteristics seen here.
The present sample of individuals with chronic mTBI had varying degrees of peripheral vestibular and oculomotor dysfunction. Therefore, while peripheral vestibular12,24 or oculomotor25,26 dysfunction is common after mTBI, the results may not be generalizable to the broader mTBI population. However, given the heterogeneity of vestibular and oculomotor disorders within our chronic mTBI sample, the significant results are unlikely to be caused by a specific dysfunction and not related to the underlying mTBI. The vestibular testing methods used here form a standard array of testing procedures, but may not have the sensitivity or specificity to be used easily to stratify or correlate with functional outcomes. Future analysis on a larger sample of individuals will attempt to stratify the chronic mTBI participants by the comorbid vestibular or oculomotor dysfunction.
Several additional limitations should be noted when interpreting these results. First, the individuals were instructed to make each turn as directed by the course, but there was not strict adherence to a specific trajectory. Participants were therefore free to perform each turn over multiple steps and to take sharper or wider turns within the confines of the course. It has been noted previously that individuals with a recent sport-related concussion take less sharp turns compared with controls,8 and it is possible that the individuals with chronic mTBI presented here performed each turn over a longer path with less curvature per turn. If so, the slower peak velocities of the pelvis may have reflected both a decrease in speed and a decrease in the change of heading angle. Such an adaptation would support our interpretation that symptomatic individuals may preferentially alter their turning movements to limit the provocation of symptoms. A course with a constrained path may have resulted in better adherence and less between-subject variability. A constrained design may have allowed for better interpretations of the physiological and biomechanical deficits during turning, but a strictly prescribed path may have also obscured compensatory strategies individuals use in everyday living. Sreenivasa and colleagues34 found a constrained path did not influence the head-trunk behavior but did impact the variability of the head-to-trunk reorientation across turning angles. The association between symptoms and HP-PVT variability suggests that variability may be an important marker relevant to both performance and self-perceived state. Therefore, the flexibility that was allowed within the course may have been advantageous in translating results obtained in a laboratory/clinical setting to patient burden. However, future studies that wish to examine the underlying mechanism of abnormal turning behavior may require a strictly defined course. Second, the turning course was not symmetric. While the course was designed to fit within a hallway and incorporate equal numbers of left and right turns of each angle, the approach and exit distances of each turn differed. It is possible that turning performance in one direction may be affected by unilateral vestibular dysfunction. Third, while we controlled for PTSD, our results do not account for other potential psychosocial factors, such as ADHD. The lack of association between turning and affective symptoms, however, suggests that self-reported psychosocial factors such as anxiety, sleep, irritability, and depression do not have a significant impact on turning velocity or coordination. Finally, the present results are based on a relatively small sample of individuals. These results demonstrate the utility of turning-based assessments and the potential importance of turning when interpreting the relationship between self-reported symptoms and mobility. We plan on repeating this analysis with a larger sample size to confirm our result.
Conclusion
Overall, the present results suggest that people with chronic mTBI and persistent symptoms exhibit significantly different turning mechanics, characterized by impaired head stabilization, in a task simulating the turns of everyday life, and that somatic symptoms, but not vestibular, affective, or cognitive symptoms, are related to this turning behavior. These preliminary results in a small sample of individuals provide important initial information and support the use of turning velocity and peak velocity timings as secondary outcome measures in larger intervention studies for persons with chronic mTBI. Future research should examine the direct relationship between symptomology and mobility, particularly in dynamic activities that require complex movements and coordination.
Acknowledgments
The authors would like to thank Clayton Swanson and Heather Belding for assistance in early data collection, Marco Jurado, AuD and Sean Kampel, AuD for performing the vestibular testing, and Jenny Wilhelm, PT for referring subjects and evaluating the clinical diagnoses of participants. This work was supported by the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-15-1-0620. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Parker T.M., Osternig L.R., Van Donkelaar P., and Chou L. (2006). Gait stability following concussion. Med. Sci. Sports Exerc. 38, 1032–1040 [DOI] [PubMed] [Google Scholar]
- 2. Buckley T.A., Munkasy B.A., Tapia-Lovler T.G., and Wikstrom E.A. (2013). Altered gait termination strategies following a concussion. Gait Posture 38, 549–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catena R.D., van Donkelaar P., and Chou L.S. (2009). Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J. Neuroeng. Rehabil. 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parker T.M., Osternig L.R., van Donkelaar P., and Chou L.S. (2008). Balance control during gait in athletes and non-athletes following concussion. Med. Eng. Phys. 30, 959–967 [DOI] [PubMed] [Google Scholar]
- 5. Mancini M., El-Gohary M., Pearson S., McNames J., Schlueter H., Nutt J.G., King L.A., and Horak F.B. (2015). Continuous monitoring of turning in Parkinson's disease: rehabilitation potential. NeuroRehabilitation 37, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glaister B.C., Bernatz G.C., Klute G.K., and Orendurff M.S. (2007). Video task analysis of turning during activities of daily living. Gait Posture 25, 289–294 [DOI] [PubMed] [Google Scholar]
- 7. Powers K.C., Kalmar J.M. and Cinelli M.E. (2014). Dynamic stability and steering control following a sport-induced concussion. Gait Posture 39, 728–732 [DOI] [PubMed] [Google Scholar]
- 8. Fino P.C., Nussbaum M.A., and Brolinson P.G. (2016). Locomotor deficits in recently concussed athletes and matched controls during single and dual-task turning gait: preliminary results. J. Neuroeng Rehabil. 13, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernardin D., Kadone H., Bennequin D., Sugar T., Zaoui M., and Berthoz A. (2012). Gaze anticipation during human locomotion. Exp. Brain Res. 223, 65–78 [DOI] [PubMed] [Google Scholar]
- 10. Raphan T., Imai T., Moore S.T., and Cohen B. (2001). Vestibular compensation and orientation during locomotion. Ann. N. Y. Acad. Sci. 942, 128–138 [DOI] [PubMed] [Google Scholar]
- 11. Taylor M., Dabnichki P., and Strike S. (2005). A three-dimensional biomechanical comparison between turning strategies during the stance phase of walking. Hum. Mov. Sci. 24, 558–573 [DOI] [PubMed] [Google Scholar]
- 12. Kolev O.I. and Sergeeva M. (2016). Vestibular disorders following different types of head and neck trauma. Funct. Neurol. 31, 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boake C., McCauley S.R., Levin H.S., Pedroza C., Contant C.F., Song J.X., Brown S.A., Goodman H., Brundage S.I., and Diaz-Marchan P.J. (2005). Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 17, 350–356 [DOI] [PubMed] [Google Scholar]
- 14. Russell K., Selci E., Chu S., Fineblit S., Ritchie L., and Ellis M.J. (2017). Longitudinal assessment of health-related quality of life following adolescent sports-related concussion. J. Neurotrauma 34,2147–2153 [DOI] [PubMed] [Google Scholar]
- 15. Schiehser D.M., Twamley E.W., Liu L., Matevosyan A., Filoteo J.V., Jak A.J., Orff H.J., Hanson K.L., Sorg S.F., and Delano-Wood L. (2015). The relationship between postconcussive symptoms and quality of life in veterans with mild to moderate traumatic brain injury. J. Head Trauma Rehabil. 30, E21–E28 [DOI] [PubMed] [Google Scholar]
- 16. Stalnacke B.M. (2007). Community integration, social support and life satisfaction in relation to symptoms 3 years after mild traumatic brain injury. Brain Inj. 21, 933–942 [DOI] [PubMed] [Google Scholar]
- 17. Alsalaheen B.A., Whitney S.L., Marchetti G.F., Furman J.M., Kontos A.P., Collins M.W., and Sparto P.J. (2016). Relationship between cognitive assessment and balance measures in adolescents referred for vestibular physical therapy after concussion. Clin. J. Sport Med. 26, 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guskiewicz K.M. (2001). Postural stability assessment following concussion: one piece of the puzzle. Clin. J. Sport Med. 11, 182–189 [DOI] [PubMed] [Google Scholar]
- 19. Fino P.C., Peterka R.J., Hullar T.E., Murchison C., Horak F.B., Chesnutt J.C., and King L.A. (2017). Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: a randomized clinical trial. BMC Neurol. 17, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvořák J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A, Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turne r M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 21. Woodson J. (2015). Traumatic Brain Injury: Updated Definition and Reporting. Department of Defense: Washington, DC [Google Scholar]
- 22. Cicerone K.D. and Kalmar K. (1995). Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 10, 1–17 [Google Scholar]
- 23. Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 24. Akin F.W. and Murnane O.D. (2011). Head injury and blast exposure: vestibular consequences. Otolaryngol. Clin. North Am. 44, 323–334, viii. [DOI] [PubMed] [Google Scholar]
- 25. Ellis M.J., Cordingley D., Vis S., Reimer K., Leiter J., and Russell K. (2015). Vestibulo-ocular dysfunction in pediatric sports-related concussion. J. Neurosurg. Pediatr. 16, 248–255 [DOI] [PubMed] [Google Scholar]
- 26. Littlefield P.D., Pinto R.L., Burrows H.L., and Brungart D.S. (2016). The vestibular effects of repeated low-level blasts. J. Neurotrauma 33, 71–81 [DOI] [PubMed] [Google Scholar]
- 27. Son E.J., Lee D.H., Oh J.H., Seo J.H., and Jeon E.J. (2015). Correlation between the dizziness handicap inventory and balance performance during the acute phase of unilateral vestibulopathy. Am. J. Otolaryngol. 36, 823–827 [DOI] [PubMed] [Google Scholar]
- 28. Kleffelgaard I., Roe C., Soberg H.L., and Bergland A. (2012). Associations among self-reported balance problems, post-concussion symptoms and performance-based tests: a longitudinal follow-up study. Disabil. Rehabil. 34, 788–794 [DOI] [PubMed] [Google Scholar]
- 29. Whitney S.L., Wrisley D.M., Brown K.E., and Furman J.M. (2004). Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol. Neurotol. 25, 139–143 [DOI] [PubMed] [Google Scholar]
- 30. Sandel N.K., Lovell M.R., Kegel N.E., Collins M.W., and Kontos A.P. (2013). The relationship of symptoms and neurocognitive performance to perceived recovery from sports-related concussion among adolescent athletes. Appl. Neuropsychol. Child 2, 64–69 [DOI] [PubMed] [Google Scholar]
- 31. Howell D.R., O'Brien M.J., Beasley M.A., Mannix R.C., and Meehan W.P., 3rd (2016). Initial somatic symptoms are associated with prolonged symptom duration following concussion in adolescents. Acta Paediatr. 105, e426–e432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu D., Carlton L.G., and Rosengren K.S. (2004). Anticipatory postural adjustments for altering direction during walking. J. Mot. Behav. 36, 316–326 [DOI] [PubMed] [Google Scholar]
- 33. Patla A.E., Adkin A., and Ballard T. (1999). Online steering: coordination and control of body center of mass, head and body reorientation. Exp. Brain Res. 129, 629–634 [DOI] [PubMed] [Google Scholar]
- 34. Sreenivasa M.N., Frissen I., Souman J.L., and Ernst M.O. (2008). Walking along curved paths of different angles: the relationship between head and trunk turning. Exp. Brain Res. 191, 313–320 [DOI] [PubMed] [Google Scholar]
- 35. Solomon D., Vijay Kumar, Jenkins R.A., and Jewell J. (2006). Head control strategies during whole-body turns. Exp. Brain Res. 173, 475–486 [DOI] [PubMed] [Google Scholar]
- 36. Grasso R., Prevost P., Ivanenko Y.P., and Berthoz A. (1998). Eye-head coordination for the steering of locomotion in humans: an anticipatory synergy. Neurosci. Lett. 253, 115–118 [DOI] [PubMed] [Google Scholar]
- 37. Hollands M.A., Patla A.E., and Vickers J.N. (2002). “Look where you're going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp. Brain Res. 143, 221–230 [DOI] [PubMed] [Google Scholar]
- 38. Hollands M.A., Sorensen K.L., and Patla A.E. (2001). Effects of head immobilization on the coordination and control of head and body reorientation and translation during steering. Exp. Brain Res. 140, 223–233 [DOI] [PubMed] [Google Scholar]
- 39. Vallis L.A. and Patla A.E. (2004). Expected and unexpected head yaw movements result in different modifications of gait and whole body coordination strategies. Exp. Brain Res. 157, 94–110 [DOI] [PubMed] [Google Scholar]
- 40. Vallis L.A., Patla A.E., and Adkin A.L. (2001). Control of steering in the presence of unexpected head yaw movements. Influence on sequencing of subtasks. Exp. Brain Res. 138, 128–134 [DOI] [PubMed] [Google Scholar]
- 41. Grasso R., Ivanenko Y.P., McIntyre J., Viaud-Delmon I., and Berthoz A. (2000). Spatial, not temporal cues drive predictive orienting movements during navigation: a virtual reality study. Neuroreport 11, 775–778 [DOI] [PubMed] [Google Scholar]
- 42. Anastasopoulos D., Ziavra N., Hollands M., and Bronstein A. (2009). Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp. Brain Res. 193, 323–336 [DOI] [PubMed] [Google Scholar]
- 43. Land M.F. (2004). The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Exp. Brain Res. 159, 151–160 [DOI] [PubMed] [Google Scholar]
- 44. Sessoms P.H., Gottshall K.R., Sturdy J., and Viirre E. (2015). Head stabilization measurements as a potential evaluation tool for comparison of persons with TBI and vestibular dysfunction with healthy controls. Mil. Med. 180, 135–142 [DOI] [PubMed] [Google Scholar]
- 45. Suppl 3, ner J.R., and DiZio P. (2005). Rapid adaptation of torso pointing movements to perturbations of the base of support. Exp. Brain Res. 165, 283–293 [DOI] [PubMed] [Google Scholar]
- 46. Haran F.J., Slaboda J.C., King L.A., Wright W.G., Houlihan D., and Norris J.N. (2016). Sensitivity of the Balance Error Scoring System and the Sensory Organization Test in the combat environment. J. Neurotrauma 33, 705–711 [DOI] [PubMed] [Google Scholar]